Abstract
Injury to the anterior cruciate ligament (ACL) is one of the most devastating and frequent injuries of the knee. Surgical reconstruction is the current standard of care for treatment of ACL injuries in active patients. The widespread adoption of ACL reconstruction over primary repair was based on early perception of the limited healing capacity of the ACL. Although the majority of ACL reconstruction surgeries successfully restore gross joint stability, post-traumatic osteoarthritis is commonplace following these injuries, even with ACL reconstruction. The development of new techniques to limit the long-term clinical sequelae associated with ACL reconstruction has been the main focus of research over the past decades. The improved knowledge of healing, along with recent advances in tissue engineering and regenerative medicine, has resulted in the discovery of novel biologically augmented ACL-repair techniques that have satisfactory outcomes in preclinical studies. This instructional review provides a summary of the latest advances made in ACL repair.
Cite this article: Bone Joint Res 2014;3:20–31.
Introduction
Dynamic knee stability is affected by both passive (ligamentous) and active (neuromuscular) joint restraints. Among the contributors to knee joint stability, the anterior cruciate ligament (ACL) has long been considered the primary passive restraint to anterior translation of the tibia with respect to the femur.1,2 Moreover, the ACL contributes to knee rotational stability in both frontal and transverse planes due to its specific orientation.3,4 The ACL has been the focus of many biomechanical/anatomical studies and is among the most frequently studied structures of the human musculoskeletal system over the past decades.
Injuries to the ACL are one of the most common and devastating knee injuries mainly sustained as a result of sports participation.5 These injuries often result in joint effusion, altered movement, muscle weakness, reduced functional performance, and may lead to the loss of an entire season or more of sports participation among young athletes.5 ACL injuries are also associated with long-term clinical sequelae that include meniscal tears, chondral lesions and an increased risk of early onset post-traumatic osteoarthritis (OA).3,6-10
The ACL has long been thought to have poor healing capacity, with a substantially high rate of failure (40% to 100%), even after surgical repair using suture.11-17 The unsatisfactory outcomes of the ACL primary repair have led to unanimous abandonment of suture repair and widespread adoption of ACL reconstruction. ACL reconstruction has remained the gold standard of care for ACL injuries, especially for young individuals and athletes who aim to return to high-level sporting activities.5,18 However, current surgical treatment of ACL injury is costly, with variable outcomes5 and is associated with high risk of post-traumatic OA within two decades of injury.9,19 While few athletes are able to resume sports at the same level without surgery,5 the surgical reconstruction is also not always successful at returning patients to their pre-injury activity level.20 Furthermore, those athletes who successfully return to activity are at high risk of a second knee injury21 with notably less favourable outcomes.22
Recent advancements in functional tissue engineering and regenerative medicine have resulted in a renewed interest in revisiting ACL repair. The promising use of novel biological/tissue engineering techniques, including growth factors, stem cells and bio-scaffolds, has been the focus of current research in ACL healing and repair. The increased number of recently published pilot clinical and basic research studies has prompted our current review of the literature, exploring the recent knowledge and indications for clinical use of these biologically enhanced techniques. In this article, we present the latest research on the biology of ACL healing and repair supplemented by a brief overview of ACL injury epidemiology, mechanism and current standard of care. Future work in this area may lead to the improvement of the current techniques along with development of novel approaches to treat this critical injury with enhanced short-term and long-term outcomes.
Search strategy and selection criteria
For the purpose of this literature review, peer-reviewed journals were consulted and the findings summarised to provide an understanding of the information gained from the current literature. Studies were identified by searching the MEDLINE, CINAHL and SPORTDiscus electronic databases. The last search was undertaken on September 15 2013. The following search terms were used: “Anterior Cruciate Ligament AND Injury”, “ACL AND Injury”, “Anterior Cruciate Ligament AND Healing”, “ACL and Healing”, “Anterior Cruciate Ligament AND Repair” and “ACL AND Repair”. Searches were repeated using the keywords as MeSH terms as well.
The search algorithm was intentionally general to maximise return. In addition to the online searches, the bibliographies of the included studies were reviewed to identify additional publications. No date limits were considered for the publications on ACL healing and repair. However, literature covering the injury epidemiology, mechanism and surgical reconstruction were deemed either seminal published works or publications after 2010. The citations identified from the searches were combined and duplicates excluded.
All in vivo and in vitro studies that focused on ACL repair following injury, not reconstruction, were considered. All titles resulting from the search criteria were reviewed and those that clearly referred to a topic other than the focus of current review were excluded. All case reports and expert opinions were excluded. Abstracts were also reviewed to confirm inclusion eligibility. Finally, full texts were obtained for the eligible studies for final review.
ACL injury epidemiology
The ACL is one of the most frequently injured ligaments of the knee, with a prevalence estimated to be 1 in 3000 in the United States (greater than 120 000 cases annually).23 Despite trivial injury incidences in the general population, ACL injury frequently affects young, active individuals, and females are at a reported two- to ten-fold greater risk than males playing the same sport (Table I).24-31 High risk of injury along with the high rate of sports participation among girls and young women over the last three decades has led to a rapid rise in ACL injuries in females. ACL injuries are mainly associated with other concomitant articular injuries, and may result in an increased risk of early onset post-traumatic OA at ten to 15 years post-injury (as high as 80%), especially in the presence of concomitant meniscal damage.6,7,9,32
Table I
Gender-specific rates of injury to the anterior cruciate ligament based on sports type
| Authors | Sports | Level of competition | ACL injury rate (female/male) |
|---|---|---|---|
| Renstrom et al30 | Basketball | Collegiate | 3.3 |
| Arendt et al24,25 | Basketball | Collegiate | 4.1 |
| Messina et al28 | Basketball | High school | 3.0 |
| Renstrom et al30 | Soccer | Collegiate | 2.5 |
| Arendt et al24,25 | Soccer | Collegiate | 2.3 |
| Lindenfeld et al27 | Soccer | Youth | 3.0 |
| Stevenson et al31 | Alpine skiing | High school | 3.1 |
| Myklebust et al29 | Handball | - | 5.0 |
| Renstrom et al30 | Lacrosse | Collegiate | 1.4 |
| Renstrom et al30 | Ice hockey | Collegiate | 2 |
| Gwinn et al26 | Military training | Collegiate | 9.7 |
In addition to pain, instability and associated long-term sequelae, ACL injury may affect the athletes’ quality of life economically as well as socially.7,32 Using a conservative cost estimate of between USD $17 000 and $25 000 per patient for surgery and rehabilitation, the estimated cost for treatment in ACL injured patients in the United States is over $1.7 billion annually. This estimate does not consider the resources necessary for non-surgical treatment, or to treat the long-term complication of post-traumatic OA associated with both the ACL-injured and ACL-reconstructed knee.33Moreover, patients who have suffered an ACL injury face long-term consequences that include lowered activity levels, high risk of re-injury and long-term disability due to post-traumatic OA.5-7,9,21,32
Injury mechanism
More than 70% of ACL injuries occur as non-contact (without a direct blow to the knee joint).2-4 They occur as a result of landing from a jump and lateral cutting maneouvres that may occur in different athletic activities such as basketball and soccer.24-31 Over the past twenty years, a myriad of research has examined potential mechanisms and associated risk factors for ACL injury using in vivo, ex vivo and in silico techniques.1-4,20,21,24-31,34-36
Neuromuscular control deficit during dynamic movements has been hypothesised to be the primary cause for both primary and secondary ACL injury risk (re-injury following ACL reconstruction).5 The deficit in dynamic active neuromuscular control manifests as excessive joint loads and leads to detrimental ACL stress/strains and ultimate failure. Non-contact ACL injury mechanisms are multi-planar in nature, involving the tibiofemoral joint articulation in all three anatomical planes.3,4,36 Previous studies have identified combined multi-planar loading including anterior tibial shear, knee valgus and internal tibial rotation to be the worst case scenario and primary mechanism of non-contact ACL injury (Fig. 1).3,4
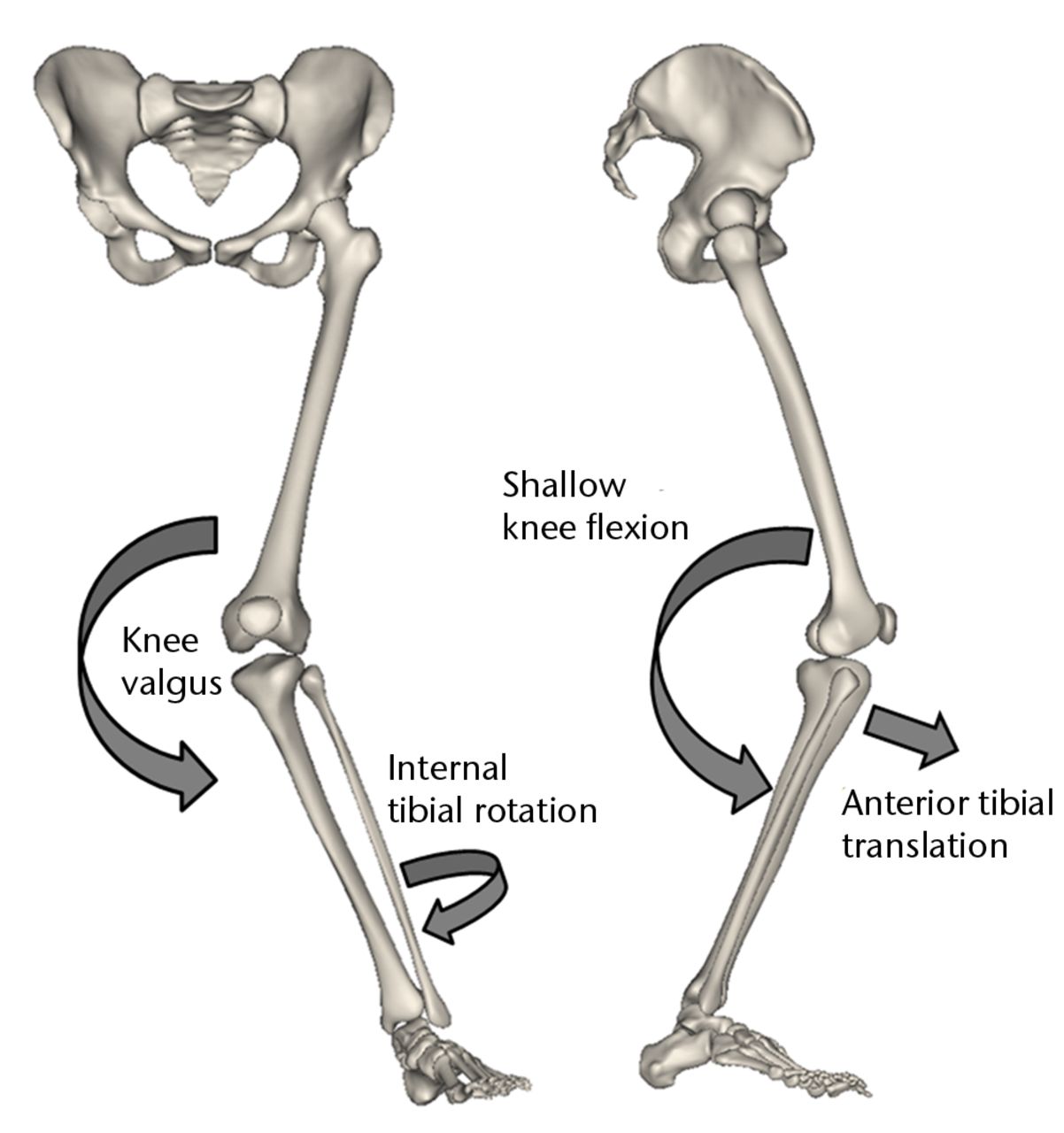
Fig. 1
Schematic showing the multi-planar loading mechanism of non-contact injury to the anterior cruciate ligament (Adapted and modified with permission from Levine et al3).
