Abstract
Objectives
The primary purpose of this meta-analysis was to determine whether statin usage could reduce the risk of glucocorticoid-related osteonecrosis in animal models.
Methods
A systematic literature search up to May 2015 was carried out using the PubMed, Ovid, EBM reviews, ISI Web of Science, EBSCO, CBM, CNKI databases with the term and boolean operators: statins and osteonecrosis in all fields. Risk ratio (RR), as the risk estimate of specific outcome, was calculated along with 95% confidence intervals (CI). The methodological quality of individual studies was assessed using a quantitative tool based on the updated Stroke Therapy Academic Industry Roundtable (STAIR) recommendations.
Results
A total of 11 eligible studies were included according to predetermined criteria. The pooled data demonstrated that animals with statin usage, either alone or combined with other treatments, were at a decreased risk of developing glucocorticoid-related osteonecrosis (RR = 2.06, 95% confidence interval (CI) 1.71 to 2.50). Moreover, subgroup analysis revealed that compared with statins alone, statins combined with other treatments significantly decreased the risk of osteonecrosis (RR = 1.23, 95% CI 1.02 to 1.47). However, we could find no significant risk difference for different gender, or for different time points.
Conclusions
The present study suggests that statins combined with other treatments are efficient in preventing the development of glucocorticoid-related osteonecrosis in animals. These results might shed light on clinical practice when glucocorticoids are prescribed, and could be further investigated in high-quality clinical trials.
Cite this article: Z. Yang, H. Liu, D. Li, X. Xie, T. Qin, J. Ma, P. Kang. The efficacy of statins in preventing glucocorticoid-related osteonecrosis in animal models: A meta-analysis. Bone Joint Res 2016;5:393–402. DOI: 10.1302/2046-3758.59.2000500.
Article focus
-
Our study aimed to determine whether statin usage reduces the risk of glucocorticoid (GC)-related osteonecrosis (ON) in animal models.
Key messages
-
Our results suggest that statin usage combined with other treatments could reduce the risk of developing GC-related ON in animal models.
Strengths and limitations
-
This is the first meta-analysis to review the efficacy of statins in preventing GC-induced ON in animal models.
-
No distinction was made for animal models and the type or dose of statins and GCs.
Introduction
Osteonecrosis (ON) is a common, progressive and devastating disease with an insidious onset. ON presents without specific clinical symptoms and signs and most commonly affects young adults in the third and fourth decade of their life.1-3 Management options1,2 for ON vary from joint salvaging procedures to joint replacement, and are based on the stages described by the Association of Research Circulation Osseous (ARCO) classification. Conservative treatment, represented by core decompression, may be efficient in the early stages and in small lesions of osteonecrosis (stages I, II). However, concerns have been raised due to the potential for core decompression to weaken the cancellous bone within and adjacent to the necrotic zones.4 With respect to joint replacement surgeries, approximately 10 000 to 20 000 new cases of ON are diagnosed in the United States every year, and it is estimated that 5% to 12% of total hip arthroplasties are performed every year in order to treat this disease.1,2
Glucocorticoid (GC) usage is the leading cause of non-traumatic ON of the femoral head.5 ON develops in 9% to 40% of patients who receive high-dose or long-term steroid therapy.6 Despite the strong association of GC with ON, the underlying mechanisms of ON have been unclear. Several hypotheses have been introduced that explain the mechanism of GC-induced ON. The proposed pathogenesis includes lipid metabolism disturbance, apoptosis, increased oxidative stress and disturbances of the coagulation-fibrinolysis system due to steroid hormones.1-3,7,8 In particular, intraosseous hypertension, intravenous fat embolisms, and compression of vessels by progressive accumulation of marrow fat store, are commonly accepted theories.9 Based on these findings, an increasing number of studies have been initiated to explore the effects of lipid-lowering agents on preventing ON.9-20
Statins (3-hydroxymethyl-3-glutaryl-CoA (HMG-CoA) reductase inhibitors), widely used for the treatment of hyperlipidemia as well as for preventing coronary artery diseases,21,22 have been likewise an attractive candidate for prevention of GC-induced ON. Beneficial effects do not only result from lowering cholesterol level, but also from pleiotropic effects including improvement of endothelial dysfunction, antioxidant effects, reduction of platelet activity and decreased bone cell apoptosis.21-23 These various effects of statins may play an important role in the prevention of GC-induced ON. Previous research studies14-16 have shown serum lipid levels were significantly lower in the statin group than in the control group, which received steroids only.
Interestingly, Iwakirietal et al15 have shown that increased CYP3A activity owing to statins is a possible mechanism for the protective effect, given the fact that extrinsic glucocorticoids are made inactivate predominantly by hepatic CYP3A. Histological examination14-16 revealed that rabbits treated with steroids and statins maintained more physiological bone marrow fat cell size and fraction of marrow filled by fat. In vitro and in vivo studies9,18 demonstrated that statin acts on bone marrow mesenchymal stem cells and modulate their differentiation by enhancing osteogenesis through increasing expression of Cbfa1/Runx2 improving activity of the osteocalcin promoter, and inhibiting the adipogenesis through decreasing expression of the adipocyte-specific genes PPARγ2 (fat cell transcription factor) and 422aP (fat-specific). Statins are also associated with an elevated bone morphogenetic protein-2 gene expression, alkaline phosphatase activity, matrix mineralisation, and enhanced osteogenesis by the bone cells in vitro.9 Although several animal and limited clinical studies indicated that statins may have a protective role against ON a recent large-scale cohort study10 revealed that ON-free survival was similar in renal transplantation patients with and without statin exposure.
It is therefore not clear if statins could effectively prevent GC-related ON. Therefore, the primary purpose of this meta-analysis is to determine whether statin usage reduces the risk of GC-related ON in animal models.
Materials and Methods
Search strategy
An electronic search was conducted online to identify relevant studies up to May 2015, using the PubMed, Ovid MEDLINE(R) (1946 to present with daily update), all EBM reviews, ISI Web of Science, Academic Search Premier and MEDLINE in EBSCO, China Biological Medicine Database, and China National Knowledge Infrastructure databases with the following terms and boolean operators: statins and osteonecrosis in all fields. In addition, bibliographies of retrieved articles were searched by hand for further pertinent studies. Furthermore, we contacted the authors of the studies to collect raw data and complete the search strategy when possible. The two investigators independently selected potential eligible studies according to predetermined criteria (Table I). No language restrictions were imposed. Any discrepancy between them was resolved by consensus. No distinction was made for animal models and the type or dose range of statins and GCs.
Table I.
