Abstract
Aims
To explore the effect of different types of articulating antibiotic-loaded cement spacers in two-stage revision for chronic hip prosthetic joint infection (PJI).
Methods
A retrospective cohort study was performed involving 36 chronic PJI patients treated with different types of articulating antibiotic-loaded cement spacers between January 2014 and December 2017. The incidence of complications and the therapeutic effects of different types of antibiotic-loaded articulating cement spacers were compared.
Results
A total of 36 patients with chronic hip PJI were included. Of these, 13 patients were treated with spacers with Kirschner wires as an endoskeleton (group I), ten patients were treated with spacers with a cemented femoral prosthesis as an endoskeleton (group II), and 13 patients were treated with cemented femoral prostheses combined with polyethylene sockets as a spacer (group III). All patients were followed for 12 to 60 months, with a mean follow-up period of 26.44 months (SEM 14.09). Infection was controlled in 34 patients (94.44%), and there were no significant differences in the eradication rate among the three groups (p = 0.705), but the risk of complications related to the spacer in group III was significantly lower than that in groups I and II (p = 0.006).
Conclusion
Articulating antibiotic-loaded cement spacers is effective in the treatment of chronic hip PJI, but we must pay attention to the occurrence of spacer fracture and dislocation, which can lead to poor joint function. The risk of spacer-related mechanical complications is low, and better joint function can be achieved when using cemented femoral prostheses combined with polyethylene sockets as spacers.
Cite this article: Bone Joint Res 2020;9(8):484–492.
Article focus
This study surveyed the effects of different types of articulating antibiotic-loaded cement spacers in two-stage revision for chronic hip prosthetic joint infection (PJI).
Key messages
The incidence of complications and the therapeutic effects of different types of antibiotic-loaded articulating cement spacers were compared, and lower spacer-related mechanical complications and better joint function can be achieved when using cemented femoral prostheses combined with polyethylene sockets as spacers.
Strengths and limitations
This study compared directly the incidence of complications and the therapeutic effects of three types of antibiotic-loaded articulating cement spacers. The study had a relatively small number of patients in the three groups, making it difficult to compare the groups. Due to the retrospective study design, details of medical histories were not always complete.
Introduction
Along with the extensive development of artificial joint arthroplasty, the incidence of prosthetic joint infection (PJI) is gradually increasing. A two-stage revision strategy has evolved as the gold standard for the treatment of chronic PJI. To facilitate reimplantation, an antibiotic-loaded spacer has been recommended between stages; it can release high doses of antibiotics to locally eradicate pathogens and control infection, which is a critical process in two-stage revision.1,2
Currently, hip spacers may be either static or articulating.3,4 Unexpected complications of static spacers such as bone loss, limb shortening, loss of soft tissue planes, disuse osteopenia, and muscle atrophy have been introduced in previous studies.5,6 Compared with static spacers, articulating spacers can fulfill the interim goals and maintain the tension of the tissue around the hip joint, increasing the range of motion (ROM) and improving functional mobilization during the interim period.5 Articulating spacers include preformed and intraoperatively custom-made spacers. In North America and Europe, preformed cement spacers, most notably spacer G ( Tecres SpA, Verona, Italy), have been widely used since the 1990s.6 The methods for intraoperatively custom-made spacers are diverse and mainly include the use of metal (including a rush pin or Kirschner wire (K-wire)) as an endoskeleton in the kneading process7 and the use of cemented femoral prostheses and polyethylene sockets, represented by prosthesis of antibiotic-loaded acrylic cement (PROSTALAC).8-10 Because commercial spacers, such as spacer G and PROSTALAC, have not been introduced to our region, we use three custom methods to make spacers, including using one to two K-wires or a small cemented femoral prosthesis as an endoskeleton and cemented femoral prostheses and polyethylene sockets as spacers. In this study, the efficacy and mechanical complications of the three types of spacers were compared to provide reference data for clinical practice.
Methods
Patient selection
Between January 2014 and December 2017, we enrolled patients diagnosed with chronic hip PJI who underwent two-stage revision surgery with different types of antibiotic-loaded articulating cement spacers at our institution. This retrospective study was approved by the ethics committee of our institution and was carried out in accordance with the international standards for human experimental ethics, Ethics No. [2014] 047. The inclusion criteria were as follows: diagnosis of hip PJI using the Musculoskeletal Infection Society (MSIS) 2014 criteria,11 and identification as type IV according to the Tsukayama classification;12 treatment with an antibiotic-loaded cement spacer for two-stage revision surgery and completion of at least 12 months of regular follow-up; and availability of complete follow-up data. The exclusion criteria were fungal infections and other sources of inflammation or malignant tumours. Demographic characteristics of patients including age, sex, body mass index (BMI), and the clinical information or laboratory examination were collected.
Surgical technique
Before the first stage of the two-stage revision strategy, we performed hip joint aspiration under ultrasound guidance. Samples were transferred to the laboratory for pathogen identification. All surgeries were performed by the same group of doctors according to a standardized procedure with the patient in the lateral position, and a posterolateral approach was routinely used. Synovial fluid (SF) was collected intraoperatively and sent for white blood cell count (WBC) determination, differential testing, and culture. At least five samples of periprosthetic tissue were obtained for microbial culture and intraoperative pathological frozen sectioning. After complete radical debridement of the synovial tissue and removal of the prosthesis, the synovial tissue was rinsed repeatedly with hydrogen peroxide and a large amount of normal saline, and the wound was soaked with povidone iodine solution for 20 minutes. All instruments were replaced and redisinfected, and surgical clothes, gloves, and waterproof towels were changed. At least 9,000 ml saline was used to rinse the wounds with a low-pressure impulse irrigator. The antibiotic-loaded articulating cement spacer was inserted. After all procedures were completed the hip joint was reduced, and the ROM of the hip joint and the stability of the cement spacer were reassessed.
