Abstract
Objective
Mortality rates reported by the National Joint Registry for England and Wales (NJR) were higher following cemented total knee replacement (TKR) compared with uncemented procedures. The aim of this study is to examine and compare the effects of cemented and uncemented TKR on the activation of selected markers of inflammation, endothelium, and coagulation, and on the activation of selected cytokines involved in the various aspects of the systemic response following surgery.
Methods
This was a single centre, prospective, case-control study. Following enrolment, blood samples were taken pre-operatively, and further samples were collected at day one and day seven post-operatively. One patient in the cemented group developed a deep-vein thrombosis confirmed on ultrasonography and was excluded, leaving 19 patients in this cohort (mean age 67.4, (sd 10.62)), and one patient in the uncemented group developed a post-operative wound infection and was excluded, leaving 19 patients (mean age 66.5, (sd 7.82)).
Results
Both groups had a similar response with regards to the levels of C-reactive protein (CRP), interleukin 6 (IL-6) and tumour necrosis factor-alpha (TNFα). CD40 levels rose significantly on the cemented group over day one to day seven compared with that of the uncemented group, which occurred over the first 24 hours. The CD14/42a levels demonstrated a statistically significant increase in the cemented group (p < 0.001 first 24 hours and p = 0.02 between days one and seven).
Conclusions
The uncemented and cemented groups demonstrated significant changes in the various parameters measured at various time points but apart from CD14/42a levels, there was no significant difference in the serum markers of inflammation, coagulation and endothelial dysfunction following cemented TKR.
Cite this article: Bone Joint Res 2014;3:108–16.
Article focus
Will cemented knee arthroplasty result in a more pronounced inflammatory response when compared with uncemented TKR?
What are the effects of TKR on the activation of selected markers of inflammation, endothelium, and coagulation?
Key messages
The uncemented and cemented groups had significant changes in the various parameters measured at various time points.
CD14/42a levels demonstrated a statistically significant increase in the cemented group.
Our current understanding of the exact role of CD14/42a is not clearly understood.
Strengths and limitations
Strength: this is a prospective case-control trial with 19 patients in the cemented and uncemented TKR groups.
Limitations: the lack of randomisation, introducing potential for bias and the small cohort size.
Introduction
Total knee replacement (TKR) is a common elective orthopaedic procedure performed in the United Kingdom. The number of primary knee replacement procedures recorded on the National Joint Registry for England and Wales (NJR) during 2011 was 79 516, 86% of which were cemented.1 Over the past three years, there has been a slight increase in cemented TKR at the expense of uncemented procedures,2 and mortality rates reported by the NJR were higher following cemented TKR.3
Polymethylmethacrylate (PMMA) was first introduced in 1943 and later adapted for orthopaedic use by Sir John Charnley,4,5 and even now remains a key component of modern orthopaedic practice. Preparation of PMMA for joint replacement results in an exothermic polymerisation process6,7; the potential risk of thermal damage to bone and surrounding tissue has been raised.8,9 PMMA has also been shown to activate the complement system,10 and activated complement particles have been implicated in osteolysis, and involvement with the macrophage response to wear debris11,12
Surgical trauma results in a series of inflammatory reactions involving interplay between several hormones, cytokines and other cellular products. The interplay among these mediators is complex and has been well documented in previous studies.13-16 The characteristics of these events have only recently become possible because of the advances in the field of molecular medicine.16 The quantification of the inflammatory response following elective orthopaedic surgery is important in light of emerging evidence supporting its association between both venous and arterial thrombosis, as well as that of atherosclerosis.17,18
To date, no systematic study has been reported comparing the effects of bone cement in elective TKR. The aim of this study is to examine and compare the effects of cemented and uncemented knee arthroplasty on the activation of selected circulating markers of inflammation, coagulation and endothelial dysfunction,19-22 and, on the activation of selected cytokines shown to be involved in the various aspects of the systemic response following injury and inflammation (Table I).
Table I
Summary of inflammatory parameters measured
| Inflammatory parameters | Role in the inflammatory response | |
|---|---|---|
| Interleukin 6 (IL-6) | Inflammatory cytokine | |
| Tumour necrosis factor alpha (TNF-α) | Inflammatory cytokine | |
| C-reactive protein (CRP) | Sensitive inflammatory marker | |
| Endothelial selectin (e-selectin) | Endothelial marker | |
| Soluable CD40 ligand (sCD40L) | General marker of inflammation | |
| Tissue plasminogen activator (tPA) | Endothelial marker | |
| von Willebrand factor (vWF) | Endothelial marker | |
| Expression of CD40 on monocytes | Inflammatory cytokine | |
| Expression of the CD14/CD42a on monocytes | Platelet-monocyte aggregate |
Materials and Methods
This was a single centre, prospective, case-controlled study. This study protocol was approved by Lanarkshire Local Research Ethics Committee, Scotland. After providing informed consent, 20 patients were recruited into the cemented TKR group and 20 in the uncemented TKR group according to the operating surgeon’s preferences.
All patients underwent an ECG prior to enrolment. They were excluded from the study if they had an abnormal or ischaemic ECG, if they had any history of previous IHD, TIA, stroke or MI, or a previous history of DVT or PE.
Post-operatively, patients were excluded if they developed chest pain with ECG changes, and unless symptomatic, patients were not routinely screened for UTIs or DVT/PE. If they were symptomatic, they were excluded from the study.
Anyone with a post-operative wound problem with positive cultures was also excluded. In the cemented group, pre-operative results showed the mean CRP count was 1.5 (sd 1.8), and the mean White Cell Count (WCC) was 7.5 (sd 1.4). In the uncemented group the pre-operative mean CRP count was 2 (sd 1), and the mean WCC was 8.1 (sd 1.5).
In the uncemented group, the mean body mass index (BMI) was 30.3 kg/m2 (sd 5.1). There were ten males, and nine females, and a total of six TKRs were carried out on the left and 13 on the right.
In the cemented group, the mean body mass index (BMI) was 30.5 kg/m2 (sd 5.2). There were six males, and 13 females, and a total of nine TKRs were carried out on the left and ten on the right. The characteristics of the two groups are summarised in Table II. None of these characteristics were significantly different between the two groups.
Table II
Demographic data on the patients and the characteristics of the two groups
| Cemented group (n = 19) | Uncemented group (n = 19) | |||
|---|---|---|---|---|
| Male | ||||
| Patients (n) | 6 | 10 | ||
| Mean (sd) age (yrs) | 60.3 (12.74) | 65.3 (7.36) | ||
| Female | ||||
| Patients (n) | 13 | 9 | ||
| Mean (sd) age (yrs) | 70.7 (8.57) | 67.8 (8.97) | ||
| Mean (sd) body mass index (kg/m2) | 30.5 (5.2) | 30.3 (5.1) | ||
| Side (n) | ||||
| Left | 9 | 6 | ||
| Right | 10 | 13 |
Blood samples were collected pre-operatively on the day of surgery, and post-operatively on day one and day seven. The variables were compared over three time periods, from pre-operative day one to day one post-operation (P1 to D1), from day one to day seven post-operation (D1 to D7) and from pre-operative day one to day seven post-operation (P1 to D7).
The peripheral blood was labelled with combinations of Fluorescein Isothiocyanate (FITC)-conjugated anti CD14; R-Phycoerythrin (PE) Conjugated anti CD42a and PE-conjugated and anti CD40 (AbD Serotec, Killington, United Kingdom). After incubation, red cells were lysed using EasyLyse (Dako Denmark A/S, Glostrup, Denmark) ammonium chloride RBC lysing solution. Data acquisition and analysis was performed on a FACS Canto II flow cytometer (Becton Dickinson, San Jose, California).
Monocytes were identified from light scatter properties and a gate applied. A total of 10 000 events within the monocyte gate were collected. Number of gated events, mean fluorescence intensity and percentage positive events were recorded. Isotype FITC and PE controls were analysed on each sample to determine background fluorescence. All samples were processed within 24 hours to 48 hours.
The citrate and serum separator tubes (SSTTM) (Narang Medical Ltd, New Delhi, India) were also taken to the haematology laboratory, where they were centrifuged at 1500 g for ten minutes. The plasma and citrate from the citrate bottle was decanted into two 1 ml aliquots. The samples were then stored in a -40°C freezer. All samples were centrifuged and stored within three hours of venepuncture; they were stored in the freezer until such time that they could be transferred by the author to the University Department of Medicine laboratory at Glasgow Royal Infirmary, for analysis.
Soluble CD40 ligand (sCD40L), e-selectin and interleukin 6 (IL6) were assayed by high-sensitivity enzyme-linked immunoasorbent assay (ELISA) kits (R& D Systems, Oxford, United Kingdom). Plasma von Willebrand Factor (vWF) antigen levels were measured with an in-house ELISA, using rabbit anti-human polyclonal antibodies (DAKO plc, High Wycombe, United Kingdom). Tissue plasminogen activator levels were measured with a commercially available ELISA (Trinity Biotech, Sulhampstead, United Kingdom).
