Abstract
Aims
Fixation of osteoporotic proximal humerus fractures remains challenging even with state-of-the-art locking plates. Despite the demonstrated biomechanical benefit of screw tip augmentation with bone cement, the clinical findings have remained unclear, potentially as the optimal augmentation combinations are unknown. The aim of this study was to systematically evaluate the biomechanical benefits of the augmentation options in a humeral locking plate using finite element analysis (FEA).
Methods
A total of 64 cement augmentation configurations were analyzed using six screws of a locking plate to virtually fix unstable three-part fractures in 24 low-density proximal humerus models under three physiological loading cases (4,608 simulations). The biomechanical benefit of augmentation was evaluated through an established FEA methodology using the average peri-screw bone strain as a validated predictor of cyclic cut-out failure.
Results
The biomechanical benefit was already significant with a single cemented screw and increased with the number of augmented screws, but the configuration was highly influential. The best two-screw (mean 23%, SD 3% reduction) and the worst four-screw (mean 22%, SD 5%) combinations performed similarly. The largest benefits were achieved with augmenting screws purchasing into the calcar and having posteriorly located tips. Local bone mineral density was not directly related to the improvement.
Conclusion
The number and configuration of cemented screws strongly determined how augmentation can alleviate the predicted risk of cut-out failure. Screws purchasing in the calcar and posterior humeral head regions may be prioritized. Although requiring clinical corroborations, these findings may explain the controversial results of previous clinical studies not controlling the choices of screw augmentation.
Article focus
-
Biomechanical investigation of the effects from cement augmentation of the screws on the predicted cyclic cut-out failure when fixing unstable multi-fragmented low-density proximal humerus fractures using a locking plate.
-
Evaluation of the optimal augmentation combinations providing the largest biomechanical benefit using validated finite element simulations.
Key messages
-
The configuration of screw augmentation strongly determines the biomechanical benefit.
-
Cement augmentation of the calcar screws and screws with posteriorly located tips is generally the most biochemically advantageous.
-
Future clinical studies should more carefully select and report the augmented screws.
Strengths and limitations
-
Validated finite element analyses allowed us to investigate a large number (> 4,600) of configurations that would have otherwise remained unfeasible experimentally or clinically.
-
A locking plate with one screw configuration was investigated for a single fracture type.
-
The results require clinical corroboration.
Introduction
The incidence of fragility fractures increases with the ageing population and the growing prevalence of osteoporosis. Therefore, it is critical to provide new solutions and procedures to manage these challenging injuries.1 Treatment of fragility fractures at the proximal humerus can be complicated by several factors, such as highly compromised bone mass and quality, complex loading conditions, multi-fragmental fractures, absent interfragmentary support, and limited surgical access. The introduction of locking plates for the management of proximal humerus fractures has improved the surgical outcome in patients with poor bone quality.2-4 Nevertheless, implant-related mechanical complications and failures, such as screw cut-out, intra-articular screw perforation, or malunion, still occur in up to 36% of patients.3-7 Moreover, some studies found no difference between surgical and conservative treatment in terms of the patient-reported outcomes.8
Various approaches have been introduced to improve the biomechanical performance of locking plate fixation for osteoporotic proximal humerus fractures.9-11 One of the available options is applying calcium phosphate or poly(methyl methacrylate) (PMMA) bone cement at the tip of cannulated locking screws. Numerous in vitro biomechanical studies have demonstrated enhanced primary stability of proximal humerus plating with cement-augmented screws.9,12 However, the clinical findings have been controversial regarding the benefit of bone cement augmentation with the proximal humerus internal locking system (PHILOS; DePuy Synthes, Zuchwil, Switzerland).13-15 These variable results may be related to heterogenicity of augmentation techniques and the screw configurations used. For example, the number of augmented screws was not standardized in those studies and ranged between two and five screws within each study.13-15 The selection of augmented screws was specified by Siebenbürger et al15 to include the most proximal row of screws and one of the two most distal rows of humeral head screws, however the augmentation patterns were not described for the other studies and may have introduced additional variability.
Augmenting all available screws would maximize stability. However, if possible, it would be advantageous to minimize the number of augmented screws and the associated cement volumes by selecting the screws that maximize primary fixation. Augmenting fewer screws could minimize additional trauma to the tissues and surgery time and may help to decrease potential risks such as cement extrusion into the fracture site or joint. The surgical technique of the PHILOS plate suggests augmenting screws from levels A (being the most proximal screw row) and E (the most distal row) to ensure a wide distribution of cement clouds in the humeral head.16 Thus far, studies have only evaluated a limited selection of the numerous possible configurations for screw augmentation. These preliminary studies concluded that augmentation should be performed in the two screws within the regions of lowest bone stock17 or the four most proximal screws.18–20
With six screws in the humeral head, there are 64 possible configurations for cement augmentation. Considering this wide array of options, an optimal augmentation strategy would be nearly impossible to derive through in vitro (cadaver) or clinical investigation. Finite element analysis (FEA) is a powerful numerical tool that enables a simulated biomechanical evaluation of a patient-specific anatomy under various physiological conditions. FEA can be used to efficiently complement or even partially replace biomechanical testing of implant fixations,21 joint restorations,22,23 and also to evaluate new fixation concepts for proximal humerus fractures.24 An automated computational test kit has been recently developed25 and validated26 for biomechanical evaluation of proximal humerus fracture fixation using locking plates. This tool allows systematic and efficient analysis under a variety of conditions such as fracture patterns, implant configuration, and loading conditions in multiple digital subjects.27–29 The computational test kit has successfully replicated previous experimental findings,19 including the increasing biomechanical benefit of augmentation with increasing severity of osteoporosis.25 Using this efficient simulation tool, the current study aimed to systematically evaluate the biomechanical effects of augmenting six PHILOS plate screws in the humeral head to stabilize a three-part proximal humerus fracture and to identify the optimal cementing strategies for low-density bones.
Methods
Sample set
In all, 24 low bone mineral density (BMD) subjects were selected from the set of digital proximal humerus models available in the computational test kit,25 as cement augmentation is intended for use in low-density bone. The bones were all left-sided and originated from 12 female and 12 male donors with a mean age of 82.9 years (SD 8.1; 64 to 98). BMD was measured in the humeral head with the method described by Krappinger et al,30 with a mean value of 107.8 mgHA/cm3 (SD 15.2; 73.5 to 129.7). Additionally, the bones needed to be large enough to accommodate the calcar screws of the PHILOS plate (i.e. row E). High-resolution peripheral quantitative CT images (HR-pQCT; XtremeCT; Scanco Medical AG, Brüttisellen, Switzerland) and surface models of the digital humeri were available in the test kit database to support finite element model generation and local material property assignment.
