Abstract
Objectives
Matrix-assisted autologous chondrocyte transplantation (MACT) has been developed and applied in the clinical practice in the last decade to overcome most of the disadvantages of the first generation procedures. The purpose of this systematic review is to document and analyse the available literature on the results of MACT in the treatment of chondral and osteochondral lesions of the knee.
Methods
All studies published in English addressing MACT procedures were identified, including those that fulfilled the following criteria: 1) level I-IV evidence, 2) measures of functional or clinical outcome, 3) outcome related to cartilage lesions of the knee cartilage.
Results
The literature analysis showed a progressively increasing number of articles per year. A total of 51 articles were selected: three randomised studies, ten comparative studies, 33 case series and five case reports. Several scaffolds have been developed and studied, with good results reported at short to medium follow-up.
Conclusions
MACT procedures are a therapeutic option for the treatment of chondral lesions that can offer a positive outcome over time for specific patient categories, but high-level studies are lacking. Systematic long-term evaluation of these techniques and randomised controlled trials are necessary to confirm the potential of this treatment approach, especially when comparing against less ambitious traditional treatments.
Introduction
The complex biomechanical features of hyaline cartilage are difficult to reproduce. An articular chondral surface has a peculiar ultrastructure, with chondrocytes sparsely distributed and minimal cell-to-cell contact, interacting in a surrounding matrix characterised by a complex framework of collagen, aggrecan and fluid.1-3Treatment options aimed at the recruitment of potential cartilage precursors allowing stem cell migration from the marrow cavity to the fibrin clot of the defect, such as abrasion, drilling and microfracture, produce predominantly type I collagen, fibrocytes and an unorganised matrix.4 This fibrous repair tissue lacks the biomechanical and viscoelastic characteristics of the original hyaline cartilage, and does not lead to durable results.5 Techniques aiming at transferring autologous osteochondral units from less weight-bearing areas to repair the lesion with a healthy tissue allow a valid articular surface to be reconstructed with good coverage of the defect and graft stability in small lesions.6 Donor site availability and technical difficulties are critical aspects that limit this approach for medium to large surfaces. An alternative option is the use of homologous osteochondral grafts, but there are concerns regarding low availability, the difficulty of preserving and managing fresh allografts and the risk of disease transmission.7,8 These concerns have reduced the indication of this procedure to large osteochondral lesions in young patients with high functional requirements, who are otherwise doomed to poor clinical outcome.9
The pioneers of this ambitious treatment approach developed and introduced the autologous chondrocyte implantation (ACI) technique in Sweden showing firstly in 1994 satisfactory results for the treatment of isolated femoral condyle lesions.10 Several studies followed and claimed both the production of a hyaline-like articular surface and a good outcome at medium to long-term follow-up: Vasiliadis et al11 used MRI to show good tissue quality despite the evidence of some osteophytes, cysts, and oedema, and Peterson et al12 reported good results in 224 cases at 13 years with 92% of patients satisfied (n = 206). However, these good results have to be weighed against several problems. From a surgical point of view, the standard ACI procedure presents various limitations related to the complexity and morbidity of the technique, including the difficulty in handling a delicate liquid suspension of chondrocytes, the need to make a hermetic periosteum seal using sutures to avoid cell leakage, the requirement of a second open surgery with a subsequent long rehabilitation period, and a high rate of complications and re-operation due to flap hypertrophy, arthrofibrosis and joint stiffness.13 From a biological point of view, critical aspects are the maintenance of the chondrocyte phenotype during the prolonged monolayer culture and the risk of a non-homogeneous distribution of the liquid cell suspension in the lesion area.13 Tissue engineering has been developed to address most of these problems, leading to the introduction into clinical practice ten years ago of the so-called matrix-assisted autologous chondrocyte transplantation (MACT) procedures.14
The aim of this systematic review is to document and analyse the available evidence in the literature on the results obtained in clinical practice by MACT techniques to address chondral and osteochondral knee lesions.
Materials and Methods
All studies on MACT procedures published in English were identified. Two reviewers performed a search of the Medline database from 2000 to March 2012, using the terms “cartilage regeneration”, “autologous chondrocyte transplantation”, “autologous chondrocyte implantation”, “second / third generation ACI”, “matrix-assisted chondrocyte implantation”, “scaffold-based repair” and “osteochondral repair”. Studies were included in our systematic review if they fulfilled the following criteria: 1) level I-IV evidence addressing the areas of interest outlined above; 2) measures of functional or clinical outcome; 3) outcome related to knee cartilage lesions. Citations from relevant studies, as well as any relevant articles captured by the search, were also examined to determine if they were suitable for inclusion. Studies not fulfilling these criteria were excluded.
Results
The search identified 187 articles. The number of articles per year increased progressively from 2000, as depicted in Figure 1. Including those with short follow-up, a total of 51 articles fulfilled the inclusion criteria: there were three randomised studies, ten comparative studies, 33 case series, and five case reports. All studies are reported and summarised in Table I15-66; those reporting clinical results at a minimum follow-up of two years are described in more detail in the following paragraphs.