Criteria for selection of studies in meta-analysis
| Criteria for inclusion | Criteria for exclusion |
|---|---|
| Animal models induced by GCs | Animal models induced by other factors |
| The exposure of interest was the usage of statin | Studies without a control group |
| The outcome was incidence of ON | Data unable to be extracted |
| Studies providing sufficient information to calculate risk estimate and corresponding 95% confidence interval |
Data extraction
Data collection was conducted by two investigators independently and the result was checked by a third investigator. Discrepancies were settled by group discussion. Collected data included the first author’s surname; publication year; study location; study design; sample size; type or dose range of statins and GC; duration of GC and statin usage; characteristics of animal models including species, animal age, weight, gender and route of delivery in which GC and statins were administrated; the absolute number of ON cases in groups with or without statin exposure and the total number of animals with or without statin usage. When various statins or dosages were present in one study, data were analysed as a single group of those exposed to statins.
Assessment of methodological quality
Two reviewers independently assessed the methodology of the included articles with use of the updated Stroke Therapy Academic Industry Roundtable (STAIR) recommendations.24 The methodological quality of individual study was scored against the following criteria: sample size calculation; inclusion and exclusion criteria; randomisation; allocation concealment; reporting of animals excluded from analysis; blinded assessment of osteonecrosis; reporting potential conflicts of interest and study funding. Each item was allocated one point for a quantitative appraisal of overall quality of the individual studies. Each study was given a quality score out of a possible total of seven points, and the group median was calculated.
Statistical analysis
The risk ratio (RR) was calculated by two investigators using the Cochrane review manager software (Version 5.3, Cochrane Collaboration, Oxford, United Kingdom). RR was calculated along with 95% confidence intervals (CI). Assessment of heterogeneity of included studies was conducted using Q and I2 statistics. Heterogeneity was not considered present with a p-value ≥ 0.1. Subgroup analyses were performed where applicable. Publication bias was accessed by funnel plots, using Egger’s tests.25 Significance was considered when a p-value was < 0.05.
Results
Search results and study characteristics
According to the aforementioned specific criteria, 11 eligible studies,13-18,23,26-29 published between 1997 and 2014 were included in our meta-analysis. Figure 1 shows the detailed process of study selection. In total, we included 554 animals consisting of 391 rabbits, 128 rats and 35 chickens. Animal model ON was induced using steroid alone in all studies except for one,23 which combined steroid with endotoxin. The study conducted by Nozaki et al13 consisted of rats with a 50% incidence of spontaneous ON of the femoral head at the age of 16 to 18 weeks, while the remaining studies consisted of healthy animals. Characteristics of the included studies are summarised in Tables II to VII. The median of the reported quality score was 3 (2 to 5).
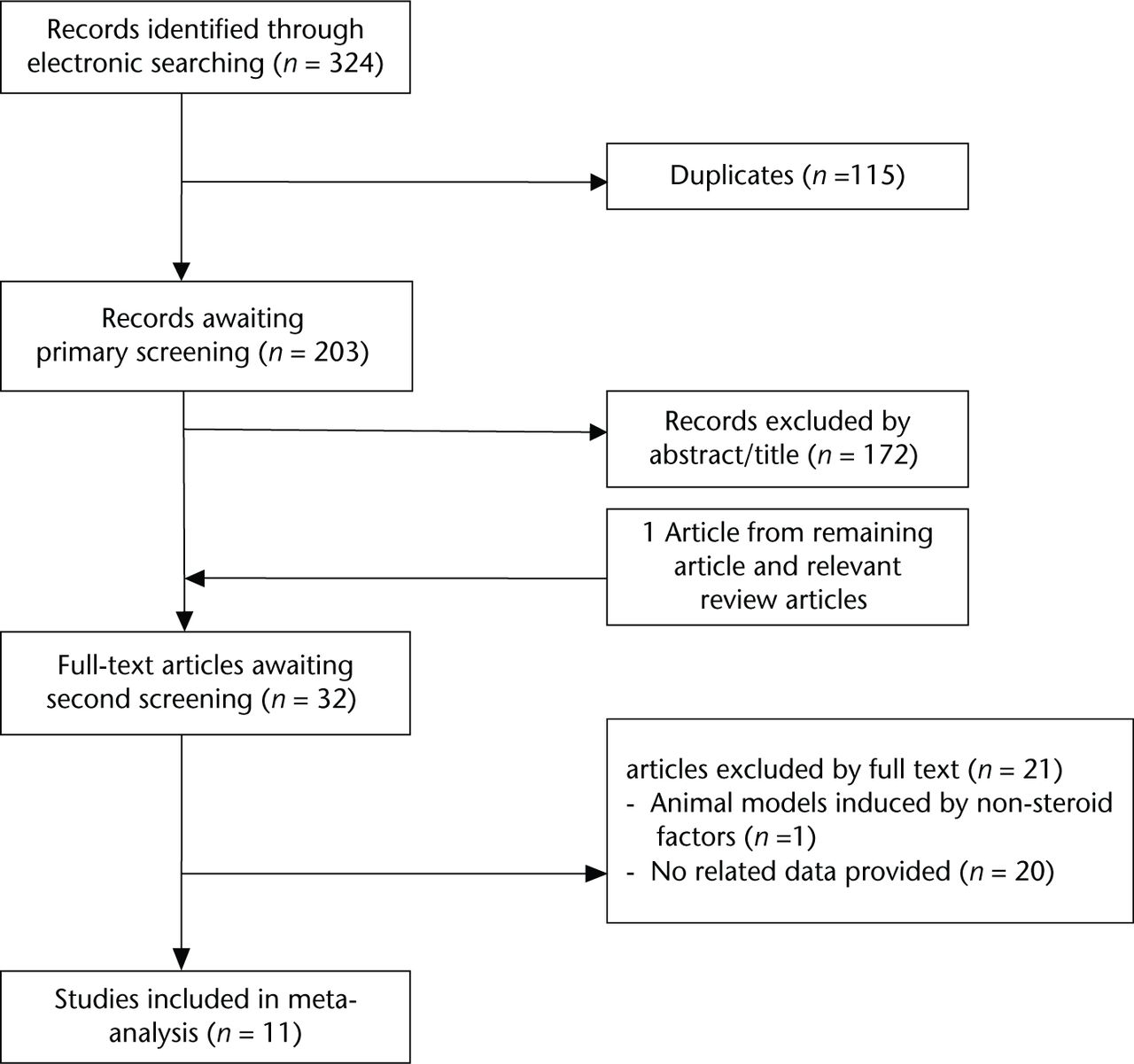
Fig. 1
Flow diagram showing the process of article selection for meta-analysis.
Table II.