Finite element simulations
An unstable, three-part fracture with medial comminution (AO/OTA 11-B3.2) was simulated on the bone models by introducing a wedge-shaped gap at the surgical neck and a 1 mm wide osteotomy to separate the greater tubercle and articular fragments. The osteotomized models were virtually instrumented with the PHILOS plate according to the surgical technique guide of the implant manufacturer.31 The plate was positioned 5 mm to 8 mm distal to the superior aspect of the greater tubercle and 2 mm to 4 mm posterior to the bicipital groove,31 using a custom-made, automatic-fitting algorithm. The plate was fixed to the humeral shaft using three bicortical screws. The proximal fragments were fixed in a frequently used screw configuration including rows A, B, and E of the PHILOS plate (Figure 1). These screws were modelled as smooth hollow cylinders, mimicking the cannulated screws of the PHILOS plate, but without the screw threads. According to the surgical technique, screw length was determined based on a distance of 8 mm between the screw tip and the subchondral bone surface, and the closest commercially available length was used. Cement augmentation was simulated by adding a spherical 0.55 ml cement cloud around the screw tip, using 0.50 ml injected cement volume as suggested by the surgical technique and assuming 10% cement porosity after setting.32 If the spherical volume extended beyond the trabecular domain, the remaining volume was distributed along the subcortical bone interface, mimicking cement flow (Figure 1). The models were meshed using Simpleware v7.0 (Simpleware, Exeter, UK) with quadratic tetrahedral elements having a mean edge length of 0.86 mm (SD 0.44) that was previously shown to meet convergence criteria.26
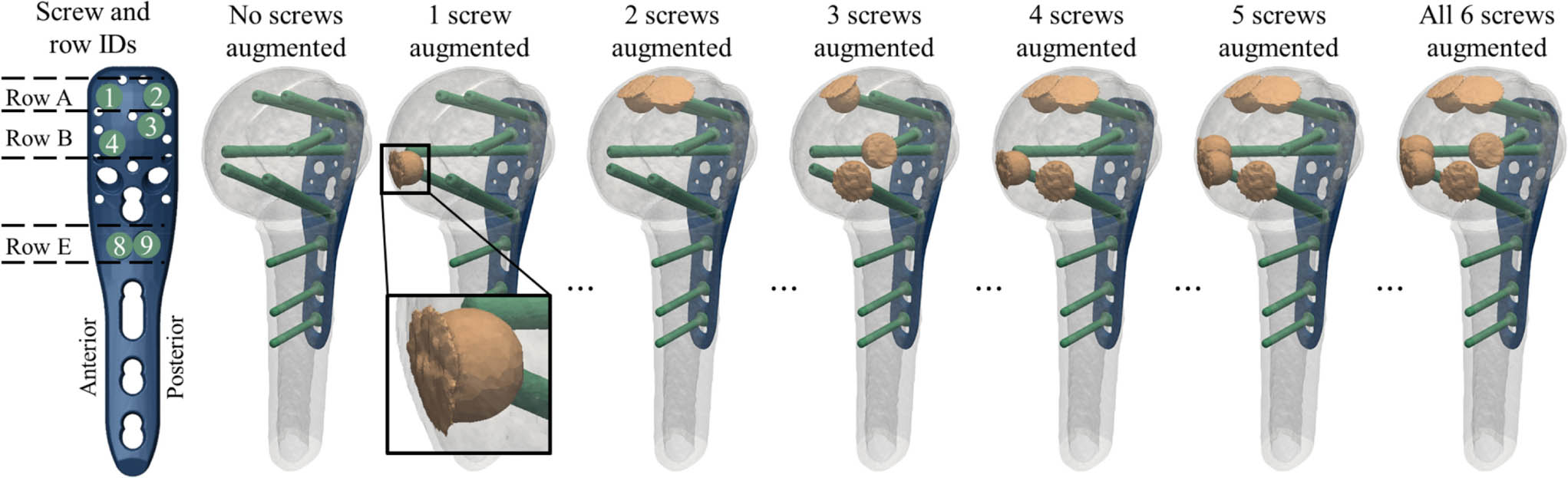
Fig. 1
Definition of the screw IDs and row IDs of the proximal humerus internal locking system (PHILOS) plate and anatomical orientation for left humeri (far left); and illustration of the fracture model with transparent fragments to show the virtual instrumentation, for exemplary configurations of zero to six augmented screws (left to right). Note that there were a total of 64 configurations for each subject. A close-up view of a cemented cloud is shown for the case of one augmented screw, demonstrating redistribution of cement material at the subchondral bone interface.
All materials were modelled as linear elastic. The titanium plate and screws were assigned a Young's modulus of 105 GPa and Poisson's ratio of 0.3. Young's moduli (E, in MPa) of the bone elements were locally mapped from the underlying HR-pQCT-based apparent BMD values (ρ, in gHA/cm3) according to the density-elasticity relationship33: E = 14,664ρ1.49. Cemented regions had an elastic modulus of 2,300 MPa and a Poisson’s ratio of 0.45, simulating the composite of trabecular bone and PMMA cement.34,35 The presence or absence of the PMMA within each screw cannula and tip was toggled on or off for the different configurations of augmenting the six humeral head screws, by changing the properties of the element group to cement or bone, respectively. The interface of the locking screw head and the plate hole was assumed to be bonded.36 Based on previous findings, the bone-screw, PMMA-bone, and screw-PMMA interfaces were modelled as tied and potential contacts between the bone and plate were disregarded.26 A sensitivity analysis in one subject confirmed that the results of a tied bone-screw interface were strongly correlated (R2 = 0.99) with a more sophisticated, and computationally more expensive, pseudothreaded interface model.37
Three physiological loading cases (45° abduction with 0° internal rotation, 45° abduction with 45° internal rotation, and 45° flexion with 0° internal rotation of the shoulder) were simulated to represent the normal postoperative range of motion based on previous musculoskeletal simulations in AnyBody 5.0.0 software (AnyBody Technology A/S, Aalborg, Denmark).25
Linear elastic analyses were performed using the standard implicit solver of Abaqus 6.13 to 3 (Simulia, Dassault Systemes, Velizy-Villacoublay, France). The outcome measure was the average principal compressive strain in the bone region around the proximal screw tips, which was previously validated as a strong predictor for the number of cycles to cut-out failure observed through in vitro experiments (R2 = 0.90).26 The principal compressive strain was evaluated for the bone elements with centres located within a quasi-cylindrical region of interest (ROI) around each screw tip. The ROIs were 15 mm in length and had a thickness of 2.5 mm measured from the bone-screw interface for nonaugmented screws and from the bone-cement interface for augmented screws. These choices assumed that the bone failure occurs in the direct vicinity of the screw for the nonaugmented screw and directly adjacent to the cement bone region for the augmented screw. The strain values of the ROIs of all six humeral head screws were averaged. To assess the benefit achieved by augmentation, the percentage reductions in the peri-implant strain were calculated for each of the augmentation configurations relative to the nonaugmented case. The quality of the local bone stock around the screw tips was evaluated by quantifying total BMD in the same ROIs based on the calibrated HR-pQCT images using previously developed methods.38
Statistical analysis
The statistical tests were performed using the R software, v3.3.324 (R Core Team, Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism v8.3.0 (GraphPad Software; San Diego, California, USA) with significance defined at an α-value of 0.05. First, to examine the relative effect of augmenting each screw on the reductions in peri-implant bone strain, repeated-measures multi-factor analysis of variance (ANOVA) was performed with the augmentation status of each screw and the loading case serving as independent, within-subjects factors (seven factors in total). Each of the six screws represented a two-level factor (augmented or not) and the load case was a three-level factor. This analysis represents an unrepeated 26 full-factorial experimental design, performed in 24 subjects under three loading conditions. The relative effect of augmenting each screw was evaluated by considering the percentage contribution to the total variance of the regression model. That is, screws with a greater contribution to the model variance indicate a larger reduction in average peri-implant bone strain of the whole construct.