Statistical analysis
Parametric data results are presented as mean, sd and standard error of the mean (SEM). Differences between the three time points (pre-operative, day one and day seven) were analysed using pair sampled t-tests. This was done for both the cemented and uncemented groups. Independent sample t-tests were used to look for differences between groups.
For the parametric data repeated measures, ANOVA was used to look for statistical difference in the variables between the two groups. A p-value of ≤ 0.05 was considered significant. Age, sex and BMI were covariates. A Bonferroni correction for multiple comparisons was performed.
In the case of the non-parametric data (e-selectin, CD1442a and CD40 count) results are presented as median and IQR. The differences between the three time points were analysed using Wilcoxon signed rank tests. Mann–Whitney U tests were used to look for differences in absolute values between the groups. All analyses were performed using SPSS version 19.0 for Windows (SPSS Inc., Chicago, Illinois).
Results
Blood cell count and CRP
The groups were compared using independent sample t-test to look for significant differences in the inflammatory and endothelial response with the use of cement. Table III summarises the results. There was a statistically significant difference in the platelet response between the cemented and uncemented groups with respect to the time period between D1 and D7 (p = 0.05), showing a higher rise in the uncemented group (Fig. 1). There was also a similar difference in the neutrophil levels, which also showed a higher rise in the uncemented group (Fig. 2). There was no difference between the two groups with respect to the changes in the WCC, monocytes and CRP between the two groups.
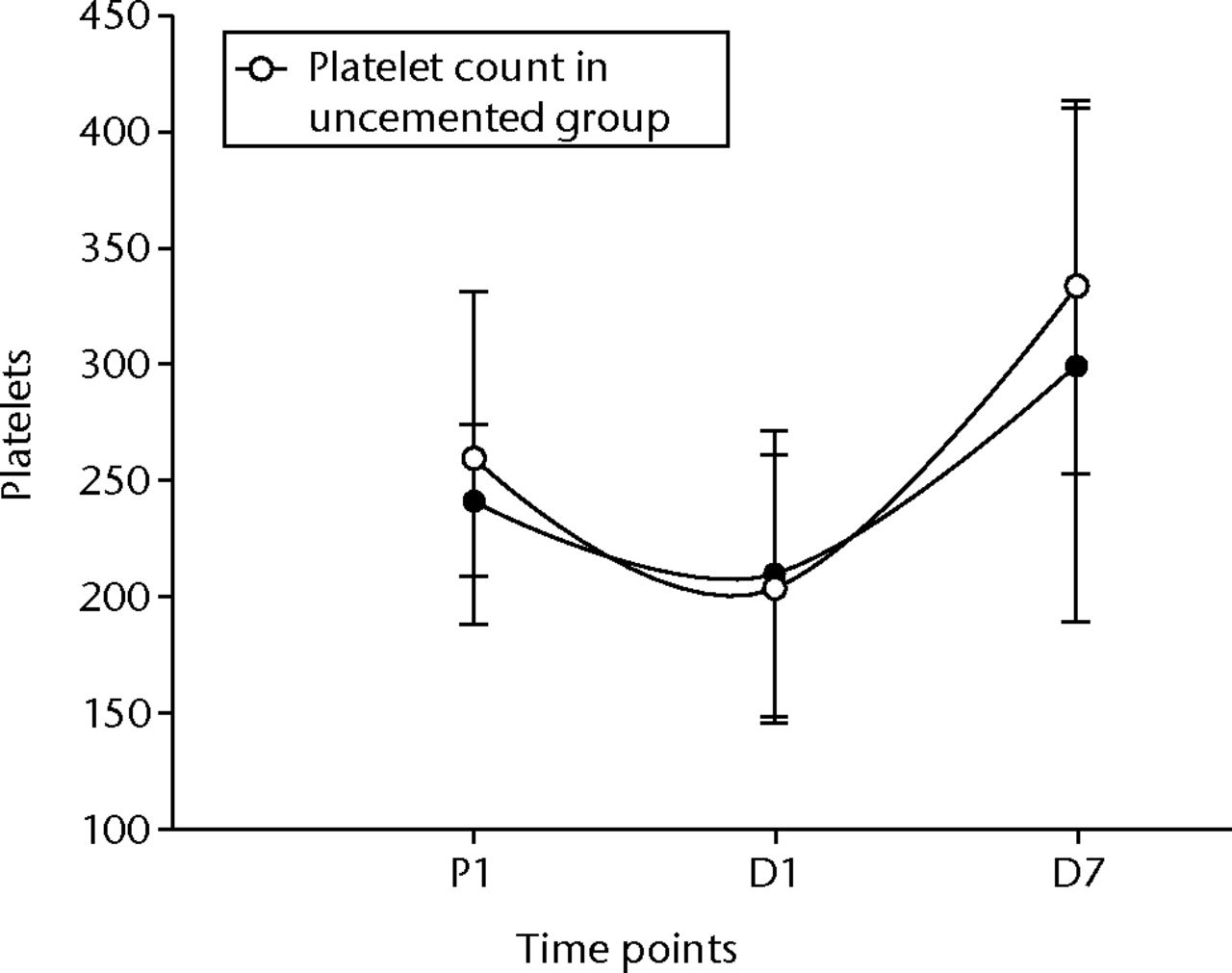
Fig. 1
Graph of changes in platelet count (x109/L) over the three time points (mean and standard deviation) in the cemented total knee replacement (TKR) group (n = 19) against the uncemented TKR group (n = 19)
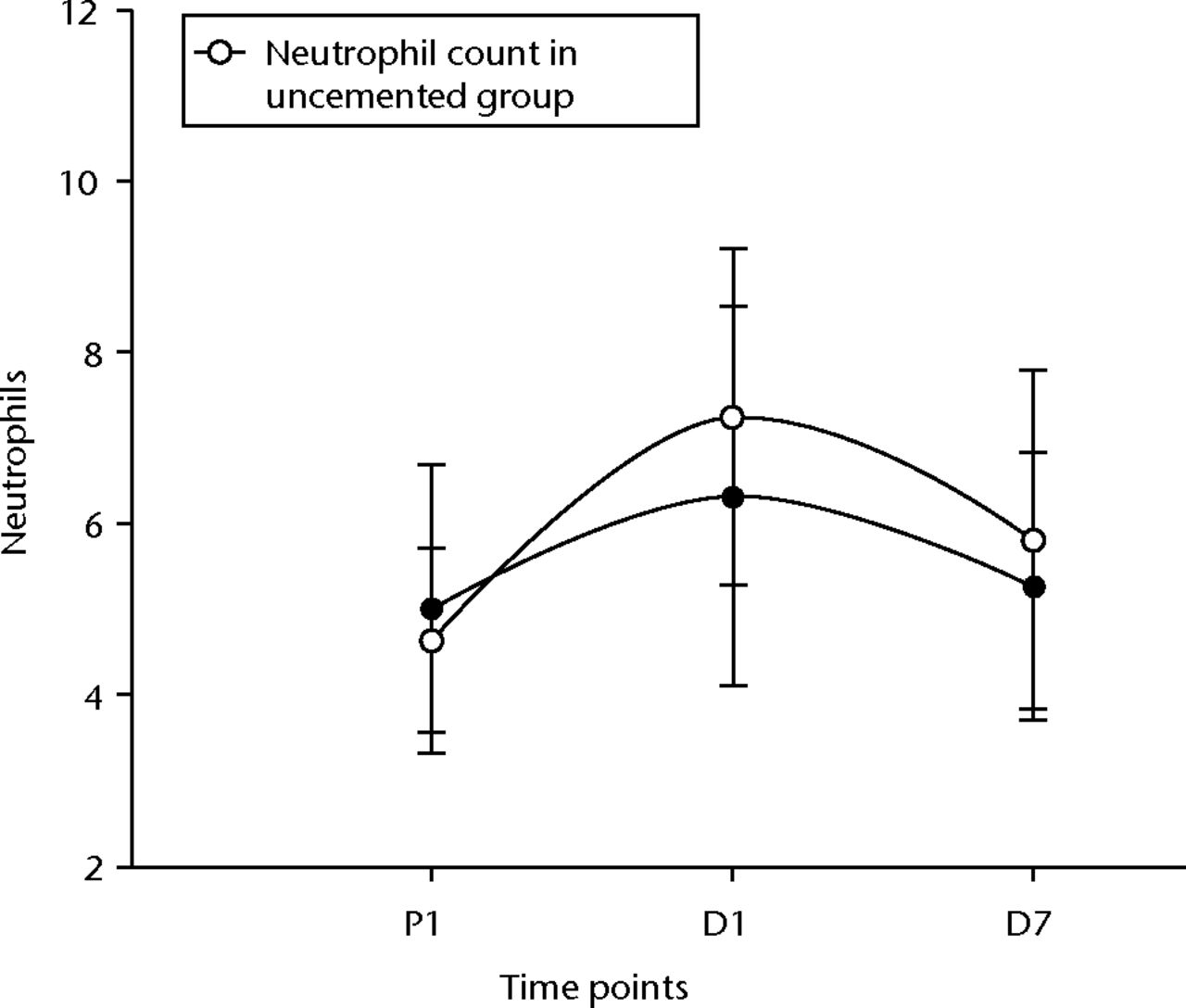
Fig. 2
Graph of changes in neutrophil count (x109/L) over the three time points (mean and standard deviation) in the cemented total knee replacement (TKR) group (n = 19) against the uncemented TKR group (n = 19)
Table III