Basic characteristics of included studies
| Study ID | Study location | Species | n (C) | n (E) | Gender | Age (wks) | Weight |
|---|---|---|---|---|---|---|---|
| Li et al 201423 | China | Rabbit | 15 | 15 | Female | 24 to 28 | 2.5 kg to 3.1 kg |
| Xie et al 201328 | China | Rat | 30 | 60 | Mixed | 8 | 250 g to 280 g |
| Nozaki et al 201213 | Japan | Rat | 23 | 15 | Male | 13 | NR |
| Li et al 201127 | China | Rabbit | 16 | 16 | Female | 28 to 32 | 2.8 kg to 3.4 kg |
| Kang et al 201014 | China | Rabbit | 24 | 52 | Male | 28 to 32 | 2.8 kg to 3.4 kg |
| Zeng et al 200926 | China | Rabbit | 9 | 20 | Mixed | Adult | 2.5 kg to 3.0 kg |
| Iwakiri et al 200815 | Japan | Rabbit | 30 | 30 | Female | 28 to 32 | 3.1 kg to 4.2 kg |
| Pengde et al 200816 | China | Rabbit | 26 | 25 | Male | 28 to32 | 2.8 kg to 3.4 kg |
| Nishida et al 200817 | Japan | Rabbit | 30 | 35 | Male | 28 to 32 | NR |
| Kang et al 200729 | China | Rabbit | 16 | 32 | Mixed | 28 to 32 | 2.7 kg to 3.3 kg |
| Cui et al 199718 | United States | Chicken | 25 | 10 | Female | Adult | NR |
-
n (C), number of animals in control group; n (E), number of animals in experimental group; NR, not reported
Table III.
Characteristics of animal models of steroid-induced osteonecrosis
| Study ID | Animal models |
||
|---|---|---|---|
| Steroids exposure | Dose (mg/kg) | Route of delivery | |
| Li et al 201423 | MPSL (+ET) | 20 (10 μg), once | i.m. (left gluteus) |
| Xie et al 201328 | MPSL | 20, daily, 4 wks | i.p. |
| Nozaki et al 201213 | MPSL | 4 mg in total | s.c. (back) |
| Li et al 201127 | MPSL | 20, once | i.m. (right gluteus) |
| Kang et al 201014 | MPSL | 20, once | i.m. (right gluteus) |
| Zeng et al 200926 | DSP | 2.5, NR | i.m. |
| Iwakiri et al 200815 | MPSL | 20, once | i.m. (right gluteus) |
| Pengde et al 200816 | MPSL | 20, once | i.m. (right gluteus) |
| Nishida et al 200817 | MPSL | 20, once | i.m. (right gluteus) |
| Kang et al 200729 | MPSL | 20, once | i.m. (right gluteus) |
| Cui et al 199718 | MPSL | 3, weekly, 12 wks | i.m. |
-
MPSL, methylprednisolone; DSP, dexamethasone sodium phosphate; ET, endotoxin; i.m., intramuscular; i.v., intravenous; i.p., intraperitoneal; s.c., subcutaneous injection; NR, not reported
Table IV.
Characteristics of statin intervention of included studies
| Study ID | Types | Statins administration |
|||
|---|---|---|---|---|---|
| Dose(mg/kg.d,) | Duration (wks) | Route of delivery | Treatment point before steroid | ||
| Li et al 201423 | atorvastatin | 2.5 | 8 | Food admixture | 0 wk |
| Xie et al 201328 | lovastatin | 25 | 14 | Gavage | 2 wks |
| Nozaki et al 201213 | pravastatin | 15 | 4 | Drinking water | 2 wks |
| Li et al 201127 | pravastatin | 2.5 | 12 | Food admixture | 0 wk |
| Kang et al 201014 | lovastatin | 5 | 14 | Food admixture | 2 wks |
| Zeng et al 200926 | simvastatin | 20mg, daily | NR | PO | 0 wk |
| Iwakiri et al 200815 | pravastatin/simvastatin | 2/5 | 6/6 | IV/IV | 3 wks/3 wks |
| Pengde et al 200816 | lovastatin | 5 | 14 | Food admixture | 2 wks |
| Nishida et al 200817 | pravastatin | 0.7 | 4 | IV | 2 wks |
| Kang et al 200729 | lovastatin | 300 | 14 | Food admixture | 2 wks |
| Cui et al 199718 | lovastatin | 20 mg, daily | NR | PO | 0 wk |
-
PO, peros; IV, intravenous; NR, not reported
Table V.
The methodological quality of individual study
| Study ID | (1) | (2) | (3) | (4) | (5) | (6) | (7) | Score |
|---|---|---|---|---|---|---|---|---|
|
Li et al 201423 |
* | * | * | 3 | ||||
| Xie et al 201328 | * | * | * | 3 | ||||
| Nozaki et al 201213 | * | * | * | * | * | 5 | ||
| Li et al 201127 | * | * | 2 | |||||
| Kang et al 201014 | * | * | * | 3 | ||||
| Zeng et al 200926 | * | * | 2 | |||||
| Iwakiri et al 200815 | * | * | * | * | 4 | |||
| Pengde et al 200816 | * | * | * | 3 | ||||
| Nishida et al 200817 | * | * | * | 3 | ||||
| Kang et al 200729 | * | * | 2 | |||||
| Cui et al 199718 | * | * | 2 |
-
*
One score
-
Studies fulfilling the criteria of (1) sample size calculation; (2) inclusion and exclusion criteria; (3) randomisation; (4) allocation concealment; (5) reporting of animals excluded from analysis; (6) blinded assessment of ON; (7) reporting potential conflicts of interest and study funding
Table VI.
Evaluation of osteonecrosis of included studies
| Study ID | Diagnosis of osteonecrosis |
||
|---|---|---|---|
| Unilateral or bilateral | Sample from | Methods | |
| Li et al 201423 | Bilateral | Femoral head | Radiological examination |
| Xie et al 201328 | NR | Femoral head | Histological examination |
| Nozaki et al 201213 | Bilateral | Proximal femur | Histological examination |
| Li et al 201127 | Unilateral | Femoral head | Histological examination |
| Kang et al 201014 | Bilateral | Femur and humerus* | Histological examination |
| Zeng et al 200926 | Bilateral | Femoral heads | Radiologicalexamination |
| Iwakiri et al 200815 | Bilateral | Proximal femur | Histological examination |
| Pengde et al 200816 | Bilateral | Femur and humerus* | Histological examination |
| Nishida et al 200817 | Bilateral | Femur and humerus* | Histological examination |
| Kang et al 200729 | Bilateral | Proximal femur | Histological examination |
| Cui et al 199718 | Bilateral | Femoral head | Histological examination |
-
*
The whole area of the proximal one third and distal condyles of both the femur and the humerus
-
NR, not reported
Table VII.