The strain reductions achieved by the best and worst combinations of the same total number of augmented screws were compared with the Wilcoxon signed-rank test for one to five augmented screws.
Next, repeated-measures two-way ANOVA with Dunnett’s correction for post-hoc comparisons was used to determine the best two-screw, three-screw, and four-screw combinations. In these analyses, the two factors were load case (three levels) and augmentation state (two levels). For example, comparing all possible two-screw configurations resulted in 15 levels for the augmentation factor.
The two screws in the regions of lowest local bone density were determined for each subject. To investigate the potential relationships between augmentation benefit and local bone density, for each subject we evaluated if the best two-screw augmentation combination coincided with the two screws having the lowest local BMD.
Results
A total of 4,608 cases (24 subjects, 64 augmentation configurations, three load cases) were simulated. Augmenting a single screw with PMMA significantly reduced mean peri-screw bone strain compared to the nonaugmented construct (14% reduction (SD 3%); p < 0.001). Augmenting more screws led to greater strain reductions, however the specific screws selected for augmentation had a large influence on the results (Figure 2a). The differences in mean strain reduction between the best and worst combinations for a given number of augmented screws were significantly different (p < 0.001 for two to five augmented screws). Consequently, the best two-screw combination (mean 23% reduction (SD 3%)) showed a similar result to the worst four-screw combination (mean 22% (SD 5%)). The best three-screw augmentation pattern (mean 29% (SD 10%)) was as good as the worst five-screw combination (mean 29% (SD 6%)). The improvement normalized to the total number of augmented screws was the highest for the first augmented screw and showed a decreasing trend among the best combination but remained low and nearly constant for the worst combinations (Figure 2b). For each of the specimen-specific best and worst augmentation combinations, there was a positive correlation between the magnitude of the strain reduction (i.e. benefit) achieved by the augmentation and the non-augmented (i.e. baseline) strain level (R2 > 0.75 for all), indicating a larger benefit for the lower BMD humeri.
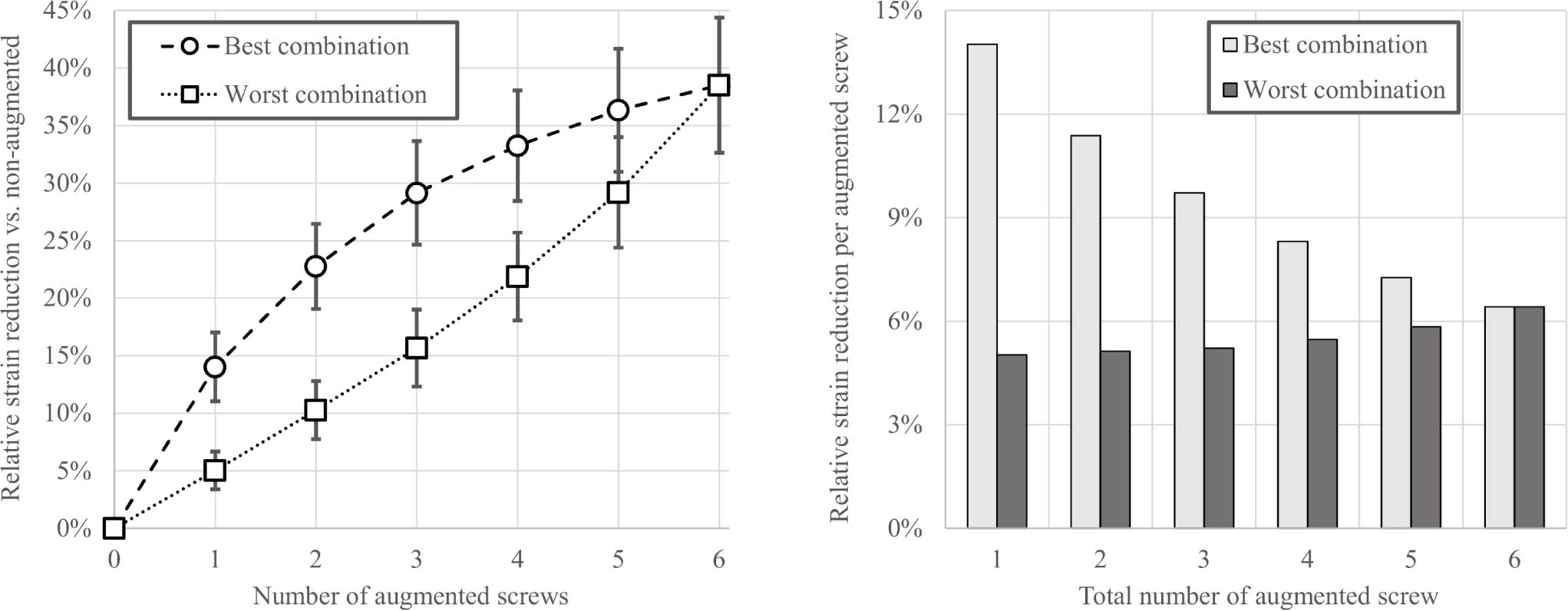
Fig. 2
Left: Mean and SD of the reduction in peri-screw strains in the 24 subjects for different augmentation combinations normalized to the non-augmented state, showing significant differences (p < 0.001, Wilcoxon signed-rank test) between the best and worst options for one to five augmented screws. Right: Mean reduction in peri-screw strains normalized to the total number of augmented screws for the best and worst combinations.
The augmentation combination that provided the largest reduction in peri-screw strain exhibited subject-to-subject variability, but included one or both calcar screws (IDs 8 and 9) for most subjects (Figure 3). While Figure 3 represents the probability of selecting the best screw combination for a given subject, the ANOVA results provided an assessment of the average magnitudes of improvement for each screw configuration. All p-values here were calculated using repeated-measures two-way ANOVA with Dunnett’s correction for post-hoc comparisons. These results indicated that the two-screw combination of screws 8 and 9 achieved significantly greater strain reduction, on average, than all other two-screw configurations (p < 0.001), except for screws 4 + 9 (p = 0.999) or screws 2 + 9 (p = 0.301). Similarly, the three-screw combination of screws 4 + 8 + 9 was significantly better than all other three-screw configurations (p < 0.008) except for 2 + 4 + 9 (p = 0.707) and 2 + 8 + 9 (p = 0.091). With four screws augmented, 2 + 4 + 8 + 9 was significantly better than every other configuration (p < 0.005) except for 3 + 4 + 8 + 9 (p = 0.099). The overall worst combinations were 1 + 3 (in 13 subjects) and 1 + 2 (seven subjects) for two-screw augmentations, 1 + 2 + 3 (13 subjects) and 1 + 3 + 4 (six subjects) for three-screw augmentations, and 1 + 2 + 3 + 4 (18 subjects) and 1 + 3 + 4 + 8 (four subjects) for four-screw augmentations. The results of all 64 configurations are summarized in Supplementary table i.