Treatment options
ACL repair by re-approximating the two ends of the ruptured ligament using suture was one of the earliest suggested methods described for treatment of ACL injuries. It was in early 1900s that Robson37 described the primary repair of ACL, a technique that was later studied and documented in detail by O’Donoghue et al.14,38 Feagin11 and Cabaud et al39,40 were the first to report the long-term outcomes of primary ACL repair in the 1970s. Feagin11 showed that the primary repair of ACL failed in over 90% of patients in a five year follow-up study. These findings were further backed up by the observations of Sandberg et al15 showing no difference in outcomes after primary repair versus conservative treatment in a randomised controlled trial. The high rates (40% to 100%) of the ACL failure to heal, even with surgical repair,11,12,14-17 have led to abandonment of suture repair and almost universal adoption of ACL reconstruction for treatment of ACL injuries.
In ACL reconstruction, the torn ACL tissue is removed from the knee surgically and replaced with an allo- or autograft tendon taken either from the medial hamstrings or the middle third of the patellar tendon. Although ACL reconstruction has become the current gold standard for restoring the gross stability of a symptomatic ACL-deficient knee, significant problems persist. In the short term, conventional ACL reconstruction fails to restore the normal joint kinematics and kinetics.41,42 This alteration in joint mechanics has been mainly associated with non-anatomic ligament insertion (location and geometry) and alignment, loss of tissue neurosensory function (proprioception), graft-tissue degeneration and neuromuscular deficit.43-45 Many studies have shown significantly greater translational and rotational laxity of the reconstructed knees relative to the contralateral uninjured sides, regardless of the graft type.46-49 Additionally, reconstruction requires tissue harvest from the knee (autograft), which is associated with tissue morbidity. Alternatively, using allografts is associated with high risk of biologic incorporation failure and disease transmission in addition to financial and tissue availability complications. Most importantly, patients remain at high risk for development of early onset OA even after surgical reconstruction. This risk has been reported to be between 66% and 100%.6,7,9,19 A meta-analysis of 33 clinical follow-up studies reported that ACL reconstruction was unable to slow the premature onset of OA following ACL tear.50
Over the last decade, substantial effort has been made to make the surgical reconstruction more anatomical by altering tunnel position and introducing the concept of a double-bundle reconstruction.17,51,52 This evolution in ACL reconstruction has resulted in an improved joint translational and rotational stability closer to the intact knee, compared with conventional, non-anatomic single-bundle reconstruction.53-56 However, no consensus has been reached on the improved clinical outcomes of anatomic double-bundle reconstruction over the traditional single-bundle technique.53,57-62 A recent randomised trial of 130 patients with a minimum four-year follow-up have reported that although anatomic double-bundle reconstruction results in improved IKDC score, it was not superior to the conventional single-bundle technique in preventing post-traumatic OA.61
The associated complications with the surgical reconstruction, despite its undeniably large success, in addition to the advent of functional tissue engineering, precipitated increased interest in bio-enhanced ACL repair as an alternative to reconstruction.63-65 However, development of a regenerative method for repair of the torn ACL begs an enhanced understanding of why the earlier primary ACL repair was largely unsuccessful. Over the past decade, researchers set out to understand the mechanisms that underlie the inability of an injured ACL to heal, a finding which is in direct contrast to the high healing capacity of extra-articular connective tissues like the medial collateral ligament (MCL).63-72 Several factors have been reported to be responsible for this discrepancy in tissue healing ability including, but not limited to, the ‘hostile’ environment of synovial fluid,66,73-75 alterations in the post-injury inflammatory response and cell metabolism,67-70,72,76-84 intrinsic cell deficiencies,71,85-92 different vascular environment,93,94 and load bearing characteristics.4,95
Biologically augmented ACL repair
The improved knowledge of ACL healing characteristics has helped researchers and clinicians to introduce novel biologic ACL repair approaches. These alternatives to the current surgical reconstruction have the potential to preserve the native insertion site and proprioceptive function, which may in turn lead to more normal joint mechanics and decreased risk of post-traumatic OA. One such approach was the ‘healing response technique’ pioneered by Steadman et al.96 In this technique, micro-holes within the femur near the ACL insertion site are created, leading to blot clot and subsequent haematoma formation. Ligament healing is then thought to be induced by the high concentration of the reparative cells near the torn ends of the ACL as a result of the created haematoma.96-99 This technique has been reported to be successful in middle-age patients with very proximal ACL tears.96 However, a recent study by Wasmaier et al100 showed no differences between patients treated by healing response technique and patients treated conservatively with regard to Lysholm and Tegner scores, normalised joint laxity, and rate of required revision surgery. Recent integration of advanced functional tissue engineering in the area of ACL repair has left researchers with multiple novel approaches to treat ACL injuries with improved outcomes. A brief overview of these methods follows.
Cell therapy
Cell therapy using mesenchymal progenitor cells (MPCs) or mesenchymal stem cells (MSCs) has been widely studied in vitro and in preclinical studies within the area of sports medicine research.101-103 MSCs harvested from mesenchymal tissues (i.e., bone marrow) can differentiate into various cell types (i.e., fibroblasts) required to regenerate different tissues such as bone, cartilage, tendon, ligament and fat.104-111 In a rat model of partial ACL tear, Kanaya et al112 showed that intra-articular injection of MSCs resulted in a healed ligament with superior histological scores and greater failure load compared with non-treated control knees. Lim et al113 and Soon et al114 have shown similar improved biomechanics in rabbit models of ACL reconstruction using autografts and allografts, respectively, all enhanced by the application of MSCs.
In a recent study, Oe et al115 used intra-articular injection of either fresh bone marrow cells (BMC) or cultured MSCs at one week after ACL transection in a rat model. They showed that the donor cells were located within the wound site and ACL exhibited almost normal histology, with more mature spindle cells with higher levels of transforming growth factor (TGF-β) in the BMC group. They concluded that the direct intra-articular BMC injection is an effective solution for the treatment of partial ACL tears,115 which was in line with previous findings of Kanaya et al.112 These findings are encouraging considering the potential of MSCs to carry and deliver therapeutic molecules in addition to the positive role of MSCs in the healing of ligaments. Despite the advantages of stem cell-based therapies, unresolved challenges exist in optimising the MSC applications in ACL repair. One such challenge is the development of proper methods to effectively differentiate these multi-pluripotent cells into specific cell types required to enhance tissue repair. Another concern is the delivery and maintenance of the stem cells within the wound site, which underscores the need for further research in this field.
Gene transfer and gene therapy
Gene transfer is a recent promising strategy to modulate durably the application of various therapeutic factors essential to the healing of injured tissues such as ligaments. Gene transfer in ligaments mainly occurs using nonviral gene delivery vectors or vectors derived from viruses with natural entry pathways in the cell (adenoviruses, lentiviruses/retroviruses) in order to alter tissue endogenous protein synthesis by mediating certain gene expression.116-124 Such gene-based approaches may have the potential to modulate the biochemical changes following an ACL injury such as variations in collagen expression, the wound contractile α–smooth muscle actin (α-SMA) markers, and nuclear factor–kB (NF-kB) markers.119,125,126 Hildebrand et al127 tested the possibility of gene transfer to normal and ACL ruptured knees in a rabbit model. They concluded that adenoviral vectors are able to express more efficiently than retroviral vectors in ACL cells and can lead to a considerably long period of gene expression in vivo (six weeks).
In a series of ex vivo and in vitro studies, Pascher et al122 confirmed the ability of vector-laden hydrogels in in situ gene delivery to the injury site for potential biological repair of the ACL. They showed increased cellularisation and collagen (I and III) deposition by in situ transfer of TGF-β1 using an adenoviral vector in a collagen hydrogel placed between the torn ends of the ACL.122 The same authors further demonstrated increased deposition of collagen (I and III), elastin, tenascin, and vimentin through in situ transfer of insulin-like growth factor-1 (IGF-1) cDNA by an adenovirus vector in the same model.123 Most recently, Madry et al119 tested the enhanced healing of the human ACL by over expression of fibroblast growth factor-2 (FGF-2) via direct recombinant adeno-associated virus (rAAV) vector–mediated gene transfer. They showed that stable FGF-2 expression using rAAV resulted in remarkable decrease in ACL lesions mainly due to increased expression of α-SMA, ligament-specific transcription factor scleraxis, and NF-kB for collagen proliferation and deposition.119
Despite these advantages, there are several issues that need to be considered during gene therapy. The loss or decrease of expression of the transferred gene after several weeks, especially in adenoviral vectors, is one of the major and most frequent challenges in gene therapy.128 Safety is also a major concern using this technique, which can lead to high risks of side effects including mutagenesis.129 Moreover, abnormal cell growth, toxicity under chronic over-expression of growth factors, and development of any malignancy are other possible side effects associated with gene-modified cell therapy. As a result this technique is a current topic of research to identify the ideal gene vectors and further optimise the current methods in an effort to overcome the difficulties associated with viral gene therapy.
Application of growth factors
The use of growth factors has gained a lot of traction in treatment of soft-tissue injuries since the late 1990s. A wide range of growth factors, including insulin-like growth factor (IGF), TGF-β, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF) and nerve growth factor (NGF) have been used previously to improve ligamentous and tendon tissue repair.78,130-140 They have shown to be able to regulate and improve the cellular activities and proliferation, extra-cellular matrix (ECM) deposition, and influence the differentiation of MSCs into fibroblasts to repair the torn ligaments. In particular, these growth factors have exhibited positive effects on various biological processes needed to improve ACL healing.