Spacer technique
The antibiotic selected for the articulating cement spacers was based on the culture results obtained from the preoperative joint aspirate. If the infecting organism was not known at the time of first-stage surgery, a combination of 4.0 g vancomycin (Lilly, Indianapolis, Indiana, USA) and 4.0 g ceftazidime (Antibióticos Do Brasil Ltda., Cosmópolis, Brazil) per 40 g of cement (Refobacin; Zimmer Biomet, Warsaw, Indiana, USA) was employed. In group I, one to two K-wires with a diameter of 5 mm and a prebent angle of 130° were used as an endoskeleton for the cement spacer (Figures 1a and 1b). In group II, a small size (length 130 mm/distal diameter 9 mm) cemented femoral prosthesis (B-C/L; Beijing Chunlizhengda Medical Instruments, Beijing, China) was used as an endoskeleton for the spacer. The endoskeletons were placed in a sterile silica gel mould, pressurized, and moulded (Figures 1c and 1d). Once the bone cement was nearly hardened, the mould was removed and excess bone cement around the spacer was also removed. After the spacer was inserted into the femoral medullary cavity, a small amount of bone cement was bonded between the spacer and the proximal femur. In group III, a proper femoral prosthesis (B-C/L; Beijing Chunlizhengda Medical Instruments; length 130 mm/150 mm, distal diameter 9/10/11/12/13/14/15 mm) and a polyethylene acetabular component (Beijing Chunlizhengda Medical Instruments; diameter 42/44/46/48/50/52/54 mm) that matched the femoral medullary cavities and acetabulum were coated with antibiotic bone cement (Figure 1e). When the bone cement had almost cured, the femoral stem and cup coated cement were inserted into the cavity with the aim of preventing microscopic bonding between the cement and the bone, thus making removal easier and avoiding unexpected bone defects. Three typical cases in the groups are shown in Figure 2).
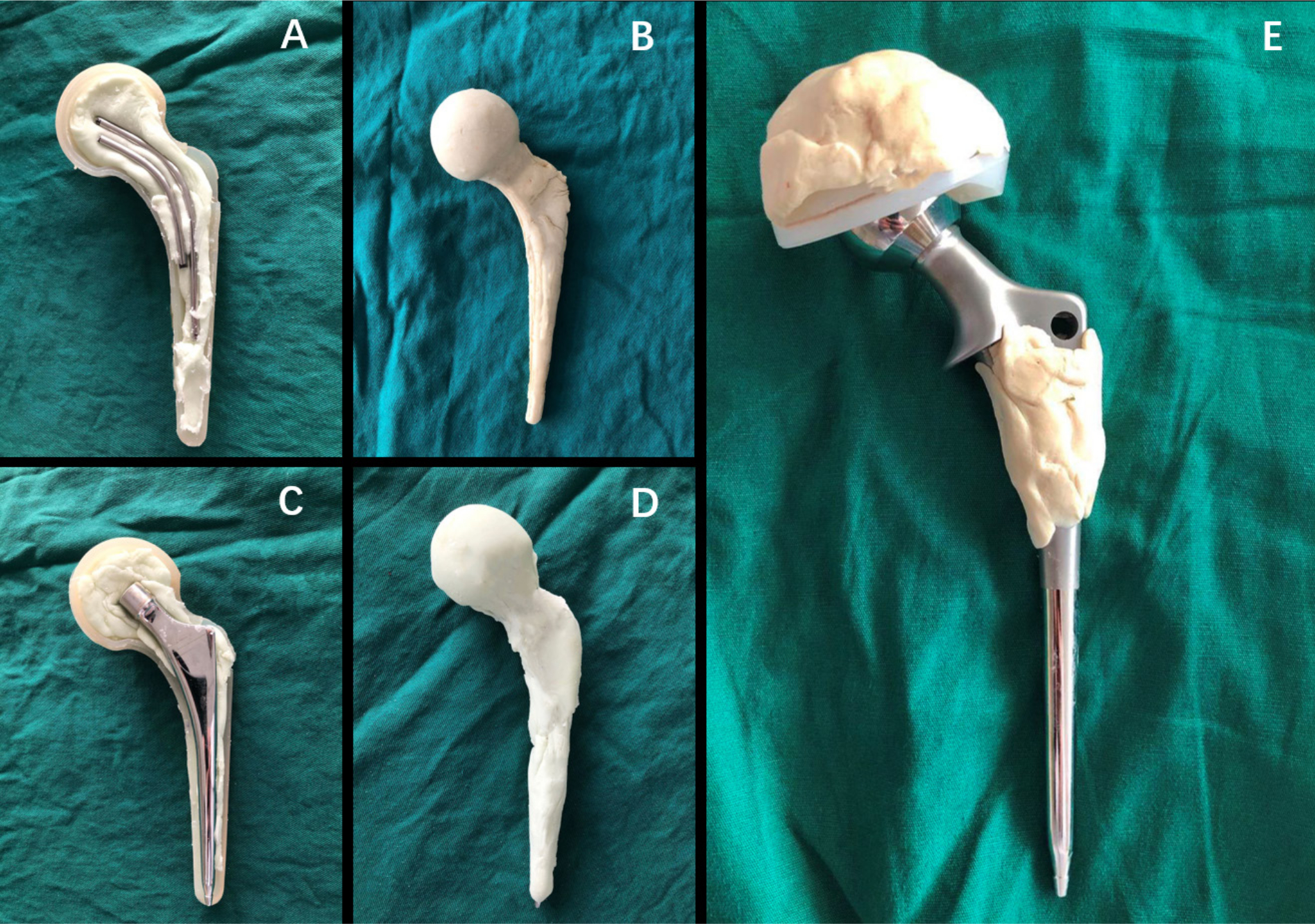
Fig. 1
a) and b) Intraoperative photograph showing the coating of two Kirschner-wires with antibiotic cement in group I. c) and d) Intraoperative photograph showing the coating of a small size femoral prosthesis with antibiotic cement in group II. e) Intraoperative photograph of the cemented prosthesis prepared and ready for insertion in group III.
