Abstract
Objectives
Injectable Bromelain Solution (IBS) is a modified investigational derivate of the medical grade bromelain-debriding pharmaceutical agent (NexoBrid) studied and approved for a rapid (four-hour single application), eschar-specific, deep burn debridement. We conducted an ex vivo study to determine the ability of IBS to dissolve-disrupt (enzymatic fasciotomy) Dupuytren’s cords.
Materials and Methods
Specially prepared medical grade IBS was injected into fresh Dupuytren’s cords excised from patients undergoing surgical fasciectomy. These cords were tested by tension-loading them to failure with the Zwick 1445 (Zwick GmbH & Co. KG, Ulm, Germany) tension testing system.
Results
We completed a pilot concept-validation study that proved the efficacy of IBS to induce enzymatic fasciotomy in ten cords compared with control in ten cords. We then completed a dosing study with an additional 71 cords injected with IBS in descending doses from 150 mg/cc to 0.8 mg/cc. The dosing study demonstrated that the minimal effective dose of 0.5 cc of 6.25 mg/cc to 5 mg/cc could achieve cord rupture in more than 80% of cases.
Conclusions
These preliminary results indicate that IBS may be effective in enzymatic fasciotomy in Dupuytren’s contracture.
Cite this article: Dr G. Rubin. A new bromelain-based enzyme for the release of Dupuytren’s contracture: Dupuytren’s enzymatic bromelain-based release. Bone Joint Res 2016;5:175–177. DOI: 10.1302/2046-3758.55.BJR-2016-0072.
Article focus
-
The aim of this study was to investigate the ability of injectable bromelain solution to dissolve-disrupt Dupuytren’s cords in an ex vivo study.
Key messages
-
The study indicates that injectable bromelain solution may be effective in enzymatic fasciotomy in Dupuytren’s contracture.
Strengths and limitations
-
This is the first preliminary report of a new potential use of injectable bromelain solution.
-
A limitation of this study is that it is an ex vivo model based on human excised Dupuytren’s cords that varied in thickness, strength and the presence of nodules, all of which may interfere with the results.
Introduction
Dupuytren’s contracture (DC) is a connective tissue disorder characterised by contractile palmar aponeurosis leading to shortening and progressive digital flexion deformity. Open partial fasciectomy has been the mainstay of treatment for DC. In recent years, there has been an increased interest in less invasive approaches, including sharp needle cordotomy/fasciotomy and enzymatic disruption by collagenase injection.1 Collagenase clostridium histolyticum is the first non-surgical, pharmacological treatment for DC with a palpable cord, approved for use in the United States (2010) and Europe (2011). Clinical trials2-8 and post-marketing studies9 have demonstrated the efficacy and favourable safety profiles of collagenase injection for treating DC.
NexoBrid is a bromelain-derivate enzyme mixture extracted from pineapple stems.10,11 It has been developed, tried and approved as an effective, fast, eschar-specific and safe debriding pharmaceutical preparation for deep burn eschar removal.12-15 The information generated during the NexoBrid development programme served to develop an injectable bromelain solution (IBS), which is a possible new approach to DC cords.
The aim of this study was to investigate the ability of IBS to dissolve-disrupt Dupuytren’s cords in an ex vivo study.
Materials and Methods
Material
Lyophilised injectable bromelain-based enzyme (MediWound Ltd, Yavne, Israel) was prepared and weighed in vials. The enzyme is dissolved in a given volume of vehicle to predefined doses. All materials are sterile medical grade pharmaceuticals.1
Tissue preparation
The first phase, concept validation study, basically followed an in vitro model that served to develop the collagenase DC enzymatic fasciotomy studies.16 After receiving Institutional Helsinki Ethical Review Board Committee approval and patient informed consent, ten fresh Dupuytren’s cords were dissected from patients undergoing routine fasciectomy. The specimens were divided in half across their long axis and were anchored to one polypropylene (Prolene) suture (Ethicon, Somerville, New Jersey) by the locking-loop, with the Krackow suture technique at both ends.17 The mean tensile strength of the suture is 8.5 sd 0.5 kg.16 Each half was injected in the middle of the cord either with 0.5 mL of 100 mg/cc dose of IBS or with a control saline injection of 0.5 mL total volume, by using a 30-gauge needle. The cords were then placed in a humidifying chamber and incubated at 37° for 24 hours before being submitted to tensile strength testing. In the second phase, we conducted a dosing study on 71 fresh cords excised from Dupuytren’s patients which were injected with descending doses of IBS from 150 mg/cc to 0.8 mg/cc (Table I).