Early in vitro studies by Marui et al141 demonstrated that the application of TGF- β1 resulted in increased collagen synthesis up to 1.5 times greater than controls in both MCL and ACL fibroblasts. Kobayashi et al142 reported the positive effect of basic-FGF (bFGF) in improved ACL tissue healing with increased vascularity compared with the control in a canine model. More recently, Kondo et al132 studied the effect of TGF- β1 in an in vivo model of ACL injury in rabbits. They showed significant improvement of biomechanical and histological healing properties of injured ACLs treated with TGF-β1 compared with controls.
In addition to the mentioned growth factors, the use of platelet-rich-plasma (PRP), which contains a multitude of growth factors, has been the centre of attention as a novel, non-invasive treatment for sports related injuries.143-149 PRP is a simple, efficient method of obtaining a high concentration of growth factors through separation of platelets from autologous blood. Platelets are the first cells reaching the injury site and are a substantial reservoir of critical growth factors and signaling molecules, including leukocyte-derived catabolic cytokines and fibrinogen.150,151 This combination of bio-active agents can mediate the tissue healing process, following an injury through both the inflammatory and remodeling phases.146,150,151 Platelets are involved in homeostasis, aggregation and clot formation steps, which finally lead to enhanced tissue healing.150 This is done by the release of PDGF, TGF-β1, VEGF, bFGF and epidermal growth factor (EGF) through degranulation of alpha granules.144,145 Among these growth factors, PDGF and TGF-β1 have been reported to be the most critical modulators in the healing process by contributing to increased fibroblast proliferation and collagen production.144
Despite the large number of research studies conducted on the role of PRP treatment on ACL reconstruction,148,152-156 the use of PRP in ACL healing and repair is not as well considered. In a series of in vivo large animal studies, Murray et al157-160 have reported improved ACL healing using a collagen-platelet hydrogel in an ACL central defect model. They demonstrated that the presence of collagen-platelet hydrogel in the wound site can result in release of growth factors with similar spatial and temporal sequence as healing extra-articular tissue. They further reported significant increases in tissue formation and mechanical properties following biologically augmented primary ACL repair.157-160
Despite these advantages, there are concerns regarding the optimised use of growth factors. One of the major concerns is the short life span of these bio-active agents, which have limited their efficacy. Delivery and maintenance of the growth factors within the wound site is another challenge using this technique for treatment of soft-tissue injuries. Therefore, safe and reproducible systems that allow sustained delivery of growth factors to the injury site are essential.
Use of bio-scaffolds
A wide range of synthetic and biologic-based scaffolds made from alginate, chitosan, collagen or hyaluronic acid have been used in functional tissue engineering and regenerative medicine.161,162 ACL tears have been previously treated with synthetic scaffolds loaded with growth factors142 and also with hyaluronic acid.163,164 Wiig et al164 reported improved healing of a ACL central defect using intra-articular injection of hyaluronic acid in a rabbit model. They showed that the group treated with hyaluronic acid showed greater angiogenic response with increased amount of reproduced type III collagen. However, these techniques are associated with critical challenges such as problems with implant–host integration, cell survival after transplantation, and short-time degradation. Alternatively, the use of collagen-based scaffolds has shown to be more effective. ACL fibroblasts have been previously shown to effectively attach, proliferate and express collagen on collage-based scaffolds.165
Porcine small intestinal submucosa (SIS) was among the first scaffolds used to enhance the regeneration and repair of ligaments and tendons.166-171 SIS is a collagen based (90% of dry weight) bio-absorbable scaffold which contains a small number of cytokines and growth factors such as FGF and TGF-β.170 In addition to the collagenous structure, which works as a provisional scaffold, it can also deliver the essential supplies (i.e., FGF-2, TGF-β, VEGF and PDGF) needed for tissue healing.166 Using a goat stifle joint model of ACL injury, Fisher et al172 reported significant improvement in tissue mechanical and histological properties using a primary repair technique supplemented with SIS bio-scaffold and hydrogel. Using a tissue-engineered collagen-I scaffold, Robayo et al64 demonstrated improved ACL fibroblasts activity (i.e., migration) in vitro supporting the collagen-based scaffolds as proper bedding for ACL tissue regeneration.
Recent in vivo work by Fleming et al173 reported no significant improvement of suture repair when supplemented with a collagen scaffold alone used for complete ACL tears in a porcine model. However, by combining a collagen scaffold with autologous platelets, Murray et al75,158,174 demonstrated significantly improved ACL repair outcomes in a series of large animal studies. They showed superior tissue mechanical properties using primary repair augmented with collagen-PRP hydrogel, compared with suture repair alone.158 It was further reported that the augmented ACL repair can result in enhanced tissue properties similar to ACL reconstruction, the current gold standard of treatment.174 Additional studies have also now demonstrated that the combination of an ECM-based collagen scaffold and PRP is substantially more effective than the application of each of these factors alone.173,175 The mechanism behind this remains unclear, but it may be due to a synergic effect between the collagen, PRP and other ECM molecules.
A new paradigm in ACL repair
The low capacity of the ACL to heal compared with other extra-articular tissues, such as the MCL, has long been attributed to the intrinsic differences in cell behaviour and insufficient blood supply following injury.71,85,86,88,90,92,94 However, extensive in vitro cell culture and in vivo histological and immunohistochemical studies of the ACL and MCL have revealed that both ligaments have a comparable proliferative vascular and neurogenic reaction to injury.66,75,176-180 It has also been shown that, similar to the MCL, collagen production continues within the ACL up to one year post-injury.179 However, germinal observations showed that the provisional scaffold (fibrin-platelet clot) found within the wound site of extra-articular ligaments was missing in the ACL (Fig. 2).178 The prevention of clot formation is mainly due to the continuous flow of the synovial fluid within the knee joint, dispersing the blood as a haemarthrosis.178 It was further demonstrated that this lack of provisional scaffold leads to a decreased presence of critical ECM proteins and cytokines such as fibrinogen, fibronectin, PDGF-A, TGF-β1, FGF-2, and von Willebrand’s factor (vWF) within the ACL wound site (Fig. 3).75,159
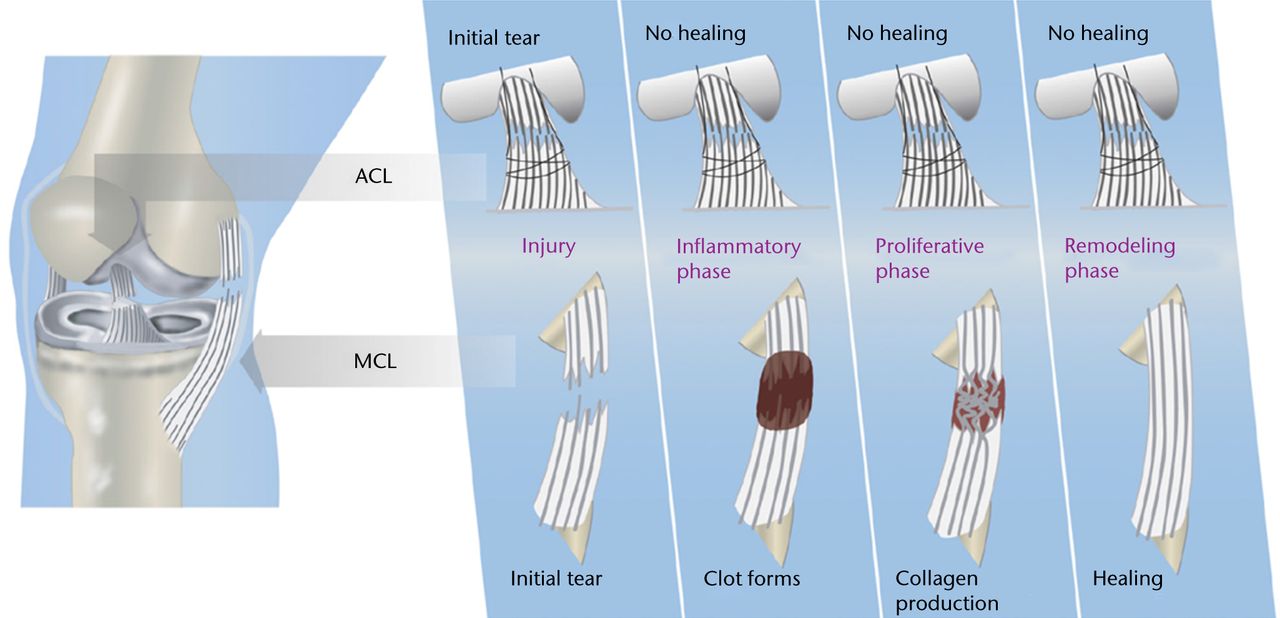
Fig. 2
Diagrams showing the differences in intrinsic healing response of the anterior cruciate ligament (ACL; top) and medial collateral ligament (MCL; bottom), highlighting the lack of provisional scaffold (blood clot) formation within the ACL wound site as the key mechanism for ACL healing failure (reproduced with permission from Murray and Fleming63).