Table I
Details of the 51 articles focusing on clinical results of matrix assisted autologous chondrocyte implantation (MACI) procedures
| Author/s | Procedure | Patients (n)* | Mean size of lesion (cm2) (range) | Mean follow-up (yrs) | Study level† |
|---|---|---|---|---|---|
| 2003 | |||||
| Marcacci et al54 | Hyalograft C | 20 | 2.9 | 1 | Prospective study |
| Cherubino et al55 | MACI | 13 | 3.5 | 6.5 mths (2 to 15) | Case series |
| Pavesio et al56 | Hyalograft C | 67 | - | 17.5 mths | Cohort study |
| 2004 | |||||
| Ronga et al57 | MACI | 1 | 2 | 1 | Case report |
| Marlovits et al58 | MACI | 16 | 4.7 (2.6 to 10.9) | 3.1 mths | Case series |
| 2005 | |||||
| Bartlett et al59 | MACI | 5 | 2.2 to 8.0 | 1 | Prospective study |
| Bartlett et al60 | MACI | 44 ACI-C/47 MACI | 6.1 | 1 | RCT |
| Marcacci et al24 | Hyalograft C | 141 | 3.5 | 38 mths | Case series |
| 2006 | |||||
| Nehrer et al25 | Hyalograft C | 36 | 1.5 to 8 | 3 | Case series |
| Behrens et al15 | MACI | 11 | 1.5 to 17.7 | 5 | Case series |
| Ronga et al16 | MACI | 1 | 5 | 2 | Case report |
| Gobbi et al26 | Hyalograft C | 32 | 4.7 | 2 | Case series |
| 2007 | |||||
| Marcacci et al28 | Hyalograft C | 70 | 2.4 | 2 to 4 | Case series |
| Manfredini et al61 | Hyalograft C | 17 ACI/10 Hyalograft C | - | 1 | Comparative study |
| Adachi et al51 | Atelocollagen | 1 | 5 and 3 | 2 | Case report |
| Ossendorf et al38 | Bioseed C | 40 | 4.6 | 2 | Case series |
| 2008 | |||||
| Ebert et al62 | MACI | 62 | - | 2 | RCT |
| Selmi et al48 | Cartipatch | 17 | 3 | 2 | Case series |
| Ferruzzi et al30 | Hyalograft C | 48 ACI I gen/50 Hyalograft C | 6.4 / 5.9 | 5 | Comparative study |
| 2009 | |||||
| Gigante et al18 | MACI | 12 | 4 | 3 | Case series |
| Kon et al5 | Hyalograft C | 40 Hyalograft C/40 MFX | 2.2 / 2.5 | 5 | Comparative study |
| Kreuz et al39 | Bioseed C | 19 | 4 | 4 | Case series |
| Crawford et al43 | Neocart | 8 | 2.2 | 2 | Case series |
| Gobbi et al27 | Hyalograft C | 34 | 4.4 | 5 | Case series |
| Salzmann et al17 | MACI | 9 OCT/9 MACI | 2.3 / 6.3 | 41 mths/42 mths | Comparative study |
| Wondrasch et al46 | Hyalograft C / CaReS | 31 | 4.8 | 2 | Case series |
| Nehrer et al29 | Hyalograft C | 42 arthrotomy/11 arthroscopy | 4.4 | 2 to 7 | Case series |
| Tohyama et al52 | Atelocollagen | 27 | 3.2 | 2 | Case series |
| Vilchez et al63 | Chondrograft | 15 | 1.5 to 8 | 1 | Prospective study |
| 2010 | |||||
| Della Villa et al33 | Hyalograft C | 31 athletes/34 non-athletes | 2.2 / 2.3 | 57 mths/52 mths | Comparative study |
| Welsch et al45 | Hyalograft C / CaReS | 10 CaReS/10 Hyalograft C | 4.6 / 4.9 | 2 | Comparative study |
| Basad et al19 | MACI | 33 MACI/15 MFX | > 4 | 2 | Randomised study |
| Kim et al50 | Chondron | 30 | 5.8 | 2 | Case series |
| Kon et al31 | Hyalograft C | 50 | 2.5 | 5 | Case series |
| Zeifang et al42 | Bioseed C | 11 Bioseed C/10 ACI I gen | 4.3 / 4.1 | 2 | Randomised study |
| Choi et al49 | Chondron | 40 | 5.2 | Minimum 2 | Case series |
| Clar et al34 | Hyalograft C | 1 | 14 | 5.5 | Case report |
| Erggelet et al41 | Bioseed C | 40 Bioseed C/42 ACI I gen | 2 to 17.5 / 2 to 15 | 2 | Comparative study |
| 2011 | |||||
| Ebert et al20 | MACI | 35 | 3.0 | 5 | Case series |
| Macmull et al64 | MACI | 24 ACI/7 MACI | 0.96 to 15.7 | 66.3 mths | Comparative study |
| Bauer et al22 | MACI | 18 | 6 | 5 | Case series |
| Kon et al32 | Hyalograft C | 22 Hyalograft C/39 MACI | 2.6 / 3.1 | 5.1/4.8 | Comparative study |
| Kreuz et al40 | Bioseed C | 52 | 4.8 | 4 | Case series |
| Enea et al65 | MACI | 30 | 5.0 | 15 mths | Case series |
| Schneider et al47 | CaReS | 116 | 5.4 | 12 to 60 mths | Case series |
| Filardo et al35 | Hyalograft C | 32 | 3 | 6 | Case series |
| Filardo et al36 | Hyalograft C | 58 | 2.3 | 6 | Case series |