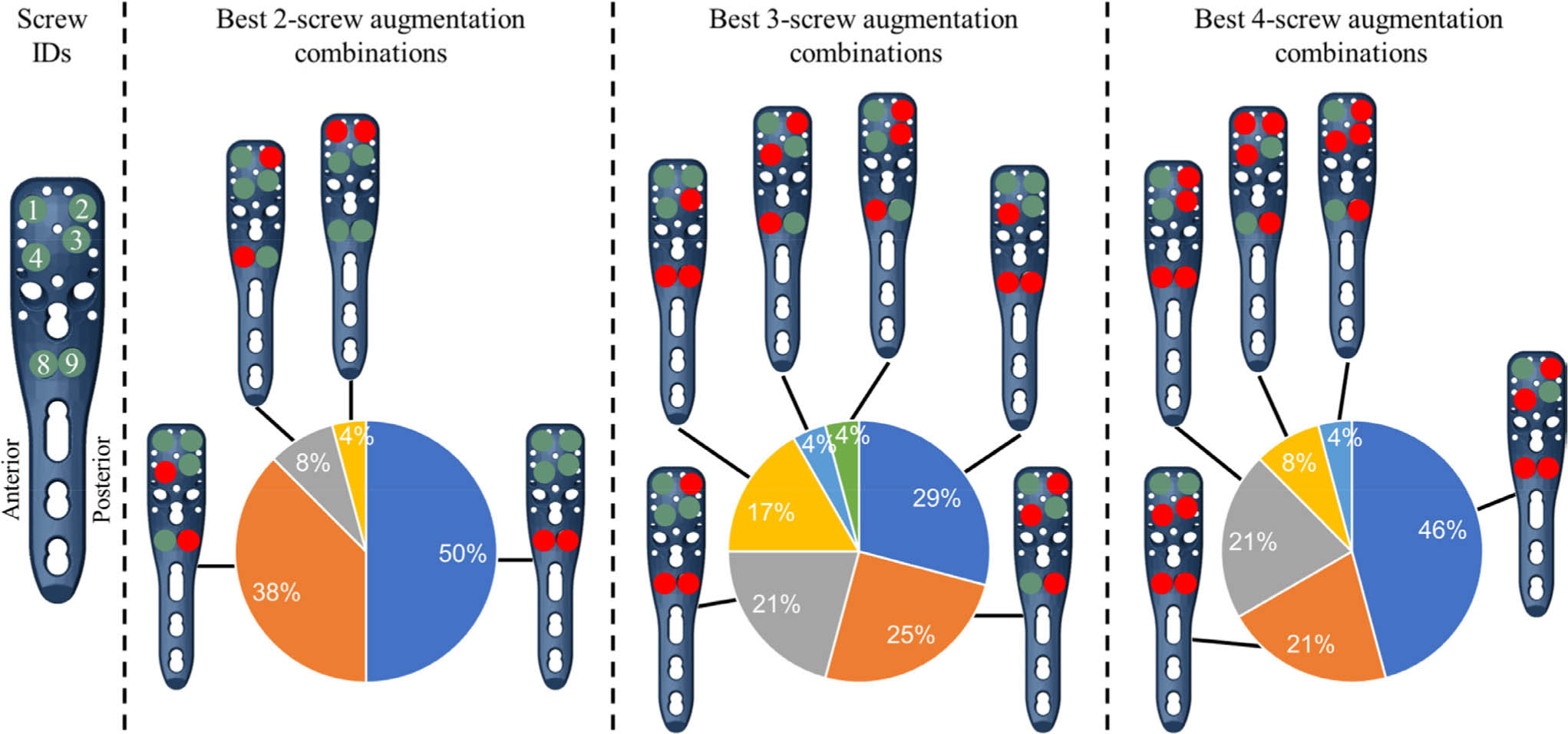
Fig. 3
The best augmentation combinations, achieving the largest reduction in the peri-screw bone strain compared to the nonaugmented condition, shown for the different number of augmented screws (2-, 3-, and 4-screw combinations). The pie charts show the percentile of the subjects (24 = 100%) where the given combination was the best. The filled circles in the plate holes indicate augmented (red) and nonaugmented (green) screws, respectively. Note that only left humeri were analyzed in this study.
The relative effect of augmenting each screw on the overall strain reduction was, in general, larger for the distal compared to proximal screw rows (Figure 4a). Further, screws with posteriorly located tips were more influential than those screws with anteriorly sited tips. Overall, 80% of the total ANOVA model variation was explained by augmentation of the six screws as factors, 9.5% was related to the subject, 1.7% was due to the load case, and the residual term of the regression model was 8.9%.
