Abstract
Objectives
The objective of this study was to determine if the use of fascia lata as a tendon regeneration guide (placed into the tendon canal following harvesting the semitendinosus tendon) would improve the incidence of tissue regeneration and prevent fatty degeneration of the semitendinosus muscle.
Materials and Methods
Bilateral semitendinosus tendons were harvested from rabbits using a tendon stripper. On the inducing graft (IG) side, the tendon canal and semitendinosus tibial attachment site were connected by the fascia lata, which was harvested at the same width as the semitendinosus tendon. On the control side, no special procedures were performed. Two groups of six rabbits were killed at post-operative weeks 4 and 8, respectively. In addition, three healthy rabbits were killed to obtain normal tissue. We evaluated the incidence of tendon tissue regeneration, cross-sectional area of the regenerated tendon tissue and proportion of fatty tissue in the semitendinosus muscle.
Results
At post-operative week 8, the distal end of the regenerated tissue reached the vicinity of the tibial insertion on the control side in two of six specimens. On the IG side, the regenerated tissue maintained continuity with the tibial insertion in all specimens. The cross-sectional area of the IG side was significantly greater than that of the control side. The proportion of fatty tissue in the semitendinosus muscle on the IG side was comparable with that of the control side, but was significantly greater than that of the normal muscle.
Conclusions
Tendon tissue regenerated with the fascia lata graft was thicker than naturally occurring regenerated tissue. However, the proportion of fatty tissue in the semitendinosus muscle was greater than that of normal muscle.
Cite this article: K. Tabuchi, T. Soejima, H. Murakami, K. Noguchi, N. Shiba, K. Nagata. Inducement of tissue regeneration of harvested hamstring tendons in a rabbit model. Bone Joint Res 2016;5:247–252. DOI: 10.1302/2046-3758.56.2000585.
Article focus
-
We hypothesised that using fascia lata tissue as a tendon regeneration guide and placing it into the tendon canal after harvesting the semitendinosus tendon would improve the incidence of tendon tissue regeneration, as well as result in a close-to-normal anatomical shape and prevent fatty degeneration of the muscle parenchyma in the associated harvested tendon.
Key messages
-
The tissue regenerated using this method was thicker than naturally occurring regenerated tissue.
-
Regardless of whether the fascia lata was implanted as a guide for regenerated tissue, the infiltration of interfibrous fat into the semitendinosus muscle was greater than that in normal muscle.
Strengths and limitations
-
Strengths: The regenerated tissue in this method was evaluated in detail both macroscopically and histologically.
-
Limitations: The mechanical strength of the regenerated harvested tissue was not assessed.
Introduction
The medial hamstring tendon is widely used as a source of grafts for anterior cruciate ligament (ACL) reconstruction. The high probability of regeneration of a harvested semitendinosus tendon was first reported by Cross et al1 in 1992. Based on the number of studies conducted subsequently, the regeneration of harvested hamstring tendons is a widely known phenomenon.2-4
In humans, the semitendinosus tendon has been demonstrated to regenerate in 63% to 82% of ACL reconstructions using hamstring tendons.2,4-6 However, decreased strength during deep knee flexion has been reported following ACL reconstructions using the medial hamstring tendon, indicating that the adverse effects of harvesting the hamstring tendon cannot be ignored.5,7 A possible reason for this limited recovery could be that many regenerated semitendinosus tendons do not achieve anatomic insertion of the pes anserinus, but instead only reach the popliteal fascia.1-3 In addition, muscle fatty degeneration occurs after the tendon is harvested.8,9 To prevent these adverse transformations, artificial inducement of regeneration has been attempted in clinical practice in recent years. To artificially guide the regeneration of the semitendinosus tendon to the pes anserinus, Murakami et al10 devised an operative procedure in which the sections of aponeurosis continuing from the branch of the semitendinosus tendon and leading to the crural fascia, which are usually discarded in the graft preparation process, are re-grafted into the canal of the tendon from which the graft was harvested. Following this procedure, MRI illustrated that the regenerated tendon reached the pes anserinus in all cases.
