Abstract
Objectives
The goal of this study was to determine whether intra-articular administration of the potentially anti-fibrotic agent decorin influences the expression of genes involved in the fibrotic cascade, and ultimately leads to less contracture, in an animal model.
Methods
A total of 18 rabbits underwent an operation on their right knees to form contractures. Six limbs in group 1 received four intra-articular injections of decorin; six limbs in group 2 received four intra-articular injections of bovine serum albumin (BSA) over eight days; six limbs in group 3 received no injections. The contracted limbs of rabbits in group 1 were biomechanically and genetically compared with the contracted limbs of rabbits in groups 2 and 3, with the use of a calibrated joint measuring device and custom microarray, respectively.
Results
There was no statistical difference in the flexion contracture angles between those limbs that received intra-articular decorin versus those that received intra-articular BSA (66° vs 69°; p = 0.41). Likewise, there was no statistical difference between those limbs that received intra-articular decorin versus those who had no injection (66° vs 72°; p = 0.27). When compared with BSA, decorin led to a statistically significant increase in the mRNA expression of 12 genes (p < 0.01). In addition, there was a statistical change in the mRNA expression of three genes, when compared with those without injection.
Conclusions
In this model, when administered intra-articularly at eight weeks, 2 mg of decorin had no significant effect on joint contractures. However, our genetic analysis revealed a significant alteration in several fibrotic genes.
Cite this article: Bone Joint Res 2014;3:82–8.
Article Focus
The goal of this study was to determine whether intra-articular administration of the potentially anti-fibrotic agent decorin influences the expression of genes involved in the fibrotic cascade and if that change ultimately leads to a decrease in clinical contractures, in an animal model.
Key Messages
When administered intra-articularly at eight weeks, 2 mg of decorin had no significant effect on joint contractures.
However, our genetic analysis revealed a significant alteration in several fibrotic genes, including a trough in decorin at approximately six hours. As such, the lack of difference in joint contractures is likely related to the timing of administration.
Strengths and limitations
Strengths: This is a validated animal model, biomechanical analysis with concurrent large-scale genetic expression investigation, studied at multiple early and late time points.
Limitations: No polymerase chain reaction (PCR) confirmatory studies have been completed.
Introduction
Joint fibrosis resulting from inflammatory or degenerative conditions, trauma, or surgery is common, of variable severity, and difficult to overcome. The intimate biological mechanisms underlying joint fibrosis remain poorly understood. However, anti-fibrotic agents could potentially be used for the prevention or treatment of joint fibrosis. Decorin is a small leucine-rich endogenous proteoglycan with known anti-fibrotic properties, but its effect on joint contractures is largely unknown.
Decorin co-localises with TGF-β and renders its effects inactive.1-5 Decorin has displayed antifibrotic effects in lung,6-9 cardiac,10 vascular,11 kidney,12,13 liver,4 brain,14 hypertrophic scar,15 conjunctival,16 and vocal cord tissues.17 In regards to orthopaedics, decorin has been shown to decrease intra-articular fibrous adhesions in a rabbit knee joint,18 minimise synovial hyperplasia in fluid from osteoarthritic knees containing mesenchymal stem cells,19 and prevent fibrosis in lacerated muscle.5 More importantly, Li et al20 have recently shown that decorin not only prevents muscle fibrosis, but also improves muscle regeneration and repair.20
We have recently developed and validated an animal model of joint contracture.21 In addition, we also created and validated a customised microarray for our rabbit model, with the goal of simultaneously evaluating changes in gene expression of hundreds of genes.22 Using this contracture model and calibrated measuring device, we assessed the anti-fibrogenic effects of decorin when compared with bovine serum albumin (BSA) (control 1) and no injection (control 2) by measuring the biomechanical properties (flexion contracture) and genetic expression of 376 genes using our customised microarray.
Materials and Methods
A total of 18 skeletally mature New Zealand white (NZW) female rabbits weighing between 2.7 kg and 3.4 kg were used for the study. As per our previously described protocols, all rabbits had their right knees operated to create 3 mm defects in the non-cartilaginous portions of the femoral condyles, with hyperextension of the joint performed to disrupt the posterior capsule and immobilisation in maximum flexion with a Kirschner (K)-wire for eight weeks (Fig. 1).21-23 The wire was removed and the rabbits were allowed activity in a cage for 16 weeks before sacrifice. After sacrifice at 24 weeks, the limbs were tested using a custom-made, calibrated joint measuring device (Fig. 2).21-23 The left knee of each animal served as a non-operative control within each group, based on our previous work, which demonstrated the validity of this control model.22 Institutional Animal Review Committee approval was obtained prior to initiation of the experiments.