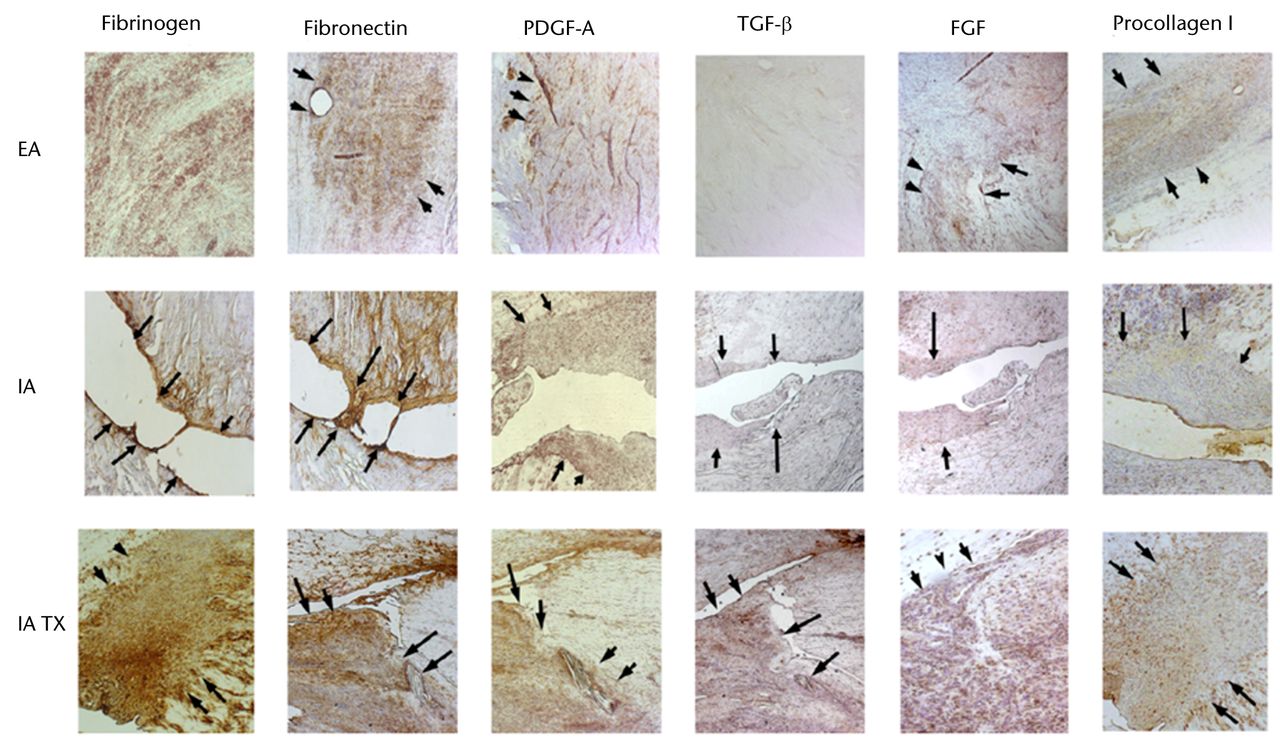
Fig. 3
Representative photomicrographs of patellar ligament wounds (extra-articular, EA), untreated anterior cruciate ligament (ACL) wounds (intra-articular, IA) and treated ACL with collagen-platelet scaffold (IA Tx) at 21 days after injury (10x). Treated ACLs show similar distribution of protein presence as the patellar ligament. The untreated ACL wounds remain with almost no substratum (PDGF-A: platelet-derived growth factor; TGF-β: transforming growth factor; FGF: fibroblast growth factor) (adapted and modified with permission from Murray et al75).
In order to test the hypothesis that the missing provisional scaffold was a key mechanism behind the failure of the ACL to heal, a collagen-based scaffold has been used to fill the gap between the two ends of the torn ligament.63 This bio-active scaffold could then be used as a carrier for cells, growth factors and enzymes required to optimise tissue healing. In the first in vivo studies, platelets maintained with their physiological plasma were placed within the collagen-based scaffold, and the loaded scaffold used to repair torn ACL using multiple established large animal models.157-160,174,181-183 These studies further demonstrated the improved biological and mechanical healing of the ACL using this novel technique (bio-enhanced repair) (Fig. 4). In a recent randomised trial in a large animal model, the biomechanical outcome of bio-enhanced ACL repair was found to be equal to that of ACL reconstruction (Fig. 5).174 More importantly, while 80% of the knees treated with ACL reconstruction developed post-traumatic OA by one year post-operatively, OA was not seen in those knees treated with bio-enhanced repair within the same time period (Fig. 6).182
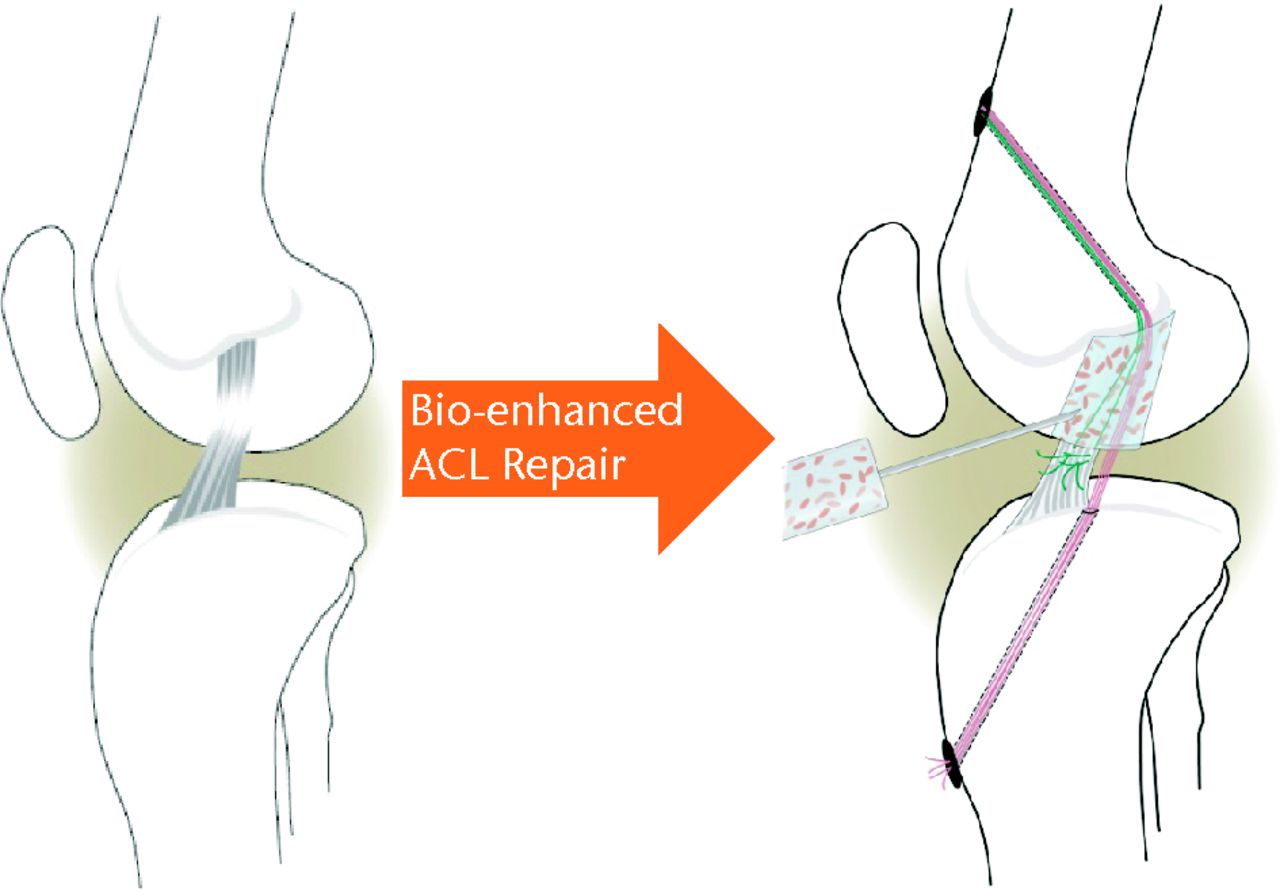
Fig. 4
Schematic of bio-enhanced anterior cruciate ligament (ACL) repair method (adapted and modified with permission from Murray and Fleming182).
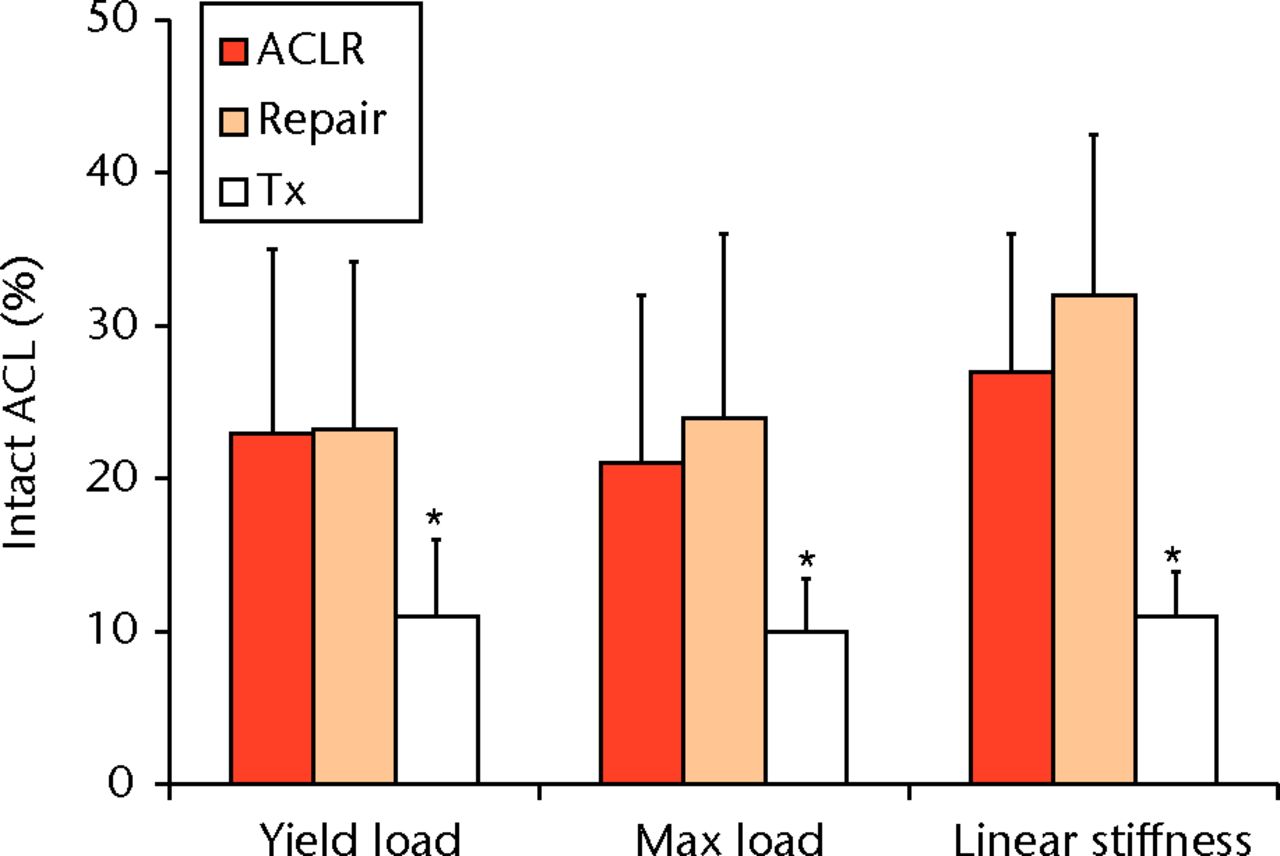
Fig. 5
Bar chart showing identical mechanical properties of bio-enhanced repaired anterior cruciate ligament (ACL) (Repair) compared with the surgically reconstructed samples (ACLR) (p > 0.6 for all comparisons), with significantly lower mechanical properties (*) within the untreated ACL rupture group (Tx) (reproduced with permission from Murray and Fleming63).