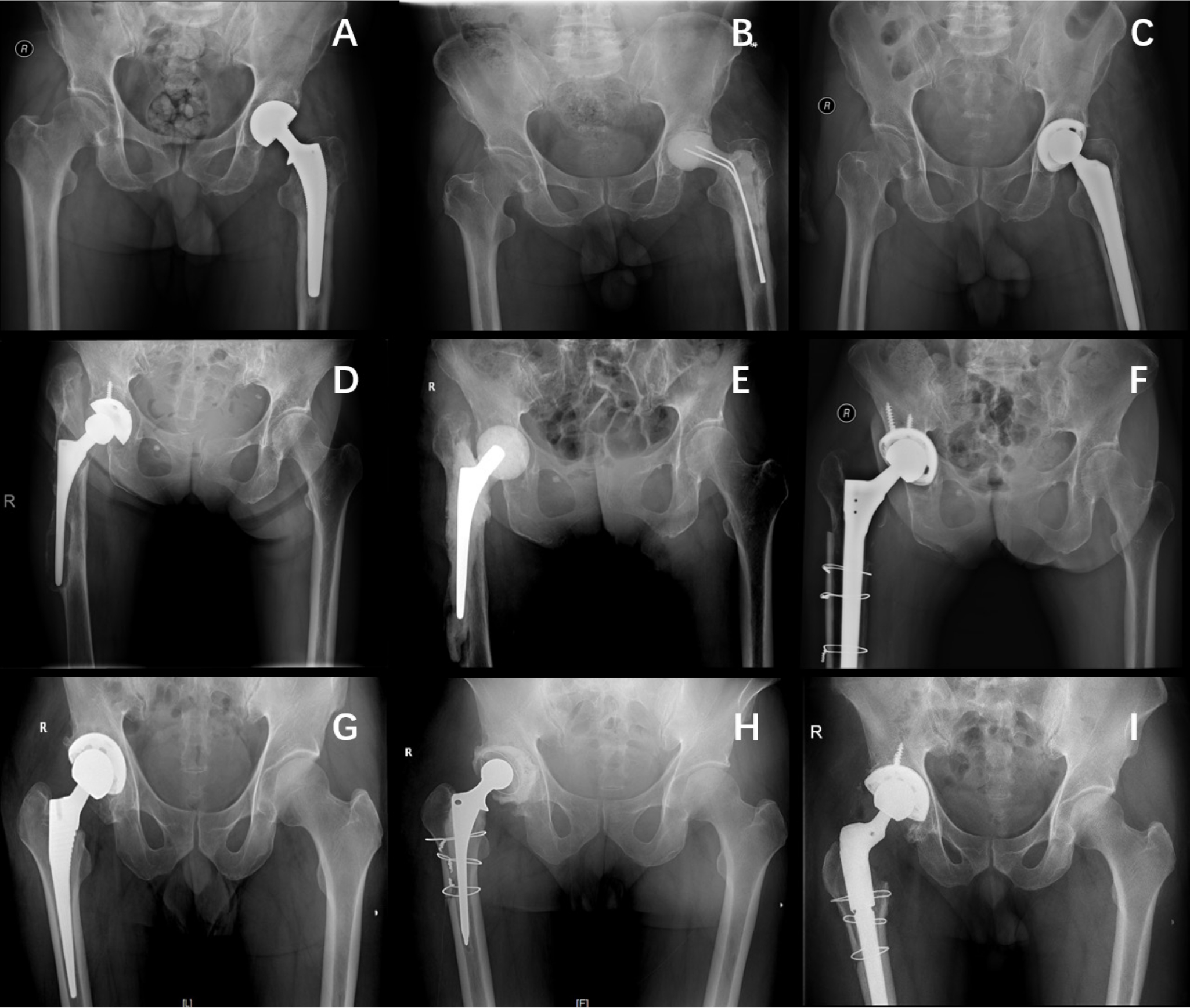
Fig. 2
Typical cases in three groups. a) to c) Two Kirschner wires as endoskeleton of spacer in group I. d) to f) A small prosthesis as endoskeleton of molded spacer in group II. g) to i) Cemented prosthesis as spacer in group III.
Perioperative and postoperative management
A total of 36 chronic hip PJI patients were given antibiotic treatment based on postoperative culture and drug sensitivity results. If the culture result was negative, vancomycin and meropenem (Sumitomo Dainippon Pharmaceutical, Suzhou, China) were empirically employed. After two weeks of intravenous antibiotic treatment following first-stage surgery, the patients received oral suppressive antibiotic therapy for four to six weeks. All 36 patients were followed through regular clinic visits, and the outpatient laboratory examination items included routine blood tests, determination of the ESR and CRP levels, and a timely re-examination of the bilateral hip radiographs and lateral hip radiographs on the affected side. The occurrence of complications such as loosening, sinking, fracture (of the spacer or the femur), acetabular wear, and deep venous thrombosis of the lower limbs was analyzed and recorded. Four to six weeks after stopping antibiotic use, if all the evidence supported infection control in the patient for more than four weeks, second-stage surgery could be performed according to the patient’s wishes and actual circumstances. In brief, after the spacer was removed, debridement was performed, and the primary or revision prosthesis was reimplanted depending on the state of the bone defects.
Definition of infection remission and follow-up evaluations
In patients who completed two-stage revision surgery, infection remission was defined as multiple negative intraoperative frozen section results (< 5/high-power field), negative joint fluid and tissue culture results, a lack of symptoms during follow-up for more than one year after the second-stage revision surgery, the absence of pain during the follow-up period, and normal ESR and CRP levels. In patients who did not complete second-stage surgery, infection remission was defined as a follow-up period of more than two years without pain and with normal ESR and CRP levels.
The visual analogue scale (VAS) score and the Harris Hip Score (HHS)13 were determined before and after first-stage and second-stage surgery for clinical assessment.