In the present study, we investigated whether artificial inducement of tendon regeneration reduces tissue degeneration caused by harvesting of the semitendinosus tendon. With this aim, we performed an experiment with rabbits, using the methods of Murakami et al10 to graft free fascia lata tissue after harvesting the semitendinosus tendon. We conducted both macroscopic and histological observations. We hypothesised that using fascia lata tissue as the guide for tendon regeneration and placing it into the tendon canal after harvesting the semitendinosus tendon would improve the incidence of tendon tissue regeneration, as well as result in a close-to-normal anatomical shape and prevent fatty degeneration of the semitendinosus muscle.
Materials and Methods
The present study was approved by the ethics board of our hospital. A total of 15 male Japanese white rabbits were used in the present study. The rabbits were anaesthetised intravenously with 0.025 mg/kg Nembutal (Hembtal, Osaka, Japan). We created 20 mm vertical incisions in the skin parallel to the femoral shaft in the lateral aspects of both thighs, with the superior border of the patella serving as the distal end. The fascia lata was harvested in three 60 mm2 sections that were used as grafts for inducing tissue regeneration (Fig. 1). Next, 10 mm skin incisions parallel to the tibial shaft were made on the medial sides of both knees. Vertical incisions of the crural fascia were made along the medial collateral ligament. We identified the semitendinosus tendon running through the deep layer of the semimembranosus muscle. The semitendinosus tendon was resected 3 mm from the tibial attachment site using a scalpel and a section was harvested with a tendon stripper designed for use in rabbits. In the right knee, one end of the fascia lata, which was used as the inducing graft (IG), was connected to the distal end of the remaining 3 mm of the semitendinosus tendon with 4-0 nylon thread (ELP, Akiyama Medical, Tokyo, Japan). The other end of the fascia lata was pulled into the tendon canal after harvesting the semitendinosus tendon. At this step, the tendon canal and tibial attachment site were connected by the IG (Fig. 2). In the left knee, no special procedures were performed after the semitendinosus tendon was harvested. The fascia and skin were sutured with 4-0 nylon thread. Moreover, a suitable dose of lidocaine hydrochloride (Xylocaine Injection 2%, AstraZeneca K.K., Osaka, Japan) was also administered via subcutaneous injection at the skin incision site as a post-operative analgesic. After the surgery, we performed no particular external fixation and allowed the rabbits to roam freely in their cages.

Fig. 1
