Abstract
Objectives
Local corticosteroid infiltration is a common practice of treatment for lateral epicondylitis. In recent studies no statistically significant or clinically relevant results in favour of corticosteroid injections were found. The injection of autologous blood has been reported to be effective for both intermediate and long-term outcomes. It is hypothesised that blood contains growth factors, which induce the healing cascade.
Methods
A total of 60 patients were included in this prospective randomised study: 30 patients received 2 ml autologous blood drawn from contralateral upper limb vein + 1 ml 0.5% bupivacaine, and 30 patients received 2 ml local corticosteroid + 1 ml 0.5% bupivacaine at the lateral epicondyle. Outcome was measured using a pain score and Nirschl staging of lateral epicondylitis. Follow-up was continued for total of six months, with assessment at one week, four weeks, 12 weeks and six months.
Results
The corticosteroid injection group showed a statistically significant decrease in pain compared with autologous blood injection group in both visual analogue scale (VAS) and Nirschl stage at one week (both p < 0.001) and at four weeks (p = 0.002 and p = 0.018, respectively). At the 12-week and six-month follow-up, autologous blood injection group showed statistically significant decrease in pain compared with corticosteroid injection group (12 weeks: VAS p = 0.013 and Nirschl stage p = 0.018; six months: VAS p = 0.006 and Nirschl p = 0.006). At the six-month final follow-up, a total of 14 patients (47%) in the corticosteroid injection group and 27 patients (90%) in autologous blood injection group were completely relieved of pain.
Conclusions
Autologous blood injection is efficient compared with corticosteroid injection, with less side-effects and minimum recurrence rate.
Article focus
The injection of autologous blood is compared with the standard practice of local corticosteroid injection for the treatment of lateral epicondylitis
Outcomes are assessed in terms of decrease in pain, rates of recurrence and complication
Key messages
This study offers encouraging results of an alternative treatment that addresses the pathophysiology of lateral epicondylitis that has been resistant to traditional conservative modalities
Strengths and limitations
This is a randomised study where a new modality of treatment is evaluated by comparing it with a commonly practiced modality of treatment
There are only a few studies of this kind
Larger studies, with longer periods of follow-up, and potentially including multiple injections over a period of time, are required
Introduction
Lateral epicondylitis, or tennis elbow, is commonly encountered in orthopaedic practice, being the second most frequently diagnosed musculoskeletal disorder in the neck and upper extremity in a primary care setting.1 It has an incidence of between four and seven per 1000 cases per year in general practice, with a peak between the ages of 35 and 54 years, and a mean age of approximately 42 years.2-5 An epidemiological study reported that 87% of cases involved the dominant arm.6
The characteristic clinical findings are pain and tenderness over the lateral epicondyle. Lateral epicondylitis has been reported to be the result of overuse from many activities. Although it is often referred to as tennis elbow, it is seen to affect non-athletes rather than athletes.7,8
The pathophysiology of the condition is a matter of controversy, and there is not enough scientific evidence to favour any particular type of treatment for acute lateral epicondylitis.9,10 Most current research has proposed degeneration of the origin of the extensor carpi radialis brevis and repeated microtrauma and incomplete healing response (tendinosis) as the cause of lateral epicondylitis.10-15 The constellation of findings has been termed angiofibroblastic hyperplasia.16
Most conservative modalities such as local injection of corticosteroid have focused on suppressing an inflammatory process that does not actually exist. It is theorised that the beneficial effects of the steroid injection result from the bleeding caused by forcing fluid through tissue planes at high pressures.17
Recently an injection of autologous blood has been reported to be effective for both intermediate and long-term outcomes for the treatment of lateral epicondylitis, with a significant decrease in pain.10,15,18 Chemical modifiers of cellular activity carried in the blood and are known to be mitomorphogenic.16 Injection of autologous blood might provide the necessary cellular and humeral mediators to induce a healing cascade.10,19-21
There are very few studies that have evaluated the injection of autologous blood for lateral epicondylitis as a treatment modality. The objective of this study was to evaluate the efficacy and role of autologous blood injection for the treatment of lateral epicondylitis, compared with the commonly used local injection of corticosteroid.22-25
Patients and Methods
This is a randomised control trial as a pilot study.