Table I.
Dose response assessment table.
| Dose (mg/cc) | n | % cord disruption success |
|---|---|---|
| 0.8 | 8 | 37.5 |
| 1.7 | 3 | 0 |
| 3.12 | 7 | 71.43 |
| 5 | 5 | 100 |
| 6.25 | 19 | 78.95 |
| 9.375 | 4 | 50 |
| 12.5 | 7 | 57.14 |
| 25 | 5 | 75 |
| 37.5 | 2 | 100 |
| 50 | 4 | 75 |
| 75 | 4 | 50 |
| 100 | 11 | 100 |
| 150 | 2 | 100 |
Mechanical testing
The cords were connected to a mechanical testing device, Zwick 1445 (Zwick GmbH & Co. KG, Ulm, Germany) via the Prolene suture and a load was applied at a constant rate of 20 mm/min displacement of the cord ends until cord or suture rupture.
Statistical analysis
In the pilot phase, Fisher’s exact test was used to explore the association between the study groups (IBS injection/control) and cord disruption. In the second study, we used the Cochran-Armitage test to explore the relationship between IBS and the probability of cord disruption.
The statistical analyses were performed using SAS 9.2. Statistical significance was considered when p < 0.05.
Results
In the pilot phase, cords had complete disruption in ten of the ten specimens in the IBS injection group. In many cases, the cords were completely dissolved to the point that, by holding one end, the other end ‘dripped’ down by its own weight. The thread-tearing threshold of 8.5 kg (sd 0.5) became the success threshold of the enzymatic efficacy. In none of the control specimens did the cords/threads break (Fisher’s exact test; p < .0001). The cords demonstrated a similar stress/elongation graph in each group (Figs 1a and 1b). In the second, multiple-dose range study, 71 cords were injected with IBS in descending doses from 150 mg/cc to0.8 mg/cc. The dosing study demonstrated that the minimal effective dose of 0.5 cc of 6.25 mg/cc to 5 mg/cc can achieve cord rupture in more than 80% of cases. The Cochran-Armitage test supports the trend hypothesis (p = 0.0021), indicating that the probability for cord rupture increases as the dose increases.
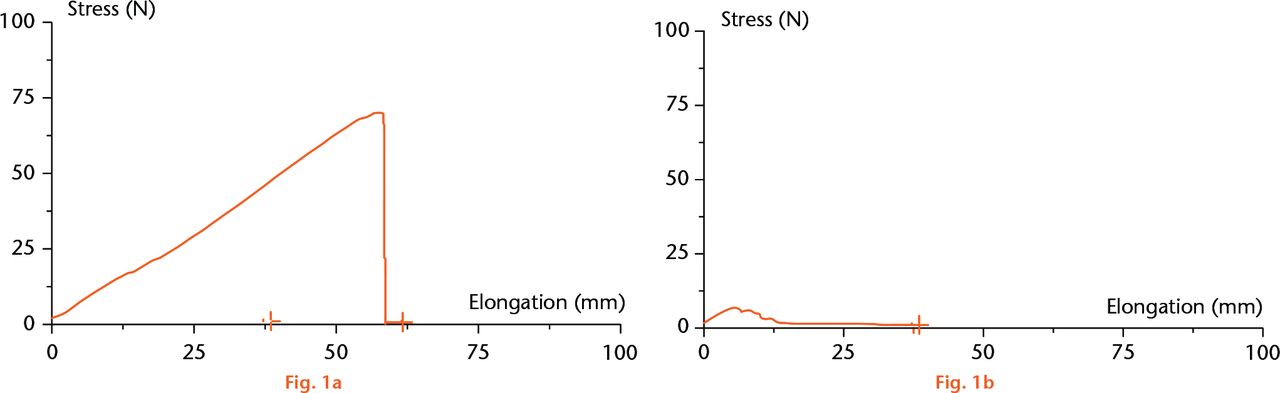
Fig.