Statistical analysis
Data were analyzed using SPSS v22.0 (IBM, Armonk, New York, USA). Data are represented as the mean, range, and standard error of the mean (SEM) for continuous variables and the number and percentage for categorical variables. Differences among the three groups were evaluated using chi-squared test or Fisher’s exact test for categorical variables. Between-group comparisons were performed using single-factor analysis of variance. A p-value ≤ 0.05 was considered statistically significant.
Results
Demographic characteristics
According to the inclusion and exclusion criteria, a total of 48 patients were selected for this study. Six patients were lost at the last follow-up, three developed fungal infections, two developed infections (such as pneumonia) at other sites during the follow-up period, and one developed a gastroenteric tumour. These patients were excluded. A total of 36 patients were finally included. The study group was composed of 16 females and 20 males with a mean age of 62.22 years (SEM 14.89). All cases were unilateral, with 17 on the left and 19 on the right. All patients had localized joint pain and limited joint activity preoperatively, 23 patients had cases that were complicated by elevated skin temperature, ten patients had a sinus associated with the joint cavity, pus, or poor incision healing, three patients had an abscess, four patients had different degrees of hepatic dysfunction, and one patient had renal dysfunction. There were no significant differences in the clinical information or laboratory examination results among the three groups (Table I).
In group I, 13 patients received a spacer with one or two K-wires as an endoskeleton. In group II, ten patients received a spacer with a cemented femoral prosthesis as an endoskeleton. In group III, 13 patients received a cement prosthesis spacer (a cemented femoral stem combined with a cemented acetabular component). The demographic data of the patients in each group are shown in Table I. There were no significant differences in the demographic parameters, such as sex and age, among the three groups (Table I). The relevant laboratory data of the patients in each group are detailed in Table I, and there were no significant differences in the CRP level, ESR, SF-WBC, synovial fluid polymorphonuclear leucocytes percentage (SF-PMN%), or other indexes among the three groups.
Table I.
Patient demographic data (before stage-I surgery).
| Parameter | Group I (n = 13) | Group II (n = 10) | Group III (n = 13) | p-value |
|---|---|---|---|---|
| Mean age, yrs (SEM) | 59.69 (10.00) | 61.10 (18.09) | 64.69 (17.93) | 0.705* |
| Sex n | 0.770† | |||
| Male | 8 | 5 | 6 | |
| Female | 5 | 5 | 7 | |
| Affected, n | 0.915† | |||
| Left | 5 | 5 | 6 | |
| Right | 8 | 5 | 7 | |
| Mean BMI, kg/m2 (SEM) | 24.32 (3.32) | 21.54 (2.88) | 21.99 (2.70) | 0.062* |
| Elevated skin temperature, n | 9 | 4 | 10 | 0.209† |
| Sinus or poor healing of incision, n | 3 | 1 | 6 | 0.168† |
| Abscess, n | 1 | 1 | 1 | 1.0† |
| Hepatic dysfunction, n | 2 | 1 | 1 | 1.0† |
| Renal dysfunction, n | 0 | 1 | 0 | 1.0† |
| Mean CRP, mg/l (SEM) | 35.24 (42.44) | 40.03 (45.38) | 37.42 (38.18) | 0.964* |
| Mean ESR, mm/hr (SEM) | 44.69 (17.57) | 66.90 (39.77) | 64.15 (37.30) | 0.197* |
| Mean SF-WBC, 10 × 6/l (SEM) | 85,968.77 (131,033.75) | 20,224 (17,006.73) | 31,693.15 (38,370.35) | 0.129* |
| Mean SF-PMN, % (SEM) | 80.87 (9.59) | 81.72 (18.78) | 85.72 (9.11) | 0.618* |
-
BMI, body mass index; PMN, polymorphonuclear leucocytes; SEM, standard error of the mean; SF, synovial fluid; WBC, white blood cell count.
-
*
One-way analysis of variance.
-
†
Chi-squared test.
Comparison of clinical efficacy
Comparative data on the curative effect in the three groups are shown in Table II. There were no significant differences in the preoperative VAS score, postoperative VAS score, preoperative Harris score, or postoperative Harris score of second-stage surgery among the three groups (Table II). There were no significant differences in the mean duration of first-stage surgery among the three groups (186.23 minutes (SEM 31.26) in group I, 174.10 minutes (SEM 48.14) in group II, and 198.92 minutes (SEM 40.80) in group III). In this study, the overall eradication rate for all patients was 94.4% (n = 34). The eradication rates for groups I, II, and III were 92.3% (n = 12), 90.0% (n = 9), and 100% (n = 13), respectively. There were no significant differences in the eradication rate among the three groups (p = 0.732). The mean Harris score after first-stage surgery in group III was 74.93 (SEM 3.43), which was significantly higher than that in group II (71.90 (SEM 4.72)) and group I (69.00 (SEM 7.33)). Of the 12 infection control patients in group I, nine (75%) eventually underwent reimplantation, and eight (88.89%) of the nine infection control patients in group II underwent reimplantation. However, only seven (53.85%) of the 13 infection control patients in group III underwent reimplantation with new prostheses. There were no significant differences in the mean HHS after second-stage surgery among the three groups (75.33 (SEM 6.27) in group I, 71.88 (SEM 5.25) in group II, 80.50 (SEM 4.51) in group III). In terms of the duration of the interim period (the period between first- and second-stage surgery), the mean interim period of group III was 8.96 months (SEM 7.29), which was significantly different from that of group II (4.17 months (SEM 10.68)) and group I (5.22 months (SEM 6.76)). There were no significant differences in mean blood loss or mean time of second-stage surgery among the three groups (318.89 ml (SEM 164.50) and 140.00 minutes (SEM 41.91) (n = 9) in group I, 293.75 ml (SEM 167.07) and 141.38 minutes (SEM 42.19) (n = 8) in group II, 232.00 ml (SEM 40.87) and 165.00 minutes (SEM 39.96) (n = 4) in group III) (Table II).