Image of a harvested fascia lata to be used as the inducing graft (IG) and the harvested semitendinosus tendon (ST).
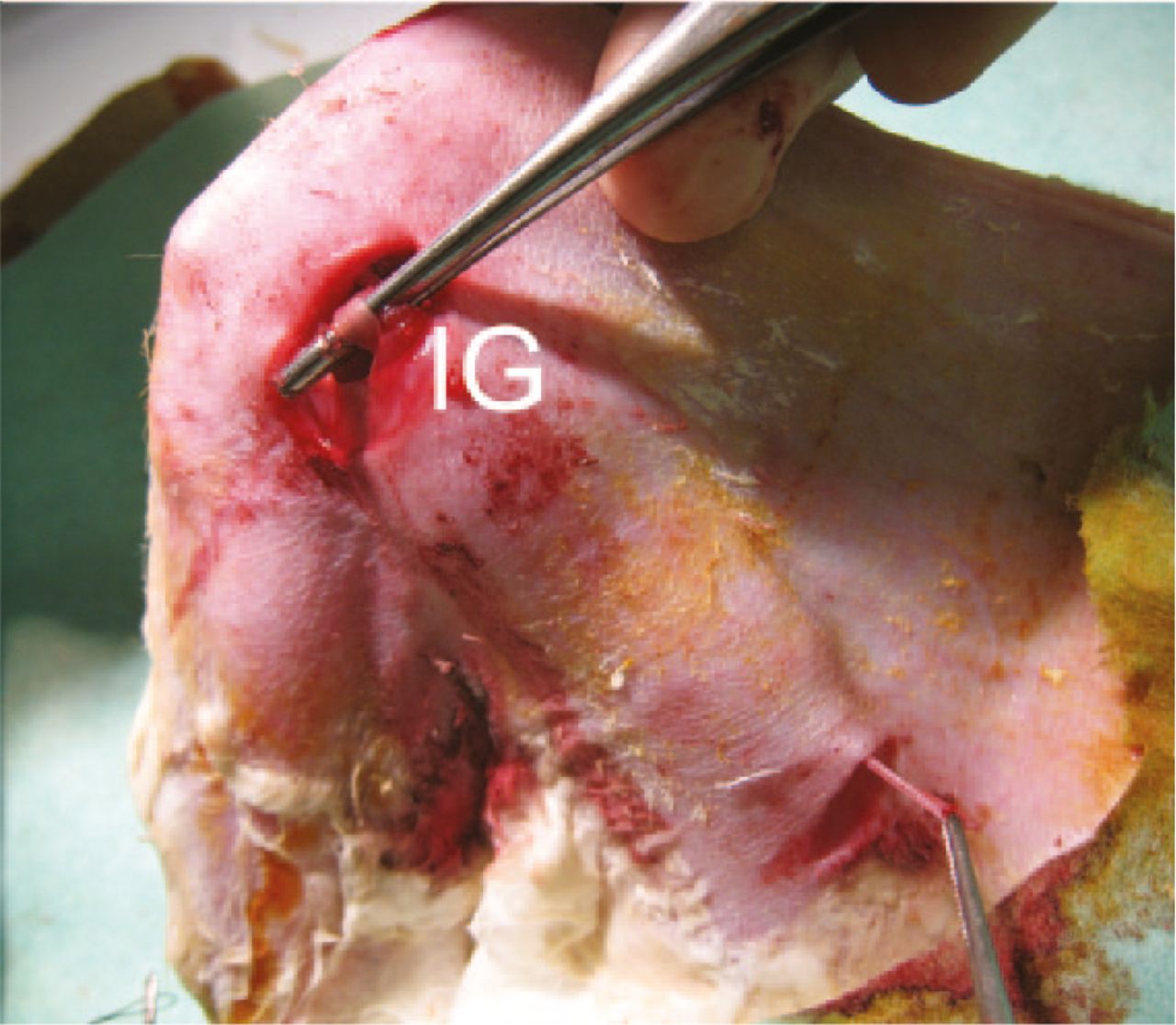
Fig. 2
Image of the fascia lata inducing graft (IG) placed into the tendon canal after harvesting the semitendinosus tendon.
Preparation of the specimens
In total, six rabbits were killed at post-operative week 4 and six at week 8. We macroscopically observed the presence or absence of regenerated tissue after semitendinosus harvesting. We then harvested the regenerated tissue and semitendinosus muscles. The harvested tissue was immediately fixed for 48 hours in 10% neutral buffered formalin. After fixation, the tissue was divided into the central portion of regenerated tissue and semitendinosus muscle belly. Each specimen was dehydrated and embedded in paraffin. In addition, three healthy rabbits matched by weight were killed to obtain normal tissue. Normal semitendinosus muscle and tendon tissues were harvested from both legs of two of the rabbits and from one leg of the third rabbit. The remaining leg was used immediately after an IG transplant to obtain macroscopic images in order to explain where the IG was transplanted.
We prepared the following tissue sections, each with a thickness of 5 μm: transverse and longitudinal sections of the central portion of the regenerated tissue and a transverse section of the semitendinosus muscle belly. These sections were observed via light microscopy using haematoxylin-eosin staining. ImageJ software (National Institutes of Health, Bethesda, Maryland) was used to measure the areas of the transverse sections (magnification, ×40) of the central portions of the regenerated tissue of the specimens at post-operative week 8. To assess muscle degeneration in transverse sections (magnification, ×100) of the semitendinosus muscle belly, the proportion of fatty tissue in the muscle (volume of fatty infiltration) was measured using ImageJ. For comparisons with normal tissue, we measured the areas of the transverse sections of the normal semitendinosus tendons (n = 6) and the percentage of fatty tissue in the normal semitendinosus muscle belly (n = 5).
The right knee was referred to as the IG side, the left knee was referred to as the control side, and the normal tendon and normal muscle belly comprised the normal side. One-way analysis of variance was used to compare the three groups in terms of the tissue section areas of the regenerated tissue/normal tendon and fatty interstitial infiltration into the semitendinosus muscle belly. The Tukey–Kramer multiple comparison test was used for post hoc comparisons. The level of statistical significance was set at p ⩽ 0.05. JMP version 9.0 software (SAS Institute Inc., Cary, North Carolina) was used in the analysis.