Our study population has a high proportion of people involved in manual labour. There is no current published data on the prevalence of lateral epicondylitis in the region where the study was conducted. Age > 15 years and a diagnosis of lateral epicondylitis were the inclusion criteria. The exclusion criteria were: 1) patients receiving steroid injections in the three months prior to study treatment; 2) history of substantial trauma; 3) previous surgery for lateral epicondylitis; 4) presence of other causes of elbow pain such as osteochondritis dessicans of capitellum, epiphyseal plate injuries, lateral compartment arthosis, varus instability, radial head arthritis, posterior interosseous nerve syndrome, cervical disc syndrome, synovitis of radiohumeral joint, cervical radiculopathy, fibromyalgia, osteoarthritis of elbow, or carpal tunnel syndrome. Each patient was assessed by history and clinical examination. In some cases radiological and imaging investigations were carried out to confirm the diagnosis and to identify any exclusion criteria.
Patients attending outpatient department were included after a diagnosis of lateral epicondylitis was established. This included interview and clinical examination comprising testing for tenderness over the lateral epicondyle or just distal to it, a positive Cozen’s test and Mill’s maneouvre.26 In 12 cases diagnosis was confirmed with radiography and in two cases by MRI, after radiographs were inconclusive. Patients were allotted sequentially into two parallel groups, A and B, of 30 cases each. Equal randomisation (1:1 allocation ratio) was undertaken according to a computer-generated randomisation table.
Procedure
Group A was designated to receive an injection of autologous blood. Patients were infiltrated with injection of 2 ml autologous blood drawn from the contralateral upper limb vein mixed with 1 ml 0.5% bupivacaine, at the lateral epicondyle according to the technique described below.10 Group B was designated to receive an injection of local cortiocosteroid. Patients were infiltrated with 2 ml of local corticosteroid (methyl prednisolone acetate 80 mg) mixed with 1 ml 0.5% bupivacaine, at the lateral epicondyle according to the same technique.
Briefly, the technique is as follows (Fig. 1).10 With the patient in supine or sitting posture the elbow is flexed to 90° with the palm facing down. The anatomical bony landmarks were identified. Under aseptic precautions the needle is introduced proximal to the lateral epicondyle along the supracondylar ridge, and gently advanced in to the undersurface of the extensor carpi radialis brevis while infiltrating. A small adhesive sterile dressing is applied. Patients are advised to rest the upper limb for three days, with no restriction of activity after that.
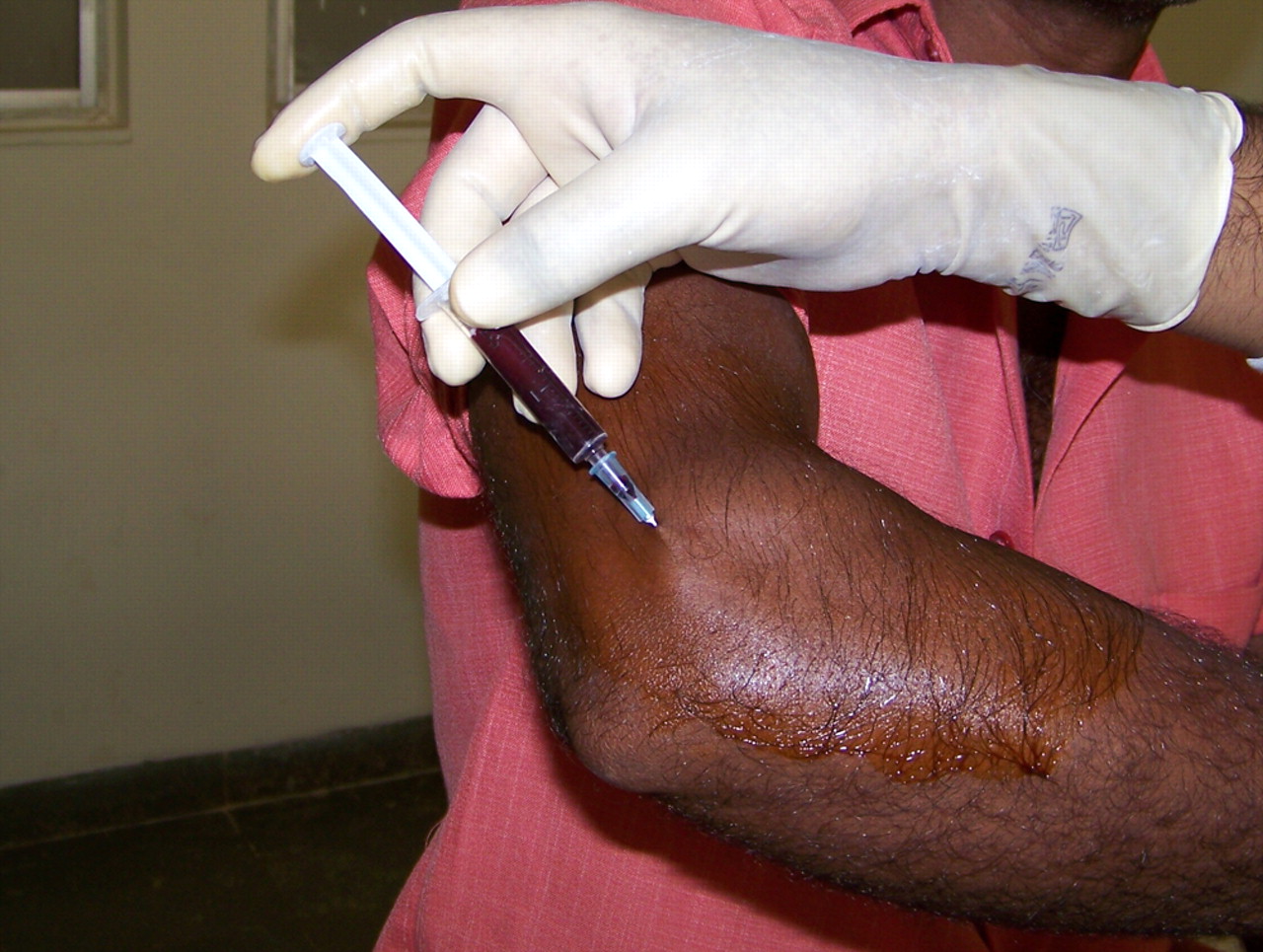