Mean difference, standard error difference and significance of blood markers between the cemented and uncemented groups. The variables were compared over three time periods, from pre-operative to day one post-operation (P1 to D1), from day one to day seven post-operation (D1 to D7) and from pre-operative to day seven post-operation (P1 to D7)
| Blood markers* | Mean difference | Standard error of the mean difference | Significance (p-value) |
|---|---|---|---|
| Platelets | |||
| P1 to D1 | -24.25 | 14.62 | 0.11 |
| D1 to D7 | 39.68 | 19.99 | 0.05 |
| P1 to D7 | 15.43 | 25.54 | 0.55 |
| White cell count | |||
| P1 to D1 | 0.56 | 0.65 | 0.39 |
| D1 to D7 | 0.63 | 0.72 | 0.93 |
| P1 to D7 | 1.06 | 0.60 | 0.09 |
| Neutrophils | |||
| P1 to D1 | 1.290 | 0.63 | 0.48 |
| D1 to D7 | -0.45 | 0.74 | 0.54 |
| P1 to D7 | 1.13 | 0.51 | 0.03 |
| Monocytes | |||
| P1 to D1 | 0.11 | 0.13 | 0.40 |
| D1 to D7 | -0.85 | 0.14 | 0.55 |
| P1 to D7 | 0.02 | 0.08 | 0.76 |
| CRP | |||
| P1 to D1 | -4.59 | 12.1 | 0.71 |
| D1 to D7 | -14.81 | 20.40 | 0.47 |
| P1 to D7 | -19.40 | 16.68 | 0.25 |
| t-PA | |||
| P1 to D1 | 0.36 | 2.27 | 0.87 |
| D1 to D7 | -2.01 | 2.69 | 0.46 |
| P1 to D7 | -0.39 | 2.11 | 0.86 |
| vWF | |||
| P1 to D1 | 42.41 | 24.05 | 0.09 |
| D1 to D7 | -36.72 | 20.89 | 0.09 |
| P1 to D7 | 2.14 | 18.51 | 0.91 |
| sCD40L | |||
| P1 to D1 | 693.99 | 713.03 | 0.34 |
| D1 to D7 | -63.29 | 698.94 | 0.93 |
| P1 to D7 | 664.63 | 591.56 | 0.27 |
| IL6 | |||
| P1 to D1 | -23.92 | 47.62 | 0.62 |
| D1 to D7 | -4.36 | 61.70 | 0.94 |
| P1 to D7 | -28.86 | 24.14 | 0.24 |
| TNF-α | |||
| P1 to D1 | -0.41 | 0.48 | 0.42 |
| D1 to D7 | -0.11 | 0.59 | 0.85 |
| P1 to D7 | -0.52 | 0.28 | 0.09 |
| e-selectin | |||
| P1 to D1 | -4.39 | 6.32 | 0.49 |
| D1 to D7 | 2.24 | 4.31 | 0.61 |
| P1 to D7 | -3.08 | 6.84 | 0.66 |
| CD14/42a | |||
| P1 to D1 | 275.53 | 235.17 | 0.25 |
| D1 to D7 | -735.60 | 322.50 | 0.03 |
| P1 to D7 | -460.07 | 277.98 | 0.11 |
| CD14/42a (%) | |||
| P1 to D1 | 5.13 | 1.72 | 0.00 |
| D1 to D7 | -8.25 | 3.39 | 0.02 |
| P1 to D7 | -3.13 | 2.76 | 0.26 |
| CD40 | |||
| P1 to D1 | 43.31 | 58.68 | 0.46 |
| D1 to D7 | 23.98 | 32.57 | 0.47 |
| P1 to D7 | 67.29 | 71.54 | 0.35 |
| CD40 (%) | |||
| P1 to D1 | 0.67 | 0.55 | 0.23 |
| D1 to D7 | -0.24 | 0.70 | 0.73 |
| P1 to D7 | 0.42 | 0.89 | 0.64 |
-
* CRP, C-reactive protein; t-PA, tissue plasminogen activator; vWF, von Willebrand Factor; sCD40L0, soluble CD40; IL6, ligand interleukin 6; TNF-α, tumour necrosis factor alpha
Circulating activation markers