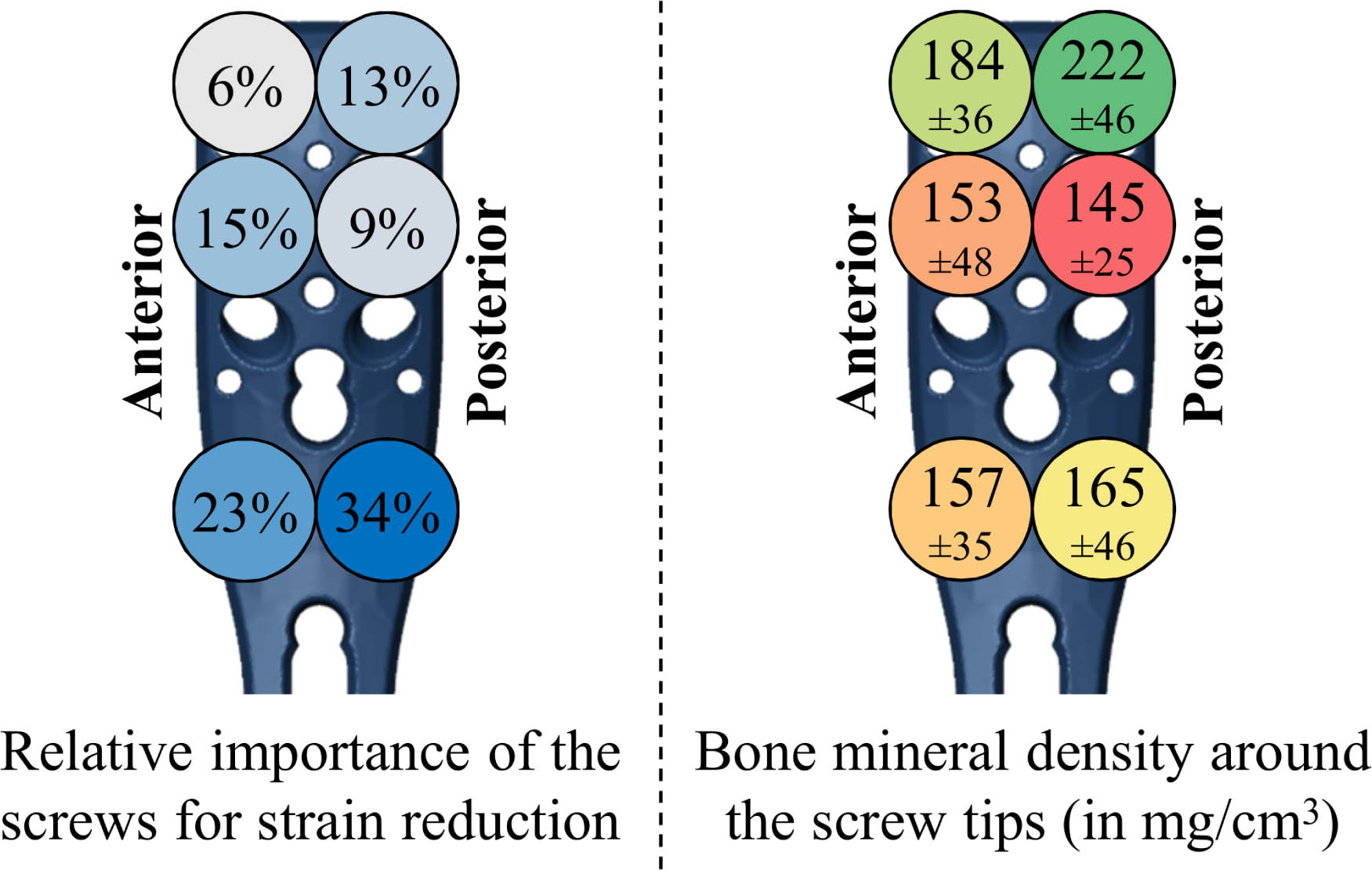
Fig. 4
Left: Results of the repeated-measure analysis of variance (ANOVA), showing the relative importance of augmenting the proximal humerus internal locking system (PHILOS) plate screws considering the reduction in average peri-screw bone strain, i.e. predicted fixation failure risk. The percentiles were normalized to the total part of the variation explained by the six screws as factors (80%). Blue colours indicate a higher strain reduction, i.e. larger mechanical benefit. Right: Local bone mineral density (mean and SD) evaluated in cylindrical regions around the tips of each screw, showing worst and best bone stock in red and green, respectively. Note that due to the oblique screw trajectories, the tip locations of the second row are flipped anteroposteriorly compared to the shown screw head positions (see Figure 1) and that only left humeri were analyzed in this study.
There was no clear trend between the influence of the screw in the ANOVA results (Figure 4a) and the BMD (Figure 4b) results. The lowest peri-screw BMD coincided with the pattern of best two-screw augmentation combination for only two subjects (8%) and partially coincided (with one screw location matching) for 14 subjects (58%).
Discussion
This computational study found significantly lower peri-screw strains, i.e. decreased predicted cut-out failure risk, with the application of PMMA at the tip of cannulated screws compared to nonaugmented constructs, which supports the concept of enhanced primary stability with augmentation. Both number and location of augmented screws had an important impact on peri-implant strain reduction in the humeral head. Augmenting the two most beneficial screws could achieve results that were equal to or better than the worst choice for augmenting four screws. Overall, the optimal screw selection for a specific number of screws was patient specific. However, on average, augmentation combinations involving the calcar screws and the screws with posteriorly oriented screw tips provided the greatest benefit.
Results from the current study do not support augmenting the four most proximal screws as the default technique for all patients, which is the configuration used in most previous biomechanical studies.18–20 This configuration was actually observed to be the worst four-screw combination in 75% of the investigated subjects. Nonaugmented screws purchasing in dense bone may benefit less from additional strengthening compared to screw tips located in poor bone stock. This assumption was used in a previous study to augment the two screws at the lowest bone stock.39 However, our results do not fully support that suggestion either, as local bone density alone did not directly explain the need for augmentation. Other factors such as the patient-specific geometry of the humeral head, proximity of screw tips based on screw length, or the spatial distribution of bone density (rather than the local density alone) may also affect the relative benefits of augmenting each specific screw. While those previous studies already reported significant biomechanical benefits, the optimal augmentation effect may not have been achieved.
When performing cement augmentation with the PHILOS plate, the surgical technique guide recommends to include four to six perforated screws, starting at the most proximal row (row A) and extending toward the more distal rows, generating a wide distribution of cement clouds in the humeral head. These recommendations are only partially supported by our findings, suggesting that the largest benefit may be achieved by prioritizing the most distal row (row E) and screws with posteriorly located tips. The best augmentation combinations found here did not include the configuration featuring the largest spread (i.e. including rows A and E). This is partially in contrast with the findings of a previous study on nonaugmented PHILOS plate fixation, indicating that the spread between the occupied screw rows should be maximized to achieve optimal primary stability.29 While that technique may apply to screw placement, it does not seem to fully translate to augmentation.
This study alone cannot define robust generalized guidelines for surgical practice, but the results indicate that augmentation of the calcar screws holds the greatest probability of achieving the largest biomechanical benefit. However, small humeri where the calcar screws cannot be implanted were not studied herein. Hence, the findings of the current study cannot address those patients. Moreover, the calcar screw tips may lie near fracture lines. During surgery, the risk of cement extrusion into the fracture site should be tested using contrast agents and avoided. It is also important to note that screw rows C and D were not evaluated in the current study, so fixation constructs that include these screws may have different results.
Clinical studies have found that low BMD,40 instability due to insufficient anatomical reduction,40–43 and absence of medial support40–44 are critical risk factors for fixation failure. The findings of our study, in line with previous computer simulations,25 support that augmentation could be more beneficial for the higher-risk patients, i.e. those with lower BMD and less fixation stability. We investigated unstable three-part fracture patterns as augmentation has been shown to provide biomechanical benefit in unstable fracture models,17–19 but no advantage in stable fracture patterns.18 Additionally, the type of loading may have an effect on the efficiency of augmentation. Kuang et al20 found improved biomechanical behaviour of PHILOS plates augmented with hydroxyapatite cement in varus bending, but not in axial rotation tests. Considering that a patient is likely to expose their shoulder to a variety of loading patterns, the current study did not focus on load-dependent differences and evaluated the three physiological loading scenarios together in an effort to identify robust results.