Stress/elongation graph of the control group (a) and the study group (b).
Discussion
DC develops as a result of increased and uncontrolled fibroblast proliferation, as well as due to the deposition of type II and type V collagen and glycosaminoglycans in the palmar aponeurosis.18 The cause of this fibrosis has not yet been elucidated. The treatment is traditionally surgery, meticulously exposing the fibrotic fascia and excising the constricting cords, thereby releasing the flexure-contracture. These procedures are demanding of skills and resources with quite a long convalescence period, mostly due to the wide dissection field, surgically-related adverse events and high recurrence rates (12% to 39%, with a mean follow-up time of 1.5 to 7.3 years).19 In order to reduce surgical trauma, enzymatic fasciotomy for DC has previously been tried. It was first introduced by Hueston in 1971, using a mixture of trypsin, hyaluronidase, and lidocaine injected into the cord during surgery.20 McCarthy21 reported long-term results with the same mixture; he noticed recurrence in seven of nine of his patients in two to three years. The options for treating patients with DC have broadened with the introduction of collagenase.2,4
IBS consists of a sterile, lyophilised, partially purified proteolytic protein mixture with specific enzymatic activity, derived from specially prepared medical grade bromelain raw material extracted from pineapple plant stems. The enzyme should be dissolved in a vehicle before injection. Bromelain, a clinically used natural pineapple product, has some reported anti-inflammatory and immunomodulatory activities;22,23 these anti-inflammatory activites, alongside its proteolytic properties, may also help reduce the recurrence rate of DC.
A limitation of this study is that it is an ex vivo model based on human excised DC that varied in thickness, strength and the presence of nodules, all of which may interfere with the results. The use of cord fixation by Prolene suture and Krackow locking-loop of mean tensile strength 8.5 sd 0.5 kg established the success/failure upper threshold of this force. Nevertheless, this is the first preliminary report of a new potential use of IBS. This study is based on an accepted in vitro model, objectively investigating IBS efficacy to dissolve DC cords.16
This preliminary study demonstrates the ability of small doses of IBS to dissolve Dupuytren’s cord tissue ex vivo. Due to the efficacy demonstrated, the possibility for treatment with minute doses and volumes, as well as the characteristics of IBS anti-inflammatory potency, there is a strong case for continuing to a full-scale development programme. However, before IBS can be considered for clinical use in DC, the safety aspects will be the focus of future studies.
Funding Statement
The study was funded by MediWound Ltd, Yavneh, Israel.