Table III is a summary of the results when we compare the tissue plasminogen activator (t-PA), vWF, soluble CD40 ligand (sCD40L0), interleukin 6 (IL6), tumour necrosis factor alpha (TNFα) and e-selectin levels. None of the variables demonstrated statistically significant changes in comparison between the cemented and uncemented groups
The t-PA levels showed a non-significant rise initially, but did become significant at D7 post-operatively in both groups (p = 0.01 in the uncemented group, p = 0.04 in the cemented group) (Fig. 3). The vWF levels showed similar changes, becoming significantly higher after 24 hours and remaining so at D7 (p < 0.001 for both groups) (Fig. 4). The sCD40L levels were significantly (p = 0.04) lower by 24 hours and remained so until day seven for the cemented group (Fig. 5).
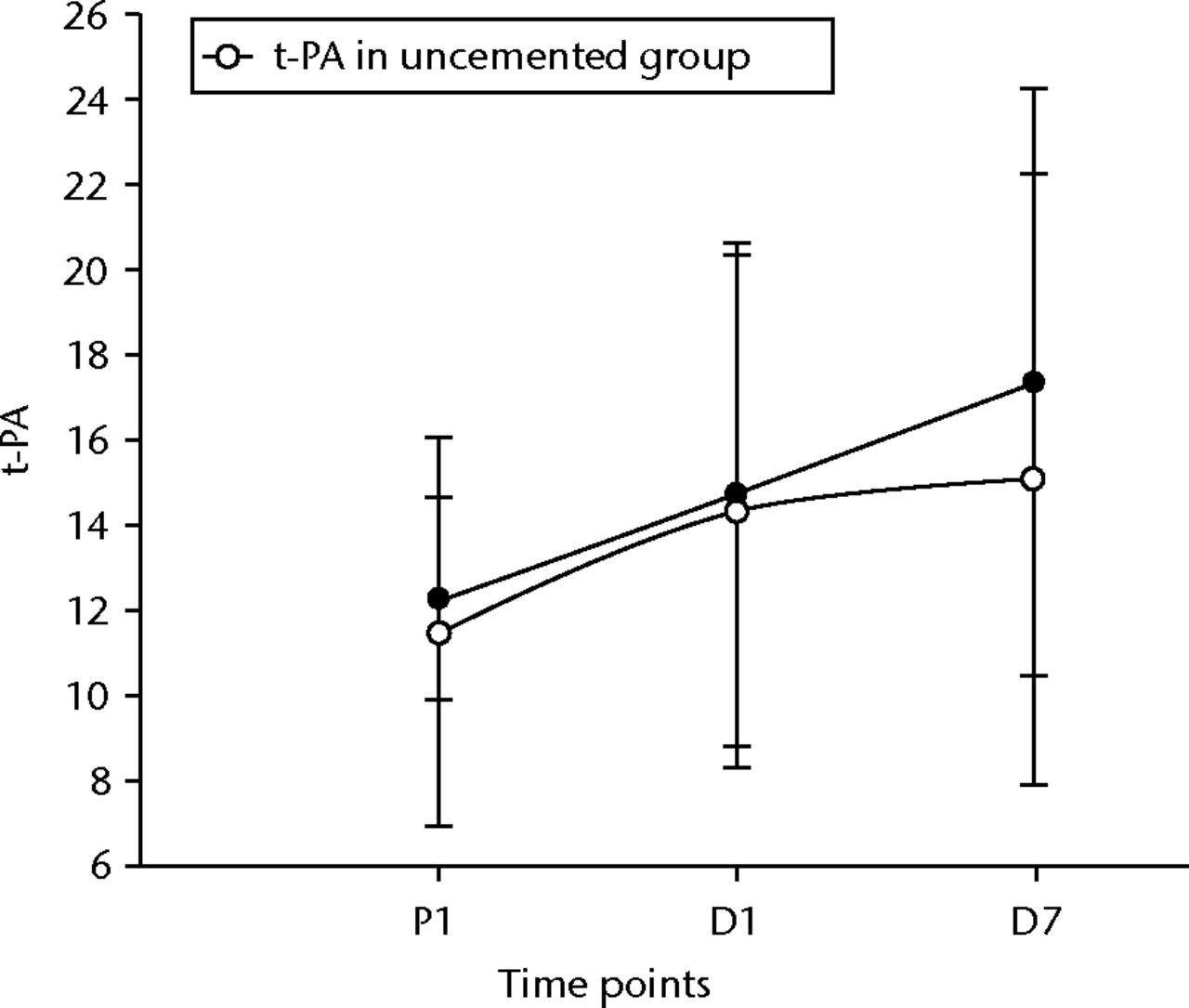
Fig. 3
Graph of changes in tissue plasminogen activator levels (t-PA) (ng/ml) over the three time points (mean and standard deviation) in the cemented total knee replacement (TKR) group (n = 19) against the uncemented TKR group (n = 19)
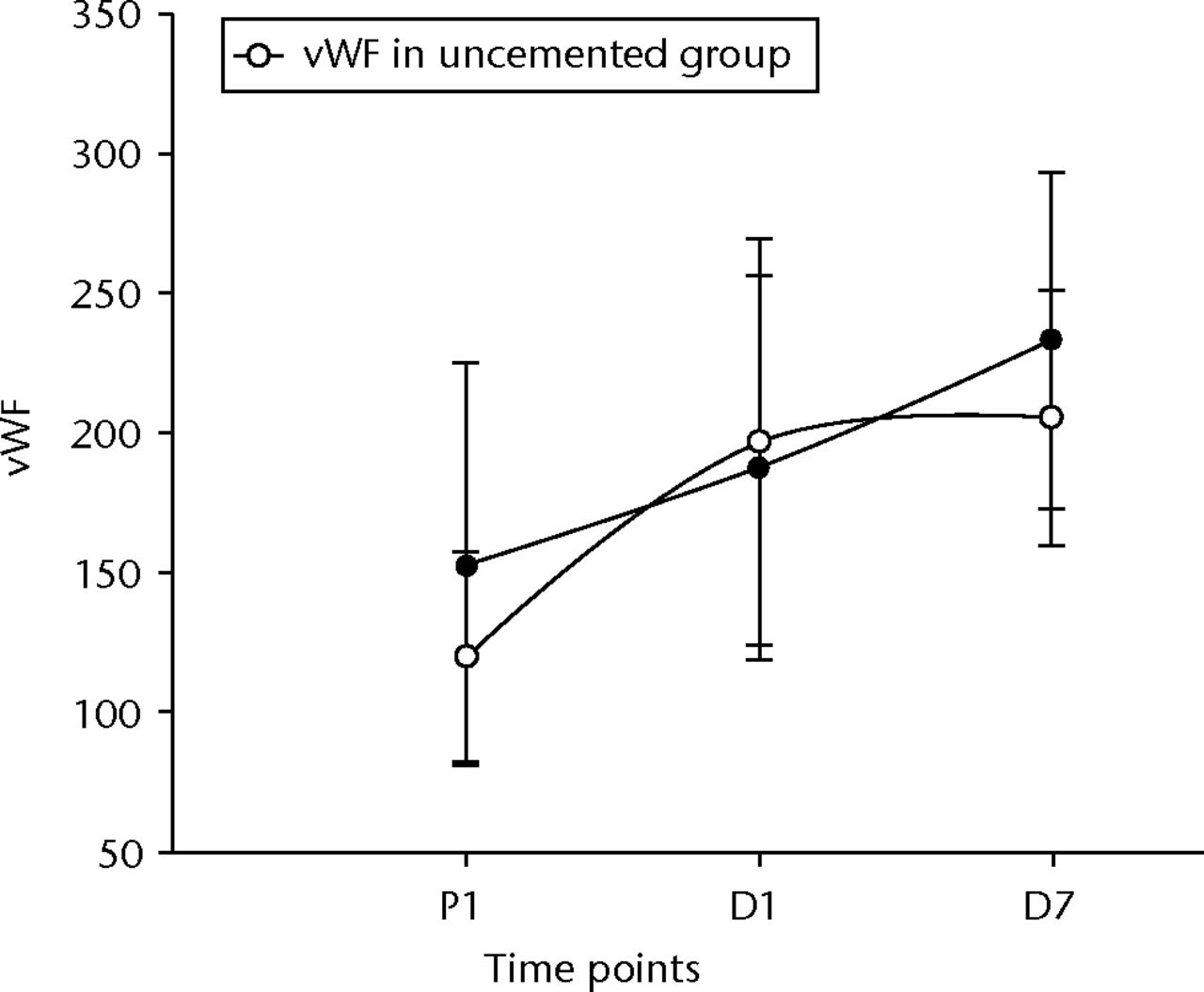
Fig. 4
Graph of changes in von Willebrand Factor (vWF) (IU/dl) over the three time points (mean and standard deviation) in the cemented total knee replacement (TKR) group (n = 19) against the uncemented TKR group (n = 19)
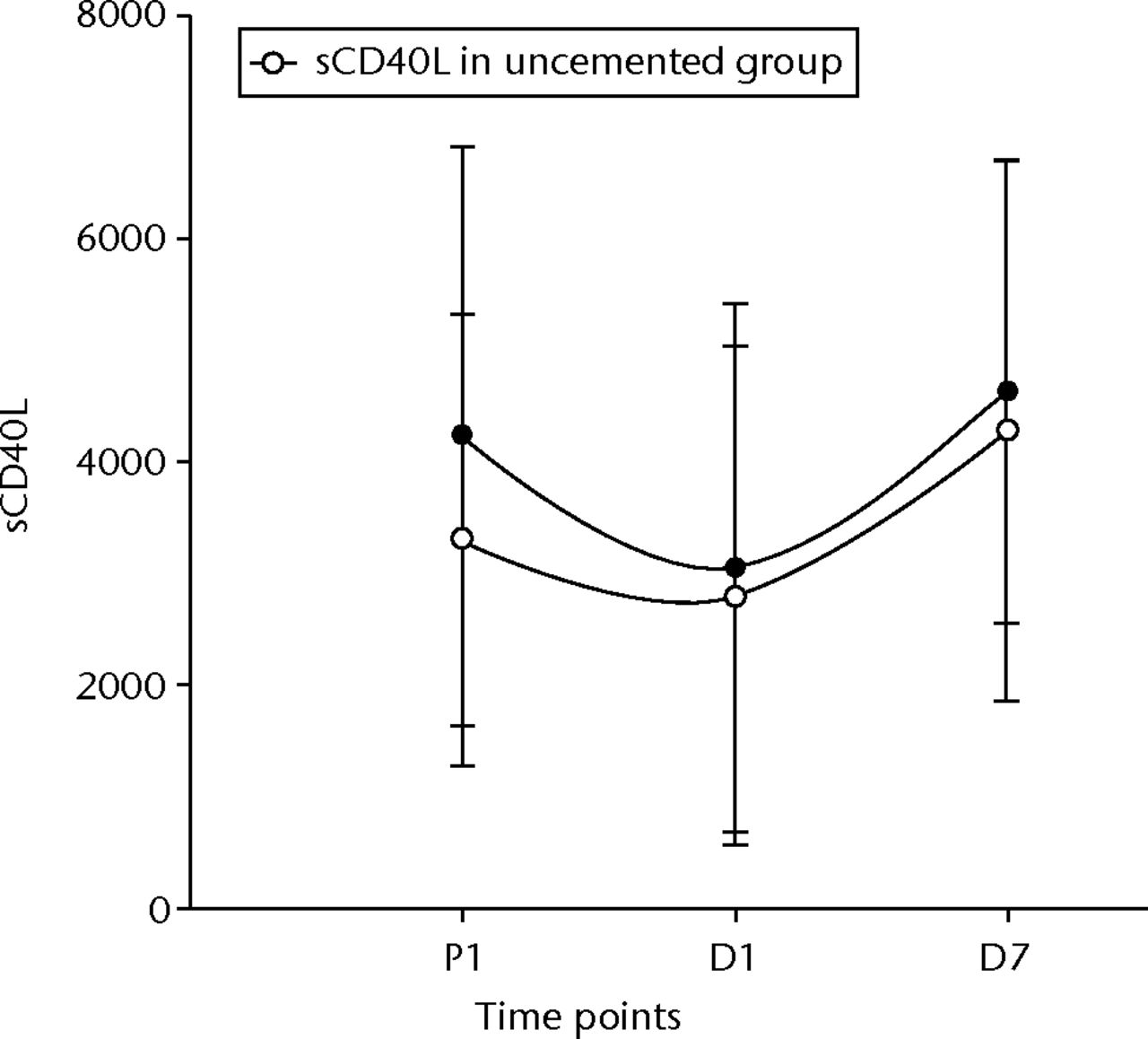
Fig. 5
Graph of changes in soluble CD40 ligand levels (sCD4OL) (pg/ml) over the three time points (mean and standard deviation) in the cemented total knee replacement (TKR) group (n = 19) against the uncemented TKR group (n = 19)
IL 6 rose and fell significantly in both groups (p < 0.001 between pre-operative values and D1 in both groups, p = 0.01 between D1 and D7 in the cemented group and p < 0.001 in the uncemented group, and p = 0.02 between pre-operative levels and D7 in the cemented group and p = 0.03 in the uncemented group) (Fig. 6). TNFα levels did not initially show a significant rise, but by D7 their levels had significantly risen compared with the pre-operative values (p = 0.05 in the cemented group) (Fig. 7). The e-selectin levels did not show any significant changes at any of the time points (p = 0.21 between P1 and D1, p = 0.40 between D1 and D7, and p = 0.12 between P1 and D7) for the uncemented group, but the levels fell significantly by D7 (p = 0.04 between P1 and D7) in the cemented group (Fig. 8).