Results
Macroscopic findings
On the control side, regenerated funicular tissue was observed at the site from which the semitendinosus tendon was harvested. This tissue originated from the cut stump of the semitendinosus muscle belly, from which the tendon was removed, and ran through the surface of the semimembranosus muscle to the popliteal fossa. This course resembled that of a normal semitendinosus tendon (Fig. 3). However, unlike a normal tendon, the regenerated tissue adhered to the surrounding semimembranosus muscle belly. Regenerated tissue was not observed in every specimen. Specifically, it was observed in two specimens at post-operative week 4 and five specimens at post-operative week 8. No regenerated tissue was observed in any of the remaining specimens. In two of the five specimens in which regenerated tissue was observed at post-operative week 8, the distal end of the regenerated tissue reached the vicinity of the tibial anatomical insertion site. In the remaining three specimens, the regenerated tissue ended at the semimembranosus muscle belly without reaching the tibia.
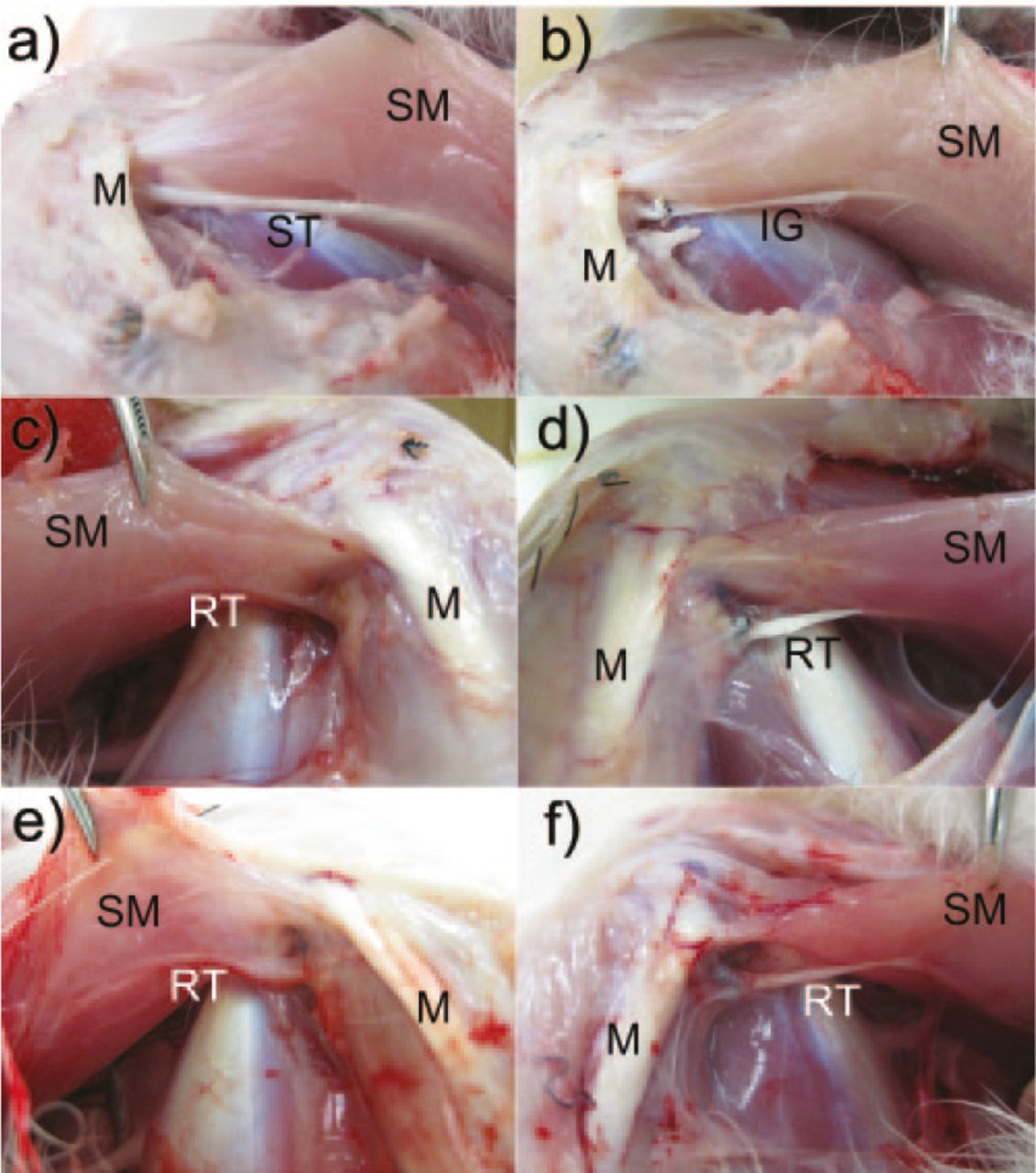
Fig. 3
Image of a) a normal semitendinosus tendon; b) inducing graft (IG) immediately after grafting; c) regenerated tissue on the control side at post-operative week 4; d) regenerated tissue on the IG side at post-operative week 4; e) regenerated tissue on the control side at post-operative week 8; and f) regenerated tissue on the IG side at post-operative week 8. (ST, semitendinosus tendon; SM, semimembranosus muscle; M, medial contralateral ligament; RT, regenerated tissue).