| Kon et al53 | Hyalograft C | 21 Hyalograft C/20 MFX | 2 | 7.5 | Comparative study |
| Filardo et al37 | Hyalograft C | 62 | 2.5 | 7 | Case series |
| 2012 | |||||
| Ventura et al23 | MACI | 53 | 4.3 | 27 mths (n = 53)/59 mths (n = 17 ) | Case series |
| Macmull et al21 | MACI | 25 ACI/23 MACI | 4.7 | 40 mths | Comparative study |
| Panagopoulos et al44 | Novocart | 11 ACI/8 MACI | 6.5 | 37.5 mths | Comparative study |
| Könst et al66 | Gel MACI | 9 | 7.1 | 9 mths | Case series |
-
* (M)ACI-C, (matrix) autologous chondrocyte implantation-collagen membrane; MFX, microfractures; CaReS, Cartilage Repair System (Ars Arthro Technology) † RCT, randomised controlled trial
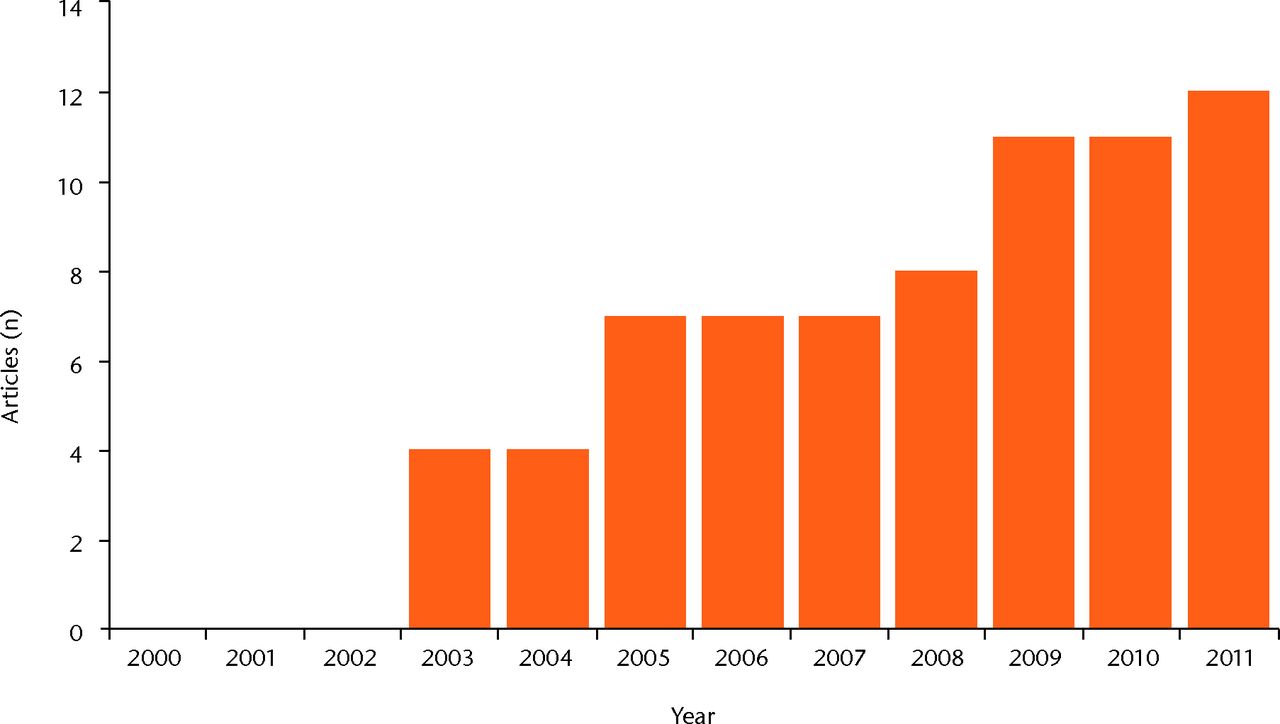
Fig. 1
Bar chart of the number of publications focusing on cartilage regeneration, showing the growing interest in the topic.
Matrix autologous chondrocyte implantation (MACI)
The first autologous chondrocyte transplantation using a porcine collagen type I/III membrane (Chondro-Gide; Geistlich Biomaterials, Wolhusen, Switzerland) was performed in 1998.67 As in every MACT procedure, the surgical technique involves two surgical steps: harvesting articular cartilage from a non-weight-bearing area and, after culturing cells for four weeks and then seeding and culturing for the remaining three days on the rough side of the collagen matrix, implantation of the bioengineered tissue into the lesion (MACI; Verigen Transplantation Service, Copenhagen, Denmark). In 2006 Behrens et al15 published a five-year prospective study, reporting that eight of 11 patients rated the function of their knee as much better or better than before. In the same year Ronga et al16 reported the successful treatment of a complex knee ligament, meniscal and chondral lesion in a 40-year-old sportsman at two years’ follow-up. Normal biomechanics of the joint were restored by performing a collagen meniscus implant and anterior cruciate ligament reconstruction during the first step, and after six months a 5 cm2 chondral lesion was treated with the second-step MACI procedure. Salzmann et al17 confirmed these good results in a comparative study, in which nine patients treated with MACI obtained a significant clinical improvement with results higher than those obtained in a matching group of patients who underwent osteochondral autograft transplantation. Gigante et al18 focused on a specific patient population affected by patellar lesion and treated with patellofemoral distal realignment and cartilage reconstruction: all 12 patients (14 knees) presented a significant improvement in all scales. Basad et al19 performed a randomised trial comparing MACI and microfracture for the treatment of lesions > 4 cm2: at two years, MACI demonstrated significantly higher and more stable results over time. Ebert et al20 evaluated 35 patients at five years, showing a clinical and MRI improvement up to two years and then stable results over time, with 35 patients (86%) satisfied with the results. More recently, Macmull et al21 evaluated the treatment of symptomatic chondromalacia patellae in 23 patients evaluated at a follow-up of 40 months: results were satisfactory and better than those obtained in a comparative ACI group. Bauer et al22combined MACI and neutralising high tibial osteotomy in patients with medial knee osteoarthritis and varus deformity, documenting good clinical and MRI results initially but a significant decline at five years. Finally, Ventura et al23 documented good results in 53 patients at two years, confirmed by the 17 patients evaluated at five years of follow-up, with improvements in function and pain and complete integration of the graft within the surrounding native cartilage in 15 patients (88%) at five years.