Table II.
Comparison of efficacy among groups.
| Parameter | Group I (n = 13) | Group II (n = 10) | Group III (n = 13) | p-value |
|---|---|---|---|---|
| Eradication rate, n (%) | 12 (92.3) | 9 (90) | 13 (100) | 0.732† |
| Mean VAS before first-stage surgery (SEM) | 6.38 (1.19) | 6.00 (1.15) | 6.46 (1.05) | 0.6* |
| Mean HHS before first-stage surgery (SEM) | 40.77 (7.08) | 35.10 (5.84) | 34.92 (7.53) | 0.071* |
| Mean VAS after first-stage surgery (SEM) | 2.31 (1.32) | 2.60 (0.97) | 2.15 (0.69) | 0.588* |
| Mean HHS after first-stage surgery (SEM) | 69.00 (7.33) | 71.90 (4.72) | 74.93 (3.43) | 0.032* |
| Mean HHS after second-stage surgery (SEM) | 75.33 (6.27) | 71.88 (5.25) | 80.50 (4.51) | 0.066* |
| Mean time of first-stage surgery, mins (SEM) | 186.23 (31.26) | 174.10 (48.14) | 198.92 (40.80) | 0.344* |
| Mean blood loss of first-stage surgery, ml (SEM) | 450.00 (232.52) | 433.00 (181.05) | 530.77 (201.60) | 0.474* |
| Mean duration of interim period, mths (SEM) | 5.22 (6.76) | 4.17 (10.68) | 8.96 (7.29) | 0.026* |
| Mean time of second-stage surgery, mins (SEM) | 140.00 (41.91) (n = 9) | 141.38 (42.19) (n = 8) | 165.00 (39.96) (n = 4) | 0.344* |
| Mean blood loss of second-stage surgery, ml (SEM) | 318.89 (164.50) (n = 9) | 293.75 (167.07) (n = 8) | 232.00 (40.87) (n = 4) | 0.555* |
-
HHS, Harris Hip Score; VAS, visual analogue scale pain score.
-
*
One-way analysis of variance.
-
†
Chi-squared test or Fisher’s exact test.
Comparison of spacer-related mechanical complications
The spacer-related complications in the three groups are shown in Table III. In terms of the incidence of complications associated with the antibiotic-loaded cement spacer, ten patients in group I had spacer-related complications, including five cases of spacer fracture (Figure 3e), three cases of spacer dislocation (Figure 3a), one case of periarticular fracture (Figure 3f), and three cases of acetabular/femoral bone wear. In group II, there were three cases of dislocation of the spacer, three cases of acetabular/femoral bone wear, and no cases of spacer fracture. Acetabular bone wear of a spacer using a small prosthesis as endoskeleton in group II was shown in the figure (Figures 3c and 3d), and a 58 mm cup and #7 femoral stem were used in second-stage surgery. In group III, there was one case of spacer dislocation (Figure 3b) and one case of periarticular fracture. Among these complications, the risk of mechanical complications associated with the articulating cement spacer in group III was significantly lower than that in groups I and II (p = 0.006, chi-squared test). The risk of spacer-related complications in group II was significantly lower than that in group I (p = 0.046, chi-squared test). All dislocation cases were successfully cured by closed reduction. All patients with spacer fractures and periarticular fracture rested in bed until second-stage surgery was performed, and no patients in the three groups underwent reoperation due to complications. However, three patients in group I and two patients in group II developed deep vein thrombosis due to long-term bed stay.
Table III.
Comparison of mechanical complications among the three groups.
| Parameter | Group I (n = 13) | Group II (n = 10) | Group III (n = 13) | p-value |
|---|---|---|---|---|
| Spacer fracture, n | 5 | 0 | 0 | 0.007* |
| Dislocation, n | 3 | 3 | 1 | 0.440* |
| Periarticular fracture, n | 1 | 0 | 1 | 1.0* |
| Acetabular/femoral bone wear, n | 3 | 3 | 0 | 0.597* |
| Reoperation due to complications, n | 0 | 0 | 0 | 0.597* |
| Overall, n | 10 | 6 | 2 | 0.006* |
-
*
Chi-squared test or Fisher’s exact test.