On the IG side, in all specimens, the IG adhered to the cut stump of the semitendinosus muscle belly on the proximal side, whereas the IG maintained continuity with the tibial side of the semitendinosus tendon on the distal end. The appearance of the IG differed from that at the time of grafting. Specifically, the IG was enveloped by the regenerated tissue, and it had become round and thick (Fig. 3).
Histological findings
On the control side at post-operative week 4, a dense group of cells was observed within the regenerated tissue. An extracellular arrangement of fibrous tissue with directionality was also observed. On the control side at post-operative week 8, a dense group of cells was observed within the regenerated tissue, similar to that observed at post-operative week 4. However, the extracellular arrangement of fibrous tissue had become more dense by this time.
On the IG side at post-operative week 4, fibrous tissue surrounded the IG, forming an oval/round structure. The central portion of the IG consisted of dense fibrous tissue in which cells were difficult to see. On the IG side at post-operative week 8, a large number of cells had infiltrated the central portion. In addition, the surrounding fibrous tissue had become more dense than that at post-operative week 4 (Fig. 4).
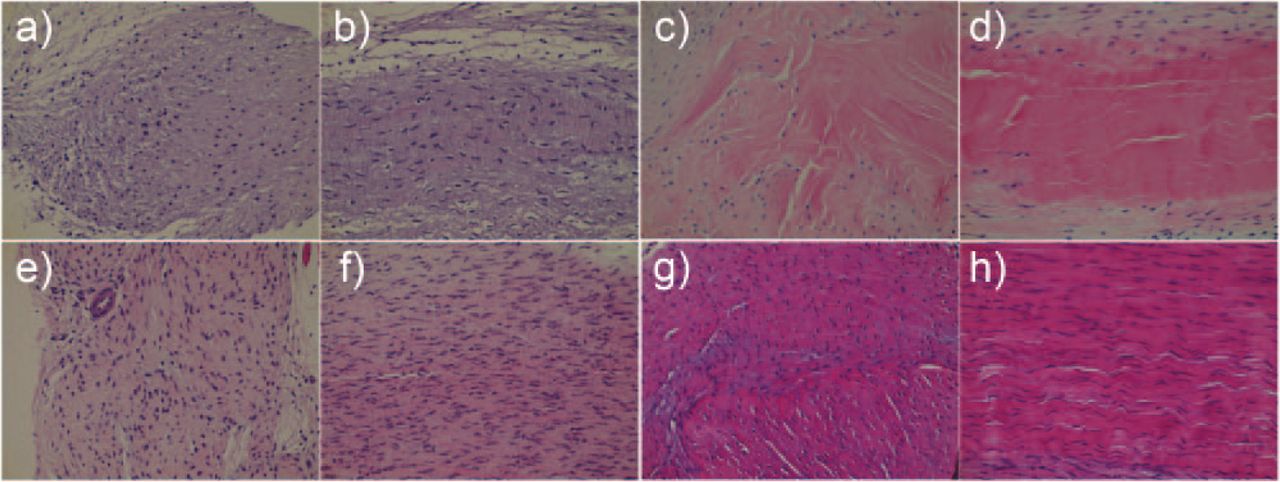
Fig. 4
Histological images of the regenerated tissue: a) and b) control side at post-operative week 4; c) and d) inducing graft (IG) side at post-operative week 4; e) and f) control side at post-operative week 8; g) and h) IG side at post-operative week 8; a), c), e), and g) are transverse sections, b), d), f), and h) are longitudinal sections. The magnification is ×400.
