Abstract
Objective
Clinical studies of patients with bone sarcomas have been challenged by insufficient numbers at individual centres to draw valid conclusions. Our objective was to assess the feasibility of conducting a definitive multi-centre randomised controlled trial (RCT) to determine whether a five-day regimen of post-operative antibiotics, in comparison to a 24-hour regimen, decreases surgical site infections in patients undergoing endoprosthetic reconstruction for lower extremity primary bone tumours.
Methods
We performed a pilot international multi-centre RCT. We used central randomisation to conceal treatment allocation and sham antibiotics to blind participants, surgeons, and data collectors. We determined feasibility by measuring patient enrolment, completeness of follow-up, and protocol deviations for the antibiotic regimens.
Results
We screened 96 patients and enrolled 60 participants (44 men and 16 women) across 21 sites from four countries over 24 months (mean 2.13 participants per site per year, standard deviation 2.14). One participant was lost to follow-up and one withdrew consent. Complete data were obtained for 98% of eligible patients at two weeks, 83% at six months, and 73% at one year (the remainder with partial data or pending queries). In total, 18 participants missed at least one dose of antibiotics or placebo post-operatively, but 93% of all post-operative doses were administered per protocol.
Conclusions
It is feasible to conduct a definitive multi-centre RCT of post-operative antibiotic regimens in patients with bone sarcomas, but further expansion of our collaborative network will be critical. We have demonstrated an ability to coordinate in multiple countries, enrol participants, maintain protocol adherence, and minimise losses to follow-up.
Cite this article: Bone Joint Res;4:154–162
Article focus
- Clinical studies of patients with bone sarcomas have been challenged by insufficient numbers at individual centres to draw valid conclusions.
- The objective of this study was to assess the feasibility of conducting a definitive multi-centre randomised controlled trial (RCT) to determine whether a five-day regimen of post-operative antibiotics in comparison to a 24-hour regimen decreases surgical site infections (SSIs) in patients undergoing endoprosthetic reconstruction for lower extremity primary bone tumours.
- The authors determined feasibility by measuring patient enrolment, completeness of follow-up, and protocol deviations for the antibiotic regimens.
Key messages
- It is feasible to conduct a definitive multi-centre RCT of post-operative antibiotic regimens in patients with bone sarcomas.
- The authors demonstrated an ability to coordinate in multiple countries, enrol participants, maintain protocol adherence, and minimise losses to follow-up.
- In total, 15% of the participants in this study experienced a SSI.
Strengths and limitations
- There is no precedent for conducting large-scale surgical trials in this field.
- Pilot studies are often essential before embarking on large clinical trials because they can demonstrate feasibility, help manage resources, and build a collaborative network.
- This pilot RCT represents the first ever multi-centre RCT in sarcoma surgery.
- Further expansion of the PARITY network will be critical moving forward
Introduction
The current standard of care for most skeletally mature patients with lower extremity bone sarcomas is limb salvage surgery, which typically involves tumour resection, followed by functional limb reconstruction with modular metallic and polyethylene endoprosthetic implants.1-3 Owing to the complexity and length of these procedures, as well as the immunocompromised nature of patients treated with chemotherapy, the risk for post-operative surgical site infection (SSI) is high.4,5
Post-operative endoprosthetic SSIs often require staged revision surgery and long-term intravenous (IV) antibiotic therapy. Even following this management, repeat infection and ultimate amputation are common.4 Patient function and quality of life can be dramatically impacted, as can healthcare costs owing to extended hospital stays and multiple re-operations.6,7 The most effective post-operative regimen of prophylactic antibiotics to prevent SSIs following endoprosthetic reconstruction for lower extremity bone tumours is unknown, and current practice among orthopaedic oncological surgeons is highly varied.8
Bone sarcomas are rare forms of cancer, and clinical studies of patients with bone sarcomas have been challenged by insufficient numbers at individual centres to draw valid conclusions.9 Sarcomas represent < 1% of all malignancies, and bone sarcomas affect just four to five patients per million persons each year.10 High-quality collaborative research that can guide clinical practice for patients with bone sarcomas has lagged behind other orthopaedic surgery subspecialties, and there have been no multi-centre randomised controlled trials (RCTs) previously conducted in the field of orthopaedic oncological surgery.
Our earlier work has demonstrated high rates of SSI following the treatment of long bone tumours by surgical excision and endoprosthetic reconstruction;11 highly varied opinion and practice among orthopaedic oncologists regarding prophylactic antibiotic regimens;8 an absence of applicable RCT evidence;9,11 and extensive support from investigators for a definitive RCT to evaluate a five-day regimen of post-operative antibiotics in comparison with a 24-hour regimen of post-operative antibiotics.8,12
In this pilot study, our primary objective was to assess the feasibility of conducting a definitive multi-centre RCT to determine whether a five-day regimen of post-operative antibiotics, in comparison with a 24-hour regimen of post-operative antibiotics, decreases the rate of SSI within one year in patients undergoing endoprosthetic reconstruction for lower extremity primary bone tumours. Our secondary objective was to determine the overall rate of SSI within one year of follow-up in patients undergoing wide resection and endoprosthetic reconstruction for lower extremity primary bone tumours.
Patients and Methods
Study design and setting
We performed a pilot international multi-centre blinded parallel two-arm RCT. Each participating site obtained local institutional research ethics board approval and all patients provided informed consent. This trial was registered at ClinicalTrials.gov (NCT01479283) and is reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement and recommendations for pilot studies.13,14 We have previously reported our study protocol in further detail.12
Participants/study subjects
We included patients who were 12 years of age or older and had lower extremity primary bone malignancies, benign aggressive tumours, or soft-tissue sarcomas which had invaded bone and required surgical excision and endoprosthetic reconstruction.
We excluded patients if they had any of the following:
- Known methicillin-resistant Staphylococcus aureus or vancomycin-resistant enterococcus skin colonisation;
- Documented anaphylaxis or angioedema to cefazolin or penicillin;
- Prior surgery within the surgical field (excluding a biopsy);
- Prior local infection within the surgical field;
- Known immunologically-deficient conditions of disease such as HIV (not including recent chemotherapy);
- Known renal insufficiency with estimated creatinine clearance (eGRF) of < 54 mL/min;
- Reconstruction planned to include allograft (bone transplant);
- Likely problems, according to the judgment of the investigators, with maintaining follow-up;
- Unable to provide consent.