Outcomes of each included study
| Study ID | Animals treated with statin and steroid |
Animals treated with steroidalone |
||
|---|---|---|---|---|
| Osteonecrosis | Total | Osteonecrosis | Total | |
| Li et al 201423 | 4 | 15 | 7 | 15 |
| Xie et al 201328 | 19 | 60 | 17 | 30 |
| Nozaki et al 201213 | 11 | 15 | 23 | 23 |
| Li et al 201127 | 5 | 16 | 10 | 16 |
| Kang et al 201014 | 13 | 52 | 16 | 24 |
| Zeng et al 200926 | 1 | 20 | 3 | 9 |
| Iwakiri et al 200815 | 9 | 30 | 25 | 30 |
| Pengde et al 200816 | 9 | 25 | 18 | 26 |
| Nishida et al 200817 | 13 | 35 | 21 | 30 |
| Kang et al 200729 | 8 | 32 | 11 | 16 |
| Cui et al 199718 | 0 | 10 | 14 | 25 |
Primary analysis
The overall estimate of the effect of statins was 2.06 (95% CI 1.71 to 2.50, p < 0.001), an approximately two-fold improvement in outcome (Fig. 2). Compared with the control group, the RR was 1.91 (95% CI 1.50 to 2.42) in studies using statins alone while the RR was 2.10 (95% CI 1.68 to 2.62) in studies using statins with other interventions such as bisphosphonate or anticoagulant. Then we further compared the effect of statin exposure alone to that of statins combined with other interventions using data from experiments containing both groups. Results revealed that combined usage significantly decreased the risk of ON (RR = 1.23, 95% CI, 1.02 to 1.47).
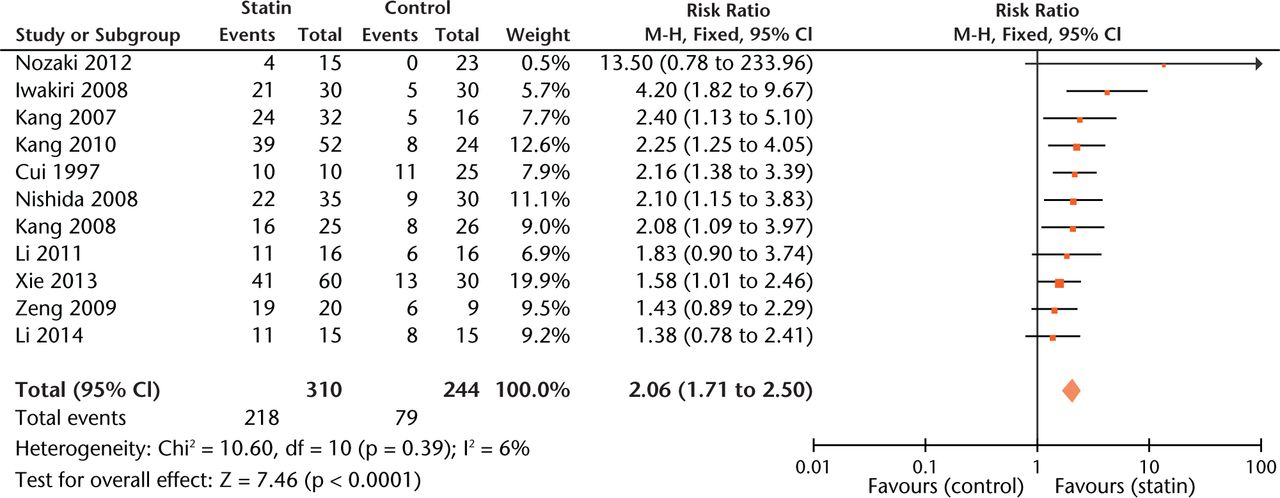
Fig. 2
Forest plot showing the overall risk estimate of osteonecrosis between groups with or without statins (M-H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom). Risk ratio (RR) on the left axis indicates statin usage decreases the risk of osteonecrosis compared with the control group, whereas RR greater than 1 indicates animals with statin usage are at increased risk of osteonecrosis. The 95% CI reveals that the result is statistically significant when “1” is not included in the interval.
Experiments with a relatively low score (< 3 points) gave more conservative estimates of effect size than those with a high score (≥ 3 points) (Fig. 3).
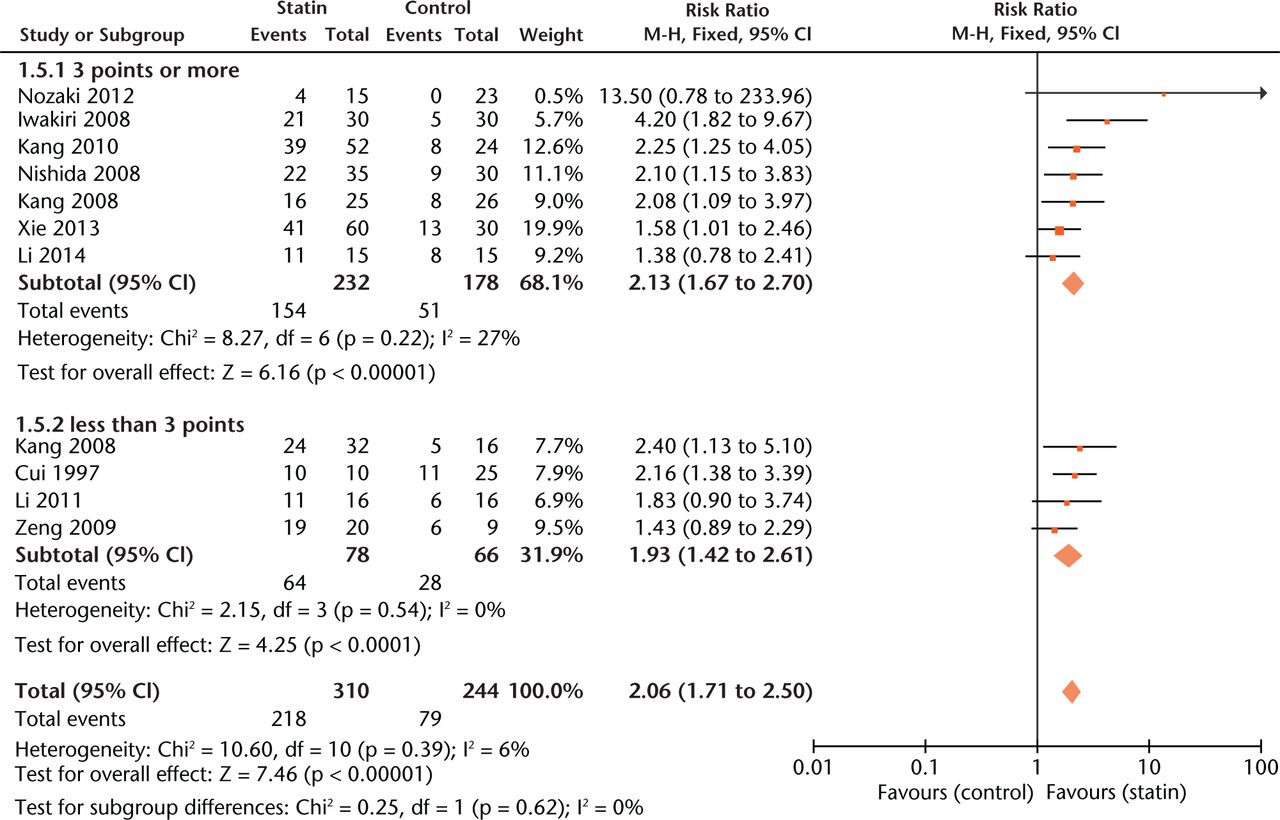
Fig. 3
Forest plot showing subgroup analysis based on study quality score (M-H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom). Risk ratio (RR) on the left axis indicates statin usage decreases the risk of osteonecrosis compared with the control group, whereas RR greater than 1 indicates animals with statin usage are at increased risk of osteonecrosis. The 95% CI reveals that the result is statistically significant when “1” is not included in the interval, and vice versa.