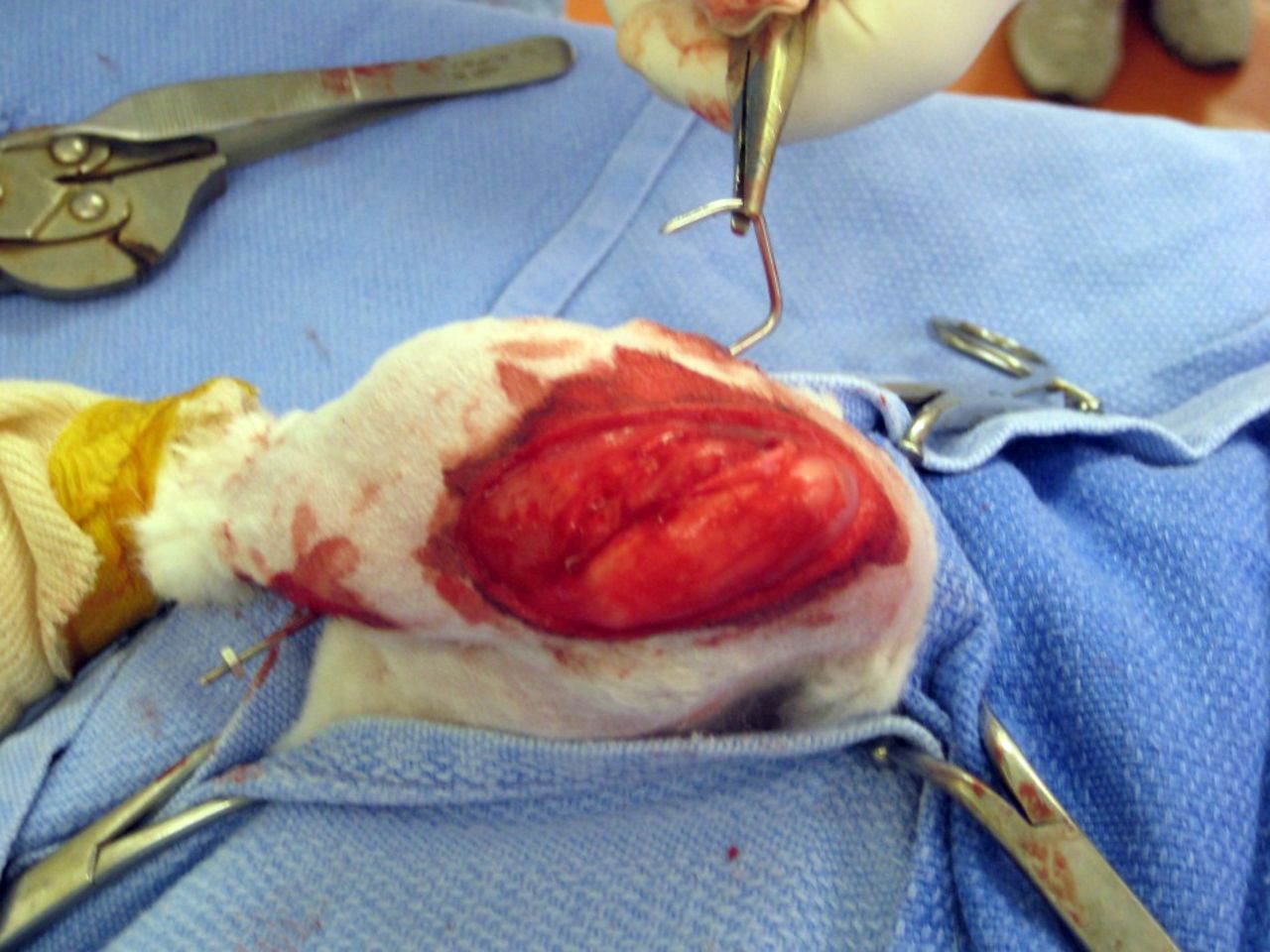
Fig. 1
Intra-operative photograph of right knee immobilisation and formation of contracture with K-wire.

Fig. 2
Photograph depicting the custom-made and validated device used to measure joint contracture angles.
The six right limbs in the experimental group (group 1) received four 500 ug/ml intra-articular injections of decorin over eight days, for a total of 2 mg (Table I), starting eight weeks from the index surgical intervention and after the initiation of the contracture. We chose the dosing and frequency based on previous literature, which implied that 2 mg of decorin is optimal to induce apoptosis.5,6,24
Table I
Table 1. Distribution of animals (N/A, Not Applicable)
| No. of animals | Intra-articular injection | Concentration (µg/mL) | Volume (mL) | |
|---|---|---|---|---|
| Group 1 | 6 | Decorin | 500 | 1 |
| Group 2 | 6 | Bovine serum albumin | N/A | 1 |
| Group 3 | 6 | None | N/A | N/A |
The six right limbs in the first control group (group 2) received four intra-articular injections of BSA over eight days, also starting at eight weeks. The six right limbs in the second control group (group 3) received no injections. Of note, all injections started at the eight-week time point and were given every other day. Injection of the solution into the knee joint was confirmed on fluoroscopy (Figs 3a and 3b). The volume injected (1 mL) was standardised and based upon the results of pilot studies.