Fig. 1
Clinical photograph showing the injection of autologous blood at the lateral epicondyle.
Outcome evaluation
Outcome in terms of pain relief was assessed using a visual analogue scale (VAS) and the Nirschl staging system.10,27
The VAS comprised a 10 cm line marked at one end with ‘no pain’ and at other end with ‘worst pain ever’. The participant is asked to indicate where on the line he or she rates the pain on the day of presentation.
The Nirschl staging system consists of seven phases in ascending order of severity of pain (Table I). It ranges from phase 1 (mild pain with exercise, resolves within 24 hours) to phase 7 (constant pain at rest, disrupts sleeps).
Table I
Details of the Nirschl staging system
| Phase | Description |
|---|---|
| 1 | Mild pain with exercise, resolves within 24 hours |
| 2 | Pain after exercise, exceeds 48 hours |
| 3 | Pain with exercise, does not alter activity |
| 4 | Pain with exercise, alters activity |
| 5 | Pain with heavy activities of daily living |
| 6 | Pain with light activities of daily living, intermittent pain at rest |
| 7 | Constant pain at rest, disrupts sleeps |
Both the VAS and the Nirschl stage were assessed by visit to the clinic pre-injection, and at one week, four weeks, 12 weeks and at the six-month final follow-up.
Statistical analysis
The Mann-Whitney U test (non-parametric test) was used to compare outcome regarding pain between the two groups. The chi-squared test was used to compare categorical variables between the groups. A p-value < 0.05 was considered to indicate statistical significance.
Results
Group A comprised 13 males and 17 female patients with a mean age of 42.9 years (22 to 67), and group B comprised 12 males and 18 females with a mean age of 42.2 years (17 to 62). The characteristics of both groups are shown in Table II. It was noted that 20 patients (33%) had diabetes mellitus.
Table II
Characteristics of group A (autologous blood injection) and group B (corticosteroid injection)
| Characteristic | Group A (n = 30) | Group B (n = 30) | p-value |
|---|---|---|---|
| Male:female | 13:17 | 12:18 | 1 |
| Mean age (yrs) (range) | 42.9 (22 to 67) | 42.2 (17 to 62) | 0.828 |
| Side (right:left) | 23:7 | 23:7 | 1 |
| Dominant side (n, %) | 25 (83) | 26 (87) | 1 |
| Mean duration of symptoms (weeks) (range) | 9.5 (2 to 54) | 7.7 (1 to 36) | 0.828 |
| Employment (n, %) | |||
| Manual | 13 (43) | 9 (30) | 0.3 |
| Non-manual | 17 (57) | 21 (70) | 0.3 |
The severity of pain was measured pre-injection and after one, four and 12 weeks, and at six months by the VAS for pain and Nirschl staging. Pre-injection, the mean VAS scores for pain were similar between group A and group B (7.7 (sd 1.3) versus 7.5 (sd 1.3), p = 0.5395), as were the mean Nirschl stages (5.4 (sd 1.1) versus 5.2 (sd 1.0), p = 0.4918) (Tables III and IV).