Several clinical studies have been performed recently to investigate the clinical effectiveness of cement augmentation of the PHILOS plate. Katthagen et al13 performed a single-centre prospective case series where they augmented a mean of three (two to five) of the mean seven (range five to nine) humeral head screws used in 24 patients, and compared outcomes to 24 historical controls. At three months follow-up, they observed significantly reduced risk of early loss of reduction and articular screw perforation when cement augmentation was used (p = 0.037, Mann-Whitney U test). However, augmentation did not affect patient-reported Constant scores at three or 12 months (p = 0.853 and 0.557 respectively, both Mann-Whitney U test).45 Siebenbürger et al15 performed a retrospective study, reporting the two-year outcomes for 39 patients who received augmentation compared to 55 patients with standard PHILOS plate fixation. They found no significant difference in rates of fixation loss between these groups, 11% versus 5% (p = 0.74, independent-samples t-test) respectively, nor in rates of avascular necrosis, 8% versus 6% (p = 0.99). It is important to note a study bias, since only the high-risk patients with low bone stock in the humeral head were assigned for augmentation while the lower-risk patients were treated without augmentation. Those findings suggest that augmentation of the higher-risk subjects was successful in achieving similar outcomes to lower-risk subjects. In that study, they augmented in two ways – always augmented both row A screws and then either both row C screws or the singular row D screw. No calcar screws (row E) were augmented. Our results show that the least benefit is seen with row A compared to other rows. While we did not include row C in our analysis, this screw row of the PHILOS plate is not recommended by the surgical guide as, firstly, the tips often lie at the same level as row A, and secondly because row C screw tips often end close to fracture lines. Hengg et al14 performed the only multicentre randomized prospective trial to date on the use of cement augmentation. However, their study was abandoned when early analysis showed the design to be underpowered based on an overestimation of failure rates seen with fixation. In the 67 patients analyzed (34 nonaugmented and 33 augmented fixations) before the trial was stopped, there was no difference in mechanical failure rates (16% augmented and 15% control; p = 1.000, two-sided Fisher's exact test) nor any differences in function, quality of life, or adverse events at the one-year follow-up. Cement augmentation was applied in two to four screws, but the exact configurations were not disclosed. Our results suggest that the pattern of augmentation could significantly affect the biomechanical benefit. Therefore, for the correct interpretation of results, it is imperative that future clinical study designs include the selection of augmented screws and their rationale. The only study providing more detailed description of the augmentation configuration was the retrospective analysis of Knierzinger et al.46 In their cohort of 24 elderly patients treated with PHILOS plate combined with screw-top augmentation, the authors found that the most commonly augmented screws were both screws of row A and one screw in row B, with all of these used in 19 patients (80%). In turn, the calcar screws were augmented for less than half of the investigated cases. While these patterns do not correspond to the optimal ones found by our study, they reported only a single patient sustaining early secondary loss of reduction. All biomechanical investigations, but only a single clinical study highlighted the improved primary stability seen with cement augmentation. The analysis of these benefits needs to go beyond a binary message, whether or not to use augmentation, given the significant variability of benefits observed in this study across configurations. Nevertheless, the limits of applicability and benefit of cement augmentation should be judged versus alternative treatment options.47
In this study, the variation between subjects and loading conditions prevents robust generalized recommendations of optimal screw choice. This indicates that treatment optimization may require a subject-specific approach. Although the simulation technique used here has been validated to predict cyclic cut-out failure (R2 = 0.90),26 the findings are based on computer simulations of a specific type of fracture pattern using one fixation system. The results may not be generalizable to other fixation systems and require prospective clinical validation. Only left humeri were simulated; the results may not hold to right bones as the PHILOS plate is not symmetric. The analysis was restricted to a single implant configuration, however other factors have been shown to influence fixation stability, including screw configuration29 and length,27 as well as plate positioning.28 The comparison screws used in the models were cannulated even if left nonaugmented and a constant tip-to-joint distance of 8 mm was used independent of augmentation. The behaviour of these screws may differ from non-cannulated screws, though their use may represent operative practice, where decisions to augment are made after screw insertion. Furthermore, the boundary conditions of the bonded screw-bone, screw-cement, and cement-bone interfaces may not be sufficiently accurate representations of the real interactions. Nevertheless, the use of the more realistic pseudothreaded screw-bone interface model did not affect the results in a sensitivity analysis. Finally, there are concerns regarding the use of PMMA bone cement, including thermal necrosis and extravasation into the joint space, although in vitro studies have provided evidence against some of these concerns.48–51 Nevertheless, partial bone necrosis around the augmented screw has been reported.15,46 Besides increasing treatment costs, cement augmentation can extend the duration of the surgery, which may be critical for elderly patients. The computational analyses in the present study were restricted to the investigation of the biomechanical effect of cement augmentation of the predicted primary stability; the biological effects were not considered.
In conclusion, the findings from this computer simulation study showed that cement augmentation of the screw tips in PHILOS plate fixation of unstable three-part fractures can alleviate the peri-implant strain, which suggests a reduced risk of cut-out failure. The number as well as the specific configuration of the augmented screws was highly influential to the achieved benefit and the optimal screw selection may be patient-specific. However, augmenting the calcar screws consistently provided the largest improvement and augmentation of the screws with posterior tips was also beneficial. Further investigation through additional simulations, in vitro testing, and prospective clinical trials involving augmentation of specific screws is required to better define optimal techniques and evaluate patient benefit.