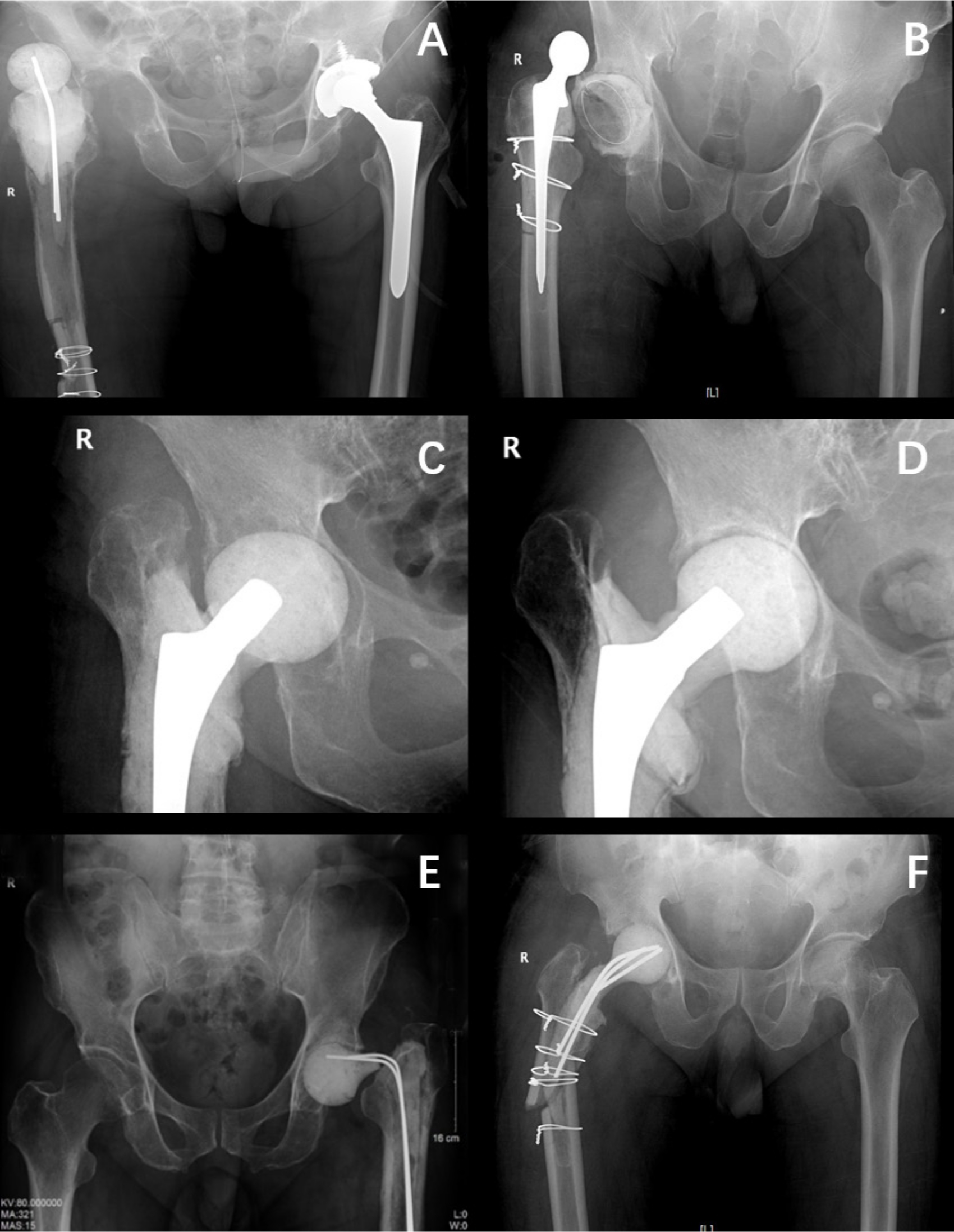
Fig. 3
Spacer-related mechanical complications. a) Dislocation of a spacer using Kirschner-wires (K-wires) as endoskeleton in group I. b) Dislocation of a spacer using cemented prosthesis as spacer in group III. c) and d) Acetabular bone wear of a spacer using a small prosthesis as endoskeleton in group II; a 58 mm cup and #7 femoral stem were used in second-stage surgery. e) Fracture of spacers using K-wires as endoskeleton in group I. f) Periarticular fracture of a spacer using K-wires as endoskeleton in group I.
Discussion
Currently, the mainstream treatment strategy in PJI two-stage revision is the use of an articulating spacer. The main drawback of preformed spacers (represented by spacer-G) is that the antibiotics used in this system are predetermined.14 In order to arrange individual antibiotics usage in bone cement according to pathogens, three types of custom-made articulating spacers were used in this study.
In group I, two 5 mm K-wires were added to the centre of the cement (Figures 1a and 1b). Because the hip joint is mainly subject to shear force, spacers with necks inevitably lead to stress concentration at the head-neck junction, so the risk of fracture of this type of spacer under full load is higher than that of other types.15,16 In this study, there were five cases of spacer fracture in group I; these did not affect the final curative effect, but the joint function significantly decreased. If the spacer breaks while the patient is walking, it may increase the risk of fracture around the spacer, as occurred in one patient in this study (Figure 3f). Therefore, patients with this type of spacer should be instructed to strictly use crutches for activities or to perform functional exercises only in bed to avoid or reduce weight-bearing and activity of the joint. Adopting these measures reduces the incidence of spacer fracture in patients using this kind of spacer but also reduces the patients’ quality of life during the interim period. Some authors have also suggested using a thickened intramedullary needle or other type of metal as an endoskeleton,7,17,18 in combination with an appropriate drug-addition ratio and vacuum-stirring technology to maintain the strength of the bone cement. In group II, a cemented prosthesis was used as skeleton to strengthen the spacers that were created (Figures 1c and 1d). This approach avoids the problem of spacer fracture but increases the size of the prosthesis. Sometimes it is difficult to implant this type of prosthesis in patients with small acetabular and medullary cavities. With the increase in the postoperative ROM, acetabular wear increases, resulting in the use of larger cups or even jumbo-sized cups in second-stage surgery. For instance, a 58 mm cup was used in second-stage surgery for one patient in group II, while only a 50 mm cup was used for primary arthroplasty (Figures 2d to 2f and Figures 3c and 3d).
The two above-described methods for producing custom-made cement spacers often do not match the actual conditions of patients because of the limits of the mould model. At the same time, to avoid spacer fracture and bone wearing patients are restricted from engaging weight-bearing activities, and joint function and quality of life in the interim cannot be guaranteed. Custom-made spacers such as PROSTALAC are recommended because they can be made in three sizes of standard-length femoral stem and three lengths of long stem to meet different host bones. It also has the advantage of maintaining length, allowing mobilization and full load.8 Since PROSTALAC prostheses are not available in our region, we have drawn lessons from this method and used less expensive cement prostheses as articulating spacers in group III. The placement method used during the operation was similar to that used for PROSTALAC prostheses (Figure 1e). Our study has proven that the hip joint function score and satisfaction in this group are generally higher than other groups after first-stage surgery. It should be noted that because of the use of a “deliberately bad placement technique” to facilitate its later removal, there is a risk of loosening or sinking of the cemented stem during the interim period, and the main problem is shortening and pain in the lower limbs. However, there were also five patients in group III in this study who declined the anticipated second-stage prosthesis reimplantation because they were satisfied with joint function with a type III spacer. Similar results could be seen in the research of Tsung et al,19 who described the use of a cement-coated Exeter stem as an antibiotic spacer, and 34 cases (44.7%) in their study retained their spacers. Although spacer-G (available in three sizes with head diameters of 46 mm, 54 mm, and 60 mm) and PROSTALAC have several sizes that can adapt to various host bones, the choices are still limited. The femoral prosthesis used in this study can be made in seven sizes with both standard length and long length. The polyethylene acetabular component can be made from a size range of 42 mm to 54 mm, which can fully adapt to the individual size of different patients. This suggests that our spacers could be a better option.