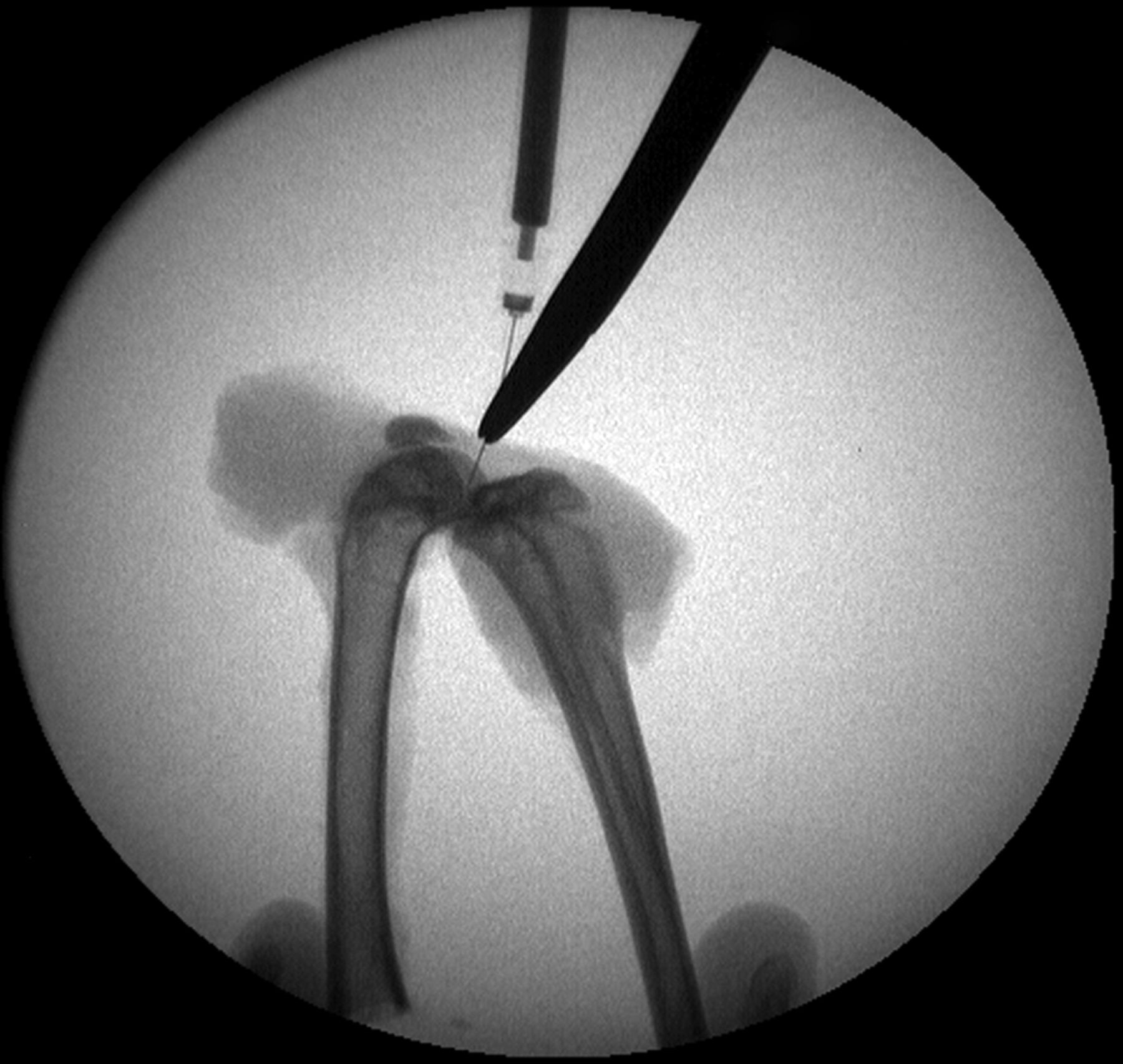
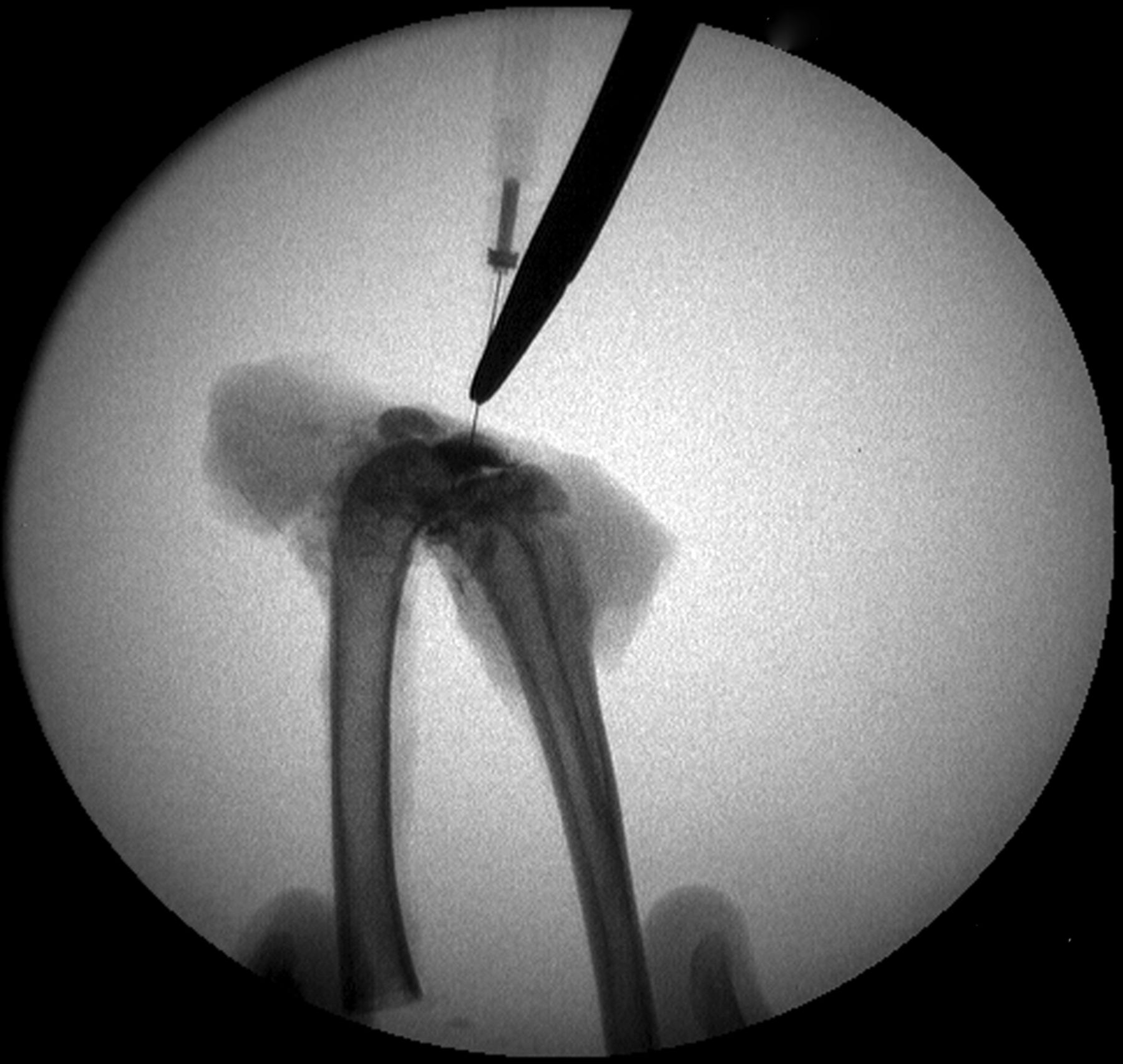
Figs. 3a - 3b
Intra-operative fluoroscopic images confirming a) intra-articular placement of needle, and b) subsequent injection of decorin or bovine serum albumin (BSA).
At 24 weeks, rabbits were sacrificed and the joint capsules were harvested. The mRNA extraction was completed as per previously described protocols.22 After mRNA extraction, a custom microarray, created in our laboratory, was used to evaluate fold changes of 376 genes, including the temporal expression of decorin.22
Statistical analysis
The sample size was calculated assuming a 5% type 1 error and 80% power to detect an effect size of 30°, with a standard deviation (sd) of 20°. The knee joint flexion angles of the operated limbs were measured and compared with the unoperated contralateral limbs in all groups. The operative limbs of group 1 were also directly compared with the limbs of groups 2 and 3, respectively. Comparison was performed using a two-sample t-test assuming unequal variances. Significance level was set at p < 0.05. Data are presented as a mean value (sd).
Gene expression profiles of the limb injected with decorin were compared with the two sets of control limbs. As previously described, the Illumina whole-genome genes expression bead chip array (Illumina, San Diego, California) was used to assay 376 transcripts.22 Six samples were run on one array and the samples from the groups were randomly allocated. Gene expression data was normalised within treatment group using the model-based normalisation method, fastlo.25 Analysis was done using log base-2 expression values and Student’s t-test was used for differences between mean expression levels. Results are presented for p < 0.01.