Randomisation
An unblinded pharmacist at each site randomised participants during surgery to either five days or 24 hours of post-operative prophylactic cefazolin. We used a central internet-based computer-generated randomisation system that concealed allocation. Our randomisation protocol included randomly permuted blocks of two or four and was stratified by location of tumour (femur vs tibia) and study centre.
The participants, surgeons, healthcare providers other than the pharmacists, data collectors, outcome assessors, data analysts, and those interpreting the results, were blinded to treatment allocation. Sites that used pre-mixed antibiotic bags kept them shrouded as there was a visible difference between pre-mixed bags and saline.
Interventions
Our protocol instructed that all participants would receive 2 g of IV cefazolin pre-operatively within 60 minutes of their procedures, and 2 g of IV cefazolin every three to four hours intra-operatively. Post-operatively, participants received either five days or 24 hours of cefazolin according to their randomised treatment assignments:
- Participants in the five-day arm received 2 g of IV cefazolin post-operatively every eight hours for five days.
- Participants in the 24-hour arm received 2 g of IV cefazolin post-operatively every eight hours for 24 hours, followed by sham doses of IV saline (placebo) for an additional four days.
- Paediatric participants received doses of cefazolin or sham that were based on 100 mg/kg/day doses, but were otherwise identical to the adult regimens.
Surgical excision and endoprosthetic reconstruction were performed according to the standard practice of the participating surgeons, which typically involved a wide extensile exposure, isolation and protection of major neurovascular structures, and resection of the segment of bone affected by tumour with a 2 cm to 3 cm bone margin. Decisions about implant selection, surgical techniques, soft-tissue reconstruction, or post-operative care other than antibiotic therapy, were left at the discretion of the treating surgeons, but were recorded in our Case Report Forms.
Follow-up
We followed participants at two and six weeks; three, six, and nine months; and one year post-operatively. Trained research personnel collected all data prospectively according to standardised procedures, and the Case Report Forms were transmitted to a central Methods Center using a secure electronic data capture system (iDataFax, Clinical DataFax Systems Inc., Hamilton, Ontario, Canada). Other communication with individual sites was conducted via email, written letters, telephone conversations, and meetings in person.
Outcome measures
In order to evaluate feasibility, we measured patient screening and enrolment, completeness of follow-up at each time point, and protocol deviations for the pre-, intra-, and post-operative antibiotic regimens.
The treating surgeons, study coordinators, and/or their delegates at each site identified potentially eligible patients at presentation and classified them as eligible and included, eligible but missed, or excluded. Completeness of follow-up was calculated relative to the number of patients eligible for follow-up at each time point.
We recorded protocol deviations whenever a pre-, intra-, or post-operative dose was missed, given incorrectly, or supplemented with additional non-study antibiotics. When patients were discharged before five days after surgery, we discontinued their study treatments early, recorded a protocol deviation for the missed doses, and no further antibiotics were given.
We pre-specified our criteria for success of the pilot as enrolment of our pilot sample within two years, 70% or greater protocol adherence, and 95% or greater completeness to follow-up.
To evaluate SSI rates, the participating surgeons diagnosed superficial, deep, or organ/space SSI according to the definition of the Centers for Disease Control and Prevention (CDC), which specifies that at least of one or the following criteria be met within one year after surgery:12,15
- Purulent drainage from the incision;
- Organisms isolated from an aseptically-obtained culture of fluid or tissue from the incision;
- Incision deliberately opened by surgeon and culture positive;
- Incision deliberately opened by surgeon or designee and not cultured, butthe patient has at least one of: pain, tenderness, localised swelling, redness, or heat;
- Diagnosis of a superficial/deep/organ space incisional SSI by surgeon.
Monitoring
Before the start of patient screening and enrolment at each site, the Methods Center collected and reviewed the following documents:
- Site Principal Investigator’s current Curriculum Vitae;
- Physician’s Clinical Trial Application;
- Confirmation of ethics approval from the local institutional ethics committee;
- Approved informed consent form(s);
- Site delegation and signature log;
- Qualified Investigator Undertaking Form, if applicable;
- Research Ethics Board Attestation Form, if applicable;
Thereafter, the Methods Center ensured that the Site Principal Investigator, Research Coordinator, and Pharmacy Designee had received appropriate study-specific training, which may have included review of the training presentation during a teleconference call, independent review of the training presentation by the site personnel, or attendance at an Investigator/Coordinator meeting.
During the participant follow-up and clinical data collection phase of the study, the Methods Center remotely conducted the following ongoing monitoring activities:
- Review quality control reports from the iDataFax system to identify sites with unacceptable amounts of missing data or unresolved queries;
- Review enrolment reports to identify sites which have not been submitting screening data;
- Review the tracking database to identify any inconsistencies between the randomisation system and the submitted Case Report Forms with respect to treatment allocation;
- Review site-specific reports on study completion and loss to follow-up;
- Conduct periodic reviews of the site regulatory binders for missing and incomplete documentation;
- Review pharmacy logs.
After all subject follow-ups were completed at a clinical site, the Methods Center conducted the following remote closeout monitoring activities:
- Ensure that any missing data have been submitted (if available) and that all remaining data queries have been resolved;
- Ensure that all required adjudication materials have been submitted;
- Review the site regulatory file for completion and request any outstanding documentation;
- Review pharmacy logs;
- Request that the site close out the study with the local ethics board and submit a copy of the confirmation from the ethics committee to Chief Executive Officer for the site regulatory file
An independent Data Safety and Monitoring Board (DSMB) comprised of three orthopaedic surgeons and a statistician monitored participant safety. The DSMB reviewed adverse events and serious adverse events data on a quarterly basis, met via teleconference at least once a year, and was governed by a DSMB charter with terms of reference and functions.
Statistical analysis and study size
Baseline characteristics, feasibility data, and SSI rates are summarised as means with standard deviation (sd) or medians with interquartile range (IQR) for continuous variables and counts (%) for categorical variables. All analyses were conducted centrally at the Methods Center. Data analysis did not occur at any of the participating sites.
We enrolled a convenience sample of 60 participants to evaluate feasibility and we considered our pilot study completed when the last enrolled participant had achieved at least three months of follow-up. We estimated that 60 patients would represent approximately 5% to 10% of the definitive RCT sample size (600 to 1200 patients), based on a pairwise comparison with an alpha of 0.05, a beta of 0.20 (80% power), a relative risk reduction of 50%,8 and a range of plausible SSI rates.12,16 We planned a priori to transition directly from our pilot RCT to a definitive RCT if feasibility was established, with the pilot event rate used to inform the definitive sample size estimation.