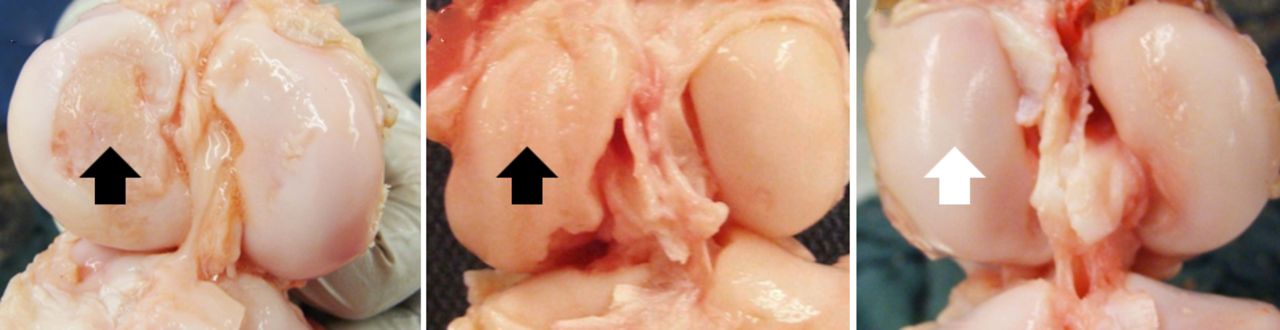
Fig. 6
Photographs showing the distal femoral cartilage at one year after a) an untreated anterior cruciate ligament (ACL) rupture, b) after conventional ACL reconstruction, and c) bio-enhanced ACL repair. Note the damage to the medial femoral condyle in the untreated and ACL-reconstructed knee (black arrows). No damage to the medial femoral condyle in the bio-enhanced ACL-repair knee (white arrow) was observed (adapted and modified with permission from Murray and Fleming 182).
Conclusion
A successful ACL repair can theoretically provide the patient with multiple advantages over surgical reconstruction, including preservation of the proprioceptive function of the ligament and the complex ligament insertion sites. However, previously reported high failure incidences of primary repair and the relative robustness of ACL reconstruction led the clinical switch to use of a graft to replace, rather than repair, the ACL. Recent advances in the area of tissue engineering and regenerative medicine coupled with an improved understanding of the requirements for ACL healing, has led to the emergence of novel biologically augmented ACL repair techniques. Despite being in their infancy, these methods have resulted in repeated stepwise improvements in ACL repair and become a promising future candidate for ACL injury treatment. One such approach, bio-enhanced repair, has shown comparable structural and biomechanical outcomes with the current gold standard of treatment, ACL reconstruction. Bio-enhanced repair using a collagen-based scaffold and autologous blood has also resulted in significant decreases in risk of post-traumatic OA, which makes it the first and so far only possible ACL injury treatment with the potential to lower the risk of OA after an ACL injury. Despite promising results obtained from in vitro and in vivo animal studies, well-controlled human trials are needed to assure the ultimate efficacy of these novel approaches. Future work should focus on further refinement of these techniques in an effort to improve the outcomes, along with successful translation to humans.
1 Butler DL , NoyesFR, GroodES. Ligamentous restraints to anterior-posterior drawer in the human knee: a biomechanical study. J Bone Joint Surg [Am]1980;62-A:259–270. Google Scholar
2 Kiapour AM, Wordeman SC, Paterno MV, et al. Diagnostic value of knee arthrometry in the prediction of anterior cruciate ligament strain during landing. Am J Sports Med 2013;Epub. Google Scholar
3 Levine JW , KiapourAM, QuatmanCE, et al.Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am J Sports Med2013;41:385–395.CrossrefPubMed Google Scholar
4 Quatman CE , KiapourAM, DemetropoulosCK, et al.Preferential loading of the ACL compared with the MCL during landing: a novel in sim approach yields the multiplanar mechanism of dynamic valgus during ACL injuries. Am J Sports Med2014;42:177–186.CrossrefPubMed Google Scholar
5 Hewett TE , Di StasiSL, MyerGD. Current concepts for injury prevention in athletes after anterior cruciate ligament reconstruction. Am J Sports Med2013;41:216–224.CrossrefPubMed Google Scholar
6 Chu CR , BeynnonBD, BuckwalterJA, et al.Closing the gap between bench and bedside research for early arthritis therapies (EARTH): report from the AOSSM/NIH U-13 Post-Joint Injury Osteoarthritis Conference II. Am J Sports Med2011;39:1569–1578.CrossrefPubMed Google Scholar
7 Lohmander LS , OstenbergA, EnglundM, RoosH. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum2004;50:3145–3152.CrossrefPubMed Google Scholar
8 Nebelung W , WuschechH. Thirty-five years of follow-up of anterior cruciate ligament-deficient knees in high-level athletes. Arthroscopy2005;21:696–702.CrossrefPubMed Google Scholar
9 von Porat A , RoosEM, RoosH. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis2004;63:269–273.CrossrefPubMed Google Scholar
10 Quatman CE , KiapourA, MyerGD, et al.Cartilage pressure distributions provide a footprint to define female anterior cruciate ligament injury mechanisms. Am J Sports Med2011;39:1706–1713.CrossrefPubMed Google Scholar
11 Feagin JA Jr , CurlWW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med1976;4:95–100.CrossrefPubMed Google Scholar
12 Kaplan N , WickiewiczTL, WarrenRF. Primary surgical treatment of anterior cruciate ligament ruptures: a long-term follow-up study. Am J Sports Med1990;18:354–358. Google Scholar
13 Marshall JL , WarrenRF, WickiewiczTL, ReiderB. The anterior cruciate ligament: a technique of repair and reconstruction. Clin Orthop Relat Res1979;143:97–106.PubMed Google Scholar
14 O’Donoghue DH , FrankGR, JeterGL, et al.Repair and reconstruction of the anterior cruciate ligament in dogs: factors influencing long-term results. J Bone Joint Surg [Am]1971;53-A:710–718. Google Scholar
15 Sandberg R , BalkforsB, NilssonB, WestlinN. Operative versus non-operative treatment of recent injuries to the ligaments of the knee: a prospective randomized study. J Bone Joint Surg [Am]1987;69-A:1120–1126. Google Scholar
16 Sherman MF , BonamoJR. Primary repair of the anterior cruciate ligament. Clin Sports Med1988;7:739–750. Google Scholar
17 Strand T , MolsterA, HordvikM, KrukhaugY. Long-term follow-up after primary repair of the anterior cruciate ligament: clinical and radiological evaluation 15-23 years postoperatively. Arch Orthop Trauma Surg2005;125:217–221.CrossrefPubMed Google Scholar
18 Musahl V , BeckerR, FuFH, KarlssonJ. New trends in ACL research. Knee Surg Sports Traumatol Arthrosc2011;19(Suppl 1):S1–S3.CrossrefPubMed Google Scholar
19 Murray JR , LindhAM, HoganNA, et al.Does anterior cruciate ligament reconstruction lead to degenerative disease?: thirteen-year results after bone-patellar tendon-bone autograft. Am. J Sports Med2012;40:404–413. Google Scholar
20 Ardern CL , WebsterKE, TaylorNF, FellerJA. Return to the preinjury level of competitive sport after anterior cruciate ligament reconstruction surgery: two-thirds of patients have not returned by 12 months after surgery. Am J Sports Med2011;39:538–543. Google Scholar
21 Shelbourne KD , GrayT, HaroM. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med2009;37:246–251.CrossrefPubMed Google Scholar
22 Spindler KP , HustonLJ, WrightRW, et al.The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. Am J Sports Med2011;39:348–359.CrossrefPubMed Google Scholar
23 Kim S , BosqueJ, MeehanJP, JamaliA, MarderR. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg [Am]2011;93-A:994–1000.CrossrefPubMed Google Scholar
24 Arendt E , DickR. Knee injury patterns among men and women in collegiate basketball and soccer: NCAA data and review of literature. Am J Sports Med1995;23:694–701. Google Scholar
25 Arendt EA , AgelJ, DickR. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train1999;34:86–92.PubMed Google Scholar
26 Gwinn DE , WilckensJH, McDevittER, RossG, KaoTC. The relative incidence of anterior cruciate ligament injury in men and women at the United States Naval Academy. Am J Sports Med2000;28:98–102.CrossrefPubMed Google Scholar
27 Lindenfeld TN , SchmittDJ, HendyMP, MangineRE, NoyesFR. Incidence of injury in indoor soccer. Am J Sports Med1994;22:364–371.CrossrefPubMed Google Scholar
28 Messina DF , FarneyWC, DeLeeJC. The incidence of injury in Texas high school basketball. A prospective study among male and female athletes. Am J Sports Med1999;27:294–299.CrossrefPubMed Google Scholar
29 Myklebust G , MaehlumS, HolmI, BahrR. A prospective cohort study of anterior cruciate ligament injuries in elite Norwegian team handball. Scand J Med Sci Sports1998;8:149–153.CrossrefPubMed Google Scholar
30 Renstrom P , LjungqvistA, ArendtE, et al.Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med2008;42:394–412.CrossrefPubMed Google Scholar
31 Stevenson H , WebsterJ, JohnsonR, BeynnonB. Gender differences in knee injury epidemiology among competitive alpine ski racers. Iowa Orthop J1998;18:64–66.PubMed Google Scholar
32 Mather RC 3rd , KoenigL, KocherMS, et al.Societal and economic impact of anterior cruciate ligament tears. J Bone Joint Surg [Am]2013;95-A:1751–1759.CrossrefPubMed Google Scholar
33 Griffin LY , AlbohmMJ, ArendtEA, et al.Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med2006;34:1512–1532.CrossrefPubMed Google Scholar
34 Kiapour A , KiapourAM, KaulV, et al.Finite element model of the knee for investigation of injury mechanisms: development and validation. J Biomech Eng2013;136:011002CrossrefPubMed Google Scholar
35 Kiapour AM , KaulV, KiapourA, et al.The effect of ligament modeling technique on knee joint kinematics: a finite element study. Appl Mathematics2013;4:91–97.CrossrefPubMed Google Scholar
36 Kiapour AM , QuatmanCE, GoelVK, et al.Timing sequence of multi-planar knee kinematics revealed by physiologic cadaveric simulation of landing: Implications for ACL injury mechanism. Clin Biomech (Bristol, Avon)2014:29:75–82.CrossrefPubMed Google Scholar
37 Robson AW . VI: ruptured crucial ligaments and their repair by operation. Ann Surg1903;37:716–718. Google Scholar
38 O’Donoghue DH , RockwoodCA Jr, FrankGR, JackSC, KenyonR. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg [Am]1966;48-A:503–519.PubMed Google Scholar
39 Cabaud HE , FeaginJA, RodkeyWG. Acute anterior cruciate ligament injury and augmented repair: experimental studies. Am J Sports Med1980;8:395–401. Google Scholar
40 Cabaud HE , RodkeyWG, FeaginJA. Experimental studies of acute anterior cruciate ligament injury and repair. Am J Sports Med1979;7:18–22.CrossrefPubMed Google Scholar