ICMJE conflict of interest
L. Rosenberg is the Chief Medical Officer of MediWound Ltd and Y. Shoham its Director of Medical Affairs.
References
1 Henry M . Dupuytren’s disease: current state of the art. Hand (N Y)2014;9:1-8. Google Scholar
2 Badalamente MA , HurstLC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am2007;32:767-774. Google Scholar
3 Gilpin D , ColemanS, HallS, et al.. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am2010;35:2027-2038.e1. Google Scholar
4 Hurst LC , BadalamenteMA, HentzVR, et al.. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med2009;361:968-979. Google Scholar
5 Warwick D , ArnerM, PajardiG, et al.. Collagenase Clostridium histolyticum in patients with Dupuytren’s contracture: results from POINT X, an open-label study of clinical and patient-reported outcomes. J Hand Surg Eur Vol2015;40:124-132. Google Scholar
6 Witthaut J , JonesG, SkrepnikN, et al.. Efficacy and safety of collagenase clostridium histolyticum injection for Dupuytren contracture: short-term results from 2 open-label studies. J Hand Surg Am2013;38:2-11.CrossrefPubMed Google Scholar
7 Peimer CA , BlazarP, ColemanS, et al.. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS study): 3-year data. J Hand Surg Am2013;38:12-22.CrossrefPubMed Google Scholar
8 Peimer CA , BlazarP, ColemanS, et al.. Dupuytren Contracture Recurrence Following Treatment With Collagenase Clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren Long-Term Evaluation of Safety Study]): 5-Year Data. J Hand Surg Am2015;40:1597-1605.CrossrefPubMed Google Scholar
9 Peimer CA , McGoldrickCA, FioreGJ. Nonsurgical treatment of Dupuytren’s contracture: 1-year US post-marketing safety data for collagenase clostridium histolyticum. Hand (N Y)2012;7:143-146. Google Scholar
10 Houck JC , ChangCM, KleinG. Isolation of an effective debriding agent from the stems of pineapple plants. Int J Tissue React1983;5:125-134.PubMed Google Scholar
11 Levine N , SeifterE, ConnertonC, LevensonSM. Debridement of experimental skin burns of pigs with bromelain, a pineapple-stem enzyme. Plast Reconstr Surg1973;52:413-424.PubMed Google Scholar
12 Krieger Y , RosenbergL, LapidO, et al.. Escharotomy using an enzymatic debridement agent for treating experimental burn-induced compartment syndrome in an animal model. J Trauma2005;58:1259-1264.CrossrefPubMed Google Scholar
13 Krieger Y , Bogdanov-BerezovskyA, GurfinkelR, et al.. Efficacy of enzymatic debridement of deeply burned hands. Burns2012;38:108-112.CrossrefPubMed Google Scholar
14 Rosenberg L , KriegerY, Bogdanov-BerezovskiA, et al.. A novel rapid and selective enzymatic debridement agent for burn wound management: A multi-center RCT. Burns2014;40:466-474.CrossrefPubMed Google Scholar
15 Taussig SJ , BatkinS. Bromelain, the enzyme complex of pineapple (Ananas comosus) and its clinical application. An update. J Ethnopharmacol1988;22:191-203.CrossrefPubMed Google Scholar
16 Starkweather KD , LattugaS, HurstLC, et al.. Collagenase in the treatment of Dupuytren’s disease: an in vitro study. J Hand Surg Am1996;21:490-495. Google Scholar
17 Krackow KA , ThomasSC, JonesLC. A new stitch for ligament-tendon fixation. Brief note. J Bone Joint Surg [Am]1986;68-A:764-766.PubMed Google Scholar
18 Tomasek JJ , VaughanMB, HaaksmaCJ. Cellular structure and biology of Dupuytren’s disease. Hand Clin1999;15:21-34. Google Scholar
19 Chen NC , SrinivasanRC, ShauverMJ, ChungKC. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand2011;6:250-255. Google Scholar
20 Hueston JT . Enzymic fasciotomy. Hand1971;3:38-40.CrossrefPubMed Google Scholar
21 McCarthy DM . The long-term results of enzymic fasciotomy. J Hand Surg Br1992;17:356.CrossrefPubMed Google Scholar
22 Fitzhugh DJ , ShanS, DewhirstMW, HaleLP. Bromelain treatment decreases neutrophil migration to sites of inflammation. Clin Immunol2008;128:66-74.CrossrefPubMed Google Scholar
23 Secor ER Jr , SzczepanekSM, CastaterCA, et al.. Bromelain Inhibits Allergic Sensitization and Murine Asthma via Modulation of Dendritic Cells. Evid Based ComplementAlternat Med2013;2013:702196. Google Scholar