Hyalograft C
Hyaluronic acid, another widely represented cartilage matrix element, is the main component of Hyalograft C, introduced into clinical practice in 1999.68 This scaffold is entirely based on the benzylic ester of hyaluronic acid (HYAFF 11; Fidia Advanced Biopolymers Laboratories, Padova, Italy) and consists of a network of 20 µm thick fibers with interstices of variable sizes. The features of this device allowed the development of an arthroscopic surgical technique,68 and in 2005 Marcacci et al24 reported the clinical results of a multicentre study on 141 patients evaluated at a minimum follow-up of two years. A mean three-year follow-up evaluation showed 129 patients (91.5%) had subjectively improved results, and cartilage repair was graded arthroscopically as normal or nearly normal in 53 of 55 knees (96.4%) that underwent second-look arthroscopy. Moreover, 12 of 22 second-look biopsies were judged as hyaline-like, and a low complication rate was recorded. In the same period, Nehrer et al25 confirmed the good short-term results in a group of 36 patients followed for three years, and Gobbi et al26 reported a positive outcome at two years in 32 patellofemoral full-thickness chondral defects. The same group of patients were described at five years,27 showing a worsening with respect to the previous study, but still good clinical and histological results. A medium-term follow-up evaluation was also performed by Marcacci et al28 and Nehrer et al29 who confirmed the significant clinical improvement with stable results over time. Ferruzzi et al30 treated 50 patients affected by osteochondritis dissecans (OCD) and traumatic lesions, and showed stable clinical results at minimum five years’ follow-up and a well-integrated cartilage tissue in 93% of the patients at the final MRI evaluation. Moreover, they also compared them with a group of patients treated with first-generation ACI and showed a similar healing potential but with fewer complications and a more rapid recovery when the arthroscopic MACT procedure was used.30 Kon et al31 followed a group of patients clinically and with MRI for five years and reported durability of the good clinical results obtained and a correlation between imaging and clinical findings. These results were also confirmed in a demanding patient population of high-level soccer players evaluated at 7.5 years: whereas microfracture allowed a faster recovery but presented a clinical deterioration over time, arthroscopic Hyalograft C delayed the return to competition but offered more durable clinical results.32Della Villa et al33 focused on the post-operative phase by evaluating highly competitive athletes, and demonstrated that an intensive rehabilitation may safely allow a faster return to competition and also positively influences the clinical outcome at medium-term follow-up. Clar et al34 reported the use of hyaluronic-based MACT as a salvage treatment for a 14 cm2 defect in a 17.5-year-old girl, due to previous steroid-induced osteonecrosis: after treatment, formation of a solid cartilage layer was observed on MRI and a continuous clinical improvement registered up to 5.5 years. Kon et al69 analysed and compared results obtained using arthroscopic Hyalograft C implantation or the mini-open MACI technique15 for the treatment of cartilage lesions in 61 patients > 40 years of age with no clear signs of osteoarthritis. Results were inferior compared with those previously found for younger populations, with a higher rate of failure, but a significant clinical improvement was still found at five years.69 In fact, this group of patients also benefited in most cases from both cartilage regenerative procedures, with the only difference being a faster recovery when the arthroscopic approach was used. Finally, in 2011 Filardo et al35-37 confirmed the good results obtained with this scaffold up to seven years of follow-up, documenting overall good and stable results over time but a lower outcome in case of degenerative lesions.
Bioseed
Bioseed C (BioTissue Technologies GmbH, Freiburg, Germany) scaffold is composed of fibrin, polyglicolic/polilactic acid and polydioxanone. It is a tissue-engineered graft that combines autologous chondrocytes, embedded in fibrin, with a 2 mm thick porous gel-like matrix in a bioresorbable polymer scaffold, and has been applied in clinical practice since 2001.38 This biomaterial can be implanted by open or arthroscopic procedure and presents a particular type of fixation: after careful debridement of the defective cartilage to a rectangular shape, the graft is fitted to the size of the defect and a strong fixation is obtained by arming the corners with resorbable threads, anchored transosseously to each corner, thus ensuring secure fixation of the graft even in defects without intact surrounding cartilage. Ossendorf et al38 reported in 2007 clinical results at two years in a group of 40 patients affected by degenerative defects, and showed good integration of the graft, formation of a cartilaginous repair tissue and a significant clinical improvement even in the more challenging osteo-arthritic lesions. Kreuz et al39 then confirmed the good results obtained in 19 patients of the same group analysed at four years, and a further evaluation of 52 patients also showed clinical improvement and moderate-to-complete filling at the MRI in the majority of the patients, even if a persisting strength deficit was found in the treated knee.40
Finally, a similar significant improvement to that achieved with the original ACI periosteum-cover technique was found independently by Erggelet et al41 in a retrospective comparative study and by Zeifang et al42 in a randomised clinical trial.
More recently, among the many scaffolds developed, a few other biomaterials have been introduced into clinical practice with a minimum follow-up of two years.
Neocart
NeoCart (Histogenics Corporation, Waltham, Massachusetts) consists of a three-dimensional (3D) type I collagen scaffold seeded with autologous chondrocytes and then undergoing development in a bioreactor.43 The resulting product is a viable proteoglycan- and glycosaminoglycan-rich tissue-like implant, which is surgically fixed to the damaged area with CT3 bioadhesive (Histogenics). Crawford et al43 reported a good clinical outcome at two years of follow-up in eight patients, describing good implant integration, defect fill, as well as progressive maturation and more organised cartilage formation.
Novocart
Novocart 3D (B. Braun-Tetec, Reutlingen, Germany) comprises autologous chondrocytes embedded in a 3D collagen-chondroitin sulfate scaffold. Results were recently reported by Panagopoulos, van Niekerk and Triantafillopoulos,44 who evaluated a cohort of either professional soldiers or athletes with large defects after classic ACI with periosteal flap in 11 cases and Novocart 3D in eight cases. At a minimum of two years, despite the overall improvement, results obtained in this demanding cohort with complex lesions were poor, with only six patients (32%) returning to previous athletic performances. A trend toward better results for Novocart 3D was found in comparison with classic ACI, but without reaching statistical significance.
CaReS
CaReS(Ars Arthro, Esslingen, Germany) consists of autologous chondrocytes seeded on 3D type-I collagen gel. The cells are isolated, mixed with collagen, and after complete gelling and two weeks of culturing, the chondrocyte-loaded gel is available for transplantation. Welsch et al45 evaluated two bioregenerative approaches: ten patients underwent CaReS implantation and were compared with ten patients treated with Hyalograft C, matched according to lesion size, site and age of patients. Although the clinical outcome at two years was comparable between the two groups, MRI analysis revealed better surface of the repair tissue in the CaReS group.45 Wondrasch et al46 applied CaReS or Hyalograft C in 31 patients, documenting an overall significant improvement at two years. The patients were randomised into either an accelerated or delayed weight-bearing protocol, which demonstrated that early weight-bearing was associated with a higher prevalence of bone marrow oedema after six months, but with no effect on clinical outcome.46 More recently, Schneider et al47 published the results of a multicentre study in which a wide population of 116 patients was evaluated at a follow-up from 12 to 60 months: overall good results were reported, with a continuous improvement towards best results at the last follow-up, regardless of lesion size, site and number of defects, whereas a greater improvement was documented in the OCD group.