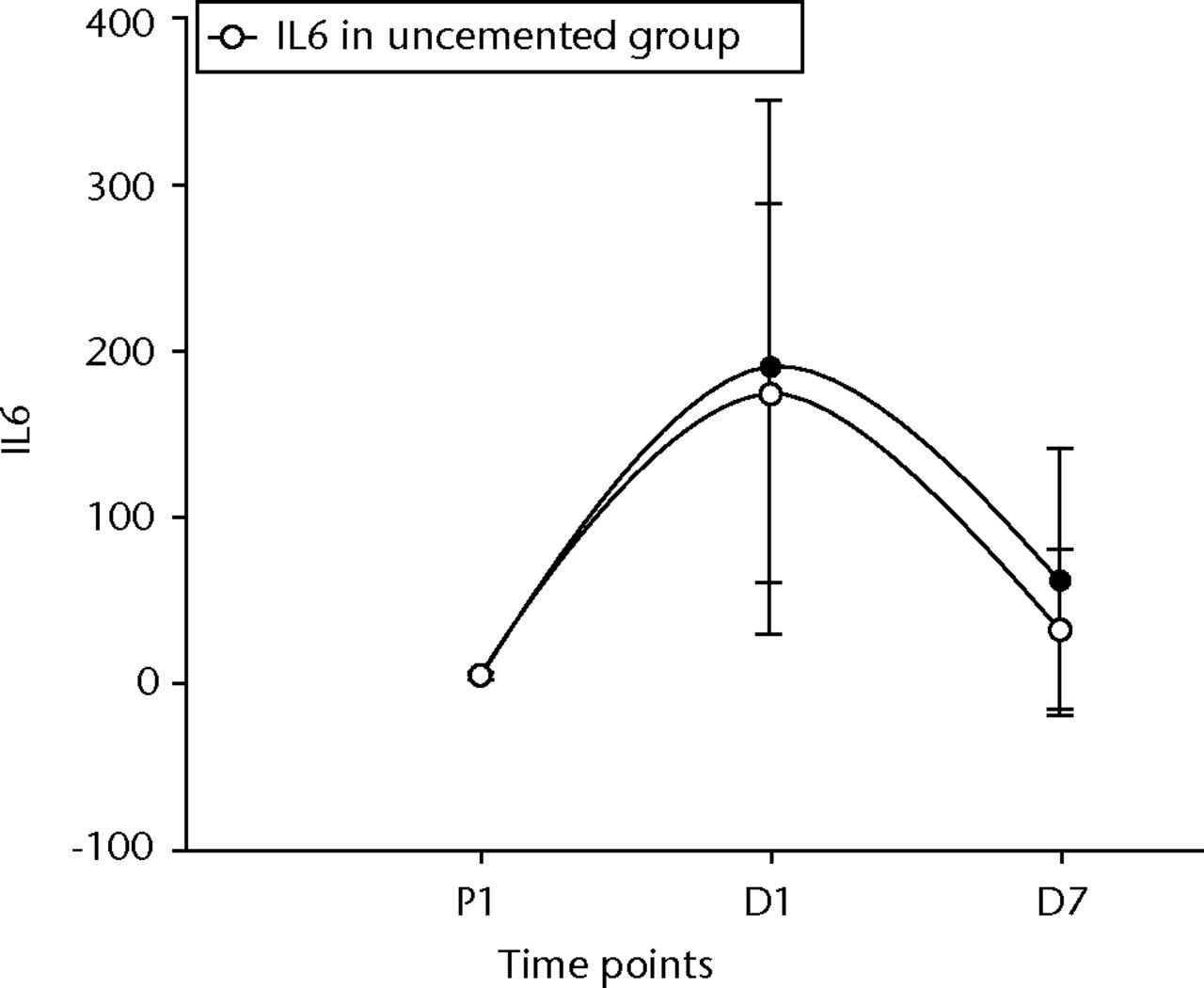
Fig. 6
Graph of changes in ligand interleukin 6 (IL-6) levels (pg/ml) over the three time points (mean and standard deviation) in the cemented total knee replacement (TKR) group (n = 19) against the uncemented TKR group (n = 19)
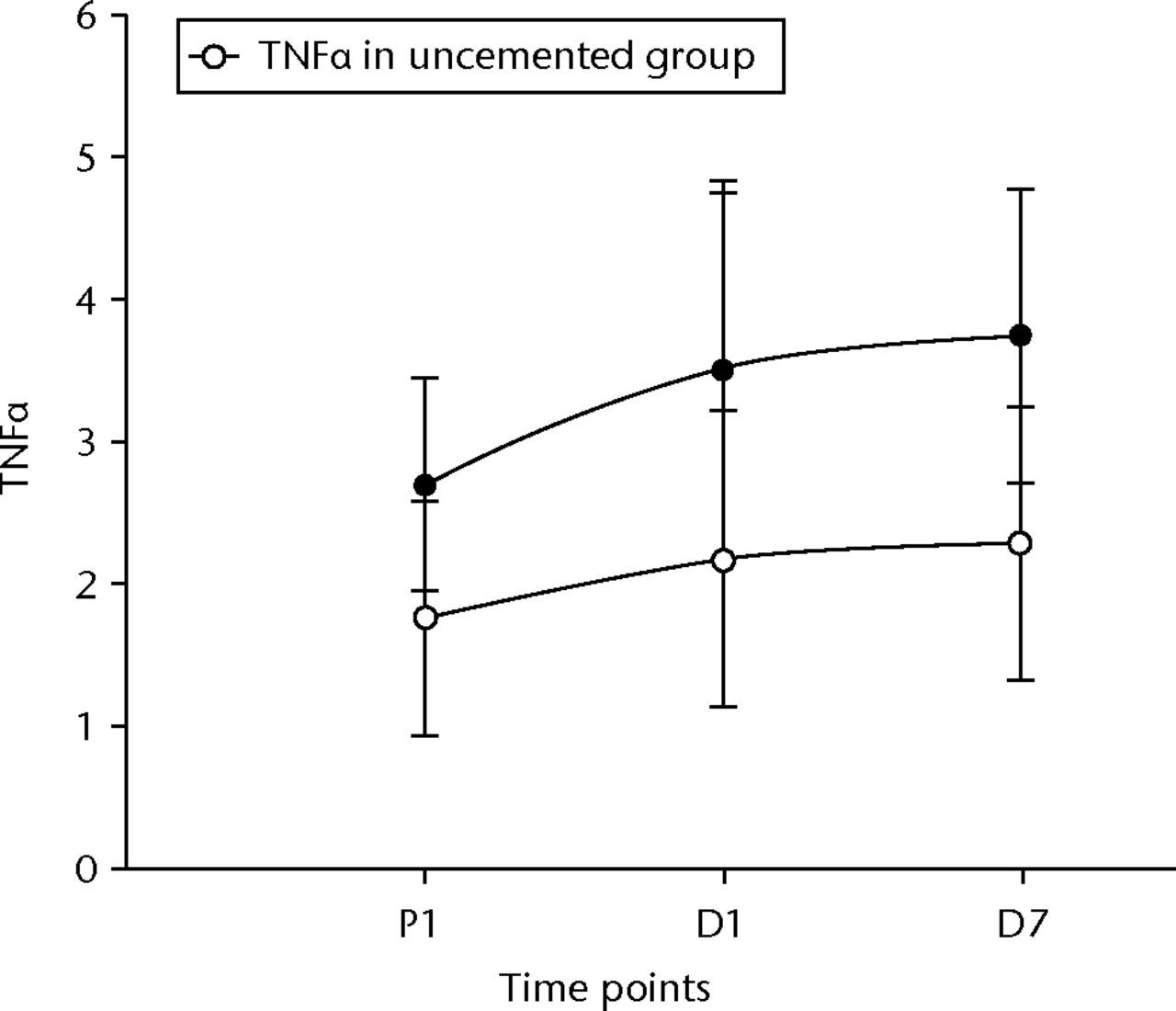
Fig. 7
Graph of changes in tumour necrosis factor alpha (TNFα) levels (pg/ml) over the three time points (mean and standard deviation) in the cemented total knee replacement (TKR) group (n = 19) against the uncemented TKR group (n = 19)
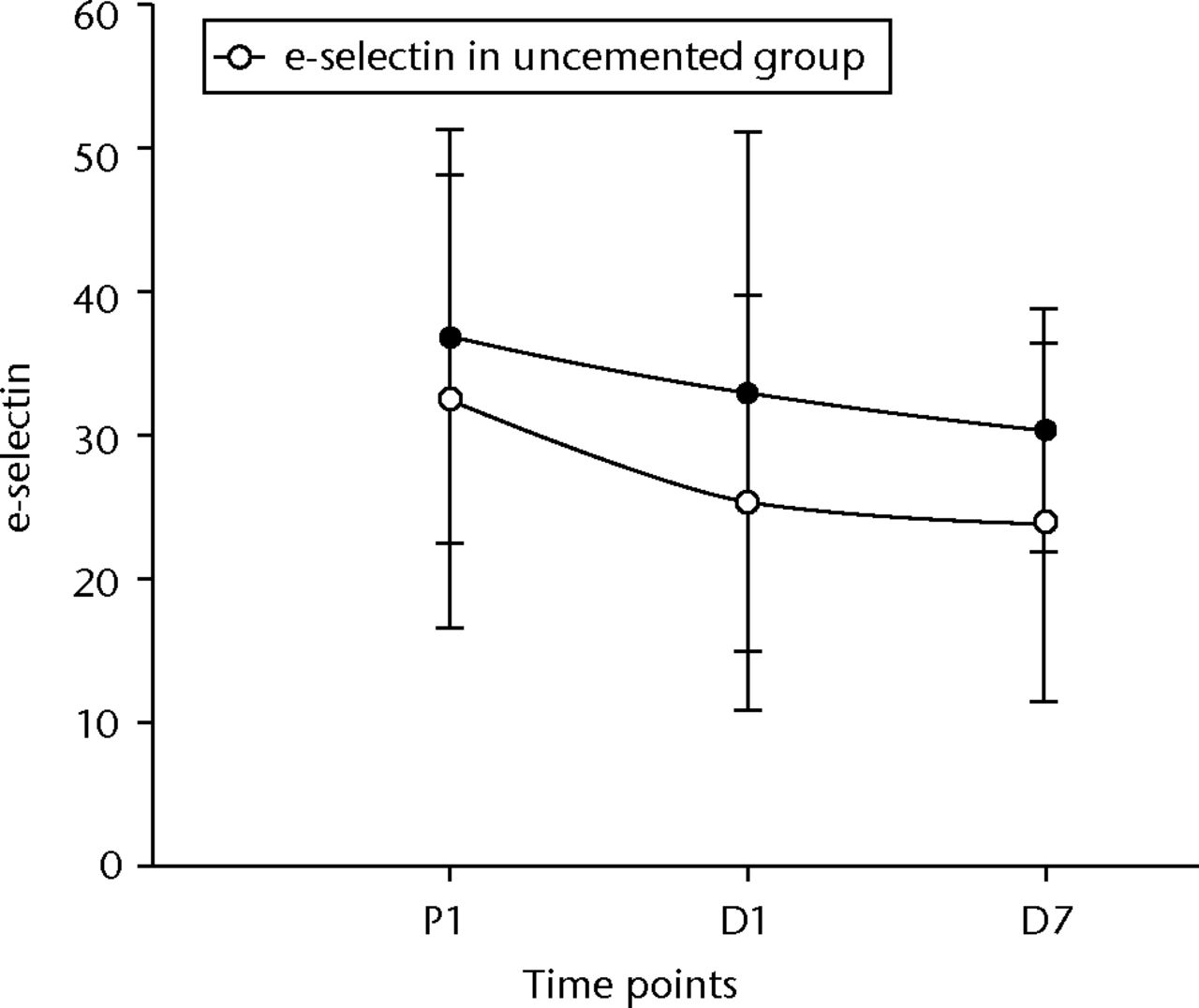
Fig. 8
Graph of changes in e-selectin levels (ng/ml) over the three time points (median and interquartile ranges) in the cemented total knee replacement (TKR) group (n = 19) against the uncemented TKR group (n = 19)
Flow cytometry assays of cell cytokine expression
The flow cytometry readings were also analysed at the three time points using pair sampled t-tests. The analysis was carried using both the absolute values and percentage values. The only statistically significant change in the uncemented group was seen in the CD40 between P1 and D1 of the