At post-operative week 8, the tissue section areas of the regenerated tissue were 1.108 mm2 (sd 0.524), 0.393 mm2 (sd 0.153), and 1.53 mm2 (sd 0.25) on the IG, control, and normal sides, respectively. Significant differences were observed between the normal and control sides and between the IG and control sides. However, no significant difference was observed between the normal and IG sides (Table I).
Table I.
Tissue section areas of the regenerated tissue/normal tendon and fatty interstitial infiltration into the semitendinosus muscle belly at post-operative week 8
| IG side (n = 6) | Control side (n = 6) | Normal side (n = 5) | p-value |
|||
|---|---|---|---|---|---|---|
| Normal vs Control | IG vs Control | Normal vs IG | ||||
| Tissue section areas of the regenerated tissue, mean sd, mm2 | 1.108 sd 0.524 | 0.393 sd 0.153 | 1.530 sd 0.250 | 0.0003 | 0.0133 | 0.1317 |
| Fatty interstitial infiltration into the semitendinosus muscle belly, mean sd, % | 30.7 sd 11.7 | 34.2 sd 15.4 | 6.16 sd 2.25 | 0.0052 | 0.8763 | 0.0125 |
-
IG, inducing graft; sd, standard deviation
At post-operative week 8, the percentages of interfibrous fat in the transverse sections of the semitendinosus muscle belly were 30.7% (sd 11.7%), 34.2% (sd 15.4%), and 6.16% (sd 2.25%) on the IG, control, and normal sides, respectively. Significant differences were observed between the normal and control sides and between the normal and IG sides. However, no significant difference was observed between the control and IG sides (Table I). The volume of fatty tissue was significantly larger on both the control and IG sides than on the normal side.
Discussion
When harvesting an autologous semitendinosus tendon for ACL reconstruction, tendon regeneration occurs. However, this regeneration is not complete, and it leads to muscle retraction and fatty degeneration of the muscle parenchyma. Thus, to ensure more complete and effective tendon regeneration, the authors of the present study placed the fascia lata into the tendon canal after the semitendinosus tendon was harvested. With this procedure, continuity of the IG with the semitendinosus muscle and tibial attachment site was maintained in all specimens, and the regenerated tissue surrounded the IG. Thus, the results supported the first hypothesis, which stated that using the fascia lata as a tendon regeneration guide and placing it into the tendon canal after tendon harvesting would improve the incidence of tendon regeneration and guide the tendon regeneration towards its original anatomical insertion site. However, the second hypothesis, which stated that degeneration of the semitendinosus muscle would be prevented, was rejected.
Gill et al11 harvested semitendinosus tendons in rabbit models using a tendon stripper and assessed tendon regeneration upon sacrificing the rabbits after nine to 12 months. Regenerated tendons were observed in 84% of the rabbits. However, almost all of the regenerated tendons were inserted more superficially than normal, and the thickness of the tendons varied. In addition, Eriksson et al2 described the mechanism of tendon regeneration as follows; the haematoma that develops in the tendon canal after tendon harvesting becomes the scaffold of fibroblast progenitor cells; these fibroblast progenitor cells infiltrate the scaffold, resulting in further proliferation and differentiation, which in turn promotes the production of collagen, through which the tendon is regenerated. Rispoli et al12 concluded that tendons regenerate along the fascial tract (along which the semitendinosus tendon runs) in the proximal to distal direction. Furthermore, Carafino and Fulkerson13 stated that the gracilis tendon helps the regenerated tissue reach the pes anserinus and induces anatomical insertion. Thus, the source of the regenerated tendon is considered to be fibrous tissue that forms in the tendon canal after the tendon is harvested and extends along the surrounding structures to the distal end.
On the control side in the present study, regenerated tissue that originated from the stump of the semitendinosus muscle belly and ran through the surface of the semimembranosus muscle to the popliteal fossa was observed in 33% (two out of six) of the specimens at post-operative week 4, and 83% (five out of six) of the specimens at post-operative week 8. However, only in 33% (two out of six) of the specimens did the distal end of the regenerated tissue ultimately reach the vicinity of the normal tibial attachment site while adhering to the surrounding semitendinosus muscle belly. The regenerated tissue ended at the semimembranosus muscle belly in 50% (three out of six) of the specimens, whereas regeneration did not occur in 17% (one out of six) of the specimens. In addition, the tissue section area of the regenerated tissue was only 25.6% of that of normal tissue, which was significantly smaller than that on the normal side. Meanwhile, on the IG side, the IG maintained continuity with the semitendinosus muscle and tibial attachment site in all specimens. By post-operative week 8, the surrounding regenerated tissue had adhered to and coated the IG.