41 Hall M , StevermerCA, GilletteJC. Gait analysis post anterior cruciate ligament reconstruction: knee osteoarthritis perspective. Gait Posture2012;36:56–60.CrossrefPubMed Google Scholar
42 Hoshino Y , FuFH, IrrgangJJ, TashmanS. Can joint contact dynamics be restored by anterior cruciate ligament reconstruction?Clin Orthop Relat Res2013;471:2924–2931.CrossrefPubMed Google Scholar
43 Jansson KA , HarilainenA, SandelinJ, et al.Bone tunnel enlargement after anterior cruciate ligament reconstruction with the hamstring autograft and endobutton fixation technique. A clinical, radiographic and magnetic resonance imaging study with 2 years follow-up. Knee Surg Sports Traumatol Arthrosc1999;7:290–295.CrossrefPubMed Google Scholar
44 Kartus J , MagnussonL, StenerS, et al.Complications following arthroscopic anterior cruciate ligament reconstruction. A 2-5-year follow-up of 604 patients with special emphasis on anterior knee pain. Knee Surg Sports Traumatol Arthrosc1999;7:2–8.CrossrefPubMed Google Scholar
45 Shelbourne KD , WilckensJH, MollabashyA, DeCarloM. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med1991;19:332–336.CrossrefPubMed Google Scholar
46 Beard DJ , MurrayDW, GillHS, et al.Reconstruction does not reduce tibial translation in the cruciate-deficient knee: an in vivo study. J Bone Joint Surg [Br]2001;83-B:1098–1103. Google Scholar
47 Georgoulis AD , PapadonikolakisA, PapageorgiouCD, MitsouA, StergiouN. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med2003;31:75–79.CrossrefPubMed Google Scholar
48 Papannagari R , GillTJ, DefrateLE, MosesJM, PetruskaAJ, LiG. In vivo kinematics of the knee after anterior cruciate ligament reconstruction: a clinical and functional evaluation. Am J Sports Med2006;34:2006–2012.CrossrefPubMed Google Scholar
49 Tashman S , KolowichP, CollonD, AndersonK, AnderstW. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res2007;454:66–73.CrossrefPubMed Google Scholar
50 Lohmander LS , RoosH. Knee ligament injury, surgery and osteoarthrosis: truth or consequences?Acta Orthop Scand1994;65:605–609. Google Scholar
51 Muller B , HofbauerM, WongcharoenwatanaJ, FuFH. Indications and contraindications for double-bundle ACL reconstruction. Int Orthop2013;37:239–246.CrossrefPubMed Google Scholar
52 Rabuck SJ , MiddletonKK, MaedaS, et al.Individualized anatomic anterior cruciate ligament reconstruction. Arthrosc Tech2012;1:23–29.CrossrefPubMed Google Scholar
53 Kondo E , YasudaK, AzumaH, TanabeY, YagiT. Prospective clinical comparisons of anatomic double-bundle versus single-bundle anterior cruciate ligament reconstruction procedures in 328 consecutive patients. Am J Sports Med2008;36:1675–1687.CrossrefPubMed Google Scholar
54 Nagai T , HeebnerNR, SellTC, et al.Restoration of sagittal and transverse plane proprioception following anatomic double-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc2013;21:2048–2056.CrossrefPubMed Google Scholar
55 Seon JK , GadikotaHR, WuJL, et al.Comparison of single- and double-bundle anterior cruciate ligament reconstructions in restoration of knee kinematics and anterior cruciate ligament forces. Am J Sports Med2010;38:1359–1367.CrossrefPubMed Google Scholar
56 Yagi M , KurodaR, NagamuneK, YoshiyaS, KurosakaM. Double-bundle ACL reconstruction can improve rotational stability. Clin Orthop Relat Res2007;454:100–107.CrossrefPubMed Google Scholar
57 Fu FH , ShenW, StarmanJS, OkekeN, IrrgangJJ. Primary anatomic double-bundle anterior cruciate ligament reconstruction: a preliminary 2-year prospective study. Am J Sports Med2008;36:1263–1274.CrossrefPubMed Google Scholar
58 Gobbi A , MahajanV, KarnatzikosG, NakamuraN. Single- versus double-bundle ACL reconstruction: is there any difference in stability and function at 3-year followup?Clin Orthop Relat Res2012;470:824–834.CrossrefPubMed Google Scholar
59 Hussein M , van EckCF, CretnikA, DinevskiD, FuFH. Prospective randomized clinical evaluation of conventional single-bundle, anatomic single-bundle, and anatomic double-bundle anterior cruciate ligament reconstruction: 281 cases with 3- to 5-year follow-up. Am J Sports Med2012;40:512–520.CrossrefPubMed Google Scholar
60 Meredick RB , VanceKJ, ApplebyD, LubowitzJH. Outcome of single-bundle versus double-bundle reconstruction of the anterior cruciate ligament: a meta-analysis. Am J Sports Med2008;36:1414–1421.CrossrefPubMed Google Scholar
61 Song EK , SeonJK, YimJH, et al.Progression of osteoarthritis after double- and single-bundle anterior cruciate ligament reconstruction. Am J Sports Med2013;41:2340–2346.CrossrefPubMed Google Scholar
62 Suomalainen P , JarvelaT, PaakkalaA, KannusP, JärvinenM. Double-bundle versus single-bundle anterior cruciate ligament reconstruction: a prospective randomized study with 5-year results. Am J Sports Med2012;40:1511–1518.CrossrefPubMed Google Scholar
63 Murray MM , FlemingBC. Biology of anterior cruciate ligament injury and repair: Kappa delta ann doner vaughn award paper 2013. J Orthop Res2013;31:1501–1506.CrossrefPubMed Google Scholar
64 Robayo LM , MoulinVJ, TremblayP, et al.New ligament healing model based on tissue-engineered collagen scaffolds. Wound Repair Regen2011;19:38–48.CrossrefPubMed Google Scholar
65 Fisher MB , LiangR, JungHJ, et al.Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrixbioscaffolds in a goat model. Knee Surg Sports Traumatol Arthrosc2012;20:1357–1365. Google Scholar
66 Woo SL , VogrinTM, AbramowitchSD. Healing and repair of ligament injuries in the knee. J Am Acad Orthop Surg2000;8:364–372.CrossrefPubMed Google Scholar
67 Xie J, Jiang J, Huang W, et al. TNF-alpha induced down-regulation of lysyl oxidase family in anterior cruciate ligament and medial collateral ligament fibroblasts. Knee 2013:Epub. Google Scholar
68 Xie J , JiangJ, ZhangY, et al.Up-regulation expressions of lysyl oxidase family in anterior cruciate ligament and medial collateral ligament fibroblasts induced by Transforming Growth Factor-Beta 1. Int Orthop2012;36:207–213.CrossrefPubMed Google Scholar
69 Xie J , WangC, HuangDY, et al.TGF-beta1 induces the different expressions of lysyl oxidases and matrix metalloproteinases in anterior cruciate ligament and medial collateral ligament fibroblasts after mechanical injury. J Biomech2013;46:890–898.CrossrefPubMed Google Scholar
70 Xie J , WangC, YinL, et al.Interleukin-1 beta influences on lysyl oxidases and matrix metalloproteinases profile of injured anterior cruciate ligament and medial collateral ligament fibroblasts. Int Orthop2013;37:495–505.CrossrefPubMed Google Scholar
71 Zhang J , YangL, TangZ, et al.Expression of MMPs and TIMPs family in human ACL and MCL fibroblasts. Connect Tissue Res2009;50:7–13.CrossrefPubMed Google Scholar
72 Zhou D , LeeHS, VillarrealF, et al.Differential MMP-2 activity of ligament cells under mechanical stretch injury: an in vitro study on human ACL and MCL fibroblasts. J Orthop Res2005;23:949–957.CrossrefPubMed Google Scholar
73 Andrish J , HolmesR. Effects of synovial fluid on fibroblasts in tissue culture. Clin Orthop Relat Res1979;279–283.PubMed Google Scholar
74 Barlow Y , WilloughbyJ. Pathophysiology of soft tissue repair. Br Med Bull1992;48:698–711.CrossrefPubMed Google Scholar
75 Murray MM , SpindlerKP, BallardP, et al.Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res2007;25:1007–1017.CrossrefPubMed Google Scholar
76 Amiel D , IshizueKK, HarwoodFL, KitabayashiL, AkesonWH. Injury of the anterior cruciate ligament: the role of collagenase in ligament degeneration. J Orthop Res1989;7:486–493.CrossrefPubMed Google Scholar
77 Beye JA , HartDA, BrayRC, McDougallJJ, SaloPT. Injury-induced changes in mRNA levels differ widely between anterior cruciate ligament and medial collateral ligament. Am J Sports Med2008;36:1337–1346.CrossrefPubMed Google Scholar
78 Lee J , HarwoodFL, AkesonWH, AmielD. Growth factor expression in healing rabbit medial collateral and anterior cruciate ligaments. Iowa Orthop J1998;18:19–25.PubMed Google Scholar
79 Menetrey J , LaumonierT, GaravagliaG, et al.alpha-Smooth muscle actin and TGF-beta receptor I expression in the healing rabbit medial collateral and anterior cruciate ligaments. Injury2011;42:735–741. Google Scholar
80 Saris DB , DhertWJ, VerboutAJ. Joint homeostasis: the discrepancy between old and fresh defects in cartilage repair. J Bone Joint Surg [Br]2003;85-B:1067–1076. Google Scholar
81 Schreck PJ , KitabayashiLR, AmielD, AkesonWH, WoodsVL Jr. Integrin display increases in the wounded rabbit medial collateral ligament but not the wounded anterior cruciate ligament. J Orthop Res1995;13:174–183. Google Scholar
82 Sung KL , KwanMK, MaldonadoF, AkesonWH. Adhesion strength of human ligament fibroblasts. J Biomech Eng1994;116:237–242.CrossrefPubMed Google Scholar
83 Tang Z , YangL, WangY, et al.Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res2009;27:243–248.CrossrefPubMed Google Scholar
84 Tang Z , YangL, XueR, et al.Differential expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in anterior cruciate ligament and medial collateral ligament fibroblasts after a mechanical injury: involvement of the p65 subunit of NF-kappaB. Wound Repair Regen2009;17:709–716.CrossrefPubMed Google Scholar
85 Gesink DS , PachecoHO, KuiperSD, et al.Immunohistochemical localization of beta 1-integrins in anterior cruciate and medial collateral ligaments of human and rabbit. J Orthop Res1992;10:596–599.CrossrefPubMed Google Scholar
86 Kobayashi K , HealeyRM, SahRL, et al.Novel method for the quantitative assessment of cell migration: a study on the motility of rabbit anterior cruciate (ACL) and medial collateral ligament (MCL) cells. Tissue Eng2000;6:29–38.CrossrefPubMed Google Scholar