Results
Biomechanical analysis
When analysing the group 1 (decorin) operative limbs at 24 weeks, the mean flexion contracture angle was 65.8° (sd 19.3°), compared with 8.2° (sd 7.4°) on the unoperated contralateral side (p = 0.0002) (Table II). When analysing the group 2 (BSA group) operative limbs at 24 weeks, the mean flexion contracture angle was 69.1° (sd 31.1°), compared with 11.8 (sd 20.3°) on the unoperated contralateral side (p = 0.0002). Similarly, the group 3 (no injection) operative limbs had a mean flexion contracture angle of 71.5° (sd 9.3°) when compared with 11.9° (sd 21.4°) on the unoperated contralateral side (p = 0.0002). In essence, all three groups formed statistically significant joint contractures, compared with their unoperated contralateral limb.
Table II
Summary of flexion contracture angles (BSA, Bovine Serum Albumin).
| Injection type | Operative (right) limb flexion contracture angle Mean (sd) (°) | Non-operated (left) limb flexion contracture angle Mean (sd) (°) | p-value (t-test) |
|---|---|---|---|
| Decorin | 65.8 (sd 19.3) | 8.2 (sd 7.4) | 0.0002 |
| BSA | 69.1 (sd 31.1) | 11.8 (sd 20.3) | 0.0002 |
| None | 71.5 (sd 9.3) | 11.9 (sd 21.4) | 0.0002 |
There was no statistical difference in the flexion contracture angles between right limbs that received intra-articular decorin versus those that received intra-articular BSA (65.8° [sd 19.3°] vs 69.1° [sd 31.1°], respectively; p = 0.41) (Table III). Likewise, there was no statistical difference between right limbs that received intra-articular decorin, as opposed to those not receiving an injection (65.8° [sd 19.3°] vs 71.5° [sd 9.3°], respectively; p = 0.27). The lack of significance remained when the control left limbs were taken into account (p > 0.40).