Significant protection by statins against ON was seen for all included species (Fig. 4) but not for gender (RR = 2.24, 1.62 to 3.09 versus RR = 2.25, 1.58 to 3.20, p = 0.98).
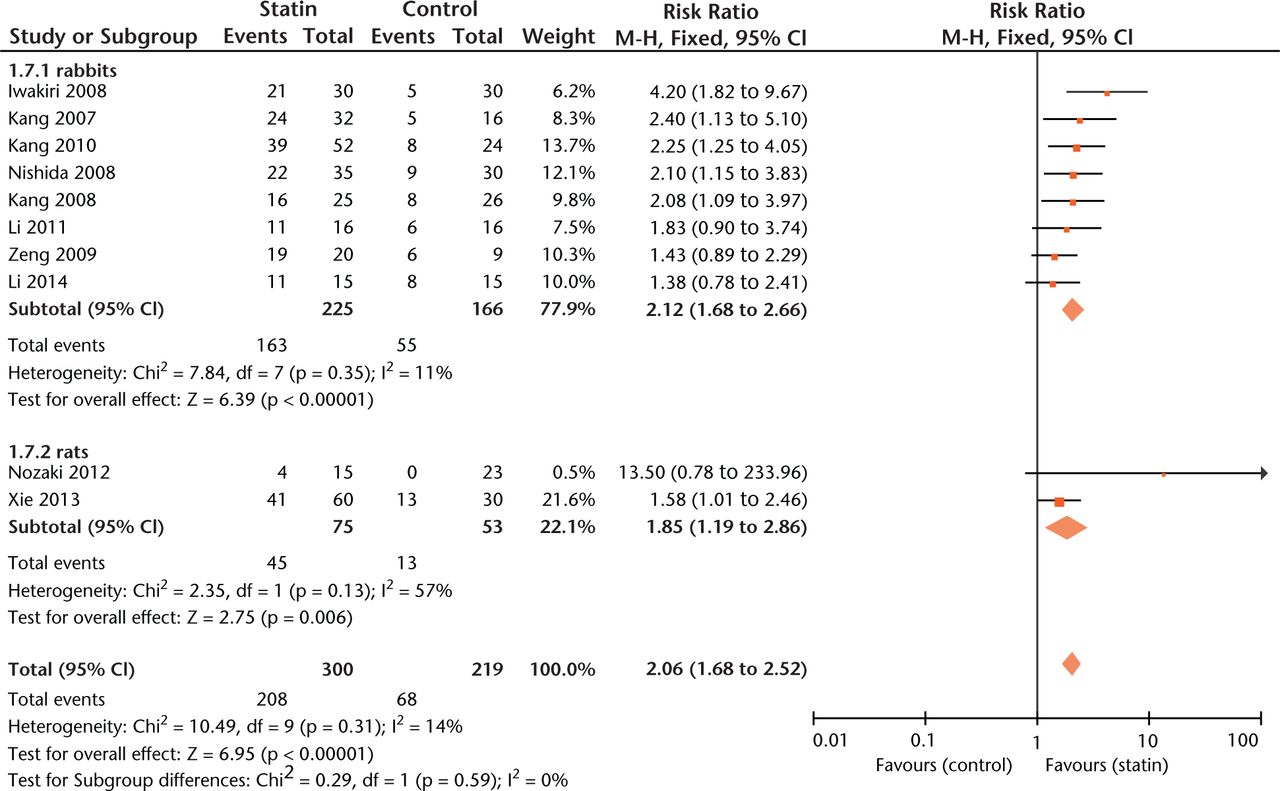
Fig. 4
Forest plot showing subgroup analysis based on species (M-H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom). Risk ratio (RR) on the left axis indicates statin usage decreases the risk of osteonecrosis compared with the control group, whereas RR greater than 1 indicates animals with statin usage are at increased risk of osteonecrosis. The 95% CI reveals that the result is statistically significant when “1” is not included in the interval, and vice versa.
Effect sizes were similar between studies where statin exposure was started at two weeks before methylprednisolone injection and simultaneously with methylprednisolone administration (Fig. 5). As to outcome measurement, experiments using radiographic examination of ON gave more conservative estimates of effect size (RR = 1.40, 0.97 to 2.02, Fig. 6) than those using histological examination (RR = 2.20, 1.77 to 2.75, Fig.6).
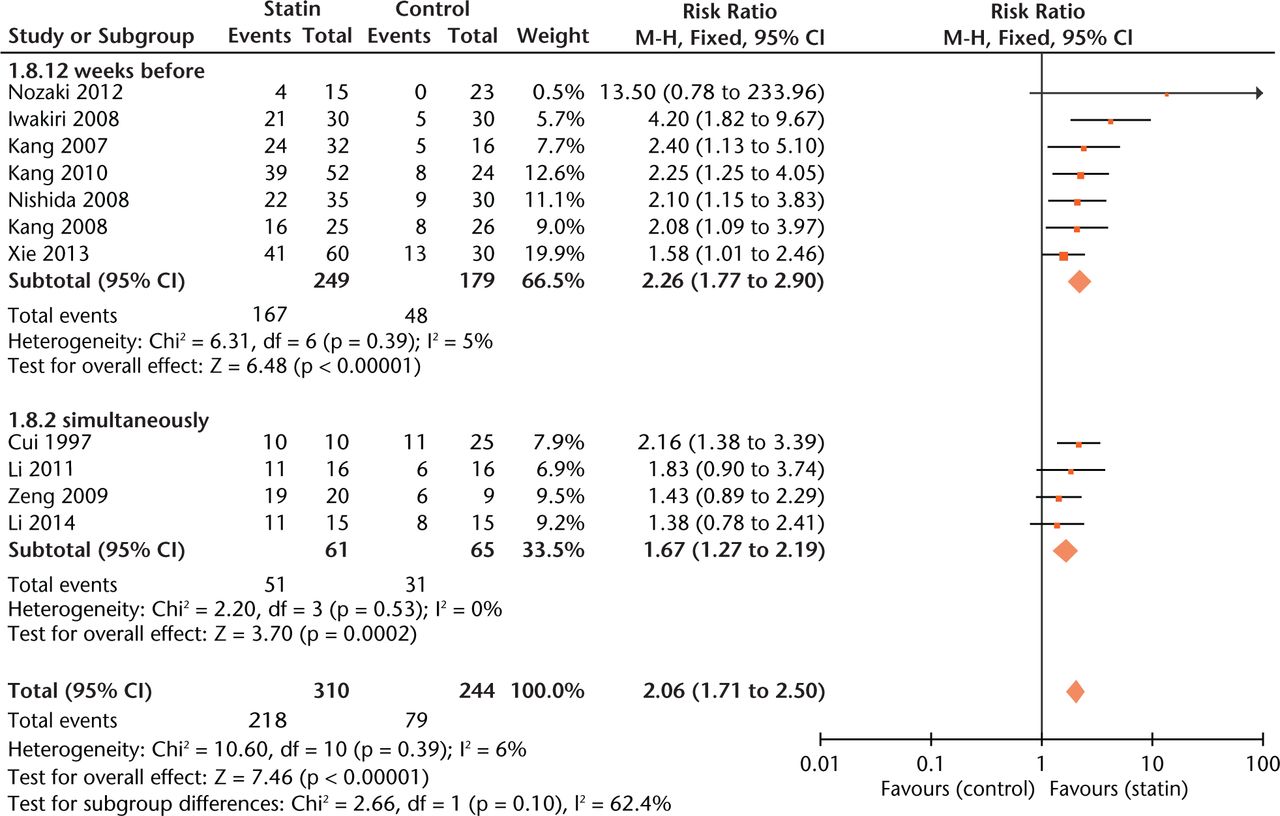
Fig. 5
Forest plot showing subgroup analysis based on treatment time point (M-H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom). Risk ratio (RR) on the right axis indicates statin usage decreases the risk of osteonecrosis compared with the control group, whereas RR greater than 1 indicates animals with statin usage are at increased risk of osteonecrosis. The 95% CI reveals that the result is statistically significant when “1” is not included in the interval, and vice versa.