Results
Recruitment
We screened 96 patients across 21 clinical sites in Canada, the United States, Australia, and Argentina between November 2012 and October 2014. Of these, 60 were eligible and included, none were eligible but missed, and 36 were excluded (Fig. 1). The number of participants enrolled at each site in this period ranged from zero (six sites) to 18 (one site), but the initiation of screening and enrolment was staggered across the participating sites because of variability in the time required to obtain ethics approval and negotiate institutional contracts (Table I). In total, 20 of the sites reported having a dedicated research nurse or research coordinator available to assist with the conduct of the trial, and 14 reported prior institutional research experience for clinical trials. Notwithstanding our inclusion and exclusion criteria, the mean estimated number of endoprosthestic reconstructions performed at each site per month was 1.8 (sd 1.7).
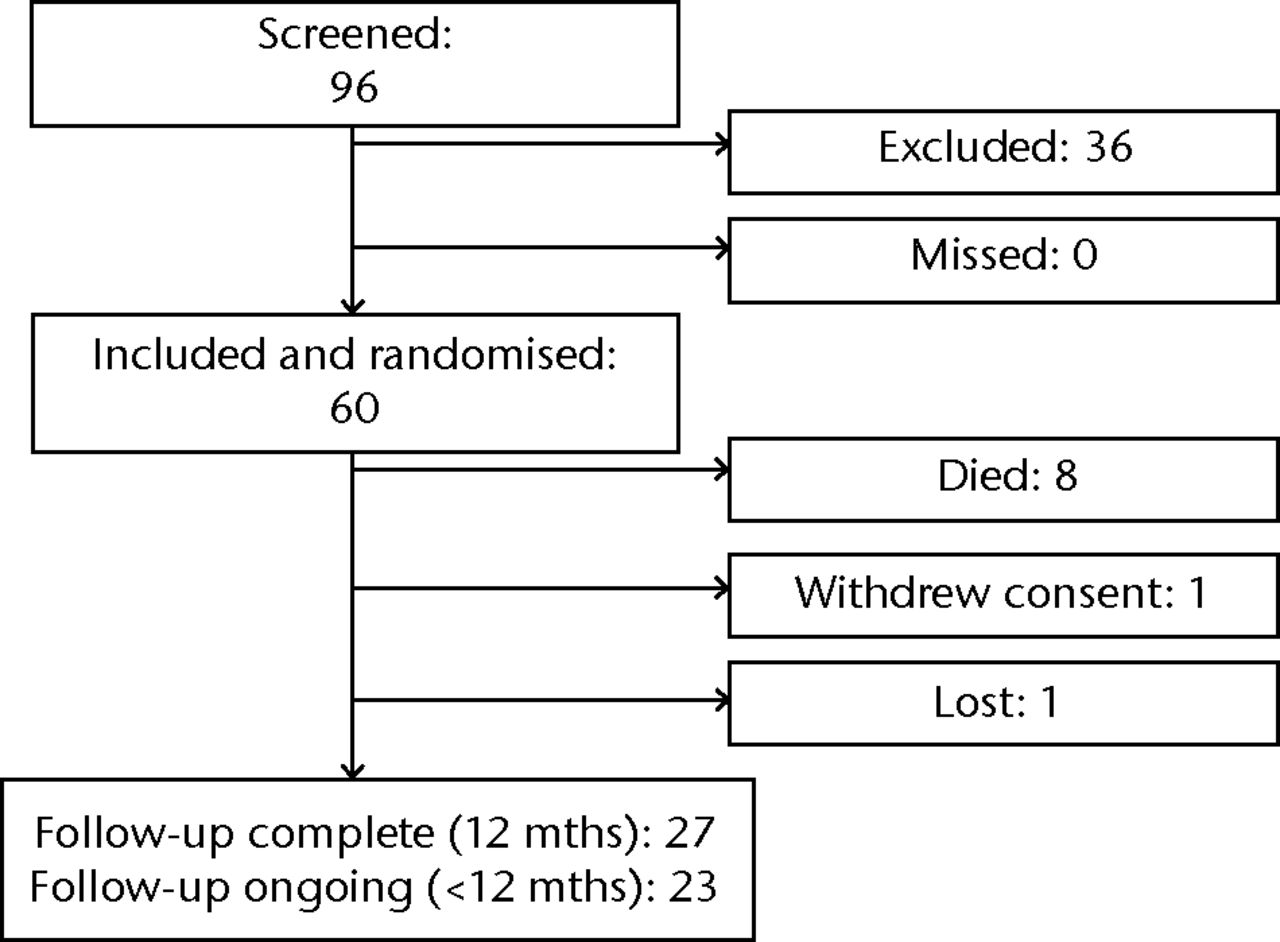
Fig. 1
Flow chart of participants in the trial.
Table I
Participating sites
| Hospital | Institution | Country | Date opened to enrolment | Estimated number of EPRs per month | Research nurse | Research assistant | Prior clinical trials experience |
|---|---|---|---|---|---|---|---|
| Mount Sinai Hospital | University of Toronto | Canada | November 2012 | 2.0 | Yes | Yes | Yes |
| The Ottawa Hospital | University of Ottawa | Canada | November 2012 | 1.0 | Yes | No | Yes |
| Vancouver General Hospital | University of British Columbia | Canada | December 2012 | 1.5 | Yes | No | Yes |
| Hôpital Maisonneuve - Rosemont | University of Montreal | Canada | December 2012 | 2.0 | Yes | Yes | No |
| Juravinski Hospital and Cancer Centre | McMaster University | Canada | January 2013 | 0.3 | Yes | Yes | No |
| McGill University Health Centre | McGill University | Canada | January 2013 | 2.0 | Yes | No | Yes |
| Huntsman Cancer Institute | University of Utah | United States | February 2013 | 5.0 | Yes | Yes | Yes |
| Vanderbilt Medical Center | Vanderbilt University School of Medicine | United States | March 2013 | 4.5 | Yes | No | No |
| Hospital Universitario Austral | Universidad Austral | Argentina | May 2013 | 1.5 | Yes | Yes | No |
| Beth Israel Deaconess Medical Center | Harvard Medical School | United States | July 2013 | 0.8 | Yes | No | Yes |
| Saint-François d’Assisse et L’Hotel-Dieu de Quebec | Laval University | Canada | November 2013 | 0.6 | Yes | Yes | Yes |
| Memorial Sloan - Kettering Cancer Center | Cornell University | United States | December 2013 | 6.0 | Yes | Yes | Yes |
| Royal Adelaide Hospital | University of Adelaide | Australia | December 2013 | 1.2 | No | No | Yes |
| Holden Comprehensive Cancer Center | University of Iowa | United States | October 2013 | 0.6 | Yes | No | No |
| Boston Children’s Hospital | Harvard Medical School | United States | February 2014 | 0.8 | Yes | No | Yes |
| University of Connecticut Health Center | University of Connecticut | United States | February 2014 | 0.3 | Yes | No | No |
| Wexner Medical Center | The Ohio State University | United States | April 2014 | 4.5 | Yes | No | Yes |
| University of Minnesota Medical Center | University of Minnesota | United States | June 2014 | 1.0 | Yes | No | Yes |
| Emory Orthopaedics and Spine Center | Emory University | United States | July 2014 | 1.7 | Yes | Yes | No |
| Stanford University Hospital and Clinics | Stanford University | United States | August 2014 | 0.6 | No | Yes | Yes |
| The Rothman Institute | Thomas Jefferson University | United States | September 2014 | 0.8 | Yes | Yes | Yes |
-
EPR, endoprosthetic reconstruction
By October 2014, 11 sites had been open for recruitment for 12 months or more, six sites had been open for six to 12 months, and four sites had been open for less than six months. The mean rate of enrolment in the trial after ‘enrolment-ready’ status was achieved was a mean of 2.13 (sd 2.4) participants per year across all sites.