Table III
Summary of flexion contracture angles (BSA, Bovine Serum Albumin).
| Injection type | Operative (right) limb flexion contracture angle mean (sd) (°) | p-value (t-test) | |
|---|---|---|---|
| First analysis | No injection vs Decorin | 71.5 (sd 9.3) / 65.8 (sd 19.3) | p = 0.27 |
| Second analysis | BSA vs Decorin | 69.1 (sd 31.1) / 65.8 (sd 19.3) | p = 0.41 |
Genetic expression profiles
Out of 376 genes, 12 had significantly increased mRNA expression in the decorin group compared with the BSA group (p < 0.01) (Table IV). Substance P, neuropeptide γ, and neurokinin A, cyclin E2, matrix metalloproteinase-9 (MMP-9), major histocompatibility complex (MHC) class II HLA-DR-α, matrix metalloproteinase-13 (MMP-13), lectin-like oxidized LDL receptor-1 (LOX-1), coagulation factor VII, eukaryotic initiation factor-2α (eIF-2α), angiotensin receptor 2, and protein kinase C-β (PKC-β) were all increased with the intra-articular injections of decorin, while hemoxygenase 1 (HO-1) was decreased. When compared with the no injection group, there were two genes that were significantly increased (GRO protein and MHC class II HLA-DR-α) and one decreased (apolipoprotein D) in the decorin group (p < 0.01) (Table V). Finally, we mapped the temporal course of decorin over time and found that it had a trough at six to 12 hours, with return to homeostatic levels at 24 hours in the fibrotic cascade (Fig. 4). These data suggest that decorin potentially has an effect on joint fibrosis, but not in the amount, timing or frequency schedule used here.
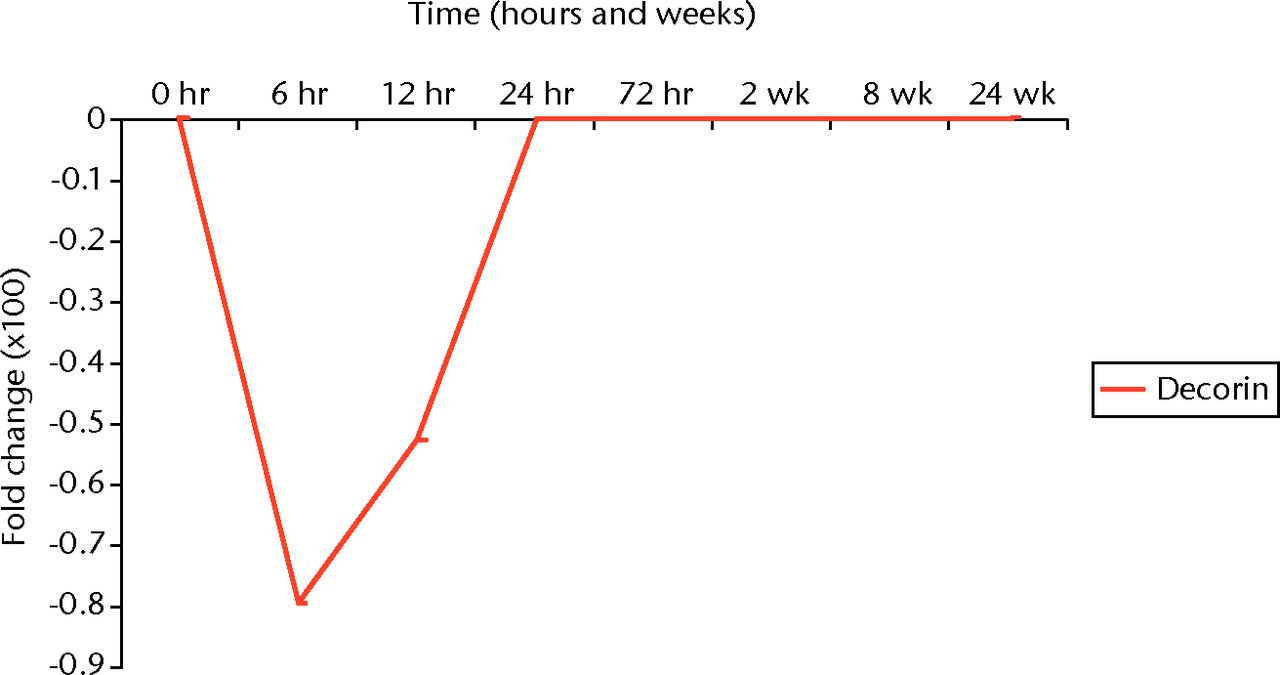
Fig. 4
Graph depicting the temporal expression of decorin mRNA expression in our rabbit model of joint contractures.