Table III
Mean visual analogue scale (VAS) for pain for group A (autologous blood injection) and group B (corticosteroid injection)
| Mean (sd) VAS | |||
|---|---|---|---|
| Follow-up | Group A (autologous blood) | Group B (corticosteroid) | p-value |
| Pre-injection | 7.7 (1.3) | 7.5 (1.3) | 0.5395 |
| 1 week | 7.2 (1.9) | 4.5 (1.9) | < 0.0001 |
| 4 weeks | 3.2 (2.4) | 1.5 (2.3) | 0.0022 |
| 12 weeks | 0.6 (1.9) | 1.5 (1.8) | 0.0127 |
| 6 months | 0.5 (1.9) | 1.8 (2.0) | 0.0058 |
Table IV
Mean Nirschl staging for group A (autologous blood injection) and group B (corticosteroid injection)
| Mean (sd) Nirschl stage | |||
|---|---|---|---|
| Follow-up | Group A (autologous blood) | Group B (corticosteroid) | p-value |
| Pre-injection | 5.4 (1.1) | 5.2 (1.0) | 0.4918 |
| 1 week | 5.1 (1.5) | 3.1 (1.4) | < 0.0001 |
| 4 weeks | 2.2 (1.6) | 1.0 (1.6) | 0.003 |
| 12 weeks | 0.43 (1.3) | 1.0 (1.3) | 0.0184 |
| 6 months | 0.36 (1.3) | 1.2 (1.4) | 0.0064 |
The results of the VAS for pain and Nirschl grades followed a remarkably similar course over the period of follow-up, as can be seen from Figure 2 and Figure 3, respectively. Initially the patients in group A reported a much smaller effect in terms of pain resolution, with group B reporting significantly lower VAS and lower Nirschl scores at one week (both p < 0.0001) and again at four weeks (p = 0.0022 and p = 0.003, respectively) (Tables III and IV, Figs 2 and 3).
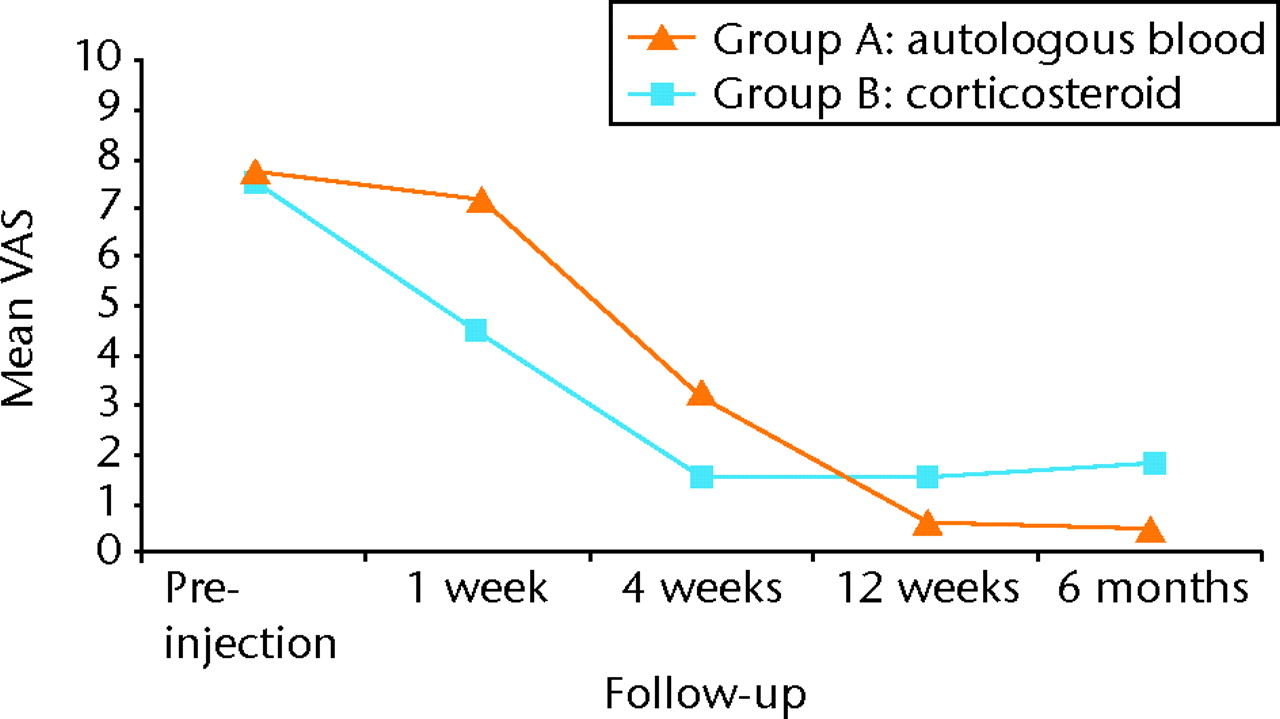
Fig. 2
Graph showing the mean visual analogue scale (VAS) of pain in both groups before treatment and at one week, four weeks, 12 weeks and six months post-injection.