Baseline characteristics
The baseline characteristics of the 60 participants are presented in Table II. There were 44 men and 16 women, and their mean age was 41.2 years (sd 23). The most common tumour location was femur (88%), and the most common tumour type was osteosarcoma (48%). In total, 47% underwent pre-operative chemotherapy, and 17% had metastatic disease at baseline.
Table II
Baseline characteristics (n (%) unless otherwise specified).
| Characteristic | Patients (n = 60) |
|---|---|
| Mean age (yrs) (sd) | 41.2 (23) |
| Women | 16 (27) |
| Mean weight (kg) (sd) | 73.2 (18) |
| Location of tumour* | |
| Femur | 53 (88) |
| Tibia | 7 (12) |
| Other (acetabulum/ilium) | 1 (2) |
| Type of tumour† | |
| Osteosarcoma | 28 (48) |
| Giant cell tumour of bone | 3 (5) |
| Non-osteogenic sarcoma of bone | 5 (8) |
| Chondrosarcoma | 12 (20) |
| Ewing’s sarcoma | 4 (7) |
| Other†¡ | 7 (12) |
| Metastases at baseline | |
| Yes | 10 (17) |
| No | 50 (83) |
| Diabetic | 4 (7) |
| Tobacco use | |
| No | 45 (75) |
| Yes | 6 (10) |
| Yes, quit | 9 (15) |
| Alcohol use | |
| Yes | 24 (40) |
| No | 36 (60) |
| Recreational IV drug use | |
| Yes | 0 (0) |
| No | 60 (100) |
| Undergone other treatment modalities | |
| No | 32 (53) |
| Yes | 28 (47) |
| Pre-operative chemotherapy | 28 |
| Pre-operative radiation | 0 |
| Other | 0 |
-
* One patient has both femur and tibia † n = 59 (one patient died before surgery, and no tumour characteristics form received) ‡ Epithelioid hemangioma with atypical features, fibrous dysplasia, high grade leiomyosarcoma, leiomyosarcoma, lymphoma of bone, pleomorphic sarcoma, synovial chondromatosis
Feasibility
As of 24 March 2015, 27 patients completed 12 months of follow-up, 23 patients had completed < 12 months but were still active in the trial, eight patients had died, one had withdrawn consent the day after surgery, and one had been lost to follow-up (Fig. 1). Of the patients that were eligible for each follow-up visit, complete data were collected for 98% at two weeks, 100% at six weeks, 86% at three months, 83% at six months, 90% at nine months, and 73% at one year (Table III). A further 17% of the patients eligible for follow-up at one year had partially complete data, and 10% had pending queries.
Table III
Summary of completeness of follow-up
| Eligible for follow-up* | Completed | Partially complete | Missed | Outstanding | |
|---|---|---|---|---|---|
| (row %) | (row %) | (row %) | (row %) | ||
| 2 weeks | 57 | 56 (98) | 1 (2) | 0 (0) | 0 (0) |
| 6 weeks | 57 | 57 (100) | 0 (0) | 0 (0) | 0 (0) |
| 3 months | 57 | 49 (86) | 7 (12) | 0 (0) | 1 (2) |
| 6 months | 47 | 39 (83) | 7 (15) | 1 (2) | 0 (0) |
| 9 months | 40 | 36 (90) | 2 (5) | 2 (5) | 0 (0) |
| 12 months | 30 | 22 (73) | 5 (17) | 0 (0) | 3 (10) |
-
* Number of participants that reached each time point and not died, been lost, or withdrawn
In total, 58 participants were randomised on the day of surgery, one was randomised on the first day after surgery, and one was randomised on the day before surgery. Protocol deviations occurred for the pre-, intra-, or post-operative antibiotic regimens of 37 participants (61%) (Table IV). Pre-operatively, three patients did not receive 2 g of IV cefazolin within 60 minutes of their procedures and three patients received other antibiotics in addition to cefazolin according to local institutional protocols, and in accordance with per-site stratification. Intra-operatively, seven patients received doses of IV cefazolin other than 2Â g every three to four hours owing to dosage adjustments for patient weight, and one patient received other antibiotics in addition to cefazolin.
Table IV
Protocol deviations for pre-, intra-, and post-operative antibiotic regimens. Data are presented as absolute numbers (%)
| Protocol deviation | Patients (n = 60) |
|---|---|
| Pre-operative antibiotics | |
| Missed dose | 3 (5) |
| Incorrect dose | 0 (0) |
| Received additional antibiotics | 3 (5) |
| Intra-operative antibiotics | |
| Missed at least one dose | 0 (0) |
| Incorrect dose | 7 (12) |
| Received additional antibiotics | 1 (2) |
| Post-operative antibiotics | |
| Missed at least one dose | 18 (30) |
| Incorrect dose | 1 (2) |
| Received additional antibiotics | 4 (7) |
Post-operatively, 18 participants missed at least one dose of cefazolin or placebo: 14 doses were missed among 11 participants because of pharmacy or nursing errors; 23 doses were missed among five participants because they were discharged before five days after surgery; 15 doses were missed in one participant who was started on alternative antibiotics after an intra-operative complication that led to a staged procedure; and 15 doses were missed in the participant who was randomised before surgery because they died before going to the operating room. The proportion of post-operative doses of cefazolin or placebo that were administered per protocol was 833 out of 900 (93%). Four patients received other antibiotics in addition to cefazolin post-operatively within the first five days after surgery.