Cartipatch
Cartipatch (TBF Tissue Engineering, Mions, France) is an autologous chondrocyte implant on a vegetal hydrogel composed of agarose and alginate. This hydrogel is mixed with isolated autologous cell suspension and can be modulated at 37°C into complex-shaped implants that solidify at approximately 25°C. Alginate provides matrix elasticity, making it easy to handle. Selmi et al48 investigated the clinical, radiological, arthroscopic and histological outcome at a minimum follow-up of two years for the treatment of chondral and osteochondral defects. Clinically, all 17 patients improved markedly, especially those with lesions > 3 cm2. Good MRI findings, arthroscopic appearance and predominantly hyaline cartilage were found in eight of 13 biopsies performed (62%).
Chondron
Another gel-type autologous chondrocyte (Chondron; Sewon Cellontech Co. Ltd, Seoul, Korea) implantation has been used by Choi et al.49 This procedure involves the injection of cultured chondrocytes mixed with fibrin (1:1) into the defect area previously prepared with debridement and multiple holes. In a multicentre study they evaluated 40 patients with follow-up > two years, showing the safety and effectiveness of this method. Fibrin gel can provide a 3D scaffold with the advantages of technical simplicity and minimal invasiveness. Kim et al50 have also shown satisfactory results in 30 patients, with significant clinical improvements, good MRI findings and nearly normal arthroscopic appearance in most patients at two years.
Atelocollagen gel
Autologous chondrocytes cultured on atelocollagen gel have been also documented. Adachi et al51 first reported a complex case in which corticosteroid-induced osteonecrosis at both condyles of the knee was treated with hydroxyapatite with interconnected pores (IP-CHA) and atelocollagen gel (3% type I collagen; Koken, Tokyo, Japan) used as a scaffold for bone-marrow expanded cells and cultured chondrocytes, and to regenerate both osseous and chondral tissues, respectively. A synovial flap was sutured to cover the lesion and secure the osteochondral implants. Despite the unremarkable arthroscopic findings at a one-year follow-up, MRI and clinical results showed a successful outcome at two years. Tohyama et al52 conducted a multicentre study on 27 patients to determine the usefulness of the atelocollagen-associated chondrocyte implantation for the repair of chondral knee defects. The first-generation ACI periosteal flap technique was used to host and protect the chondrocyte-atelocollagen gel. Both clinical and arthroscopic outcomes were positive, with a marked improvement and 23 knees (92%) presented normal or nearly normal arthroscopic appearance.
Discussion
Research in bioengineering offers new technologies and new surgical treatment options for cartilage lesions. The use of 3D structures for cell growth has been shown to allow the maintenance of a chondrocyte differentiated phenotype70 and to overcome most of the biological and surgical concerns raised by the first-generation methods.13,14,71 Thus, the interest in this scaffold-based cartilage regenerative approach is constantly growing, as shown by the increasing number of publications every year that focus on this topic (Fig. 1).
The rationale of using a scaffold is to have a 3D biodegradable structure for the in vitro growth of living cells and their subsequent implantation. An ideal scaffold should mimic the biology, architecture and structural properties of the native tissue, thus facilitating cell infiltration, attachment, proliferation and differentiation. Other important properties include biocompatibility and biodegradability through safe biochemical pathways at suitable time intervals to support the first phases of tissue formation and then the gradual replacement by the regenerating tissue.
Following these principles, many scaffolds have been developed and, as reported in this systematic review, introduced in the clinical practice with promising results. As polymers can be designed to have a wide range of properties and are easily modified depending on the biological/surgical strategy, many more are being developed. Several other natural and synthetic scaffolds for cartilage regeneration are under investigation and will be available in clinical practice.13,14,71 In particular, hydrogels have recently been developed as an attractive evolution of cartilage tissue engineering. Another important source of innovation comes from photopolymerisation: liquid or gel scaffolds can be injected into the site of cartilage injury, thus requiring a less invasive procedure, and then polymerised by exposure to ultraviolet light. It is also possible to encapsulate cells within the gels, thus obtaining a scaffold with uniformly distributed cells, offering both surgical and biological potential advantages.48
MACT was introduced into clinical practice in Europe between 1998 and 1999 and a considerable number of clinical studies have been published. However, since introduction into clinical practice is recent, it is difficult to have a long-term follow-up, and most of the papers report case series; up to now only ten non-randomised and three randomised controlled studies have been published, and the few comparative studies available are not conclusive.
Moreover, in the United States the Food and Drug Administration has not yet approved MACT, but different alternative solutions are being developed, avoiding manipulation of cells and regulatory obstacles. In fact, there is an increasing awareness that the role of scaffolds is not only to deliver cells to enhance tissue regeneration, and the use of cell-free scaffolds has been proposed and is gaining popularity. Some scaffolds may have a potential themselves to promote chondral or osteochondral regeneration by exploiting the self-regenerative potential of the body.53,72 One-step cell-free approaches have been developed to avoid the problems related to the ex vivo chondrocyte culture and expansion in a scaffold, with marked advantages both from the surgical and economic points of view.
In fact, an ideal graft would be an off-the-shelf product. The possibility of a cell-free implant that is ‘smart’ enough to provide the joint with the appropriate stimuli to induce orderly and durable tissue regeneration is an attractive prospect, and new biomaterials and surgical strategies have been recently proposed to induce in situ cartilage regeneration after direct transplantation onto the defect site.
Finally, the increasing awareness on the role of the subchondral bone has led to the development of some new biphasic products: the bilayer structure allows the entire osteochondral unit to be treated, which is important in particular in cases of large chondral or osteochondral articular defects, reproducing the different biological and functional requirements for guiding the growth of both bone and cartilage tissues.53,73
Promising results have been reported with all of these procedures,27,53,74,75 but the properties of the healthy cartilage tissue are still unmatched by any available substitute. Moreover, despite the thousands of patients treated and the published studies suggesting good clinical results, at the present time there is no agreement about the effective superiority of the regenerative approach over the others, and both results and indications remain controversial. One explanation of the contradictory and inconclusive findings in the literature might be that regenerative procedures may lead to a hyaline-like tissue through a remodeling process, thus leading to superior clinical results only detectable at two to three years of follow-up.76 Unfortunately, due to the recent development of these techniques, only a few studies report medium to long-term results,13,14 and up to now only a few comparative trials have been performed. Thus, medium to long-term comparative studies are mandatory to confirm the positive findings reported and to determine the real potential of the bioengineered approach with respect to the more traditional and less ambitious procedures.