References
1. Kammerlander C , Erhart S , Doshi H , Gosch M , Blauth M . Principles of osteoporotic fracture treatment . Best Pract Res Clin Rheumatol . 2013 ; 27 ( 6 ): 757 – 769 . Crossref PubMed Google Scholar
2. Hirschmann MT , Fallegger B , Amsler F , Regazzoni P , Gross T . Clinical longer-term results after internal fixation of proximal humerus fractures with a locking compression plate (PHILOS) . J Orthop Trauma . 2011 ; 25 ( 5 ): 286 – 293 . Crossref PubMed Google Scholar
3. Röderer G , Erhardt J , Kuster M , et al. Second generation locked plating of proximal humerus fractures--a prospective multicentre observational study . Int Orthop . 2011 ; 35 ( 3 ): 425 – 432 . Crossref PubMed Google Scholar
4. Yang H , Li Z , Zhou F , Wang D , Zhong B . A prospective clinical study of proximal humerus fractures treated with a locking proximal humerus plate . J Orthop Trauma . 2011 ; 25 ( 1 ): 11 – 17 . Crossref PubMed Google Scholar
5. Brunner F , Sommer C , Bahrs C , et al. Open reduction and internal fixation of proximal humerus fractures using a proximal humeral locked plate: a prospective multicenter analysis . J Orthop Trauma . 2009 ; 23 ( 3 ): 163 – 172 . Crossref PubMed Google Scholar
6. Südkamp N , Bayer J , Hepp P , et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Results of a prospective, multicenter, observational study . J Bone Joint Surg Am . 2009 ; 91-A ( 6 ): 1320 – 1328 . Crossref PubMed Google Scholar
7. Panagiotopoulou VC , Varga P , Richards RG , Gueorguiev B , Giannoudis PV . Late screw-related complications in locking plating of proximal humerus fractures: a systematic review . Injury . 2019 ; 50 ( 12 ): 2176 – 2195 . Crossref PubMed Google Scholar
8. Rangan A , Handoll H , Brealey S , et al. Surgical vs nonsurgical treatment of adults with displaced fractures of the proximal humerus: the PROFHER randomized clinical trial . JAMA . 2015 ; 313 ( 10 ): 1037 – 1047 . Crossref PubMed Google Scholar
9. Biermann N , Prall WC , Böcker W , Mayr HO , Haasters F . Augmentation of plate osteosynthesis for proximal humeral fractures: a systematic review of current biomechanical and clinical studies . Arch Orthop Trauma Surg . 2019 ; 139 ( 8 ): 1075 – 1099 . Crossref PubMed Google Scholar
10. Laux CJ , Grubhofer F , Werner CML , Simmen H-P , Osterhoff G . Current concepts in locking plate fixation of proximal humerus fractures . J Orthop Surg Res . 2017 ; 12 ( 1 ): 137 . Crossref PubMed Google Scholar
11. Schliemann B , Wähnert D , Theisen C , et al. How to enhance the stability of locking plate fixation of proximal humerus fractures? an overview of current biomechanical and clinical data . Injury . 2015 ; 46 ( 7 ): 1207 – 1214 . Crossref PubMed Google Scholar
12. Jabran A , Peach C , Ren L . Biomechanical analysis of plate systems for proximal humerus fractures: a systematic literature review . Biomed Eng Online . 2018 ; 17 ( 1 ): 47 . Crossref PubMed Google Scholar
13. Katthagen JC , Lutz O , Voigt C , Lill H , Ellwein A . Cement augmentation of humeral head screws reduces early implant-related complications after locked plating of proximal humeral fractures . Obere Extrem . 2018 ; 13 ( 2 ): 123 – 129 . Crossref PubMed Google Scholar
14. Hengg C , Nijs S , Klopfer T , et al. Cement augmentation of the proximal humerus internal locking system in elderly patients: a multicenter randomized controlled trial . Arch Orthop Trauma Surg . 2019 ; 139 ( 7 ): 927 – 942 . Crossref PubMed Google Scholar
15. Siebenbürger G , Helfen T , Biermann N , Haasters F , Böcker W , Ockert B . Screw-tip augmentation versus standard locked plating of displaced proximal humeral fractures: a retrospective comparative cohort study . J Shoulder Elbow Surg . 2019 ; 28 ( 7 ): 1326 – 1333 Crossref PubMed Google Scholar
16. No authors listed . PHILOS with augmentation. Surgical Guide . DePuy Synthes . 2016 . http://synthes.vo.llnwd.net/o16/LLNWMB8/INT%20Mobile/Synthes%20International/Product%20Support%20Material/legacy_Synthes_PDF/DSEM-TRM-0614-0087-3_LR.pdf (date last accessed 19 August 2020 ). Google Scholar
17. Röderer G , Scola A , Schmölz W , Gebhard F , Windolf M , Hofmann-Fliri L . Biomechanical in vitro assessment of screw augmentation in locked plating of proximal humerus fractures . Injury . 2013 ; 44 ( 10 ): 1327 – 1332 . Crossref PubMed Google Scholar
18. Kathrein S , Kralinger F , Blauth M , Schmoelz W . Biomechanical comparison of an angular stable plate with augmented and non-augmented screws in a newly developed shoulder test bench . Clin Biomech . 2013 ; 28 ( 3 ): 273 – 277 . Crossref PubMed Google Scholar
19. Unger S , Erhart S , Kralinger F , Blauth M , Schmoelz W . The effect of in situ augmentation on implant anchorage in proximal humeral head fractures . Injury . 2012 ; 43 ( 10 ): 1759 – 1763 . Crossref PubMed Google Scholar
20. Kuang GM , Wong TM , Wu J , et al. Augmentation of a locking plate system using bioactive bone Cement—Experiment in a proximal humeral fracture model . Geriatr Orthop Surg Rehabil . 2018 ; 9 ( 1 ): 215145931879531 . Google Scholar
21. MacLeod A , Simpson AHRW , Pankaj P . Experimental and numerical investigation into the influence of loading conditions in biomechanical testing of locking plate fracture fixation devices . Bone Joint Res . 2018 ; 7 ( 1 ): 111 – 120 . Crossref PubMed Google Scholar
22. Kang K-T , Son J , Suh D-S , Kwon SK , Kwon O-R , Koh Y-G . Patient-Specific medial unicompartmental knee arthroplasty has a greater protective effect on articular cartilage in the lateral compartment: a finite element analysis . Bone Joint Res . 2018 ; 7 ( 1 ): 20 – 27 . Crossref PubMed Google Scholar
23. Danese I , Pankaj P , Scott CEH . The effect of malalignment on proximal tibial strain in fixed-bearing unicompartmental knee arthroplasty . Bone Joint Res . 2019 ; 8 ( 2 ): 55 – 64 . Crossref PubMed Google Scholar
24. Acklin YP , Zderic I , Inzana JA , et al. Biomechanical evaluation of a new gliding screw concept for the fixation of proximal humeral fractures . Bone Joint Res . 2018 ; 7 ( 6 ): 422 – 429 . Crossref PubMed Google Scholar
25. Varga P , Inzana JA , Gueorguiev B , Südkamp NP , Windolf M . Validated computational framework for efficient systematic evaluation of osteoporotic fracture fixation in the proximal humerus . Med Eng Phys . 2018 ; 57 : 29 – 39 . Crossref PubMed Google Scholar
26. Varga P , Grünwald L , Inzana JA , Windolf M . Fatigue failure of plated osteoporotic proximal humerus fractures is predicted by the strain around the proximal screws . J Mech Behav Biomed Mater . 2017 ; 75 : 68 – 74 . Crossref PubMed Google Scholar
27. Fletcher JWA , Windolf M , Grünwald L , Richards RG , Gueorguiev B , Varga P . The influence of screw length on predicted cut-out failures for proximal humeral fracture fixations predicted by finite element simulations . Arch Orthop Trauma Surg . 2019 ; 139 ( 8 ): 1069 – 1074 . Crossref PubMed Google Scholar