SSI
Nine participants (15%) experienced SSI: six were organ/space, two were deep, and one was superficial. All eight of the organ/space or deep SSIs were treated with a re-operation (13%), and the superficial SSI was treated without a re-operation. Three of the SSIs were recorded at the six-week follow-up visits, two SSIs each were recorded at the three- and six-months visits, and one SSI each was recorded at the two-week and one-year visits.
Discussion
The rarity of bone sarcomas dictates that multi-centre international collaboration is necessary in order to power surgical trials adequately in orthopaedic oncology. There is no precedent for conducting large-scale surgical trials in this field, however, and this pilot RCT represents the first ever multi-centre RCT in sarcoma surgery.9 Pilot studies are often essential before embarking on large clinical trials because they can demonstrate feasibility, help manage resources, and build a collaborative network.14 In this study, we established the feasibility of conducting a definitive large multi-centre RCT by enrolling of our pilot sample within two years, and demonstrating high protocol adherence with minimal losses to follow-up.
Limitations
We required 24 months to enrol 60 patients across 21 sites from four countries. After accounting for variability in the timing of start-up at the clinical sites, our mean enrolment rate was 2.13 participants per site per year. Our screening data suggest that every eligible patient was enrolled (no patients were missed), but it is also possible that some eligible patients were not screened at all. For example, the most common type of tumour in our study was osteosarcoma, and the United States Cancer Statistics Working Group reported nearly equivalent incidence rates of osteosarcoma in men and women (5.0 per million vs 5.1 per million, respectively),10 however, we enrolled more men than women in our study, which raises the possibility of selection bias at the participating sites. Data from the Surveillance, Epidemiology, and End Results database, however, suggested higher incidence of osteosarcoma in men (5.4 per million vs 4.0 per million, respectively).17
Nevertheless, the most likely factor leading to the imbalance of male versus female participants is our small sample size and the subsequent likelihood of chance alone leading to the uneven gender distribution.18 This factor further highlights the critical importance of conducting large randomised trials as they ensure a balance of both known and unknown prognostic variables through the randomisation process.
Our recruitment data suggest that the most critical factor for the success of our definitive trial will be further expansion of our collaborative network. At the time of manuscript submission, the PARITY network consisted of 66 sites: 28 sites are open to enrolment, 30 sites are in various stages of ethics review or contracts negotiation, and eight further sites have expressed interest. With the addition of these sites to the collaborative network, we anticipate that the pace of enrolment will continue to accelerate.
The primary outcome for our definitive trial will be the rate of SSIs in each arm within one year after surgery, an endpoint that relies heavily on subjective clinical judgments.19 Although the surgeons in our pilot were blinded, it is possible our pilot event rate could be biased towards under reporting, given their involvement in the cases or that there could have been variability in the way that the CDC criteria were applied. In order to minimise bias and variability in the definitive PARITY trial, the definitive trial will implement a blinded Central Adjudication Committee in order to evaluate all potential SSIs according to pre-defined criteria.20
Feasibility
Sarcoma patients require intense oncological follow-up to monitor for disease relapse, and we anticipated only minimal losses to follow-up in our pilot sample, but we also implemented several procedures in order to minimise losses.21 Although only 73% of the patients eligible for one-year follow-up had complete data available, we consider that our data support feasibility because there were only three patients with outstanding queries at 12 months, and a further 17% of eligible patients had at least partial data available. Given that some of the pilot patients are still within their one-year follow-up period, we expect to resolve most or all of these outstanding queries and missing data. Only one patient was lost to follow-up, although one additional patient withdrew consent on the first day after surgery. With respect to data quality in relation to participating site characteristics, we believe that in the pilot phase of this trial there was a learning curve for all sites, and reporting of data quality by site at this stage would be premature.
More than half of the participants experienced minor protocol deviations related to their peri-operative antibiotics, but the importance of these deviations to the validity and feasibility of a definitive trial is doubtful. Ten participants did not receive the correct regimens of pre- and intra-operative antibiotics, but it is implausible that these errors could introduce systematic bias, because allocation was concealed. Three participants received additional antibiotics pre-operatively according to local institutional protocols, but stratification should randomly distribute these deviations evenly between groups. Trials with protocol deviations can be considered within a ‘mechanistic-practical’ framework of design and interpretation, whereby mechanistictrials address the impact of interventions administered under ideal testing circumstances, and practicaltrials address the impact of interventions administered in ‘real world’ clinical practice.22 Given that most of the protocol deviations in our study reflected typical clinical practice, and that 93% of all post-operative doses were administered per protocol, these protocol deviations are unlikely to compromise the practical applicability of our results.
Furthermore, many previous trials in orthopaedic surgery have not adequately reported protocol deviations.23 However, we reported according to the CONSORT statement and recommendations for pilot studies in order to improve the design and conduct of a subsequent large definitive trial.14 The doses missed because of pharmacy, nursing, or randomisation errors will be used as feedback to guide our future standard operating procedures.
We coordinated an expert panel of six orthopaedic oncology surgeons and three infectious disease specialists in preparation for this study.8,12,24 Based on our survey data, expert opinion, and standard of care, we determined that the most appropriate antibiotic for this study was cefazolin. Our choice of five days as the long duration reflects consensus that an even longer duration would increase the risk for resistant organisms without providing further antimicrobial effectiveness.25 We standardised the pre- and intra-operative antibiotic regimens in order to limit differential co-interventions.
Rates of SSI
In total, 15% of the participants in this study experienced a SSI. This rate exceeds the weighted mean of 9.5% identified in our systematic review (95% confidence interval (CI) 8.1 to 11.0), but this finding is not surprising, because most of the studies in our systematic review were retrospective case series at risk for selection bias or under reporting, owing to outcomes assessment bias. Prior studies have also used widely varying diagnostic criteria, and only two reported use of the CDC criteria.11
The minimum follow-up in this pilot was just three months, but others havereported a median interval from implantation to infection of 8.5 months.26 Therefore, it is possible that we could have detected an absolute risk of infection higher than 15% if all patients were followed up to one year. To the extent that this is true, a sample size calculation based on our pilot data might conservatively over power our definitive PARITY RCT, which would reduce the likelihood of reporting a spuriously negative result. Our pilot data support the idea that minimal or no adjustments to the definitive sample size calculation are required for losses to follow-up.