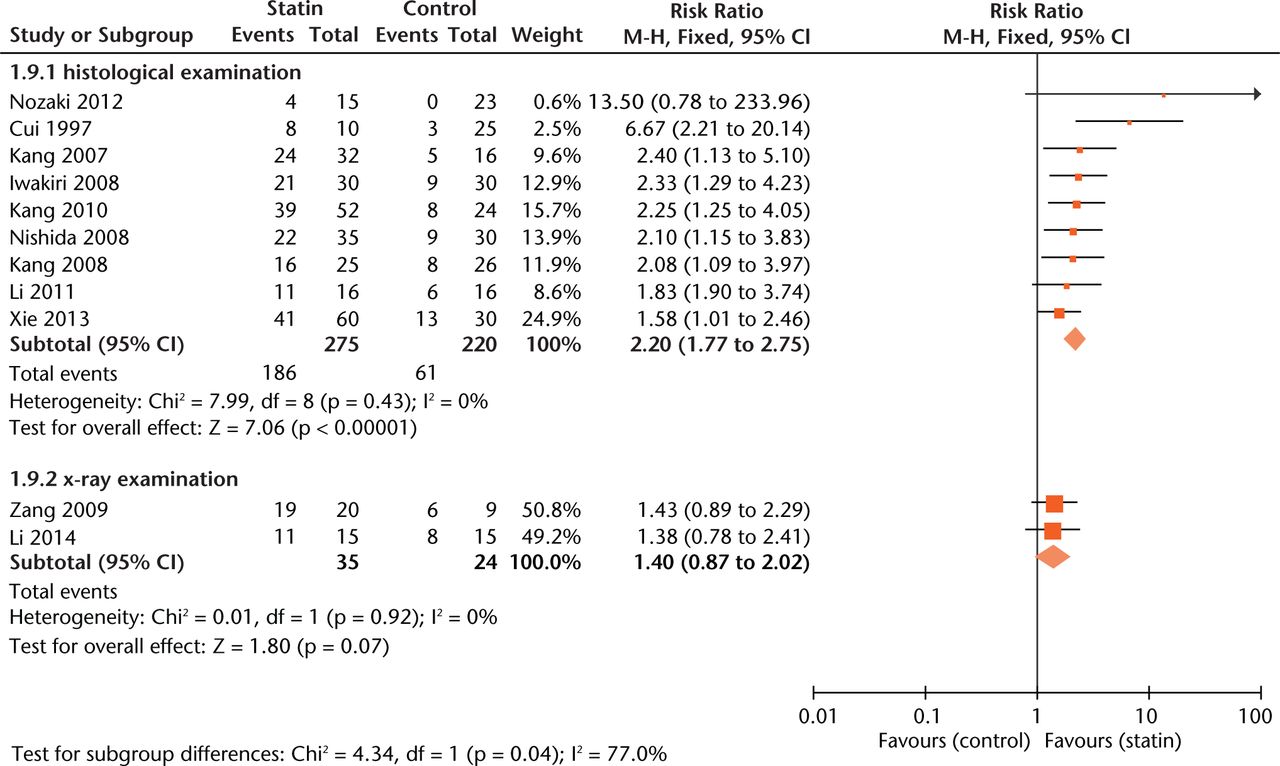
Fig. 6
Forest plot showing subgroup analysis based on measurement (M-H, Mantel–Haenszel; CI, confidence interval; df, degrees of freedom). Risk ratio (RR) on the left axis indicates statin usage decreases the risk of osteonecrosis compared with the control group, whereas RR greater than 1 indicates animals with statin usage are at increased risk of osteonecrosis. The 95% CI reveals that the result is statistically significant when “1” is not included in the interval, and vice versa.
A funnel plot (Fig. 7) analysis showed no evident publication bias towards positive studies, further confirmed by Egger’s regression asymmetry test (p > 0.05).