Table IV
Summary of genes most up- and down-regulated with decorin compared with bovine serum albumin (BSA) (in descending order of p-value)
| Decorin vs BSA | Log2 fold change | p-value (t-test) | |
|---|---|---|---|
| ↑ Tachykinin family (substance P, neuropeptide γ, neurokinin A) | 0.25 | 0.00044 | |
| ↑ Cyclin E2 (CCNE2) | 0.95 | 0.00057 | |
| ↑ Matrix metalloproteinase-9 (MMP-9) | 0.34 | 0.00098 | |
| ↑ Major histocompatibility complex (MHC) class II HLA-DR-α | 0.22 | 0.00118 | |
| ↑ Matrix metalloproteinase-13 (MMP-13) | 0.52 | 0.00218 | |
| ↑ Lectin-like oxidised LDL receptor (LOX-1) | 1.8 | 0.00249 | |
| ↑ Coagulation factor VII | 0.28 | 0.00346 | |
| ↓ Hemoxygenase-1 (HO-1) | -1.3 | 0.00419 | |
| ↑ Eukaryotic initiation factor-2α (eIF-2α) | 0.65 | 0.00488 | |
| ↑ Angiotensin receptor 2 | 0.31 | 0.00566 | |
| ↑ Protein kinase C-β (PKC-β) | 0.68 | 0.00832 |
Table V
Summary of genes most up- and down-regulated with decorin compared with no injection (in descending order of p-value)
| Decorin vs no injection | Log2 fold change | p-value (t-test) |
|---|---|---|
| ↑ GRO protein (GRO) | 0.489 | 0.00237 |
| ↓ Apolipoprotein D | -0.74 | 0.00435 |
| ↑ Major histocompatibility complex (MHC) class II HLA-DR-α | 0.27 | 0.00474 |
Discussion
Joint contractures are detrimental to the functional capability of patients. Once a joint becomes stiff, physical therapy and surgery may be required, but restoration of normal movement is not achieved in many individuals. Pharmacologic blockade of the fibrotic cascade could be extremely useful for the prevention or treatment of joint stiffness in orthopaedic surgery. However, to date there are no known effective pharmacologic treatments and/or preventative agents for arthrofibrosis. This may be in part related to the fact that while advances in operative treatment of joint stiffness have continued, the pathophysiology of arthrofibrosis remains poorly understood. Furthermore, some models of joint contractures do not produce a severe or permanent contracture, making it difficult to detect any effects from pharmacological treatments.26 An animal model leading to severe and permanent joint contracture must be used to detect differences in joint stiffness when various pharmacologic agents are tested.21
Several pharmacologic agents have been investigated for the prevention and treatment of fibrosis in different conditions. Decorin is a small leucine-rich endogenous proteoglycan. It was selected to be tested in this study as it is a promising anti-fibrotic agent in musculoskeletal conditions. Furthermore, its endogenous nature, lack of systemic side effects, ability to bind selectively and inactivate TGF-β, and its demonstrated anti-fibrotic effect in other organ systems, makes it an attractive consideration.
Numerous studies have demonstrated that decorin is an apoptotic agent.24,27,28 The relationship between myofibroblasts and apoptosis has been investigated in other fibrotic disease processes. For instance, as hypertrophic scars remodel, the number of fibroblasts and myofibroblasts reduce, possibly by apoptosis.28 These data supported the previous work of Desmouliere et al,29 showing that myofibroblasts appear in hypertrophic scars prior to the development of high levels of apoptosis, and may be differentiated fibroblasts committed to apoptosis.29 Garbin et al30 demonstrated a correlation between expression of α-SMA and apoptosis. The authors suspect that a delay in apoptosis may be an important factor in the pathophysiology of joint contracture.28,31 To our knowledge, such a theory of apoptosis and myofibroblasts in the resolution stages of arthrofibrosis has not been studied or described. Furthermore, to our knowledge, the effects of recombinant decorin on joint stiffness, myofibroblasts, and apoptosis has not been studied or described, making it an exciting target for the potential treatment of established joint contractures.
In our study, we found no clinically or statistically significant difference in joint contractures between animals receiving decorin versus placebo (either BSA or no injection). This may be due to the route of administration, dosing, frequency, and most importantly, timing. We chose to use intra-articular injections based on the notion that this directly translates to the practicing orthopaedic surgeon. Dosing and frequency were based upon previous literature implying that 2 mg of decorin was optimal to induce fibrotic apoptosis.5,6,24 However, what was not appreciated at the initiation of the study, and only apparent through our subsequent genetic analyses, was the temporal course of decorin. Our mRNA expression data showed that decorin expression has the lowest levels at about six to 12 hours during the fibrotic cascade, with complete return to normal at 24 hours. As such, it is likely that the lack of clinical resolution of the contractures with decorin is secondary to the fact that it was administered when the contracture was already fully established; eight weeks after index surgery. This finding highlights the essential nature of completing both biomechanical and genetic analyses.
Interestingly, our genetic data demonstrated significant alteration in the mRNA expression of several genes when decorin was administered. This is novel, given that a custom microarray for rabbits had to be created and validated, combined with the use of several controls (BSA, no injection, and the unoperated contralateral limb). Moreover, advanced biostatistical analysis was required to ensure accuracy and reproducibility. To our knowledge, this is the first study to investigate the genetic expression profile of arthrofibrosis when treated with a potential anti-fibrotic agent, particularly over time and on such an expansive level as to include > 300 genes. In our model, the highest differences in mRNA expression were for the following genes: substance P, neuropeptide γ, and neurokinin A, cyclin E2, MMP-9, MHC class II HLA-DR-α, MMP-13, LOX-1, coagulation factor VII, eIF-2α, angiotensin receptor 2, PKC-β, HO-1, GRO protein, and apolipoprotein D.
In humans, elbows with post-traumatic contractures have been shown to have increased mRNA expression of MMP-9 and MMP-13.32As such, it is intriguing that these were two of the genes most up-regulated by the administration of decorin. There may be several explanations for this. Foremost, it may be that the decorin was injected far too late in the fibrotic cascade. As such, the repeat trauma of the injections and intra-articular fluid may have perpetuated the process. In addition, while MMP-9 is indeed involved in matrix turnover, it has other functions. It can modulate chemokines and cytokines, which may have an effect on fibrosis by recruiting macrophages and fibroblasts to the area of injury. Mirastschijski et al33 found that MMP-9 levels were significantly increased in the presence of an inhibitor, suggesting that MMP-9 production can overcome the inhibition of other pathways to prevent scarring.