In conclusion, it is feasible to conduct a definitive multi-centre RCT of post-operative antibiotic regimens in patients with bone sarcomas, but further expansion of our collaborative network will be critical for study completion. We have demonstrated an ability to coordinate in multiple countries, enrol participants, maintain protocol adherence, and minimise losses to follow-up. The overall goal of PARITY is to provide high-quality evidence that can be used in the development of clinical guidelines. This pilot study has established a successful network and will support the rigorous design, organisation, and execution of a definitive RCT.
1 Ilyas I , YoungeD, PantR, MoreauP. Limb salvage for proximal tibial tumours using a modular prosthesis. Int Orthop .2000;24:208–211.CrossrefPubMed Google Scholar
2 Reddy KIA , WafaH, GastonCL, et al.Does amputation offer any survival benefit over limb salvage in osteosarcoma patients with poor chemonecrosis and close margins?Bone Joint J2015;97-B:115–120.CrossrefPubMed Google Scholar
3 Ruggieri P , MavrogenisAF, MercuriM. Quality of life following limb-salvage surgery for bone sarcomas. Expert Rev Pharmacoecon Outcomes Res2011;11:59–73.CrossrefPubMed Google Scholar
4 Jeys L , GrimerR. The long-term risks of infection and amputation with limb salvage surgery using endoprostheses. Recent Results Cancer Res2009;179:75–84.CrossrefPubMed Google Scholar
5 Jeys LM , GrimerRJ, CarterSR, TillmanRM. Risk of amputation following limb salvage surgery with endoprosthetic replacement, in a consecutive series of 1261 patients. Int Orthop2003;27:160–163.CrossrefPubMed Google Scholar
6 Akahane T , ShimizuT, IsobeK, et al.Evaluation of postoperative general quality of life for patients with osteosarcoma around the knee joint. J Pediatr Orthop B2007;16:269–272.CrossrefPubMed Google Scholar
7 Gutowski CJ , ZmistowskiBM, ClydeCT, ParviziJ. The economics of using prophylactic antibiotic-loaded bone cement in total knee replacement. Bone Joint J2014;96-B:65–69.CrossrefPubMed Google Scholar
8 Hasan K , RacanoA, DeheshiB, et al.Prophylactic antibiotic regimens in tumor surgery (PARITY) survey. BMC Musculoskelet Disord2012;13:91.CrossrefPubMed Google Scholar
9 Evaniew N , NuttallJ, FarrokhyarF, BhandariM, GhertM. What are the levels of evidence on which we base decisions for surgical management of lower extremity bone tumors?Clin Orthop Relat Res2014;472:8–15.CrossrefPubMed Google Scholar
10 Ottaviani G , JaffeN. The epidemiology of osteosarcoma. Cancer Treat Res2009;152:3–13.CrossrefPubMed Google Scholar
11 Racano A , PazionisT, FarrokhyarF, DeheshiB, GhertM. High infection rate outcomes in long-bone tumor surgery with endoprosthetic reconstruction in adults: a systematic review. Clin Orthop Relat Res2013;471:2017–2027.CrossrefPubMed Google Scholar
12 Ghert M , DeheshiB, HoltG, et al.Prophylactic antibiotic regimens in tumour surgery (PARITY): protocol for a multicentre randomised controlled study. BMJ Open2012;2:002197.CrossrefPubMed Google Scholar
13 Moher D , HopewellS, SchulzKF, et al.CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. J Clin Epidemiol2010;63:1–37.CrossrefPubMed Google Scholar
14 Thabane L , MaJ, ChuR, et al.A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol2010;10:1.CrossrefPubMed Google Scholar
15 No authors listed. CDC C for DC and P. Definition of Surgical Site Infection. http://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf (date last cccessed 23 September 2015). Google Scholar
16 Chow S- C . Sample size calculations for clinical trials. Wiley Interdiscip Rev Comput Stat2011;3:414–427.CrossrefPubMed Google Scholar
17 Linabery AM , RossJA. Trends in childhood cancer incidence in the U.S. (1992-2004). Cancer2008;112:416–432.CrossrefPubMed Google Scholar
18 Slobogean GP , SpragueS, BhandariM. The tactics of large randomized trials. J Bone Joint Surg [Am]2012;94-A(suppl 1):19–23.CrossrefPubMed Google Scholar
19 Parvizi J , GhanemE, SharkeyP, et al.Diagnosis of infected total knee: findings of a multicenter database. Clin Orthop Relat Res2008;466:2628–2633.CrossrefPubMed Google Scholar
20 Vannabouathong C , SacconeM, SpragueS, SchemitschEH, BhandariM. Adjudicating outcomes: fundamentals. J Bone Joint Surg [Am]2012;94-A(suppl 1):70–74.CrossrefPubMed Google Scholar
21 Sprague S , LeeceP, BhandariM, et al.Investigators. Limiting loss to follow-up in a multicenter randomized trial in orthopedic surgery. Control Clin Trials2003;24:719–725. Google Scholar
22 Karanicolas PJ , MontoriVM, DevereauxPJ, SchünemannH, GuyattGH. A new 'mechanistic-practical" framework for designing and interpreting randomized trials. J Clin Epidemiol2009;62:479–484.CrossrefPubMed Google Scholar
23 Chan S , BhandariM. The quality of reporting of orthopaedic randomized trials with use of a checklist for nonpharmacological therapies. J Bone Joint Surg [Am]2007;89-A:1970–1978.CrossrefPubMed Google Scholar
24 Johnson R , JamesonSS, SandersRD, et al.Reducing surgical site infection in arthroplastyof the lower limb: a multi-disciplinary approach. Bone Joint Res2013;2:58–65. Google Scholar
25 Dunkel N , PittetD, TovmirzaevaL, et al.Short duration of antibiotic prophylaxis in open fractures does not enhance risk of subsequent infection. Bone Joint J2013;95-B:831–837. Google Scholar
26 Jeys LM , GrimerRJ, CarterSR, TillmanRM. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J Bone Joint Surg [Am]2005;87-A:842–849.CrossrefPubMed Google Scholar
Funding statement:
This work was supported by grants from Physicians’ Services Incorporated, Orthopaedic Research and Education Fund/Musculoskeletal Tumor Society, and Canadian Cancer Society Research Institute.
This study was coordinated by the Center for Evidence-Based Orthopaedics at McMaster University
ICMJE Conflict of Interest:
None declared
©2015 The Parity Investigators. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.
Acknowlegements:
The PARITY Investigators - the following persons participated in the PARITY pilot study.
Study Co-Principal Investigators: M. Ghert, M. Bhandari.