Conclusions
MACT procedures have been reported in the literature in the last decade with promising results, and the growing interest on this scaffold based regenerative approach is confirmed by the growing number of publications documented in this review. Different types of scaffolds have been applied in the clinical practice and shown a good outcome at short and medium-term follow-up, but well-designed studies are lacking. Systematic long-term evaluation of these techniques and randomised controlled studies are necessary to confirm the potential of this tissue-engineered approach, especially compared with the available traditional treatments.
1 Buckwalter JA , MankinHJ. Articular cartilage. Part I: tissue design and chondrocyte-matrix interactions. J Bone Joint Surg [Am]1997;79-A:600–611. Google Scholar
2 Buckwalter JA , MankinHJ. Articular cartilage. Part II: degeneration and osteoarthrosis, repair, regeneration, and tranplantation. J Bone Joint Surg [Am]1997;79-A:612–632. Google Scholar
3 Changoor A , NeleaM, MéthotS, et al.Structural characteristics of the collagen network in human normal, degraded and repair articular cartilages observed in polarized light and scanning electron microscopies. Osteoarthritis Cartilage2011;19:1458–1468.CrossrefPubMed Google Scholar
4 Nehrer S , SpectorM, MinasT. Hystologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res1999;365:149–162. Google Scholar
5 Kon E , GobbiA, FilardoG, et al.Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med2009;37:33–41.CrossrefPubMed Google Scholar
6 Marcacci M , KonE, DelcoglianoM, et al.Arthroscopic autologous osteochondral grafting for cartilage defects of the knee: prospective study results at a minimum 7-year follow-up. Am J Sports Med2007;35:2014–2021.CrossrefPubMed Google Scholar
7 Bugbee W , CavalloM, GianniniS. Osteochondral allograft transplantation in the knee. J Knee Surg2012;25:109–116.CrossrefPubMed Google Scholar
8 Demange M , GomollAH. The use of osteochondral allografts in the management of cartilage defects. Curr Rev Musculoskelet Med2012;53:229–235.CrossrefPubMed Google Scholar
9 Gross AE , ShashaN, AubinP. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res2005;435:79–87.CrossrefPubMed Google Scholar
10 Brittberg M , LindahlA, NilssonA, et al.Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med1994;331:889–895.CrossrefPubMed Google Scholar
11 Vasiliadis HS , DanielsonB, LjungbergM, et al.Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med2010;38:943–949.CrossrefPubMed Google Scholar
12 Peterson L , VasiliadisHS, BrittbergM, LindahlA. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med2010;38:1117–1124.CrossrefPubMed Google Scholar
13 Kon E , VerdonkP, CondelloV, et al.Matrix-assisted autologous chondrocyte transplantation for the repair of cartilage defects of the knee: systematic clinical data review and study quality analysis. Am J Sports Med2009;37(Suppl):156S–166S.CrossrefPubMed Google Scholar
14 Kon E , DelcoglianoM, FilardoG, MontapertoC, MarcacciM. Second generation issues in cartilage repair. Sports Med Arthrosc2008;16:221–229.CrossrefPubMed Google Scholar
15 Behrens P , BitterT, KurzB, RussliesM. Matrix-associated autologous chondrocyte transplantation/implantation (MATC/MACI): 5-year follow-up. Knee2006;13:194–202. Google Scholar
16 Ronga M , GrassiFA, ManelliA, BulgheroniP. Tissue engineering techniques for the treatment of a complex knee injury. Arthroscopy2006;22:576–571.CrossrefPubMed Google Scholar
17 Salzmann GM , PaulJ, BauerJS, et al.T2 assessment and clinical outcome following autologous matrix-assisted chondrocyte and osteochondral autograft transplantation. Osteoarthritis Cartilage2009;17:1576–1582.CrossrefPubMed Google Scholar
18 Gigante A , EneaD, GrecoF, et al.Distal realignment and patellar autologous chondrocyte implantation: mid-term results in a selected population. Knee Surg Sports Traumatol Arthrosc2009;17:2–10.CrossrefPubMed Google Scholar
19 Basad E , IshaqueB, BachmannG, StürzH, SteinmeyerJ. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc2010;18:519–527.CrossrefPubMed Google Scholar
20 Ebert JR , RobertsonWB, WoodhouseJ, et al.Clinical and magnetic resonance imaging-based outcomes to 5 years after matrix-induced autologous chondrocyte implantation to address articular cartilage defects in the knee. Am J Sports Med2011;39:753–763.CrossrefPubMed Google Scholar
21 Macmull S, Jaiswal PK, Bentley G, et al. The role of autologous chondrocyte implantation in the treatment of symptomatic chondromalacia patellae. Int Orthop 2012:Epub. Google Scholar
22 Bauer S, Khan RJ, Ebert JR, et al. Knee joint preservation with combined neutralising High Tibial Osteotomy (HTO) and Matrix-induced Autologous Chondrocyte Implantation (MACI) in younger patients with medial knee osteoarthritis: a case series with prospective clinical and MRI follow-up over 5years. Knee 2011:Epub. Google Scholar
23 Ventura A , MemeoA, BorgoE, et al.Repair of osteochondral lesions in the knee by chondrocyte implantation using the MACI technique. Knee Surg Sports Traumatol Arthrosc2012;20:121–126. Google Scholar
24 Marcacci M , BerrutoM, BrocchettaD, et al.Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res2005;435:96–105.CrossrefPubMed Google Scholar
25 Nehrer S , DomayerS, DorotkaR, et al.Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol2006;57:3–8.CrossrefPubMed Google Scholar
26 Gobbi A , KonE, BerrutoM, et al.Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, and histologic review. Am J Sports Med2006;34:1763–1773.CrossrefPubMed Google Scholar