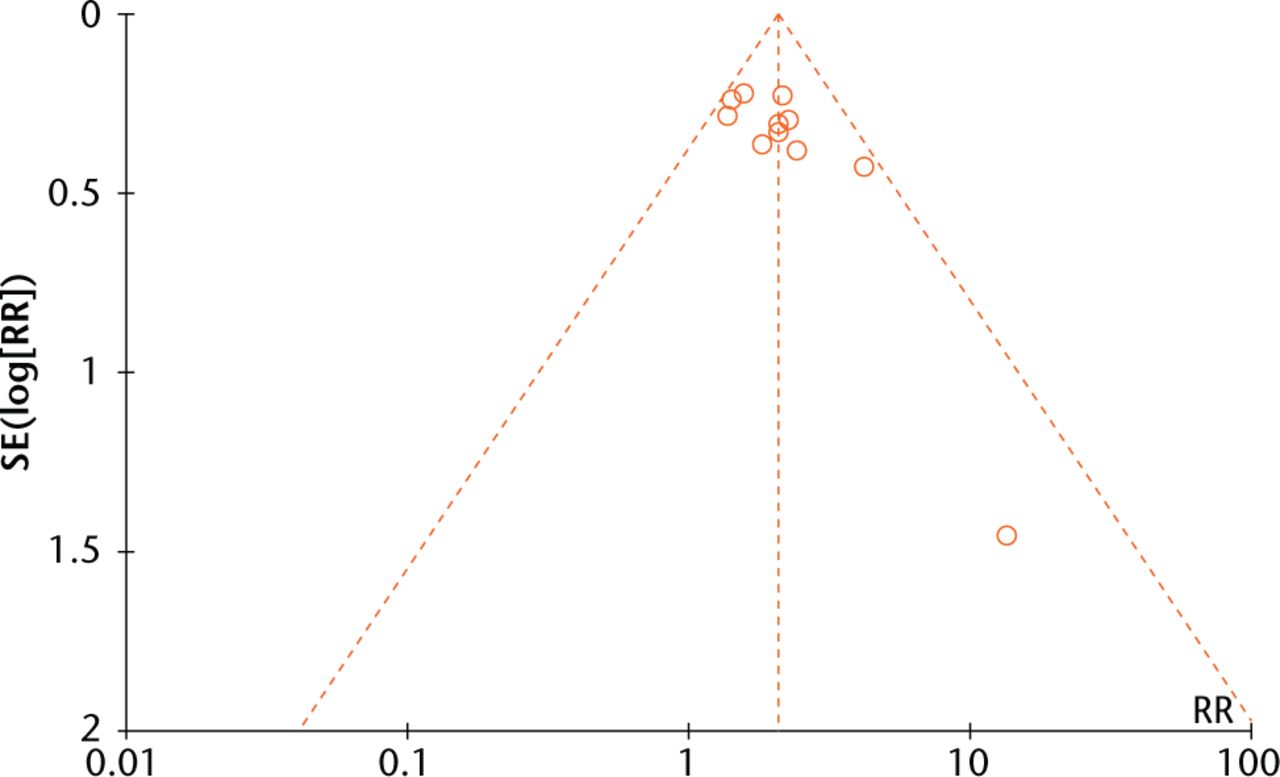
Fig. 7
Funnel plots evaluating publication bias among included studies.
Discussion
This is the first meta-analysis to review the efficacy of statins in preventing GC induced ON in animal models. Our study indicated that animals with statin usage, either alone or combined with other treatment, were at decreased risk of developing GC-related ON. Moreover, subgroup analysis revealed that compared with statin alone, statin usage combined with other treatments significantly decreased the risk of ON (RR = 1.23, 95% CI 1.02 to 1.47). However, we could find no significant risk difference for different gender or for different treatment points (two weeks before or simultaneously with GC injection).
The overall risk estimate of statins in the present study supports the findings of previous work conducted by Pritchett11 who found that only three patients (3/284, 1%) treated with statins developed ON, much less than the 3% to 20% incidence usually reported for patients receiving high-dose steroids, However, our findings do not correlate with those of the cohort study by Ajmal et al,10 in which 2881 renal transplantation patients were evaluated and 4.4% (15/338) of patients on statins developed ON compared with 7% (180/2543) of patients who were not on statins. The authors concluded that statin usage does not appear to lower the risk of ON. There are several possible explanations for the discrepancy in the results. Firstly, the dosage of GC administrated to patients following renal transplantation was uncertain at baseline. Higher doses, even of short duration, present greater risks.2 Secondly, the indication for statin treatment was confined to patients with hypercholesterolaemia. And thirdly, the measure of ON was not reported and it is also unknown whether the measurement was taken identically for each patient.
In a randomised double-blinded placebo-controlled study, Lydonet, Schweitzer and Belmont30 also found that the incidence of newly developed ON in patients with 40 mg daily of atorvastatin was not statistically different to that found in those administered hydroxychloroquine or bisphosphonates. However, it must be noted that the patients in the control group were administrated bisphosphonates, which may reduce the risk of developing ON to some degree.4,26,28
Our results also revealed that statin usage in combination with bisphosphonate or anticoagulant significantly decreased the risk of ON, compared with statins alone, suggesting a protective role for bisphosphonates or anticoagulants against developing GC-induced ON. These findings have been supported elsewhere in the literature.14,20,28,29,31
In this current study, we could find no significant difference in risk between animal gender. This differs from the published studies conducted by Ajmal et al,10 who found that male gender is associated with an increased risk (34%) of ON compared with female patients. The reason for this finding is not clear but it may be related to different mechanisms of fat metabolisms between genders.32,33
With regard to animal species, risk estimate was lower in rabbits than in rats. To our knowledge, this has not been reported previously. A possible explanation for this might be that animal models used in Nozaki et al’s study13 consist of rats with a 50% incidence of spontaneous ON of the femoral head, rather than rats in healthy condition.
Both of the intervention procotols (statin exposure started at two weeks before versus simultaneously with methylprednisolone injection) demonstrated protective action against development of ON, but there was no significant difference in effect size between the two groups. To the best of our knowledge, this comparison has not been reported previously. A possible explanation for this finding might be that different statin dosage and species were used across studies. It also may be due to a possible threshold effect for development of ON.14,29
As for assessment of ON, it was more conservatively estimated with the use of radiographic examination than with the use of histological examination. Our findings are consistent with those of Kang et al34 who found no significant changes of ON examined using radiographs during two two to 12-week periods. Accordingly, the difference in risk estimate between various measurements could be explained by the lower detection rate of radiographic examination. Given the small sample size of animals examined using radiographs, however, the results should be considered with caution.
Several limitations must be considered in interpreting our findings. First, the time point at which the outcome was measured varied from study to study. However, ON was evaluated in all included studies at a time point more than two weeks after steroid injection, since two weeks is a time point that has been reported to be crucial in the development of ON.7,16 Secondly, the sites measured for diagnosis of ON were not controlled for all included studies. The more sites per animal evaluated, the greater risk for the detection of ON. Thirdly, effect size might be weakened in animals with comorbidities Finally, the preventive effects of different statin dosage, and various types of statins on the development of ON, were not analysed.
In conclusion, the present study revealed that statin usage could reduce the risk of GC-related ON in animal models, suggesting statins are efficacious in preventing GC-induced ON to some degree in animals. However, while the overall results of this meta-analysis suggest that statins have substantial effect size, concerns should be raised that the true efficacy of statins might be substantially lower than reported here because of the possible influence of publication or other bias. Furthermore, given the limited application of experimental studies to humans, the heterogeneity among studies and lack of high quality evidence, the results should be extrapolated to the clinical setting with great caution.
Supplementary material
A table showing the search strategy is available alongside this article at www.bjr.boneandjoint.org.uk
Funding Statement
This work was supported by the China Health Ministry Program (Grants 201302007), the National Natural Science Fund of China (Grants 81271976/H0605 and 81171763) and the Sichuan Province Science and Technology Support Program (Grant 2013JY0151).