The main concern of cemented prothesis as spacer is the formation of biofilms when using polyethylene as the weight-bearing surface.20–22 However, it should be noted that there is no evidence that the biofilm formation rate of polyethylene is higher than that of polymethyl methacrylate.23 Through follow-up observations, most studies in the literature have reported that the eradication rate with this type of spacer is 89% to 96%, which is not lower than that of cement spacers.24,25 In our study, the overall eradication rate with these three types of spacers was 94.40% (n = 34), and there were no significant differences in the eradication rate among the groups. This rate is close to the eradication rate of the articulating spacers in various case series reported in the literature,10,19,26 indicating that the type of spacer may not affect the control of infection. Interestingly, there were no cases of infection recurrence in group III in our study, while there were two patients with recurrent infection in both group I and group II. The pathogens were multidrug-resistant gram-negative bacteria in these two patients. Although we used an antibiotic cement regimen that covered gram-negative bacteria, its thermal stability and drug release concentration may not be as good as that of vancomycin, which may be the cause of recurrence. Recurrence may not be related to the type of spacer but to the type of bacteria.
In this study, there were seven cases (26.09%) of spacer dislocation. The higher dislocation rates in groups I and II may be related to the fact that the spacer was made using a mould that was inconsistent with the residual acetabular bone, and in some cases there were severe acetabular defects. It has been reported that there is a 10% to 23% risk of dislocation in the treatment of hip joint infection with a mould-made spacer.27–29 In group III, there was only one case of dislocation, mainly due to weakness of the gluteus medius. Therefore, the occurrence of complications of dislocation can also be reduced by using cemented prostheses.
In conclusion, the use of an articulating antibiotic-loaded cement spacer as part of a two-stage revision strategy for treating hip PJI can yield good clinical results. This study compared directly the incidence of complications and the therapeutic effects of three types of antibiotic-loaded articulating cement spacers and found similar eradication rates; therefore, we believe that using cemented femoral prostheses and polyethylene sockets directly as a spacer leads to better functional outcomes during the interim period and a lower incidence of mechanical complications, and should be recommended. Moreover, this study suggests that all commercial cemented prostheses can be used as the spacer in the interim period.
References
1. Fink B , Vogt S , Reinsch M , Büchner H . Sufficient release of antibiotic by a spacer 6 weeks after implantation in two-stage revision of infected hip prostheses . Clin Orthop Relat Res . 2011 ; 469 ( 11 ): 3141 – 3147 . Crossref PubMed Google Scholar
2. Masri BA , Duncan CP , Beauchamp CP . Long-term elution of antibiotics from bone-cement: An in vivo study using the prosthesis of antibiotic-loaded acrylic cement (PROSTALAC) system . J Arthroplasty . 1998 ; 13 ( 3 ): 331 – 338 . Crossref PubMed Google Scholar
3. Pivec R , Naziri Q , Issa K , et al. Systematic review comparing static and articulating spacers used for revision of infected total knee arthroplasty . J Arthroplasty . 2014 ; 29 ( 3 ): 553 – 557 . Crossref PubMed Google Scholar
4. Fehring TK , Odum S , Calton TF , Mason JB . Articulating versus static spacers in revision total knee arthroplasty for sepsis . Clin Orthop Relat Res . 2000 ; 380 : 9 – 16 . Crossref PubMed Google Scholar
5. Emerson RH , Muncie M , Tarbox TR , Higgins LL . Comparison of a static with a mobile spacer in total knee infection . Clin Orthop Relat Res . 2002 ; 404 : 132 – 138 . Crossref PubMed Google Scholar
6. Romanò CL , Romanò D , Meani E , et al. Two-stage revision surgery with preformed spacers and cementless implants for septic hip arthritis: a prospective, non-randomized cohort study . BMC Infect Dis . 2011 ; 11 ( 1 ): 129 . Crossref PubMed Google Scholar
7. Barrack RL . Rush pin technique for temporary antibiotic-impregnated cement prosthesis for infected total hip arthroplasty . J Arthroplasty . 2002 ; 17 ( 5 ): 600 – 603 . Crossref PubMed Google Scholar
8. Younger AS , Duncan CP , Masri BA , McGraw RW . The outcome of two-stage arthroplasty using a custom-made interval spacer to treat the infected hip . J Arthroplasty . 1997 ; 12 ( 6 ): 615 – 623 . Crossref PubMed Google Scholar
9. Sandiford NA , Duncan CP , Garbuz DS , Masri BA . Two-stage management of the infected total hip arthroplasty . Hip Int . 2015 ; 25 ( 4 ): 308 – 315 . Crossref PubMed Google Scholar
10. Chalmers BP , Mabry TM , Abdel MP , et al. Two-Stage Revision Total Hip Arthroplasty With a Specific Articulating Antibiotic Spacer Design: Reliable Periprosthetic Joint Infection Eradication and Functional Improvement . J Arthroplasty . 2018 ; 33 ( 12 ): 3746 – 3753 . Crossref PubMed Google Scholar
11. Parvizi J , Zmistowski B , Berbari EF , et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society . Clin Orthop Relat Res . 2011 ; 469 ( 11 ): 2992 – 2994 . Crossref PubMed Google Scholar
12. Tsukayama DT , Estrada R , Gustilo RB . Infection after total hip arthroplasty . J Bone Jt Surg . 1996 : 512 – 523 . Google Scholar