Moreover, several members of the tachykinin family showed significant increases in expression with decorin administration. This is a crucial finding, as multiple studies have shown that increased levels of tachykinin genes, particularly substance P, are significantly associated with musculoskeletal contractures.34 Furthermore, this highlights the need for correct timing of pharmacologic intervention. If tachykinin expression occurs early in the fibrotic cascade, then delivery of decorin at a later stage may not be sufficient to overcome the earlier process. The significant up-regulation of several other genes in response to the inhibition of TGF-β highlights the complexity of the fibrotic pathway. It is likely that blocking one potential target may lead to an increase in several other pathways, resulting in a counter-intuitive response.
There are limitations to the current study. Foremost, we did not complete polymerase chain reaction (PCR) confirmatory studies. PCR would allow us to confirm the microarray with higher fidelity. However, that is our next line of investigation. In addition, the custom microarray only allowed us to investigate a subset of genes. While not optimal, the current technology in an animal model remains in its infancy. Future studies must focus on all potential genes of interest.
In conclusion, 2 mg of decorin administered intra-articularly after an experimental joint contracture that had been established over eight weeks, led to alterations in the mRNA expression of a number of genes likely implicated in arthrofibrosis. Although these changes in mRNA expression did not translate to clinically relevant differences in joint range of movement in this experiment, it does suggest that further studies investigating the route of administration, dosing, frequency, and timing are warranted. In particular, we recommend intra-articular administration of decorin using the same experimental model, but at the very early stages after the initial injury (i.e. the six-hour time point).
Acknowledgments: We thank D. A. Schultz, BS, for assistance with the genetic microarrays.
1 Tredget EE , NedelecB, ScottPG, GhaharyA. Hypertrophic scars, keloids, and contractures. The cellular and molecular basis for therapy. Surg Clin North Am1997;77:701–730.CrossrefPubMed Google Scholar
2 Scott PG , DoddCM, TredgetEE, GhaharyA, RahemtullaF. Immunohistochemical localization of the proteoglycans decorin, biglycan and versican and transforming growth factor-beta in human post-burn hypertrophic and mature scars. Histopathology1995;26:423–431.CrossrefPubMed Google Scholar
3 Yokozeki M , MoriyamaK, ShimokawaH, KurodaT. Transforming growth factor-beta 1 modulates myofibroblastic phenotype of rat palatal fibroblasts in vitro. Exp Cell Res1997;231:328–336.CrossrefPubMed Google Scholar
4 Tiggelman AM , LinthorstC, BoersW, BrandHS, ChamuleauRA. Transforming growth factor-beta-induced collagen synthesis by human liver myofibroblasts is inhibited by alpha2-macroglobulin. J Hepatol1997;26:1220–1228.CrossrefPubMed Google Scholar
5 Fukushima K , BadlaniN, UsasA, et al.The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med2001;29:394–402.CrossrefPubMed Google Scholar
6 Giri SN , HydeDM, BraunRK, et al.Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol1997;54:1205–1216.CrossrefPubMed Google Scholar
7 Kolb M , MargettsPJ, GaltT, et al.Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am J Respir Crit Care Med2001;163:770–777.CrossrefPubMed Google Scholar
8 Kolb M , MargettsPJ, SimePJ, GauldieJ. Proteoglycans decorin and biglycan differentially modulate TGF-beta-mediated fibrotic responses in the lung. Am J Physiol Lung Cell Mol Physiol2001;280:1327–1334.CrossrefPubMed Google Scholar
9 Nakatani T , HondaE, HayakawaS, et al.Effects of decorin on the expression of alpha-smooth muscle actin in a human myofibroblast cell line. Mol Cell Biochem2008;308:201–207.CrossrefPubMed Google Scholar
10 Jahanyar J , JoyceDL, SouthardRE, et al.Decorin-mediated transforming growth factor-beta inhibition ameliorates adverse cardiac remodeling. J Heart Lung Transplant2007;26:34–40.CrossrefPubMed Google Scholar
11 Fischer JW , KinsellaMG, ClowesMM, et al.Local expression of bovine decorin by cell-mediated gene transfer reduces neointimal formation after balloon injury in rats. Circ Res2000;86:676–683.CrossrefPubMed Google Scholar
12 Isaka Y , BreesDK, IkegayaK, et al.Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nat Med1996;2:418–423.CrossrefPubMed Google Scholar
13 Border WA , NobleNA, YamamotoT, et al.Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature1992;360:361–364.CrossrefPubMed Google Scholar
14 Logan A , BerryM. Transforming growth factor-beta 1 and basic fibroblast growth factor in the injured CNS. Trends Pharmacol Sci1993;14:337–342.CrossrefPubMed Google Scholar