87 Lo IK , OuY, RattnerJP, et al.The cellular networks of normal ovine medial collateral and anterior cruciate ligaments are not accurately recapitulated in scar tissue. J Anat2002;200:283–296. Google Scholar
88 Lyon RM , AkesonWH, AmielD, KitabayashiLR, WooSL. Ultrastructural differences between the cells of the medical collateral and the anterior cruciate ligaments. Clin Orthop Relat Res1991;272:279–286.PubMed Google Scholar
89 McKean JM , HsiehAH, SungKL. Epidermal growth factor differentially affects integrin-mediated adhesion and proliferation of ACL and MCL fibroblasts. Biorheology2004;41:139–152.PubMed Google Scholar
90 Nagineni CN , AmielD, GreenMH, BerchuckM, AkesonWH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res1992;10:465–475.CrossrefPubMed Google Scholar
91 Wiig ME , AmielD, IvarssonM, et al.Type I procollagen gene expression in normal and early healing of the medial collateral and anterior cruciate ligaments in rabbits: an in situ hybridization study. J Orthop Res1991;9:374–382.CrossrefPubMed Google Scholar
92 Yoshida M , FujiiK. Differences in cellular properties and responses to growth factors between human ACL and MCL cells. J Orthop Sci1999;4:293–298.CrossrefPubMed Google Scholar
93 Bray RC , LeonardCA, SaloPT. Vascular physiology and long-term healing of partial ligament tears. J Orthop Res2002;20:984–989.CrossrefPubMed Google Scholar
94 Bray RC , LeonardCA, SaloPT. Correlation of healing capacity with vascular response in the anterior cruciate and medial collateral ligaments of the rabbit. J Orthop Res2003;21:1118–1123.CrossrefPubMed Google Scholar
95 Andersen HN , AmisAA. Review on tension in the natural and reconstructed anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc1994;2:192–202.CrossrefPubMed Google Scholar
96 Steadman J , CameronM, BriggsK, RodkeyW. Healing-response treatment for ACL injuries. Orthop Tech Rev2002;3:7. Google Scholar
97 Gobbi A , BathanL, BoldriniL. Primary repair combined with bone marrow stimulation in acute anterior cruciate ligament lesions: results in a group of athletes. Am J Sports Med2009;37:571–578.CrossrefPubMed Google Scholar
98 Steadman JR , Cameron-DonaldsonML, BriggsKK, RodkeyWG. A minimally invasive technique (“healing response”) to treat proximal ACL injuries in skeletally immature athletes. J Knee Surg2006;19:8–13. Google Scholar
99 Steadman JR , MathenyLM, BriggsKK, RodkeyWG, CarreiraDS. Outcomes following healing response in older, active patients: a primary anterior cruciate ligament repair technique. J Knee Surg2012;25:255–260.CrossrefPubMed Google Scholar
100 Wasmaier J , Kubik-HuchR, PfirrmannC, et al.Proximal anterior cruciate ligament tears: the healing response technique versus conservative treatment. J Knee Surg2013;26:263–271.CrossrefPubMed Google Scholar
101 Caplan AI . Mesenchymal stem cells. J Orthop Res1991;9:641–650.CrossrefPubMed Google Scholar
102 Caplan AI . Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol2007;213:341–347.CrossrefPubMed Google Scholar
103 Caplan AI . Mesenchymal stem cells: the past, the present, the future. Cartilage2010;1:6–9.CrossrefPubMed Google Scholar
104 Awad HA , BoivinGP, DresslerMR, et al.Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res2003;21:420–431.CrossrefPubMed Google Scholar
105 Awad HA , ButlerDL, BoivinGP, et al.Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng1999;5:267–277.CrossrefPubMed Google Scholar
106 106 BruderSP, JaiswalN, HaynesworthSE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem1997;64:278–294.CrossrefPubMed Google Scholar
107 Ge Z , GohJC, LeeEH. The effects of bone marrow-derived mesenchymal stem cells and fascia wrap application to anterior cruciate ligament tissue engineering. Cell Transplant2005;14:763–773.CrossrefPubMed Google Scholar
108 Haynesworth SE , GoshimaJ, GoldbergVM, CaplanAI. Characterization of cells with osteogenic potential from human marrow. Bone1992;13:81–88.CrossrefPubMed Google Scholar
109 Johnstone B , HeringTM, CaplanAI, GoldbergVM, YooJU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res1998;238:265–272.CrossrefPubMed Google Scholar
110 Pittenger MF , MackayAM, BeckSC, et al.Multilineage potential of adult human mesenchymal stem cells. Science1999;284:143–147.CrossrefPubMed Google Scholar
111 Young RG , ButlerDL, WeberW, et al.Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res1998;16:406–413.CrossrefPubMed Google Scholar
112 Kanaya A , DeieM, AdachiN, et al.Intra-articular injection of mesenchymal stromal cells in partially torn anterior cruciate ligaments in a rat model. Arthroscopy2007;23:610–617.CrossrefPubMed Google Scholar
113 Lim JK , HuiJ, LiL, et al.Enhancement of tendon graft osteointegration using mesenchymal stem cells in a rabbit model of anterior cruciate ligament reconstruction. Arthroscopy2004;20:899–910.CrossrefPubMed Google Scholar
114 Soon MY , HassanA, HuiJH, GohJC, LeeEH. An analysis of soft tissue allograft anterior cruciate ligament reconstruction in a rabbit model: a short-term study of the use of mesenchymal stem cells to enhance tendon osteointegration. Am J Sports Med2007;35:962–971.CrossrefPubMed Google Scholar
115 Oe K , KushidaT, OkamotoN, et al.New strategies for anterior cruciate ligament partial rupture using bone marrow transplantation in rats. Stem Cells Dev2011;20:671–679.CrossrefPubMed Google Scholar
116 Gerich TG , KangR, FuFH, RobbinsPD, EvansCH. Gene transfer to the rabbit patellar tendon: potential for genetic enhancement of tendon and ligament healing. Gene Ther1996;3:1089–1093.PubMed Google Scholar
117 Hildebrand KA , FrankCB, HartDA. Gene intervention in ligament and tendon: current status, challenges, future directions. Gene Ther2004;11:368–378.CrossrefPubMed Google Scholar
118 Huard J , LiY, PengH, FuFH. Gene therapy and tissue engineering for sports medicine. J Gene Med 2003;5:93–108.CrossrefPubMed Google Scholar
119 Madry H , KohnD, CucchiariniM. Direct FGF-2 gene transfer via recombinant adeno-associated virus vectors stimulates cell proliferation, collagen production, and the repair of experimental lesions in the human ACL. Am J Sports Med2013;41:194–202.CrossrefPubMed Google Scholar
120 Menetrey J , KasemkijwattanaC, DayCS, et al.Direct-, fibroblast- and myoblast-mediated gene transfer to the anterior cruciate ligament. Tissue Eng1999;5:435–442.CrossrefPubMed Google Scholar
121 Nixon AJ , WattsAE, SchnabelLV. Cell- and gene-based approaches to tendon regeneration. J Shoulder Elbow Surg2012;21:278–294.CrossrefPubMed Google Scholar
122 Pascher A , SteinertAF, PalmerGD, et al.Enhanced repair of the anterior cruciate ligament by in situ gene transfer: evaluation in an in vitro model. Mol Ther2004;10:327–336.CrossrefPubMed Google Scholar
123 Steinert AF , WeberM, KunzM, et al.In situ IGF-1 gene delivery to cells emerging from the injured anterior cruciate ligament. Biomaterials2008;29:904–916.CrossrefPubMed Google Scholar
124 Woo SL , JiaF, ZouL, GabrielMT. Functional tissue engineering for ligament healing: potential of antisense gene therapy. Ann Biomed Eng2004;32:342–351.CrossrefPubMed Google Scholar
125 Attia E , BrownH, HenshawR, GeorgeS, HannafinJA. Patterns of gene expression in a rabbit partial anterior cruciate ligament transection model: the potential role of mechanical forces. Am J Sports Med2010;38:348–356.CrossrefPubMed Google Scholar
126 Wang Y , TangZ, XueR, et al.TGF-beta1 promoted MMP-2 mediated wound healing of anterior cruciate ligament fibroblasts through NF-kappaB. Connect Tissue Res2011;52:218–225. Google Scholar
127 Hildebrand KA , DeieM, AllenCR, et al.Early expression of marker genes in the rabbit medial collateral and anterior cruciate ligaments: the use of different viral vectors and the effects of injury. J Orthop Res1999;17:37–42.CrossrefPubMed Google Scholar
128 Bonniaud P , MargettsPJ, KolbM, et al.Adenoviral gene transfer of connective tissue growth factor in the lung induces transient fibrosis. Am J Respir Crit Care Med2003;168:770–778.CrossrefPubMed Google Scholar
129 Crystal RG . Transfer of genes to humans: early lessons and obstacles to success. Science1995;270:404–410.CrossrefPubMed Google Scholar
130 Amiel D , NagineniCN, ChoiSH, LeeJ. Intrinsic properties of ACL and MCL cells and their responses to growth factors. Med Sci Sports Exerc1995;27:844–851.PubMed Google Scholar
131 Azuma H , YasudaK, TohyamaH, et al.Timing of administration of transforming growth factor-beta and epidermal growth factor influences the effect on material properties of the in situ frozen-thawed anterior cruciate ligament. J Biomech2003;36:373–381.CrossrefPubMed Google Scholar
132 Kondo E , YasudaK, YamanakaM, MinamiA, TohyamaH. Effects of administration of exogenous growth factors on biomechanical properties of the elongation-type anterior cruciate ligament injury with partial laceration. Am J Sports Med2005;33:188–196.CrossrefPubMed Google Scholar
133 Meaney Murray M , RiceK, WrightRJ, SpectorM. The effect of selected growth factors on human anterior cruciate ligament cell interactions with a three-dimensional collagen-GAG scaffold. J Orthop Res2003;21:238–244.CrossrefPubMed Google Scholar
134 Molloy T , WangY, MurrellG. The roles of growth factors in tendon and ligament healing. Sports Med2003;33:381–394.CrossrefPubMed Google Scholar
135 Moreau JE , ChenJ, BramonoDS, et al.Growth factor induced fibroblast differentiation from human bone marrow stromal cells in vitro. J Orthop Res2005;23:164–174.CrossrefPubMed Google Scholar