absolute numbers and percentage changes (p = 0.01 and p = 0.004, respectively). The CD40 counts in the cemented group, on the other hand, only showed a significant change in the absolute values between D1 and D7 (p = 0.03) (Fig. 9). There was no difference between the two groups for the CD40 levels.
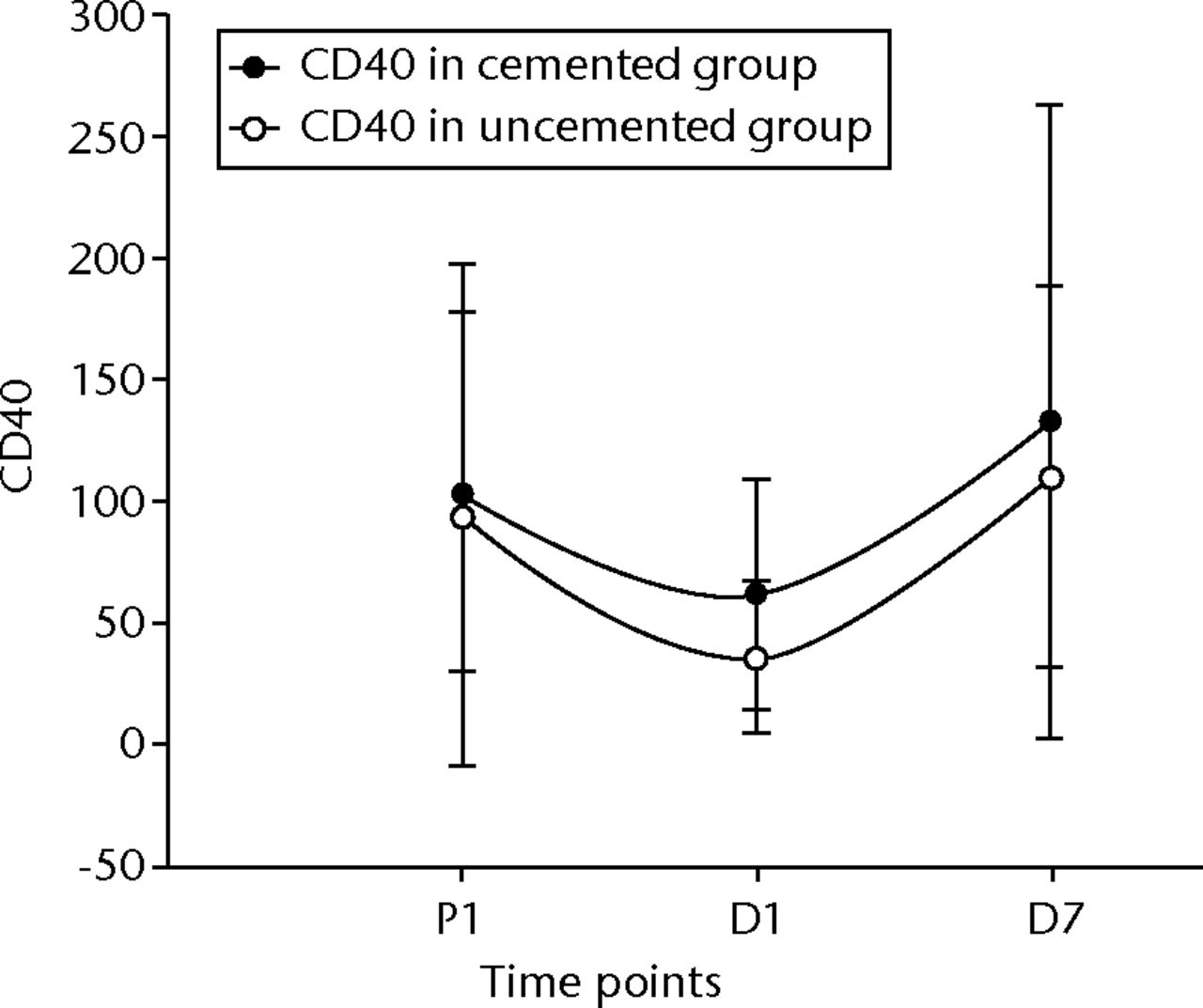
Fig. 9
Graph of changes in CD40 counts over the three time points (median and interquartile ranges) in the cemented total knee replacement (TKR) group (n = 19) against the uncemented TKR group (n = 19)
In the cemented group, the CD14/42a counts showed a statistically significant fall in the first 24 hours (p = 0.022), and a significant rise at D7 when compared with D1 and P1 levels (p = 0.006 and p = 0.02, respectively) (Fig. 10). Comparing the flow cytometry counts between the two groups (Table III and Table IV) showed a significant rise in the CD1442a levels both for the absolute values and percentage changes from D1 to D7 (p = 0.03 and p = 0.02, respectively) (Fig. 10). There were also significant differences in the first 24 hours (P1 to D1) when we look at the percentage changes (p < 0.001).