13. Harris WH . Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation . J Bone Joint Surg Am . 1969 ; 51-A ( 4 ): 737 – 755 . PubMed Google Scholar
14. Magnan B , Regis D , Biscaglia R , et al. Preformed acrylic bone cement spacer loaded with antibiotics after total replacement . Acta Orthop Scand . 2001 ; 72 ( 6 ): 591 – 594 . Google Scholar
15. Citak M , Masri BA , Springer B , et al. Are Preformed Articulating Spacers Superior To Surgeon-Made Articulating Spacers in the Treatment Of PJI in THA? A Literature Review . Open Orthop J . 2015 ; 9 : 255 – 261 . Crossref PubMed Google Scholar
16. Faschingbauer M , Reichel H , Bieger R , Kappe T . Mechanical complications with one hundred and thirty eight (antibiotic-laden) cement spacers in the treatment of periprosthetic infection after total hip arthroplasty . Int Orthop . 2015 ; 39 ( 5 ): 989 – 994 . Crossref PubMed Google Scholar
17. Anagnostakos K . Therapeutic Use of Antibiotic-loaded Bone Cement in the Treatment of Hip and Knee Joint Infections . J Bone Jt Infect . 2017 ; 2 ( 1 ): 29 – 37 . Crossref PubMed Google Scholar
18. Jacobs C , Christensen CP , Berend ME . Static and mobile antibioticimpregnated cement spacers for the management of prosthetic joint infection . J Am Acad Orthop Surg . 2009 ; 17 ( 6 ): 356 – 368 . Google Scholar
19. Tsung JD , Rohrsheim JAL , Whitehouse SL , et al. Management of periprosthetic joint infection after total hip arthroplasty using a custom made articulating spacer (CUMARS); the Exeter experience . J Arthroplasty . 2014 ; 29 ( 9 ): 1813 – 1818 . Crossref PubMed Google Scholar
20. Shida T , Koseki H , Yoda I , et al. Adherence ability of Staphylococcus epidermidis on prosthetic biomaterials: an in vitro study . Int J Nanomedicine . 2013 ; 8 : 3955 – 3961 . Crossref PubMed Google Scholar
21. Lass R , Giurea A , Kubista B , et al. Bacterial adherence to different components of total hip prosthesis in patients with prosthetic joint infection . Int Orthop . 2014 ; 38 ( 8 ): 1597 – 1602 . Crossref PubMed Google Scholar
22. Pitto RP , Sedel L . Periprosthetic Joint Infection in Hip Arthroplasty: Is There an Association Between Infection and Bearing Surface Type? Clin Orthop Relat Res . 2016 ; 474 ( 10 ): 2213 – 2218 . Crossref PubMed Google Scholar
23. Gibon E , Córdova LA , Lu L , et al. The biological response to orthopedic implants for joint replacement. II: Polyethylene, ceramics, PMMA, and the foreign body reaction . J Biomed Mater Res B Appl Biomater . 2017 ; 105 ( 6 ): 1 – 15 . Crossref PubMed Google Scholar
24. Biring GS , Kostamo T , Garbuz DS , et al. Two-stage revision arthroplasty of the hip for infection using an interim articulated Prostalac hip spacer: a 10- to 15-year follow-up study . J Bone Joint Surg Br . 2009 ; 91-B ( 11 ): 1431 – 1437 . Crossref PubMed Google Scholar
25. Evans RP . Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method . Clin Orthop Relat Res . 2004 ; 427 : 37 – 46 . Crossref PubMed Google Scholar
26. Lee WY , Hwang DS , Kang C , et al. Usefulness of Prosthesis Made of Antibiotic-Loaded Acrylic Cement as an Alternative Implant in Older Patients With Medical Problems and Periprosthetic Hip Infections: A 2- to 10-Year Follow-Up Study . J Arthroplasty . 2017 ; 32 ( 1 ): 228 – 233 . Crossref PubMed Google Scholar
27. Durbhakula SM , Czajka J , Fuchs MD , Uhl RL . Spacer endoprosthesis for the treatment of infected total hip arthroplasty . J Arthroplasty . 2004 ; 19 ( 6 ): 760 – 767 . Crossref PubMed Google Scholar
28. Jung J , Schmid NV , Kelm J , et al. Complications after spacer implantation in the treatment of hip joint infections . Int J Med Sci . 2009 ; 6 ( 5 ): 265 – 273 . Crossref PubMed Google Scholar
29. Anagnostakos K , Duchow L , Koch K . Two-stage protocol and spacer implantation in the treatment of destructive septic arthritis of the hip joint . Arch Orthop Trauma Surg . 2016 ; 136 ( 7 ): 899 – 906 . Crossref PubMed Google Scholar
Author contributions
W. Zhang: Designed the study, Acquired, analyzed, and interpreted the data, Wrote and edited the manuscript.
X. Fang: Designed the study, Acquired, analyzed, and interpreted the data, Wrote and edited the manuscript.
T. Shi: Designed the study, Acquired, analyzed, and interpreted the data, Wrote and edited the manuscript.
Y. Cai: Acquired and analyzed the data, Edited the manuscript.
Z. Huang: Designed the study, Edited the manuscript.
C. Zhang: Designed the study, Edited the manuscript.
J. Lin: Designed the study, Edited the manuscript.
W. Li: Designed the study, Analyzed and interpreted the data, Edited the manuscript.
W. Zhang, X. Fang and T. Shi contributed equally to this work.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
No competing financial interests exist.
Acknowledgements
This study was supported by the Natural Science Foundation of Fujian Province (2018I0006) and the University Cooperation Project, which is supported by the Natural Science Foundation of Fujian Province (2018Y4003).
Ethical review statement
This study was approved by the ethics committee of the First Affiliated Hospital of Fujian Medical University and was carried out in accordance with the international standards for human experimental ethics, ethics no. [2014] 047.
W. Zhang, X. Fang and T. Shi contributed equally to this work.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.