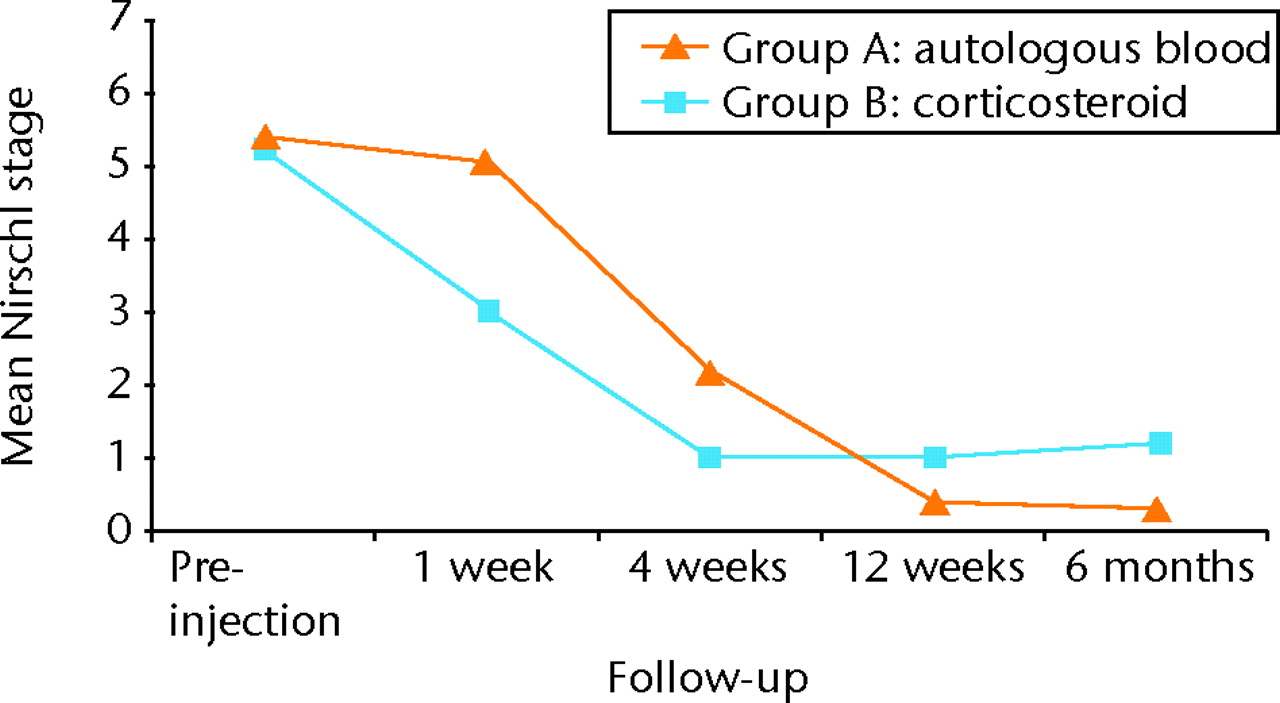
Fig. 3
Graph showing the mean Nirschl stage in both groups before treatment and at one week, four weeks, 12 weeks and six months post-injection.
However, at 12 weeks, the scores for group B had slowed, and the VAS and Nirschl scores were significantly lower in group A (p = 0.0127 and p = 0.0184, respectively). This difference was maintained at the final six-month follow-up, at which point the pain scores for group B had begun to rise compared with the four- and 12-week scores (Tables III and IV, Figs 2 and 3). At six months after injection, the pain scores were significantly lower in group A compared with group B (VAS: 0.5 (sd 1.9) versus 1.8 (sd 2.0), p = 0.0058; Nirschl grade: 0.36 (sd 1.3) versus 1.2 (sd 1.4), p = 0.0064).
At the six-month follow-up, a total of 27 patients (90%) in group A were completely relieved of pain, compared with 14 patients (47%) in group B (p < 0.001, chi-squared test). However, at the four-week assessment, 19 patients (63%) in group B had complete relief of pain, and many of these patients reported recurrences at 12 weeks and at six months, resulting in a rate of recurrence in this group of 36.8%. In comparison, only five patients (17%) in group A were pain-free at four weeks, but there was a no recurrence by six months, which reached statistical significance (p < 0.001, chi-squared test).