28. Fletcher JWA , Windolf M , Richards RG , Gueorguiev B , Buschbaum J , Varga P . Importance of locking plate positioning in proximal humeral fractures as predicted by computer simulations . J Orthop Res . 2019 ; 37 ( 4 ): 957 – 964 . Crossref PubMed Google Scholar
29. Fletcher JWA , Windolf M , Richards RG , Gueorguiev B , Varga P . Screw configuration in proximal humerus plating has a significant impact on fixation failure risk predicted by finite element models . J Shoulder Elbow Surg . 2019 ; 28 ( 9 ): 1816 – 1823 . Crossref PubMed Google Scholar
30. Krappinger D , Roth T , Gschwentner M , et al. Preoperative assessment of the cancellous bone mineral density of the proximal humerus using CT data . Skeletal Radiol . 2012 ; 41 ( 3 ): 299 – 304 . Crossref PubMed Google Scholar
31. No authors listed . Lcp percutaneous aiming system 3.5 for PHILOS. for less invasive surgery at the proximal humerus . DePuy Synthes . 2016 . http://synthes.vo.llnwd.net/o16/LLNWMB8/INT%20Mobile/Synthes%20International/Product%20Support%20Material/legacy_Synthes_PDF/LCP%20Percutaneous%20Aiming%20System%203.5%20for%20PHILOS%20Technique%20Guide%20-%20DSEMTRM02160617.pdf (date last accessed 19 August 2020 ). Google Scholar
32. Kinzl M , Boger A , Zysset PK , Pahr DH . The effects of bone and pore volume fraction on the mechanical properties of PMMA/bone biopsies extracted from augmented vertebrae . J Biomech . 2011 ; 44 ( 15 ): 2732 – 2736 . Crossref PubMed Google Scholar
33. Dragomir-Daescu D , Op Den Buijs J , McEligot S , et al. Robust QCT/FEA models of proximal femur stiffness and fracture load during a sideways fall on the hip . Ann Biomed Eng . 2011 ; 39 ( 2 ): 742 – 755 . Crossref PubMed Google Scholar
34. Orr JF , Dunne NJ , Quinn JC . Shrinkage stresses in bone cement . Biomaterials . 2003 ; 24 ( 17 ): 2933 – 2940 . Crossref PubMed Google Scholar
35. Race A , Mann KA , Edidin AA . Mechanics of bone/PMMA composite structures: an in vitro study of human vertebrae . J Biomech . 2007 ; 40 ( 5 ): 1002 – 1010 . Crossref PubMed Google Scholar
36. Synek A , Chevalier Y , Baumbach SF , Pahr DH . The influence of bone density and anisotropy in finite element models of distal radius fracture osteosynthesis: evaluations and comparison to experiments . J Biomech . 2015 ; 48 ( 15 ): 4116 – 4123 . Crossref PubMed Google Scholar
37. Inzana JA , Varga P , Windolf M . Implicit modeling of screw threads for efficient finite element analysis of complex bone-implant systems . J Biomech . 2016 ; 49 ( 9 ): 1836 – 1844 . Crossref PubMed Google Scholar
38. Varga P , Grünwald L , Windolf M . The prediction of cyclic proximal humerus fracture fixation failure by various bone density measures . J Orthop Res . 2018 : 2250 – 2258 . Crossref PubMed Google Scholar
39. Röderer G , Brianza S , Schiuma D , et al. Mechanical assessment of local bone quality to predict failure of locked plating in a proximal humerus fracture model . Orthopedics . 2013 ; 36 ( 9 ): e1134 – e1140 . Crossref PubMed Google Scholar
40. Krappinger D , Bizzotto N , Riedmann S , Kammerlander C , Hengg C , Kralinger FS . Predicting failure after surgical fixation of proximal humerus fractures . Injury . 2011 ; 42 ( 11 ): 1283 – 1288 . Crossref PubMed Google Scholar
41. Hardeman F , Bollars P , Donnelly M , Bellemans J , Nijs S . Predictive factors for functional outcome and failure in angular stable osteosynthesis of the proximal humerus . Injury . 2012 ; 43 ( 2 ): 153 – 158 . Crossref PubMed Google Scholar
42. Kralinger F , Blauth M , Goldhahn J , et al. The influence of local bone density on the outcome of one hundred and fifty proximal humeral fractures treated with a locking plate . J Bone Joint Surg Am . 2014 ; 96-A ( 12 ): 1026 – 1032 . Crossref PubMed Google Scholar
43. Thanasas C , Kontakis G , Angoules A , Limb D , Giannoudis P . Treatment of proximal humerus fractures with locking plates: a systematic review . J Shoulder Elbow Surg . 2009 ; 18 ( 6 ): 837 – 844 . Crossref PubMed Google Scholar
44. Gardner MJ , Weil Y , Barker JU , et al. The importance of medial support in locked plating of proximal humerus fractures . J Orthop Trauma . 2007 ; 21 ( 3 ): 185 – 191 . Crossref PubMed Google Scholar
45. Constant CR , Gerber C , Emery RJH , et al. A review of the constant score: modifications and guidelines for its use . J Shoulder Elbow Surg . 2008 ; 17 ( 2 ): 355 – 361 . Crossref PubMed Google Scholar
46. Knierzinger D , Crepaz-Eger U , Hengg C , Kralinger F . Does cement augmentation of the screws in angular stable plating for proximal humerus fractures influence the radiological outcome: a retrospective assessment . Arch Orthop Trauma Surg . 2020 . (Epub ahead of print). CrossrefPubMed Google Scholar
47. Johnson JP , Norris G , Giannoudis PV . Bone augmentation: Is it really needed? Injury . 2018 ; 49 ( 8 ): 1367 – 1372 . Crossref PubMed Google Scholar
48. Blankstein M , Widmer D , Götzen M , et al. Assessment of intraosseous femoral head pressures during cement augmentation of the perforated proximal femur nail antirotation blade . J Orthop Trauma . 2014 ; 28 ( 7 ): 398 – 402 . Crossref PubMed Google Scholar
49. Blazejak M , Hofmann-Fliri L , Büchler L , Gueorguiev B , Windolf M . In vitro temperature evaluation during cement augmentation of proximal humerus plate screw tips . Injury . 2013 ; 44 ( 10 ): 1321 – 1326 . Crossref PubMed Google Scholar
50. Goetzen M , Hofmann-Fliri L , Arens D , et al. Does metaphyseal cement augmentation in fracture management influence the adjacent subchondral bone and joint cartilage?: an in vivo study in sheep stifle joints . Medicine . 2015 ; 94 ( 3 ): e414 . Crossref PubMed Google Scholar
51. Goetzen M , Windolf M , Schmoelz W . Augmented screws in angular stable plating of the proximal humerus: what to do when revision is needed? Clin Biomech . 2014 ; 29 ( 9 ): 1023 – 1026 . Crossref PubMed Google Scholar
Author contributions
P. Varga: Acquired and interpreted the data, Wrote the manuscript.
J. A. Inzana: Acquired and interpreted the data, Wrote the manuscript.
J. W. A. Fletcher: Wrote the manuscript.
L. Hofmann-Fliri: Wrote the manuscript.
A. Runer: Provided critical revision.
N. P. Südkamp: Provided critical revision.
M. Windolf: Provided critical revision.
P. Varga and J. A. Inzana contributed equally to this work.
Funding statement
This study was performed with the assistance of the AO Foundation via the AOTRAUMA Network (Grant No.: AR2013_01). J. A. Inzana reports a Whitaker International Program Post-doctoral Research Scholarship related to this study.
ICMJE COI statement
The authors are not compensated and there are no other institutional subsidies, corporate affiliations, or funding sources supporting this work unless clearly documented and disclosed.
Supplementary material
Table presenting all 64 investigated augmentation configurations, ordered according to the mean strain reduction.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