27 Gobbi A , KonE, BerrutoM, et al.Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med2009;37:1083–1092. Google Scholar
28 Marcacci M , KonE, ZaffagniniS, et al.Arthroscopic second generation autologous chondrocyte implantation. Knee Surg Sports Traumatol Arthrosc2007;15:610–619.CrossrefPubMed Google Scholar
29 Nehrer S , DorotkaR, DomayerS, StelzenederD, KotzR. Treatment of full-thickness chondral defects with hyalograft C in the knee: a prospective clinical case series with 2 to 7 years’ follow-up. Am J Sports Med2009;37(Suppl 1):81S–87S. Google Scholar
30 Ferruzzi A , BudaR, FaldiniC, et al.Autologous chondrocyte implantation in the knee joint: open compared with arthroscopic technique: comparison at a minimum follow-up of five years. J Bone Joint Surg [Am]2008;90-A(Suppl 4):90–101. Google Scholar
31 Kon E , Di MartinoA, FilardoG, et al.Second-generation autologous chondrocyte transplantation: MRI findings and clinical correlations at a minimum 5-year follow-up. Eur J Radiol2011;79:382–388.CrossrefPubMed Google Scholar
32 Kon E , FilardoG, BerrutoM, et al.Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am J Sports Med2011;39:2549–2557.CrossrefPubMed Google Scholar
33 Della Villa S , KonE, FilardoG, et al.Does intensive rehabilitation permit early return to sport without compromising the clinical outcome after arthroscopic autologous chondrocyte implantation in highly competitive athletes?Am J Sports Med2010;38:68–77.CrossrefPubMed Google Scholar
34 Clar H , PascherA, KastnerN, et al.Matrix-assisted autologous chondrocyte implantation into a 14cm(2) cartilage defect, caused by steroid-induced osteonecrosis. Knee2010;17:255–257.CrossrefPubMed Google Scholar
35 Filardo G, Kon E, Berruto M, et al. Arthroscopic second generation autologous chondrocytes implantation associated with bone grafting for the treatment of knee osteochondritis dissecans: results at 6 years. Knee 2011:Epub. Google Scholar
36 Filardo G, Kon E, Di Martino A, et al. Second-generation arthroscopic autologous chondrocyte implantation for the treatment of degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc 2011:Epub. Google Scholar
37 Filardo G , KonE, Di MartinoA, IaconoF, MarcacciM. Arthroscopic second-generation autologous chondrocyte implantation: a prospective 7-year follow-up study. Am J Sports Med2011;39:2153–2160.CrossrefPubMed Google Scholar
38 Ossendorf C , KapsC, KreuzPC, et al.Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther2007;9:R41.CrossrefPubMed Google Scholar
39 Kreuz PC , MüllerS, OssendorfC, KapsC, ErggeletC. Treatment of focal degenerative cartilage defects with polymer-based autologous chondrocyte grafts: four year clinical results. Arthritis Res Ther2009;11:R33. Google Scholar
40 Kreuz PC , MüllerS, FreymannU, et al.Repair of focal cartilage defects with scaffold-assisted autologous chondrocyte grafts: clinical and biomechanical results 48 months after transplantation. Am J Sports Med2011;39:1697–1705.CrossrefPubMed Google Scholar
41 Erggelet C , KreuzPC, MrosekEH, et al.Autologous chondrocyte implantation versus ACI using 3D-bioresorbable graft for the treatment of large full-thickness cartilage lesions of the knee. Arch Orthop Trauma Surg2010;130:957–964.CrossrefPubMed Google Scholar
42 Zeifang F , OberleD, NierhoffC, et al.Autologous chondrocyte implantation using the original periosteum-cover technique versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med2010;38:924–933.CrossrefPubMed Google Scholar
43 Crawford DC , HeveranCM, CannonWD Jr, FooLF, PotterHG. An autologous cartilage tissue implant NeoCart for treatment of grade III chondral injury to the distal femur: prospective clinical safety trial at 2 years. Am J Sports Med2009;37:1334–1343.CrossrefPubMed Google Scholar
44 Panagopoulos A , van NiekerkL, TriantafillopoulosI. Autologous chondrocyte implantation for knee cartilage injuries: moderate functional outcome and performance in patients with high-impact activities. Orthopedics2012;35:6–14.CrossrefPubMed Google Scholar
45 Welsch GH , MamischTC, ZakL, et al.Evaluation of cartilage repair tissue after matrix-associated autologous chondrocyte transplantation using a hyaluronic-based or a collagen-based scaffold with morphological MOCART scoring and biochemical T2 mapping: preliminary results. Am J Sports Med2010;38:934–942.CrossrefPubMed Google Scholar
46 Wondrasch B , ZakL, WelschGH, MarlovitsS. Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle on radiographic and clinical outcome after 2 years: a prospective, randomized controlled pilot study. Am J Sports Med2009;37(Suppl 1):88S–96S.CrossrefPubMed Google Scholar
47 Schneider U , RackwitzL, AndereyaS, et al.A prospective multicenter study on the outcome of type I collagen hydrogel-based autologous chondrocyte implantation (CaReS) for the repair of articular cartilage defects in the knee. Am J Sports Med2011;39:2558–2565.CrossrefPubMed Google Scholar
48 Selmi TA , VerdonkP, ChambatP, et al.Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg [Br]2008;90-B:597–604.CrossrefPubMed Google Scholar
49 Choi NY , KimBW, YeoWJ, et al.Gel-type autologous chondrocyte (Chondron) implantation for treatment of articular cartilage defects of the knee. BMC Musculoskelet Disord2010;11:103.CrossrefPubMed Google Scholar
50 Kim MK , ChoiSW, KimSR, OhIS, WonMH. Autologous chondrocyte implantation in the knee using fibrin. Knee Surg Sports Traumatol Arthrosc2010;18:528–534.CrossrefPubMed Google Scholar
51 Adachi N , OchiM, DeieM, IshikawaM, ItoY. Osteonecrosis of the knee treated with a tissue-engineered cartilage and bone implant: a case report. J Bone Joint Surg [Am]2007;89-A:2752–2757. Google Scholar