15 Zhang Z , LiXJ, LiuY, et al.Recombinant human decorin inhibits cell proliferation and downregulates TGF-beta1 production in hypertrophic scar fibroblasts. Burns2007;33:634–641.CrossrefPubMed Google Scholar
16 Grisanti S , SzurmanP, WargaM, et al.Decorin modulates wound healing in experimental glaucoma filtration surgery: a pilot study. Invest Ophthalmol Vis Sci2005;46:191–196.CrossrefPubMed Google Scholar
17 Krishna P , RosenCA, BranskiRC, WellsA, HebdaPA. Primed fibroblasts and exogenous decorin: potential treatments for subacute vocal fold scar. Otolaryngol Head Neck Surg2006;135:937–945.CrossrefPubMed Google Scholar
18 Fukui N , NakajimaK, TashiroT, OdaH, NakamuraK. Neutralization of fibroblast growth factor-2 reduces intraarticular adhesions. Clin Orthop Relat Res2001;383:250–258.CrossrefPubMed Google Scholar
19 Zhang S , MunetaT, MoritoT, MochizukiT, SekiyaI. Autologous synovial fluid enhances migration of mesenchymal stem cells from synovium of osteoarthritis patients in tissue culture system. J Orthop Res2008;26:1413–1418.CrossrefPubMed Google Scholar
20 Li Y , LiJ, ZhuJ, et al.Decorin gene transfer promotes muscle cell differentiation and muscle regeneration. Mol Ther2007;15:1616–1622.CrossrefPubMed Google Scholar
21 Nesterenko S , MorreyME, AbdelMP, et al.New rabbit knee model of posttraumatic joint contracture: indirect capsular damage induces a severe contracture. J Orthop Res2009;27:1028–1032.CrossrefPubMed Google Scholar
22 Abdel MP , MorreyME, GrillDE, et al.Effects of joint contracture on the contralateral unoperated limb in a rabbit knee contracture model: a biomechanical and genetic study. J Orthop Res2012;30:1581–1585.CrossrefPubMed Google Scholar
23 Abdel MP , MorreyME, BarlowJD, et al.Myofibroblast cells are preferentially expressed early in a rabbit model of joint contracture. J Orthop Res2012;30:713–719.CrossrefPubMed Google Scholar
24 Seidler DG , GoldoniS, AgnewC, et al.Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem2006;281:26408–26418.CrossrefPubMed Google Scholar
25 Ballman KV , GrillDE, ObergAL, TherneauTM. Faster cyclic loess: normalizing RNA arrays via linear models. Bioinformatics2004;20:2778–2786.CrossrefPubMed Google Scholar
26 Hildebrand KA , SutherlandC, ZhangM. Rabbit knee model of post-traumatic joint contractures: the long-term natural history of motion loss and myofibroblasts. J Orthop Res2004;22:313–320.CrossrefPubMed Google Scholar
27 Wu H , WangS, XueA, et al.Overexpression of decorin induces apoptosis and cell growth arrest in cultured rat mesangial cells in vitro. Nephrology (Carlton)2008;13:607–615.CrossrefPubMed Google Scholar
28 Nedelec B , ShankowskyH, ScottPG, GhaharyA, TredgetEE. Myofibroblasts and apoptosis in human hypertrophic scars: the effect of interferon-alpha2b. Surgery2001;130:798–808.CrossrefPubMed Google Scholar
29 Desmouliere A , BadidC, Bochaton-PiallatML, GabbianiG. Apoptosis during wound healing, fibrocontractive diseases and vascular wall injury. Int J Biochem Cell Biol1997;29:19–30.CrossrefPubMed Google Scholar
30 Garbin S , PittetB, MontandonD, GabbianiG, DesmoulièreA. Covering by a flap induces apoptosis of granulation tissue myofibroblasts and vascular cells. Wound Repair Regen1996;4:244–251.CrossrefPubMed Google Scholar
31 Tredget EE, Shankowsky HA, Pannu R, et al. Transforming growth factor-beta in thermally injured patients with hypertrophic scars: effects of interferon alpha-2b. Plast Reconstr Surg 1998;102:1317-28; discussion 1329-30. Google Scholar
32 Hildebrand KA , ZhangM, HartDA. High rate of joint capsule matrix turnover in chronic human elbow contractures. Clin Orthop Relat Res2005;439:228–234.CrossrefPubMed Google Scholar
33 Mirastschijski U , SchnabelR, ClaesJ, et al.Matrix metalloproteinase inhibition delays wound healing and blocks the latent transforming growth factor-beta1-promoted myofibroblast formation and function. Wound Repair Regen2010;18:223–234.CrossrefPubMed Google Scholar
34 Franceschi F , LongoUG, RuzziniL, et al.Circulating substance P levels and shoulder joint contracture after arthroscopic repair of the rotator cuff. Br J Sports Med2008;42:742–745.CrossrefPubMed Google Scholar
Funding statement:
None declared.
Author contributions:
M. P. Abdel: Study design, Experimental studies, Synthesising the data, Drafting the manuscript, Reviewing the manuscript
M. E. Morrey: Study design, experimental studies, Synthesising the data, Reviewing the manuscript
J. D. Barlow: Synthesising the data, Reviewing the manuscript
D. E. Grill: Statistical analysis
C. P. Kolbert: Genetic studies
K. N. An: Study design, Drafting the manuscript, Reviewing the manuscript
S. P. Steinmann: Study design, Drafting the manuscript, Reviewing the manuscript
B. F. Morrey: Study design, Drafting the manuscript, Reviewing the manuscript
J. Sanchez-Sotelo: Study design, Drafting the manuscript, Reviewing the manuscript
ICMJE Conflict of Interest:
None declared.
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