Steering Committee: M. Ghert (Co-Chair), M. Bhandari (Co-Chair), B. Deheshi, G. Guyatt, G. Holt, T. O’Shea, R. L. Randall, L. Thabane, and J. Wunder.
Writing Committee: N. Evaniew, P. McKay, P. Schneider, M. Bhandari, and M. Ghert.
Central Adjudication Committee: M. Ghert (Chair), T. O’Shea, R. L. Randall, and R. Turcotte.
PARITY Methods Center Staff: (McMaster University, Hamilton, Ontario, Canada): M. Ghert, N. Evaniew, P. McKay, P. Schneider, K. Madden, T. Scott, S. Sprague, N. Simunovic, M. Swinton, A. Racano, D. Heels-Ansdell, and L. Buckingham.
Data Safety Monitoring Board: P. Rose (Chair), B. Brigman, and E. Pullenayegum.
Clinical Sites – pilot study: Juravinski Hospital and Cancer Centre (Hamilton, Ontario): M. Ghert, N. Evaniew, P. McKay, P. Schneider, G. Sobhi, R. Chan, and M. Biljan; Mount Sinai Hospital (Toronto, Ontario): P. Ferguson, J. Wunder, A. Griffin, I. Mantas, A. Wylie, A. Han, and G. Grewal; McGill University Health Centre (Montreal, Quebec): R. Turcotte, K. Goulding, F. Dandachli, and G. Matte; The Ottawa Hospital (Ottawa, Ontario): J. Werier, H. Abdelbary, K. Paquin, H. Cosgrove, A-M. Dugal, S. Fetzer, and W. Aikens; Vancouver General Hospital (Vancouver, British Columbia): P. Clarkson, B. Wang, L. Kondo, and J. Yip; Hôpital Maisonneuve-Rosemont (Montreal, Quebec): M. Isler, S. Mottard, J. Barry, H. St. Yves, M. Quach, H. Assayag, K. Daoust, K. Goyette, and D. Projean; CHU de Québec (Québec, Quebec): N. Dion, A. Arteau, S. Turmel, A. Bertrand, N. Gagnon, and V. Labbé; Vanderbilt University Medical Center (Nashville, Tennessee): G. Holt, J. Halpern, H. Schwartz, A. Atkinson, J. Daniels, and M. S. Moore; Beth Israel Deaconess Medical Center (Boston, Massachusetts): M. Anderson, M. Gebhardt, K. Wagner, H. Patel and H. Jolin; Boston Children’s Hospital (Boston, Massachusetts): M. Anderson, M. Gebhardt, B. Allar, M. Naqvi, J. Bennett, and S. Albuquerque; Huntsman Cancer Institute (Salt Lake City, Utah): R. L. Randall, K. Jones, S. Crabtree, R. Davis, and S. Sorenson; Memorial Sloan-Kettering Cancer Center (New York City, New York): J. H. Healey, J. Galle, G. O’Neill, B. Del Corral, and S. Lopez; Universidad Austral (Pilar, Buenos Aires): M. Galli Serra, W. Parizzia, A. Podrzaj, and M. Foa Torres; Royal Adelaide Hospital (Adelaide, South Australia): M. Clayer, Y. Chai, and P. Slobodian; University of Connecticut Health Center (Farmington, Connecticut): T. Balach, K. Coyle, and R. LaCasse; The Rothman Institute at Jefferson (Philadelphia, Pennsylvania): J. Abraham, T. Morrison, M. Angelos, L. Sailor, and R. Sadaka; Holden Comprehensive Cancer Center (Iowa City, Iowa): B. Miller, M. Milhem, N. McCurdy, J. Kain, J. Nohr, K. Johnson, and A. Merriss; University of Minnesota Health Center (Minneapolis, Minnesota): E. Cheng and D. G. Luke; Wexner Medical Center (Columbus, Ohio): T. J. Scharschmidt, M. K. Crist, A. DiMeo, and L. Marmon; Emory University Orthopedics and Spine Center (Atlanta, Georgia): N. Reimer, D. Monson, S. Oskouei, C. Lomba, and S. Rogers; Stanford University Health Care (Redwood City, California): R. Avedian, L. Jordan, S. Chinn, and M. Hamilton.
Clinical Sites – definitive study, enrolling: Juravinski Hospital and Cancer Centre (Hamilton, Ontario): M. Ghert, N. Evaniew, P. McKay, P. Schneider, G. Sobhi, R. Chan, and M. Biljan; Mount Sinai Hospital (Toronto, Ontario): P. Ferguson, J. Wunder, A. Griffin, I. Mantas, A. Wylie, A. Han, and G. Grewal; McGill University Health Centre (Montreal, Quebec): R. Turcotte, K. Goulding, F. Dandachli, and G. Matte; The Ottawa Hospital (Ottawa, Ontario): J. Werier, H. Abdelbary, K. Paquin, H. Cosgrove, A-M. Dugal, S. Fetzer, and W. Aikens; Vancouver General Hospital (Vancouver, British Columbia): P. Clarkson, B. Wang, L. Kondo, and J. Yip; Hôpital Maisonneuve-Rosemont (Montreal, Quebec): M. Isler, S. Mottard, J. Barry, H. St. Yves, M. Quach, H. Assayag, K. Daoust, Kristine Goyette, and D. Projean; CHU de Québec (Québec, Quebec): N. Dion, A. Arteau, S. Turmel, A. Bertrand, N. Gagnon, and V. Labbé; Vanderbilt University Medical Center (Nashville, Tennessee): G. Holt, J. Halpern, H. Schwartz, A. Atkinson, J. Daniels, and M. S. Moore; Beth Israel Deaconess Medical Center (Boston, Massachusetts): M. Anderson, M. Gebhardt, K. Wagner, H. Patel and H. Jolin; Boston Children’s Hospital (Boston, Massachusetts): M. Anderson, M. Gebhardt, B. Allar, M. Naqvi, J. Bennett, and S. Albuquerque; Huntsman Cancer Institute (Salt Lake City, Utah): R. L. Randall, K. Jones, S. Crabtree, R. Davis, and S. Sorenson; Memorial Sloan-Kettering Cancer Center (New York City, New York): J. H. Healey, J. Galle, G. O’Neill, B. Del Corral, and S. Lopez; Universidad Austral (Pilar, Buenos Aires): M. Galli Serra, W. Parizzia, A. Podrzaj, and M. Foa Torres; Royal Adelaide Hospital (Adelaide, South Australia): M. Clayer, N. Tran, and P. Slobodian; University of Connecticut Health Center (Farmington, Connecticut): T. Balach, K. Coyle, and R. LaCasse; The Rothman Institute at Jefferson (Philadelphia, Pennsylvania): J. Abraham, T. Morrison, M. Angelos, L. Sailor, and