52 Tohyama H , YasudaK, MinamiA, et al.Atelocollagen-associated autologous chondrocyte implantation for the repair of chondral defects of the knee: a prospective multicenter clinical trial in Japan. J Orthop Sci2009;14:579–588.CrossrefPubMed Google Scholar
53 Kon E , DelcoglianoM, FilardoG, et al.Novel nano-composite multilayered biomaterial for osteochondral regeneration: a pilot clinical trial. Am J Sports Med2011;39:1180–1190.CrossrefPubMed Google Scholar
54 Marcacci M , KonE, ZaffagniniS, et al.New cell-based technologies in bone and cartilage tissue engineering. II. Cartilage regeneration. Chir Organi Mov2003;88:42–47.PubMed Google Scholar
55 Cherubino P , GrassiFA, BulgheroniP, RongaM. Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg (Hong Kong)2003;11:10–15.CrossrefPubMed Google Scholar
56 Pavesio A , AbatangeloG, BorrioneA, et al.Hyaluronan-based scaffolds (Hyalograft C) in the treatment of knee cartilage defects: preliminary clinical findings. Novartis Found Symp2003;249:203–217.PubMed Google Scholar
57 Ronga M , GrassiFA, BulgheroniP. Arthroscopic autologous chondrocyte implantation for the treatment of a chondral defect in the tibial plateau of the knee. Arthroscopy2004;20:79–84.CrossrefPubMed Google Scholar
58 Marlovits S , StriessnigG, Kutscha-LissbergF, et al.Early postoperative adherence of matrix-induced autologous chondrocyte implantation for the treatment of full-thickness cartilage defects of the femoral condyle. Knee Surg Sports Traumatol Arthrosc2005;13:451–457.CrossrefPubMed Google Scholar
59 Bartlett W , GoodingCR, CarringtonRW, et al.Autologous chondrocyte implantation at the knee using a bilayer collagen membrane with bone graft: a preliminary report. J Bone Joint Surg [Br]2005;87-B:330–332. Google Scholar
60 Bartlett W , SkinnerJA, GoodingCR, et al.Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg [Br]2005;87-B:640–645.CrossrefPubMed Google Scholar
61 Manfredini M , ZerbinatiF, GildoneA, FacciniR. Autologous chondrocyte implantation: a comparison between an open periosteal-covered and an arthroscopic matrix-guided technique. Acta Orthop Belg2007;73:207–218.PubMed Google Scholar
62 Ebert JR , RobertsonWB, LloydDG, et al.Traditional vs accelerated approaches to post-operative rehabilitation following matrix-induced autologous chondrocyte implantation (MACI): comparison of clinical, biomechanical and radiographic outcomes. Osteoarthritis Cartilage2008;16:1131–1140.CrossrefPubMed Google Scholar
63 Vilchez F , LaraJ, Alvarez-LozanoE, et al.Knee chondral lesions treated with autologous chondrocyte transplantation in a tridimensional matrix: clinical evaluation at 1-year follow-up. J Orthop Traumatol2009;10:173–177.CrossrefPubMed Google Scholar
64 Macmull S , ParrattMT, BentleyG, et al.Autologous chondrocyte implantation in the adolescent knee. Am J Sports Med2011;39:1723–1730.CrossrefPubMed Google Scholar
65 Enea D , CecconiS, BusilacchiA, et al.Matrix-induced autologous chondrocyte implantation (MACI) in the knee. Knee Surg Sports Traumatol Arthrosc2012;20:862–869.CrossrefPubMed Google Scholar
66 Könst YE , BeninkRJ, VeldstraR, et al.Treatment of severe osteochondral defects of the knee by combined autologous bone grafting and autologous chondrocyte implantation using fibrin gel. Knee Surg Sports Traumatol Arthrosc2012;20:2263–2269.CrossrefPubMed Google Scholar
67 Behrens P, Ehlers EM, Köchermann KU, et al. New therapy procedure for localized cartilage defects: encouraging results with autologous chondrocyte implantation. MMW Fortschr Med 1999;141:49–51 (in German). Google Scholar
68 Marcacci M , ZaffagniniS, KonE, et al.Arthroscopic autologous chondrocyte transplantation: technical note. Knee Surg Sports Traumatol Arthrosc2002;10:154–159.CrossrefPubMed Google Scholar
69 Kon E , FilardoG, CondelloV, et al.Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am J Sports Med2011;39:1668–1675.CrossrefPubMed Google Scholar
70 Grigolo B , LisignoliG, PiacentiniA, et al.Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAff 11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials2002;23:1187–1195.CrossrefPubMed Google Scholar
71 Sharma B , ElisseeffJH. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng2004;32:148–159.CrossrefPubMed Google Scholar
72 Gille J , SchuseilE, WimmerJ, et al.Mid-term results of Autologous Matrix-Induced Chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc2010;18:1456–1464.CrossrefPubMed Google Scholar
73 Carmont MR , Carey-SmithR, SaithnaA, et al.Delayed incorporation of a TruFit plug: perseverance is recommended. Arthroscopy2009;25:810–814.CrossrefPubMed Google Scholar
74 Giannini S , BudaR, VanniniF, CavalloM, GrigoloB. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res2009;467:3307–3320.CrossrefPubMed Google Scholar
75 Kon E , DelcoglianoM, FilardoG, et al.A novel nano-composite multi-layered biomaterial for treatment of osteochondral lesions: technique note and an early stability pilot clinical trial. Injury2010;41:693–701.CrossrefPubMed Google Scholar
76 Saris DB , VanlauweJ, VictorJ, et al.Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med2009;37:10S–19S.CrossrefPubMed Google Scholar
Funding statement:
This research has been financed by the FIRB project: The efficacy of a new scaffold for osteochondral regeneration.
Author contributions:
E. Kon: Writing the manuscript
G. Filardo: Writing the manuscript
B. Di Matteo: Literature research
F. Perdisa: Literature research
M. Marcacci: Senior consultant and editor of text
ICMJE Conflict of Interest:
E. Kon is a consultant for CartiHeal Ltd, Tel Aviv, Israel.
©2013 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