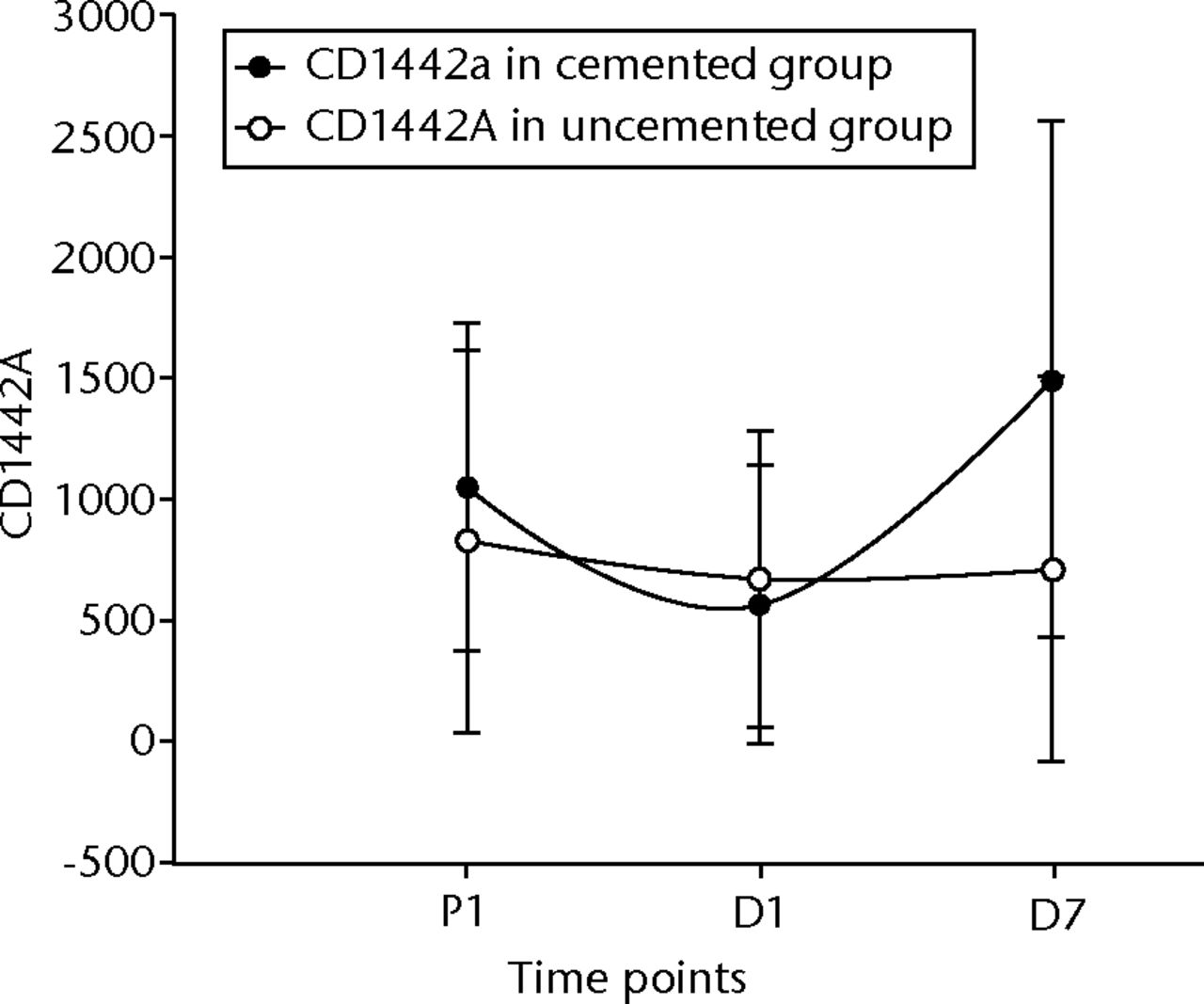
Fig. 10
Graph of changes in CD14/42a counts over the three time points (median and interquartile ranges) in the cemented total knee replacement (TKR) group (n = 19) against the uncemented TKR group (n = 19)
Table IV
Mann–Whitney U test for e-selectin (ng/ml), CD1442a, and CD40 absolute values between the cemented total knee replacement (TKR) group (n = 19) and uncemented TKR group (n = 19)
| Percentiles | ||||
|---|---|---|---|---|
| Blood markers | 25th | 50th (Median) | 75th | Significance (p-value) |
| E-selectin | ||||
| P1 to D1 | -6.3 | -4.3 | 1.5 | 0.84 |
| D1 to D7 | -3.4 | 0.1 | 3.6 | 0.82 |
| P1 to D7 | -9.9 | -2.7 | 1.9 | 0.84 |
| CD14/42a | ||||
| P1 to D1 | -719 | -177 | 41 | 0.15 |
| D1 to D7 | -112 | 358 | 1206 | 0.04 |
| P1 to D7 | -286 | 171 | 720 | 0.07 |
| CD40 | ||||
| P1 to D1 | -114 | -54 | -4 | 0.93 |
| D1 to D7 | 16 | 64 | 169 | 0.17 |
| P1 to D7 | -32 | 19 | 130 | 0.35 |
Discussion
The safety of cement has been questioned following its use in total hip replacements (THR) and in the surgical management of intracapsular hip fractures. Data from the National Joint Registry for England and Wales (NJR) has suggested increased mortality rates following cemented TKR and THR. Ritter, Gioe and Sieber23 demonstrated that the addition of PMMA had a significant impact on the overall mortality following THR and TKR.
McMinn et al24 examined the results from the NJR database, and found a significant difference in mortality between cemented and uncemented THR after adjustment for diagnosis, age, gender, diagnosis, complexity, and fitness based on the American Society of Anesthesiologists (ASA) grade25 with a significant adjusted mortality rate ratio for cemented compared with uncemented procedures as 1.11 (95% confidence interval 1.07 to 1.16) (p < 0.001). Adverse cardiopulmonary results have been widely documented following the use of PMMA bone cement.26-30
This is the first prospective study to compare the response of inflammatory mediators following cemented and uncemented TKRs. Both the uncemented and cemented groups showed significant changes in the parameters, measured at various time points as explained above.
The aim of this study was to determine if the changes seen both in uncemented and cemented groups were more statistically significant than for any of the measured parameters. Both groups had a similar response with regards to the CRP, IL6 and TNFα levels. The IL6 levels reflect the degree of surgical trauma31 and in most instances the secretion of IL6 is brief and self-limiting, lasting from 48 hours to 72 hours,19 but this demonstrates that the stimulus of the surgical trauma continues at least up to seven days post-operatively.
As expected, following the surgical trauma to the tissues and vessels, the vascular endothelium becomes procoagulant, with the release of several factors including vWF. In response to the thrombus, formation t-PA is released from the endothelium32 and our results reflect this, with significant levels of both vWF and t-PA in the first 24 hours and levels remaining significantly elevated up to at least seven days.
The only parameter which demonstrated a more significant change in the cemented group compared with that of the uncemented group was CD14/42a. This showed a more statistically significant change not only in the first 24 hours, but also in the period between D1 and D7 (p < 0.001 and p = 0.02, respectively).
CD14 is a membrane glycoprotein, which was identified as a receptor for the endotoxin lipopolysaccharide.33 Changes in CD14 expression and serum sCD14 have been associated with several disorders.34,35 The more mature the subpopulation of monocytes, the more CD14+/CD16+ are known to expand in infectious diseases such as sepsis, tuberculosis, the critically ill and in uraemic patients without infections.35-37 It has also been suggested that they have a role as promoters of the inflammatory process in vascular lesions in the development of atherosclerosis.38,39
CD42a belongs to the mucin family, and is a small membrane glycoprotein found on the surface of platelets.40 The CD14/42a dyad is a measure of the platelet-monocyte aggregate. Current evidence suggests that patients with acute coronary syndromes not only have increased interactions between platelets (homotypic aggregates), but also between platelets and leukocytes (heterotypic aggregates), in circulating blood.40 Early evidence suggested that these heterotypic aggregates form in inflammatory states.40
There is growing evidence demonstrating the importance of platelet-leucocyte aggregation in atherothrombosis and acute coronary syndromes. Increased circulating platelet-monocyte aggregates were found in patients with chronic obstructive pulmonary disease (COPD) compared with well-matched controls in a study, and the authors suggested this mechanism as an increased cardiovascular risk in COPD, and recommended platelet inhibition as a plausible therapeutic target.41
The exact role of platelet-monocyte aggregates in acute coronary syndrome is unknown, whether they are a reflection of the inflammatory and thrombotic process or directly contribute to the ongoing athero-thrombogenesis.
Limitations to this study include the lack of randomisation, introducing potential for bias and the small cohort size of 19 patients in each group. The two treatment groups, however, did not differ in their demographic data. We can therefore conclude from these results that the use of bone cement causes a statistically significant rise in CD14/42a levels in this group of patients, which occurs in the first 24 hours and continues to at least day seven post-operatively. Our current understanding of the exact role of CD14/42a is not clearly understood. It appears that the use of bone cement here has a significant effect on its levels, albeit the relevance at this current time is unknown. As our understanding of the role of cytokines such that of C14/42a evolves, as will our understanding of the real effect of TKR on our patients at the cellular level.