136 Nagumo A , YasudaK, NumazakiH, et al.Effects of separate application of three growth factors (TGF-beta1, EGF, and PDGF-BB) on mechanical properties of the in situ frozen-thawed anterior cruciate ligament. Clin Biomech (Bristol, Avon)2005;20:283–290.CrossrefPubMed Google Scholar
137 Sakai T , YasudaK, TohyamaH, et al.Effects of combined administration of transforming growth factor-beta1 and epidermal growth factor on properties of the in situ frozen anterior cruciate ligament in rabbits. J Orthop Res2002;20:1345–1351.CrossrefPubMed Google Scholar
138 Scherping SC Jr , SchmidtCC, GeorgescuHI, et al.Effect of growth factors on the proliferation of ligament fibroblasts from skeletally mature rabbits. Connect Tissue Res1997;36:1–8.CrossrefPubMed Google Scholar
139 Spindler KP , MurrayMM, DetwilerKB, et al.The biomechanical response to doses of TGF-beta 2 in the healing rabbit medial collateral ligament. J Orthop Res2003;21:245–249.CrossrefPubMed Google Scholar
140 Vavken P , SaadFA, FlemingBC, MurrayMM. VEGF receptor mRNA expression by ACL fibroblasts is associated with functional healing of the ACL. Knee Surg Sports Traumatol Arthrosc2011;19:1675–1682.CrossrefPubMed Google Scholar
141 Marui T , NiyibiziC, GeorgescuHI, et al.Effect of growth factors on matrix synthesis by ligament fibroblasts. J Orthop Res1997;15:18–23.CrossrefPubMed Google Scholar
142 Kobayashi D , KurosakaM, YoshiyaS, MizunoK. Effect of basic fibroblast growth factor on the healing of defects in the canine anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc1997;5:189–194.CrossrefPubMed Google Scholar
143 Cole BJ , SeroyerST, FilardoG, BajajS, FortierLA. Platelet-rich plasma: where are we now and where are we going?Sports Health2010;2:203–210.CrossrefPubMed Google Scholar
144 Eppley BL , WoodellJE, HigginsJ. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg2004;114:1502–1508.CrossrefPubMed Google Scholar
145 McCarrel T , FortierL. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J Orthop Res2009;27:1033–1042.CrossrefPubMed Google Scholar
146 Mishra A , WoodallJ Jr, VieiraA. Treatment of tendon and muscle using platelet-rich plasma. Clin Sports Med2009;28:113–125.CrossrefPubMed Google Scholar
147 Schnabel LV , MohammedHO, MillerBJ, et al.Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res2007;25:230–240.CrossrefPubMed Google Scholar
148 Vavken P , SadoghiP, MurrayMM. The effect of platelet concentrates on graft maturation and graft-bone interface healing in anterior cruciate ligament reconstruction in human patients: a systematic review of controlled trials. Arthroscopy2011;27:1573–1583.CrossrefPubMed Google Scholar
149 Weibrich G , KleisWK, HafnerG, HitzlerWE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg2002;30:97–102.CrossrefPubMed Google Scholar
150 Borregaard N , CowlandJB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood1997;89:3503–3521.PubMed Google Scholar
151 Velnar T , BaileyT, SmrkoljV. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res2009;37:1528–1542.CrossrefPubMed Google Scholar
152 Nin JR , GasqueGM, AzcarateAV, BeolaJD, GonzalezMH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing?Arthroscopy2009;25:1206–1213.CrossrefPubMed Google Scholar
153 Orrego M , LarrainC, RosalesJ, et al.Effects of platelet concentrate and a bone plug on the healing of hamstring tendons in a bone tunnel. Arthroscopy2008;24:1373–1380.CrossrefPubMed Google Scholar
154 Sanchez M , AnituaE, AzofraJ, et al.Ligamentization of tendon grafts treated with an endogenous preparation rich in growth factors: gross morphology and histology. Arthroscopy2010;26:470–480.CrossrefPubMed Google Scholar
155 Silva A , SampaioR. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing?Knee Surg Sports Traumatol Arthrosc2009;17:676–682.CrossrefPubMed Google Scholar
156 Vogrin M , RuprehtM, DinevskiD, et al.Effects of a platelet gel on early graft revascularization after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind, clinical trial. Eur Surg Res2010;45:77–85.CrossrefPubMed Google Scholar
157 Joshi SM , MastrangeloAN, MagarianEM, FlemingBC, MurrayMM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med2009;37:2401–2410.CrossrefPubMed Google Scholar
158 Murray MM , SpindlerKP, AbreuE, et al.Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res2007;25:81–91.CrossrefPubMed Google Scholar
159 Murray MM , SpindlerKP, DevinC, et al.Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res2006;24:820–830.CrossrefPubMed Google Scholar
160 Spindler KP , MurrayMM, DevinC, NanneyLB, DavidsonJM. The central ACL defect as a model for failure of intra-articular healing. J Orthop Res2006;24:401–406.CrossrefPubMed Google Scholar
161 Drury JL , MooneyDJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials2003;24:4337–4351.CrossrefPubMed Google Scholar
162 Kim BS , PutnamAJ, KulikTJ, MooneyDJ. Optimizing seeding and culture methods to engineer smooth muscle tissue on biodegradable polymer matrices. Biotechnol Bioeng1998;57:46–54.PubMed Google Scholar
163 Berry SM , GreenMH. Hyaluronan: a potential carrier for growth factors for the healing of ligamentous tissues. Wound Repair Regen1997;5:33–38.CrossrefPubMed Google Scholar
164 Wiig ME , AmielD, VandeBergJ, et al.The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: an experimental study in rabbits. J Orthop Res1990;8:425–434.CrossrefPubMed Google Scholar
165 Dunn MG , LieschJB, TikuML, ZawadskyJP. Development of fibroblast-seeded ligament analogs for ACL reconstruction. J Biomed Mater Res1995;29:1363–1371.CrossrefPubMed Google Scholar
166 Badylak S , ArnoczkyS, PlouharP, et al.Naturally occurring extracellular matrix as a scaffold for musculoskeletal repair. Clin Orthop Relat Res1999;367(Suppl):S333–S343.CrossrefPubMed Google Scholar
167 Badylak SF , ParkK, PeppasN, McCabeG, YoderM. Marrow-derived cells populate scaffolds composed of xenogeneic extracellular matrix. Exp Hematol2001;29:1310–1318.CrossrefPubMed Google Scholar
168 Badylak SF , TulliusR, KokiniK, et al.The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res1995;29:977–985.CrossrefPubMed Google Scholar
169 Dejardin LM , ArnoczkySP, EwersBJ, HautRC, ClarkeRB. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa: histologic and mechanical evaluation in dogs. Am J Sports Med2001;29:175–184. Google Scholar
170 McDevitt CA , WildeyGM, CutroneRM. Transforming growth factor-beta1 in a sterilized tissue derived from the pig small intestine submucosa. J Biomed Mater Res A2003;67:637–640.CrossrefPubMed Google Scholar
171 Musahl V , AbramowitchSD, GilbertTW, et al.The use of porcine small intestinal submucosa to enhance the healing of the medial collateral ligament: a functional tissue engineering study in rabbits. J Orthop Res2004;22:214–220. Google Scholar
172 Fisher MB , LiangR, JungHJ, et al.Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surg Sports Traumatol Arthrosc2012;20:1357–1365.CrossrefPubMed Google Scholar
173 Fleming BC , MagarianEM, HarrisonSL, PallerDJ, MurrayMM. Collagen scaffold supplementation does not improve the functional properties of the repaired anterior cruciate ligament. J Orthop Res2010;28:703–709. Google Scholar
174 Vavken P , FlemingBC, MastrangeloAN, MachanJT, MurrayMM. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy2012;28:672–680.CrossrefPubMed Google Scholar
175 Murray MM , PalmerM, AbreuE, et al.Platelet-rich plasma alone is not sufficient to enhance suture repair of the ACL in skeletally immature animals: an in vivo study. J Orthop Res2009;27:639–645. Google Scholar
176 Frank C , AmielD, AkesonWH. Healing of the medial collateral ligament of the knee: a morphological and biochemical assessment in rabbits. Acta Orthop Scand1983;54:917–923. Google Scholar
177 Lo IK , MarchukLL, HartDA, FrankCB. Comparison of mRNA levels for matrix molecules in normal and disrupted human anterior cruciate ligaments using reverse transcription-polymerase chain reaction. J Orthop Res1998;16:421–428.CrossrefPubMed Google Scholar
178 Murray MM , MartinSD, MartinTL, SpectorM. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg [Am]2000;82-A:1387–1397.CrossrefPubMed Google Scholar
179 Spindler KP , ClarkSW, NanneyLB, DavidsonJM. Expression of collagen and matrix metalloproteinases in ruptured human anterior cruciate ligament: an in situ hybridization study. J Orthop Res1996;14:857–861.CrossrefPubMed Google Scholar
180 Witonski D , Wagrowska-DanilewiczM. Distribution of substance-P nerve fibers in intact and ruptured human anterior cruciate ligament: a semi-quantitative immunohistochemical assessment. Knee Surg Sports Traumatol Arthrosc2004;12:497–502.CrossrefPubMed Google Scholar
181 Magarian EM , FlemingBC, HarrisonSL, et al.Delay of 2 or 6 weeks adversely affects the functional outcome of augmented primary repair of the porcine anterior cruciate ligament. Am J Sports Med2010;38:2528–2534. Google Scholar
182 Murray MM , FlemingBC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med2013;41:1762–1770.CrossrefPubMed Google Scholar
183 Vavken P , ProffenB, PetersonC, et al.Effects of suture choice on biomechanics and physeal status after bioenhanced anterior cruciate ligament repair in skeletally immature patients: a large-animal study. Arthroscopy2013;29:122–132.CrossrefPubMed Google Scholar
Funding statement:
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under award numbers 1R01-AR056834 and 2R01-AR054099. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions:
A. M. Kiapour: Writing the article, Literature search
M. M. Murray: Supervision, Review and editing of the manuscript
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