In histological terms, the IG temporarily lost nearly all of its cell components at post-operative week 4. However, cell infiltration from the side of the regenerated tissue, which coats the IG that extends from the semitendinosus fascial side, was observed at post-operative week 8. In addition, no significant difference in the tissue section area of the regenerated tissue was observed between the IG and normal sides. However, the thickness of the regenerated tissue was 2.8-fold greater on the IG side than on the control side.
Thus, we demonstrated that the IG is a useful guide for inducing tendon regeneration. At the same time, the biological reactions of necrosis and cell infiltration occur in the IG, and the IG becomes the biological scaffold of the regenerated tendon. Regarding post-tenotomy muscle changes, Meyer et al8 loosened the infraspinatus tendon in sheep models by approximately 5 cm, followed by retention 40 weeks later. Various irreversible changes were reported at week 35 after retention (75 weeks after loosening), such as an increased pennation angle of the muscle fibres, gap formation in the muscle fibres, and infiltration of fatty tissue (33%, sd 8.7%) and fibrous tissue (12.9%, sd 3.2%). In addition, Frey et al9 stated that post-tenotomy muscle retraction results in differentiation of muscle-derived stem cells into adipocytes. In human clinical trials, semitendinosus muscle retraction is known to occur as a consequence of harvesting the semitendinosus tendon.14,15 The present study also found that the semitendinosus muscle retracted after the tendon graft was harvested (data not shown). At post-operative week 8, the percentage of interfibrous fat in the transverse sections of the semitendinosus muscle belly on the control side was 34.2% (sd 15.4%). This result is fully consistent with the findings reported by Meyer at al.8 In the present study, the percentage of interfibrous fat in the transverse sections of the semitendinosus muscle belly on the IG side was 30.7% (sd 11.7%), nearly identical to that on the control side, which was not expected. Compared with that on the normal side (6.16%, sd 2.25%), the percentage of interfibrous fat amounted to a 5.6-fold increase on the control side and a fivefold increase on the IG side. Although no significant difference was observed between the control and IG sides, both sides had a significantly higher percentage of fatty tissue than the normal side. These results indicate that the IG had no effect on muscle retraction or muscle fatty degeneration occurring after the tendon was harvested.
The present study had some limitations. The first was the small number of outcome measures. Second, the observation period was short. It is possible that different results pertaining to fatty degeneration of the attachment site or muscle of the regenerated tissue would have been obtained from a longer experiment. However, macroscopic and histological findings revealed differences between the IG and control sides during the eight-week observation period. Third, our model was a small animal that does not possess the same anatomical hamstring tendon structure as humans. Nonetheless, the results of the present study pertaining to the incidence of regenerated tendons, the form of those tendons, and the degeneration of muscle tissue were consistent with those of previous studies using human and animal models larger than rabbits. Therefore, we believe that the results of the present study are valid. Fourth, because the present study was an animal experiment, the muscle strength of the muscle-regenerated tendon complex was not evaluated. Regarding this limitation, examination of muscle strength using a method such as electrical stimulation may be necessary in the future. Fifth, the mechanical strength of the regenerated harvested tissue was not assessed. Thus, an additional mechanical experiment may be necessary in the future. Nevertheless, the mechanical properties of regenerated tissues (mechanical strength per tissue section area) are normally inferior to those of normal tissues. Therefore, if the tissue section area is the same, then it may be valid to assume that the mechanical strength of regenerated tissues is lower than normal.
In conclusion, we used a fascia lata graft as a guide for the regeneration of tissue produced after harvesting the semitendinosus tendon. The grafted fascia guided the regenerated tendon from the stump of the muscle from which the tendon was removed to the tibia. As a result, the tissue regenerated using this method was thicker than naturally occurring regenerated tissue. However, regardless of whether the fascia was implanted as a guide for tissue regeneration, the infiltration of interfibrous fat into the semitendinosus muscle was greater than that observed in normal muscle. In the future, the possibility of enabling regenerated tendons to become histologically normal tendons that possess sufficient strength to withstand use as a graft material should be investigated.