More work is also required in examining the role of inflammatory markers following cementation in orthopaedic surgery and in THR, where during reaming and the cement pressurisation processes fat, marrow, air, particles of bone, cement, and aggregates of platelets and fibrin, are driven into the systemic circulation.26
1 Schaad UB , McCrackenGH Jr, NelsonJD. Pyogenic arthritis of the sacroiliac joint in pediatric patients. Pediatrics1980;66:375–379.PubMed Google Scholar
2 Cotton FJ . Fender fracture of the tibia of the knee. N Engl J Med1929;201:989–995. Google Scholar
3 No authors listed. National Joint Registry for England and Wales 9th Annual report. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/9th_annual_report/NJR%209th%20Annual%20Report%202012.pdf (date last accessed 6 December 2013). Google Scholar
4 Charnley J. Low friction arthroplasty of the hip: theory and practice.Berlin, Heidelberg: Springer-Verlag, 1979. Google Scholar
5 Charnley J. Acrylic cement in orthopaedic surgery.Edinburgh: Churchill Livingstone, 1972. Google Scholar
6 Nussbaum DA , GailloudP, MurphyK. The chemistry of acrylic bone cements and implications for clinical use in image-guided therapy. J Vasc Interv Radiol2004;15:121–126.CrossrefPubMed Google Scholar
7 Kuhn KD. Bone cement: up-to-date comparison of physical and chemical properties of commercial interests.Berlin: Springer, 2000. Google Scholar
8 DiPisa JA , SihGS, BermanAT. The temperature problem at the bone-acrylic cement interface of the total hip replacement. Clin Orthop Relat Res1976;121:95–98.PubMed Google Scholar
9 Mjoberg B , PetterssonH, RosenqvistR, RydholmA. Bone cement, thermal injury and the radiolucent zone. Acta Orthop Scand1984;55:597–600.CrossrefPubMed Google Scholar
10 Noordin S , ShortkroffS, SledgeCB, SpectorM. Investigation of the activation of a human serum complement protein, C3, by orthopedic prosthetic particulates. Biomaterials2004;25:5347–5352.CrossrefPubMed Google Scholar
11 Baldwin L , FlanaganBF, HuntJA. Flow cytometric measurement of phagocytosis reveals a role for C3b in metal particle uptake by phagocytes. J Biomed Mater Res2005;73:80–85.CrossrefPubMed Google Scholar
12 Rakshit DS , LimJTE, LyK, et al.Involvement of complement receptor 3 (CR3) and scavenger receptor in macrophage responses to wear debris. J Orthop Res2006;24:2036–2044.CrossrefPubMed Google Scholar
13 Cipolle MD , PasqualeMD, CerraFB. Secondary organ dysfunction: from clinical perspectives to molecular mediators. Crit Care Clin1993;9:261–298. Google Scholar
14 Pastores SM , ThakkarA, GennisP, KatzDP, KvetanV. Posttraumatic multiple-organ dysfunction syndrome: role of mediators in systemic inflammation and subsequent organ failure. Acad Emerg Med1996;3:611–622.CrossrefPubMed Google Scholar
15 Giannoudis PV , SmithRM, BanksRE, et al.Stimulation of inflammatory markers after blunt trauma. Br J Surg1998;85:986–990.CrossrefPubMed Google Scholar
16 Giannoudis PV . Current concepts of the inflammatory response after major trauma: an update. Injury2003;34:397–404.CrossrefPubMed Google Scholar
17 Lie SA , EngesaeterLB, HavelinLI, GjessingHK, VollsetSE. Mortality after total hip replacement: 0-10-year follow-up of 39,543 patients in the Norwegian Arthroplasty Register. Acta Orthop Scand2000;71:19–27.CrossrefPubMed Google Scholar
18 Mantilla CB , HorlockerTT, SchroederDR, BerryDJ, BrownDL. Frequency of myocardial infarction, pulmonary embolism, deep venous thrombosis, and death following primary hip or knee arthroplasty. Anesthesiology2002;96:1140–1146. Google Scholar
19 Cruickshank AM , FraserWD, BurnsHJ, Van DammeJ, ShenkinA. Response of serum interleukin-6 in patients undergoing elective surgery of varying severity. Clin Sci (Lond)1990;79:161–165.CrossrefPubMed Google Scholar
20 Nygaard OP , UnnebergK, ReikeråsO, OsterudB. Thromboplastin activity of blood monocytes after total hip replacement. Scand J Clin Lab Invest1990;50:183–186.CrossrefPubMed Google Scholar
21 Dahl OE , PedersenT, KierulfP, et al.Sequential intrapulmonary and systemic activation of coagulation and fibrinolysis during and after total hip replacement surgery. Thromb Res1993;70:451–458.CrossrefPubMed Google Scholar
22 Ray MJ , CalabroLJ, SirisenaT, et al.Pre-operative platelet-bound CD40 ligand is probably associated with peri-operative cardiac events in hip and knee arthroplasty. Eur J Clin Invest2010;40:497–503.CrossrefPubMed Google Scholar
23 Ritter MA , GioeTJ, SieberJM. Systemic effects of polymethylmethacrylate. Increased serum levels of gamma-glutamyltranspeptidase following arthroplasty. Acta Orthop Scand1984;55:411–413.CrossrefPubMed Google Scholar
24 McMinn DJW , SnellKIE, DanielJ, et al.Mortality and implant revision rates of hip arthroplasty in patients with osteoarthritis: registry based cohort study. BMJ2012;344:3319.CrossrefPubMed Google Scholar
25 No authors listed. American Society of Anesthesiologists: New classification of physical status (ASA) Anesthesiology1963;24:111. Google Scholar
26 Donaldson AJ , ThomsonHE, HarperNJ, KennyNW. Bone cement implantation syndrome. BrJ Anaesth2009;102:12–22.CrossrefPubMed Google Scholar
27 Byrick RJ . Cement implantation syndrome: a time limited embolic phenomenon. Can J Anaesth1997;44:107–111.CrossrefPubMed Google Scholar
28 Edmonds CR , BarbutD, HagerD, SharrockNE. Intraoperative cerebral arterial embolization during total hip arthroplasty. Anesthesiology2000;93:315–318.CrossrefPubMed Google Scholar
29 Patterson BM , HealeyJH, CornellCN, SharrockNE. Cardiac-arrest during hip-arthroplasty with a cemented long-stem component - a report of 7 cases. J Bone Joint Surg [Am]1991;73-A:271–277. Google Scholar
30 Peebles DJ , EllisRH, StrideSDK, SimpsonBRJ. Cardiovascular effects of methylmethacrylate cement. Br Med J1972;1:349–351.CrossrefPubMed Google Scholar
31 Taga T , HibiM, HirataY, et al.Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell1989;58:573–581.CrossrefPubMed Google Scholar
32 Emeis JJ . Regulation of the acute release of tissue-type plasminogen activator from the endothelium by coagulation activation products. Ann N Y Acad Sci1992;667:249–258.CrossrefPubMed Google Scholar
33 Wright SD , RamosRA, TobiasPS, UlevitchRJ, MathisonJC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science1990;249:1431–1433.CrossrefPubMed Google Scholar
34 Scherberich JE , NockherWA. CD14++ monocytes, CD14+/CD16+ subset and soluble CD14 as biological markers of inflammatory systemic diseases and monitoring immunosuppressive therapy. Clin Chem Lab Med1999;37:209–213.CrossrefPubMed Google Scholar
35 Brauner A , LuY, HalldenG, HylanderB, LundahlJ. Difference in the blood monocyte phenotype between uremic patients and healthy controls: its relation to monocyte differentiation into macrophages in the peritoneal cavity. Inflammation1998;22:55–66.CrossrefPubMed Google Scholar
36 Fingerle G , PforteA, PasslickB, et al.The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood1993;82:3170–3176.PubMed Google Scholar
37 Fingerle-Rowson G , AuersJ, KreuzerE, et al.Expansion of CD14(+)CD16(+) monocytes in critically ill cardiac surgery patients. Inflammation1998;22:367–379. Google Scholar
38 Schlitt A , HeineGH, BlankenbergS, et al.CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost2004;92:419–424.CrossrefPubMed Google Scholar
39 Schmitz G , HerrAS, RotheG. T-lymphocytes and monocytes in atherogenesis. Herz1998;23:168–177.CrossrefPubMed Google Scholar
40 Arber N , BerlinerS, PrasE, et al.Heterotypic leukocyte aggregation in the peripheral blood of patients with leukemia, inflammation and stress. Nouv Rev Fr Hematol1991;33:251–255.PubMed Google Scholar
41 Maclay JD , McAllisterDA, JohnstonS, et al.Increased platelet activation in patients with stable and acute exacerbation of COPD. Thorax2011;66:769–774.CrossrefPubMed Google Scholar
Funding statement:
None declared
Author contributions:
K. Cheng: Set-up of study and data collection, Data analysis, Writing of thesis from which data and paper is based
D. Giebaly: Writing the paper
A. Campbell: Help set-up study, Supervisor
A. Rumley: Analysis of samples
G. Lowe: Supervisor of study
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