R. Sadaka; Holden Comprehensive Cancer Center (Iowa City, Iowa): B. Miller, M. Milhem, N. McCurdy, J. Kain, J. Nohr, K. Johnson, and A. Merriss; University of Minnesota Health Center (Minneapolis, Minnesota): E. Cheng and D. G. Luke; Wexner Medical Center (Columbus, Ohio): T. J. Scharschmidt, M. K. Crist, A. DiMeo, and L. Marmon; Emory University Orthopedics and Spine Center (Atlanta, Georgia): N. Reimer, D. Monson, S. Oskouei, C. Lomba, and S. Rogers; Montefiore Medical Center (Bronx, New York): D. Geller, B. Hoang, J. Tingling, and C. Solorzano; Stanford University Health Care (Redwood City, California): R. Avedian, L. Jordan, S. Chinn, and M. Hamilton; Foothills Medical Center (Calgary, Alberta): S. Puloski, M. Monument, K. Carcary, and C. Cameron; Sinai Hospital of Baltimore (Baltimore, Maryland): A. Aboulafia, M. Loomis, J. Bosley, R. Bonvegna, and M. Kassa; SUNY Upstate University Hospital (East Syracuse, New York): T. Damron, T. Craig, and M. Reale; Maimonides Medical Center (Brooklyn, New York): H. J. Goodman, M. Deza Culbertson, P. Caruso, and E. Garling; Massachusetts General Hospital (Boston, Massachusetts): J. Schwab, A. Fiore, R. Phukan, C. Park, and L. Joshi; Franklin Square Medical Center (Baltimore, Maryland): A. Aboulafia, M. Wallace, J. Flack, K. Vaughan, A. Avergas, M. Brady, S. Brown, N. Schadie, and R. Battersby; University of Pittsburgh Medical Center (Pittsburgh, Pennsylvania): K. Weiss, M. Goodman, A. Heyl, C. A. Yeschke, P. Sumic, M. Dudgeon, R. Prosser, and C. Korenoski; Albany Medical Center (Albany, New York): M. DiCaprio, B. Palmer, E. Cioppa and T. M. Schaeffer.
Clinical Sites – definitive study, planned: Misericordia Hospital (Edmonton, Alberta): P. Paul and J. Toreson; Specialty Orthopaedic Surgery (Phoenix, AZ): J. Cummings, L. Schwartz, and B. Zahner; Johns Hopkins Hospital (Baltimore, MD): C. Morris and V. Laljani; The Cleveland Clinic (Cleveland, Ohio): N. Mesko, M. Joyce, and S. Lietman; University of California San Francisco Baker Cancer Center (San Francisco, California): R. Wustrack, R. O’Donnell, and C. Stevenson; Strong Memorial Hospital (Rochester, New York): E. Carmody, W. Tyler, and A. McIntyre; University of Florida Health Shands Hospital (Gainesville, Florida): A. Spiguel, M. Scarborough, C. P. Gibbs, J. Steshyn, and B. Nunn; University of Kansas Cancer Center (Overland Park, Kansas): H. Rosenthal and K. Haynes; Medical University of South Carolina Health Orthopaedics (Charleston, South Carolina): L. Leddy and Z. Walton; Oregon Health and Science University Hospital (Portland, Oregon): Y-C. Doung and J. Hayden; Hospital Universitario Vall d’Hebron (Barcelona, Spain): R. Velez, M. Aguirre, M. Perez, S. Barrera and A. García López; Royal Orthopaedic Hospital NHS Trust (Birmingham, United Kingdom): R. Grimer, K. Dunn, and H. Virdee; Newcastle Upon Tyne Hospital NHS Trust (Newcastle upon Tyne, United Kingdom): K. Rankin, T. Beckingsale, C. Gerrand, I. Campbell, and M. Allen; All India Institute of Medical Sciences (New Delhi, India): S. Alam Khan, S. Bakshi, S. Rastogi, R. Poudel, V. Sampath Kumar, and A. Rai; Orthopaedic Oncology Group of the University of São Paulo (São Paulo, Brazil): A. M. Baptista and O. P. de Camargo; Grey’s Hospital (Pietermaritzburg, South Africa): L. Marais, R. Rodseth, N. Ferreira, C. Rajah, and S. Gumede; Tel Aviv Sourasky Medical Center (Tel Aviv, Israel): Y. Gortzak, A. Sternheim, J. Bickels, Y. Kolander, and S. Lev; National University Hospital, Rigshospitalet (Copenhagen, Denmark): W. Hettwer, M. M. Petersen, and T. Grum-Schwensen; University of Groningen, University Medical Center Groningen (Groningen, Netherlands): P. Jutte, J. J.W. Ploegmakers, and M. Stevens; Nuffield Health Glasgow Hospital (Glasgow, United Kingdom): A. Mahendra and S. Gupta ; LKH – Universitätsklinikum Graz (Graz, Austria): M. Bergovec and A. Leithner; AKH – Wein (Vienna, Austria): P. Funovics; Leiden University Medical Center (Leiden, Netherlands): P. D. S. Dijkstra, M. Van De Sande, A. Hoogenstraaten, and N. Leijerzapf; Princess Alexandra Hospital (Brisbane, Australia): P. Steadman; Lady Cilento Children’s Hospital (Brisbane, Australia): P. Steadman; CTO Hospital AO Città della Salute e della Scienza (Torino, Italy): M. Boffano, R. Piana, S. Marone, U. Albertini, E. Boux, and A. Maiello; Hospital of Traumatology and Orthopaedics (Riga, Latvia): L. Repsa and S. Zile; Royal National Orthopaedic Hospital (Stanmore, United Kingdom): W. Aston and R. Pollock; The Robert Jones and Agnes Hunt Orthopaedic Hospital (Oswestry, United Kingdom): P. Cool; Oxford University Hospital (Oxford, United Kingdom): M. Gibbons, D. Whitwell, T. Cosker, and J. Hemingway; Royal Infirmary of Edinburgh (Edinburgh, United Kingdom): D. Porter and S. Patton; Institute of Osteoarticular Diseases (Cali, Colombia): J. Navia and A. F. Betancur; University Hospital of Tampere (Tampere, Finland): M. Laitenen, T-K. Pakarinen, J. Nieminen, S. Ylä-Mononen, and S. Rautiainen; Hôpital Dupuytren (Limoges, France): F. Fiorenza.