Funding Statement
The authors have no sources of financial support.
ICMJE conflict of interest
None declared.
References
1 Cross MJ , RogerG, KujawaP, AndersonIF. Regeneration of the semitendinosus and gracilis tendons following their transection for repair of the anterior cruciate ligament. Am J Sports Med1992;20:221-223.CrossrefPubMed Google Scholar
2 Eriksson K , LarssonH, WredmarkT, HambergP. Semitendinosus tendon regeneration after harvesting for ACL reconstruction. A prospective MRI study. Knee Surg Sports Traumatol Arthrosc1999;7:220-225.CrossrefPubMed Google Scholar
3 Papandrea P , VulpianiMC, FerrettiA, ConteducaF. Regeneration of the semitendinosus tendon harvested for anterior cruciate ligament reconstruction. Evaluation using ultrasonography. Am J Sports Med2000;28:556-561.CrossrefPubMed Google Scholar
4 Nakamura E , MizutaH, KadotaM, et al.. Three-dimensional computed tomography evaluation of semitendinosus harvest after anterior cruciate ligament reconstruction. Arthroscopy2004;20:360-365.CrossrefPubMed Google Scholar
5 Tadokoro K , MatsuiN, YagiM, et al.. Evaluation of hamstring strength and tendon regrowth after harvesting for anterior cruciate ligament reconstruction. Am J Sports Med2004;32:1644-1650.CrossrefPubMed Google Scholar
6 Okahashi K , SugimotoK, IwaiM, et al.. Regeneration of the hamstring tendons after harvesting for arthroscopic anterior cruciate ligament reconstruction: a histological study in 11 patients. Knee Surg Sports Traumatol Arthrosc2006;14:542-545.CrossrefPubMed Google Scholar
7 Nakamura N , HoribeS, SasakiS, et al.. Evaluation of active knee flexion and hamstring strength after anterior cruciate ligament reconstruction using hamstring tendons. Arthroscopy2002;18:598-602.CrossrefPubMed Google Scholar
8 Meyer DC , HoppelerH, von RechenbergB, GerberC. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res2004;22:1004-1007.CrossrefPubMed Google Scholar
9 Frey E , RegenfelderF, SussmannP, et al.. Adipogenic and myogenic gene expression in rotator cuff muscle of the sheep after tendon tear. J Orthop Res2009;27:504-509.CrossrefPubMed Google Scholar
10 Murakami H , SoejimaT, InoueT, et al.. Inducement of semitendinosus tendon regeneration to the pes anserinus after its harvest for anterior cruciate ligament reconstruction-A new inducer grafting technique. Sports Med Arthrosc Rehabil Ther Technol2012;4:17.CrossrefPubMed Google Scholar
11 Gill SS , TurnerMA, BattagliaTC, et al.. Semitendinosus regrowth: biochemical, ultrastructural, and physiological characterization of the regenerate tendon. Am J Sports Med2004;32:1173-1181.CrossrefPubMed Google Scholar
12 Rispoli DM , SandersTG, MillerMD, MorrisonWB. Magnetic resonance imaging at different time periods following hamstring harvest for anterior cruciate ligament reconstruction. Arthroscopy2001;17:2-8.CrossrefPubMed Google Scholar
13 Carofino B , FulkersonJ. Medial hamstring tendon regeneration following harvest for anterior cruciate ligament reconstruction: fact, myth, and clinical implication. Arthroscopy2005;21:1257-1265.CrossrefPubMed Google Scholar
14 Nishino A , SanadaA, KanehisaH, FukubayashiT. Knee-flexion torque and morphology of the semitendinosus after ACL reconstruction. Med Sci Sports Exerc2006;38:1895-1900.CrossrefPubMed Google Scholar
15 Nakamae A , DeieM, YasumotoM, et al.. Three-dimensional computed tomography imaging evidence of regeneration of the semitendinosus tendon harvested for anterior cruciate ligament reconstruction: a comparison with hamstring muscle strength. J Comput Assist Tomogr2005;29:241-245.CrossrefPubMed Google Scholar