ICMJE conflict of interest
None declared
References
1 Malizos KN , KarantanasAH, VaritimidisSE, et al.. Osteonecrosis of the femoral head: etiology, imaging and treatment. Eur J Radiol2007;63:16-28.CrossrefPubMed Google Scholar
2 Parsons SJ , SteeleN. Osteonecrosis of the femoral head: Part 1—Aetiology, pathogenesis, investigation, classification. Curr Orthop2007;21:457-463. Google Scholar
3 Assouline-Dayan Y , ChangC, GreenspanA, ShoenfeldY, GershwinME. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum2002;32:94-124.PubMed Google Scholar
4 Parsons SJ , SteeleN. Osteonecrosis of the femoral head: Part 2 Options for treatment. Curr Orthop2008;22:349-358. Google Scholar
5 Fukushima W , FujiokaM, KuboT, et al.. Nationwide epidemiologic survey of idiopathic osteonecrosis of the femoral head. Clin Orthop Relat Res2010;468:2715-2724.CrossrefPubMed Google Scholar
6 Weinstein RS . Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med2011;365:62-70.CrossrefPubMed Google Scholar
7 Miyanishi K , YamamotoT, IrisaT, et al.. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone2002;30:185-190.CrossrefPubMed Google Scholar
8 Ichiseki T , MatsumotoT, NishinoM, KaneujiA, KatsudaS. Oxidative stress and vascular permeability in steroid-induced osteonecrosis model. J Orthop Sci2004;9:509-515.CrossrefPubMed Google Scholar
9 Jiang Y , ZhangY, ZhangH, et al.. Pravastatin prevents steroid-induced osteonecrosis in rats by suppressing PPARγ expression and activating Wntsignaling pathway. Exp Biol Med (Maywood2014;239:347-355. Google Scholar
10 Ajmal M , MatasAJ, KuskowskiM, ChengEY. Does statin usage reduce the risk of corticosteroid-related osteonecrosis in renal transplant population?Orthop Clin North Am2009;40:235-239.CrossrefPubMed Google Scholar
11 Pritchett JW . Statin therapy decreases the risk of osteonecrosis in patients receiving steroids. Clin Orthop Relat Res2001;386:173-178.CrossrefPubMed Google Scholar
12 Belmont HM , LydonE. Avascular necrosis prevention with lipitor in lupus erythematosus. Lupus2005;14:869-870.CrossrefPubMed Google Scholar
13 Nozaki Y , KumagaiK, MiyataN, NiwaM. Pravastatin reduces steroid-induced osteonecrosis of the femoral head in SHRSP rats. Acta Orthop2012;83:87-92.CrossrefPubMed Google Scholar
14 Kang P , GaoH, PeiF, et al.. Effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits. Int J Exp Pathol2010;91:235-243.CrossrefPubMed Google Scholar
15 Iwakiri K , OdaY, KaneshiroY, et al.. Effect of simvastatin on steroid-induced osteonecrosis evidenced by the serum lipid level and hepatic cytochrome P4503A in a rabbit model. J Orthop Sci2008;13:463-468.CrossrefPubMed Google Scholar
16 Pengde K , FuxingP, BinS, JingY, JingqiuC. Lovastatin inhibits adipogenesis and prevents osteonecrosis in steroid-treated rabbits. Joint Bone Spine2008;75:696-701.CrossrefPubMed Google Scholar
17 Nishida K , YamamotoT, MotomuraG, JingushiS, IwamotoY. Pitavastatin may reduce risk of steroid-induced osteonecrosis in rabbits: a preliminary histological study. Clin Orthop Relat Res2008;466:1054-1058.CrossrefPubMed Google Scholar
18 Cui Q , WangGJ, SuCC, BalianG. The Otto Aufranc Award. Lovastatin prevents steroid induced adipogenesis and osteonecrosis. Clin Orthop Relat Res1997;344:8-19.PubMed Google Scholar
19 Wang GJ , RawlesJG, HubbardSL, StampWG. Steroid-induced femoral head pressure changes and their response to lipid-clearing agents. Clin Orthop Relat Res1983;174:298-302.PubMed Google Scholar
20 Motomura G , YamamotoT, MiyanishiK, JingushiS, IwamotoY. Combined effects of an anticoagulant and a lipid-lowering agent on the prevention of steroid-induced osteonecrosis in rabbits. Arthritis Rheum2004;50:3387-3391.CrossrefPubMed Google Scholar
21 Jasińska M , OwczarekJ, Orszulak-MichalakD. Statins: a new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol Rep2007;59:483-499.PubMed Google Scholar
22 Vaughan CJ , MurphyMB, BuckleyBM. Statins do more than just lower cholesterol. Lancet1996;348:1079-1082.CrossrefPubMed Google Scholar
23 Li TS , XiaoZM, HuangQP, et al.. Impact of lipid lowering and anticoagulant combined application on the femoral bone cell apoptosis in the rabbits with steroid-induced necrosis. Journal of China Medical University2014;43:441-445. Google Scholar
24 Fisher M , FeuersteinG, HowellsDW, et al.. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke2009;40:2244-2250.CrossrefPubMed Google Scholar
25 Egger M , Davey SmithG, SchneiderM, MinderC. Bias in meta-analysis detected by a simple, graphical test. BMJ1997;315:629-634.PubMed Google Scholar
26 Zeng XJ , YangSH, YuZH. Preventive effects of Simvastatin combined alendronate on steroid-induced osteonecrosis of the femoral head in rabbits. J YMC2009;28:237-239. Google Scholar
27 Li TS , XiaoZM, ZhanXL. Effects of pravastatin combined with warfarin on steroid-induced femoral head necrosis in rabbits. Chinese Journal of Pathophysiology2011;27:1603-1608. Google Scholar
28 Xie X , KangP, PeiF, et al.. Effect of alendronate and lovastatin in preventing early glucocorticoids-induced osteonecrosis of femoral head in rats by micro-CT. Orthopedic Journal of China2013;21:82-86. Google Scholar
29 Kang P , ShenB, YangJ, et al.. Prevention of steroids-induced osteonecrosis of the femoral head with anticoagulant and lipid-lowering management in rabbits. Chin J Joint Surg2007;30:56-61. Google Scholar
30 Lydon E , SchweitzerM, BelmontHM. Atorvostatin to prevent avascular necrosis of bone in systemic lupus erythematosus. Arthritis Rheum2006;54:S432. Google Scholar
31 Yamamoto T , MiyanishiK, MotomuraG, et al.. Animal models for steroid-induced osteonecrosis. Clin Calcium2007;17:879-886. Google Scholar
32 Mittendorfer B . Sexual dimorphism in human lipid metabolism. J Nutr2005;135:681-686.CrossrefPubMed Google Scholar
33 Lima JJ , MaurasN, KissoonN, et al.. Influence of sex and beta2 adrenergic receptor haplotype on resting and terbutaline-stimulated whole body lipolysis. Metabolism2005;54:492-499.CrossrefPubMed Google Scholar
34 Kang P , YangJ, ShenB, et al.. An experimental dynamic study on the relationship between histopathological and radiological changes in steroid-induced femoral head osteonecrosis of rabbits. Chin J Orthop2010;30:92-97. Google Scholar