In group A, 18 patients (60%) complained of an increase in pain immediately (and during the following few days) after the injection, compared with eight (26%) in group B (p = 0.009). Only two patients (6.6%) had local skin atrophy in group B while no patient in group A had this problem, but this did not reach statistical significance (p = 0.150, chi-squared test); demonstrating that the local steroid infiltration done with proper care minimises this complication. No patients reported elbow stiffness, infection, reflex sympathetic dystrophy, post-injection flare, facial flushing, neurovascular damage or tendon rupture or other untoward complications.
Discussion
The mean age of the patients included in our study was 42.6 years (17 to 67), with a peak incidence in the fourth decade. A study by Hamilton2 included a population with age ranging between 14 and 78 years with a mean age of 45 years. Other studies have reported mean ages of approximately 42 years.3-5
There has been much controversy over the pathophysiology of this disorder.28,29 The most widely held theory proposes macro- or microscopic tears in the common tendon, coupled with an incomplete healing response.30-32
The origin of the extensor carpi radialis brevis is the primary site of this injury, and pathological changes have been consistently documented at this location.12,31,33-35 Histopathological studies have demonstrated that tennis elbow is not an inflammatory condition; rather, it is a fibroblastic and vascular response called angiofibroblastic degeneration, now more commonly known as tendinosis.27,36 Thus, the terms epicondylitis and tendinitis are misnomers.7,14,28,33
Assendelft et al,37 in their 1996 systematic review, compared the validity and outcome of randomised controlled trials of corticosteroid injections for lateral epicondylitis. Pooled analysis indicated short-term effectiveness only (two to six weeks). At follow-up > six weeks, no difference was found between corticosteroid injection and other treatments, including placebo. No conclusions could be made about the most suitable corticosteroid, dose, injection interval or volume.37
Bisset et al38 concluded that physiotherapy combining elbow manipulation and exercise has a superior benefit to ‘wait-and-see’ in the first six weeks and to corticosteroid injections after six weeks. The significant short-term benefits of corticosteroid injection are paradoxically reversed after six weeks, with high recurrence rates, implying that this treatment should be used with caution in the management of tennis elbow.38 These are comparable to our results, which by 12 weeks were showing a slight reversal of the early pain relief in the corticosteroid group, with high rates of recurrence.
Studies on animal models have shown that intratendinous corticosteroid adversely affect the biomechanical properties of tendons.39-42 Corticosteroid injection has also been associated with side-effects such as sepsis, tendon rupture, post-injection pain, local skin atrophy, facial flushing, post-injection flare, hyperglycaemia and hypersensitivity reactions.8,24,30,42-44
Resuscitation facilities should be available in case patients have a rare severe reaction.
Edwards and Calandruccio,10 investigating autologous blood injections in 28 patients in whom conservative therapy had failed to resolve symptoms of lateral epicondylitis, found that 22 patients (79%) had a reduction in pain over 9.5 months post-injection. All patients maintained their maximal benefit throughout the course of their follow-up evaluation, with no recurrence.10
There are very few studies in the literature comparing the efficacy of injection of autologous blood with injection of local corticosteroid for lateral epicondylitis.45,46 This is a pilot study to evaluate the efficacy and role of single autologous blood injection with single local corticosteroid injection for treatment of lateral epicondylitis.
There are studies with more than one injection of autologous blood and local corticosteroid in patients who had suboptimal relief of symptoms after the initial injection.10,18,22,37,38 The optimal interval between autologous blood injections is unknown, but postulated to be six weeks, with up to three injections. The time required to reach maximal benefit after the repeat injection was on average shorter (one to two weeks) than after the initial injection – possibly because the healing cascade was already underway.10,18 However, in this study the role of only one injection of autologous blood or corticosteroid was used, as the follow-up was limited to six months.
In our study group B showed a statistically significant decrease in VAS score and Nirschl stage at one and four weeks compared with group A. Hay et al30 found similar results when local corticosteroid injection was compared with oral naproxen.
At six months, we found that significantly more of group A had complete relief of pain compared with group B (90% versus 47%, p < 0.001). Edwards and Calandruccio10 found that 22 of 28 patients (79%) were relieved completely of pain with autologous blood injections, with the mean Nirschl stage decreasing from 6.5 to 2.0 at a mean of 9.5 months.
Despite good early results, with a rate of complete pain relief of 63% at four weeks, we found the corticosteroid group more likely to experience recurrence of pain, with a rate of recurrence of 37% by final follow-up at six months. Bisset et al38 described 72% recurrence after three to six weeks on longer follow-up.
In conclusion, autologous blood injection demonstrated statistically significant lower pain compared with corticosteroid injection group at long-term follow-up (six months), with 90% of patients in this group having complete relief of pain. Corticosteroid injection group showed early decrease in pain compared to autologous blood injection group but the short-term benefits of corticosteroid injection were followed by high rates of recurrence (Figs 2 and 3).
This study offers encouraging results of an alternative treatment that addresses the pathophysiology of lateral epicondylitis, which has failed to resolve with traditional nonsurgical modalities. We feel with larger control trials with one or more than one injection and with a longer follow-up period, a fair conclusion can be drawn with regard to the efficacy and otherwise of this treatment modality.
1 Haahr JP , AndersenJH. Physical and psychosocial risk factors for lateral epicondylitis: a population based case-referent study. Occup Environ Med2003;60:322–329.CrossrefPubMed Google Scholar
2 Hamilton PG . The prevalence of humeral epicondylitis: a survey in general practice. J R Coll Gen Pract1986;36:464–465.PubMed Google Scholar
3 Verhaar JAN. Tennis elbow [thesis]. Maastricht: Maastricht University Press, 1992. Google Scholar
4 Gruchow HW , PelletierD. An epidemiologic study of tennis elbow: incidence, recurrence, and effectiveness of prevention strategies. Am J Sports Med1979;7:234–238. Google Scholar
5 Nirschl RP . Tennis elbow. Prim Care1977;4:367–382.CrossrefPubMed Google Scholar
6 Bernhang AM . The many causes of tennis elbow. N Y State J Med1979;79:1363-6.PubMed Google Scholar
7 Teitz CC , GarrettWE, MiniaciA, LeeMH, MannRA. Instuctional Course Lectures. The American Academy of Orthopaedic Surgeons: tendon problem in athletic individuals. J Bone Joint Surg [Am]1997;79-A:138–152. Google Scholar
8 Terry CS, Beaty JH. Shoulder and elbow injuries. In: Terry CS, ed. Campbell’s operative orthopaedics. 11th ed. Vol. 3. Mosby: Elsevier, 2008:2634–2638. Google Scholar
9 Bowen RE , DoreyFJ, ShapiroMS. Efficacy of nonoperative treatment for lateral epicondylitis. Am J Orthop (Belle Mead NJ)2001;30:642–646.PubMed Google Scholar
10 Edwards SG , CalandruccioJH. Autologous blood injections for refractory lateral epicondylitis. J Hand Surg Am2003;28-A:272–278.CrossrefPubMed Google Scholar
11 Leadbetter WB . Cell-matrix response in tendon injury. Clin Sports Med1992;11:533–578.PubMed Google Scholar
12 Regan W , WoldLE, CoonradR, MorreyBF. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med1992;20:746–749.CrossrefPubMed Google Scholar
13 Józsa LG, Kannus P. Overuse injuries of tendons. In: Human tendons: anatomy, physiology and pathology. Champaign: Human Kinetics, 1997:164–253. Google Scholar
14 Kraushaar BS , NirschlRP. Tendinosis of the elbow (tennis elbow): clinical features and findings of histological, immunohistochemical, and electron microscopy studies. J Bone Joint Surg [Am]1999;81-A:259–278. Google Scholar
15 Taylor MA , NormanTL, ClovisNB, BlahaJD. The response of rabbit patellar tendons after autologous blood injection. Med Sci Sports Exerc2002;34:70–73.CrossrefPubMed Google Scholar
16 Iwasaki M , NakaharaH, NakataK, et al.Regulation of proliferation and osteochondrogenic differentiation of periosteum-derived cells by transforming growth factor-B and basic fibroblast growth factor. J Bone Joint Surg [Am]1995;77-A:543–554. Google Scholar
17 Balasubramaniam P , PrathapK. The effect of injection of hydrocortisone into rabbit calcaneal tendons. J Bone Joint Surg [Br]1972;54-B:729–734.PubMed Google Scholar
18 Connell DA , AliKE, AhmadM, et al.Ultrasound guided autologous blood injection for tennis elbow. Skeletal Radiol2006;35:371–377. Google Scholar
19 Anitua E , AndíaI, SanchezM, et al.Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J Orthop Res2005;23:281–286.CrossrefPubMed Google Scholar
20 Wadsworth TG . Lateral epicondylitis (tennis elbow). Lancet1972;1:959–960. Google Scholar
21 Baumgard SH , SchwartzDR. Percutaneous release of the epicondylar muscles for humeral epicondylitis. Am J Sports Med1982;10:233–236.CrossrefPubMed Google Scholar
22 Smidt N , van der WindtDA, AssendelftWJ, et al.Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet2002;359:657–662.CrossrefPubMed Google Scholar
23 Nirschl RF , SobelJ. Conservative treatment of tennis elbow. Phys Sports Med1981;9:43–54.CrossrefPubMed Google Scholar
24 Baily RA , BrockBH. Hydrocortisone in tennis elbow: a controlled series. Proc R Soc Med1957;50:389–390. Google Scholar
25 Leadbetter WB. Corticosteroid injection therapy in sports injuries. In: Leadbetter WB, Buckwalter JA, Gordon SL, eds. Sports-induced inflammation. Rosemont: American Academy of Orthopaedic Surgeons, 1990:527–545. Google Scholar
26 Zouzias IC , ByramIR, ShillingfordJN, LevineWN. A primer for physical examination of the elbow. Phys Sports Med2012;40:51–61.CrossrefPubMed Google Scholar
27 Nirschl RP . Elbow tendinosis/tennis elbow. Clin Sports Med1992;11:851–870.PubMed Google Scholar
28 Labelle H , GuibertR, JoncasJ, et al.Lack of scientific evidence for the treatment of lateral epicondylitis of the elbow: an attempted meta-analysis. J Bone Joint Surg [Br]1992;74-B:646–651. Google Scholar
29 Boyer MI , HastingsH. Lateral tennis elbow: “is there any science out there?”. J Shoulder Elbow Surg1999;8:481–491. Google Scholar
30 Hay EM , PatersonSM, LewisM, HosieG, CroftP. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ1999;319:964–968.CrossrefPubMed Google Scholar
31 Cyriax JH . The pathology and treatment of tennis elbow. J Bone Joint Surg1936;18:921–940. Google Scholar
32 Greenbaum B , ItamuraJ, VangsnessCT, TiboneJ, AtkinsonR. Extensor carpi radialis brevis: an anatomical analysis of its origin. J Bone Joint Surg [Br]1999;81-B:926–929. Google Scholar
33 Nirschl RP . Tennis elbow tendinosis: pathoanatomy, nonsurgical and surgical management. In: Gordon SL, Blair SJ, Fine LJ, eds. Repetitive motion disorders of the upper extremity . Rosemont: American Academy of Orthopaedic Surgeons, 1995:467–479. Google Scholar
34 Coonrad RW , HooperWR. Tennis elbow: its course, natural history, conservative and surgical management. J Bone Joint Surg [Am]1973;55-A:1177–1182.PubMed Google Scholar
35 Goldie I . Epicondylitis lateralis humeri (epicondylalgia or tennis elbow): a pathogenetical study. Acta Chir Scand Suppl1964;57:339. Google Scholar
36 Nirschl RP , PettroneFA. Tennis elbow. J Bone Joint Surg [Am]1979;61-A:832–839.CrossrefPubMed Google Scholar
37 Assendelft WJ , HayEM, AdsheadR, BouterLM. Corticosteroid injections for lateral epicondylitis: a systematic overview. Br J Gen Pract1996;46:209–216.PubMed Google Scholar
38 Bisset L , BellerE, JullG, et al.Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomised trial. BMJ2006;333:939.CrossrefPubMed Google Scholar
39 Kapetanos G . The effect of the local corticosteroids on the healing and biomechanical properties of the partially injured tendon. Clin Orthop Relat Res1982;163:170–179.PubMed Google Scholar
40 Ketchum LD . Effects of triamcinalone on tendon healing and function: a laboratory study. Plast Reconstr Surg1971;47:471–482. Google Scholar
41 Unverferth LJ , OlixML. The effect of local steroid injections on tendon. J Sports Med1973;1:31–37.CrossrefPubMed Google Scholar
42 Gottlieb NL , RiskinWG. Complications of local corticosteroid injections. JAMA1980;243:1547–1548.CrossrefPubMed Google Scholar
43 Haslock I , MacFarlaneD, SpeedC. Intra-articular and soft tissue injections: a survey of current practice. Br J Rheumatol1995;34:449–452.CrossrefPubMed Google Scholar
44 No authors listed. Articular and periarticular corticosteroid injections Drugs Ther Bull1995;33:67–70. Google Scholar
45 Wolf JM , OzerK, ScottF, GordonMJ, WilliamsAE. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg Am2011;36:1269–1272.CrossrefPubMed Google Scholar
46 Kazemi M , AzmaK, TavanaB, Rezaiee MoghaddamF, PanahiA. Autologous blood versus corticosteroid local injection in the short-term treatment of lateral elbow tendinopathy: a randomized clinical trial of efficacy. Am J Phys Med Rehabil2010;89:660–667.CrossrefPubMed Google Scholar
Funding statement:
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author contributions:
C. M. Dojode: Study design, Data collection, Data analysis, Writing the paper
ICMJE Conflict of Interest:
None declared
©2012 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










