Abstract
Introduction
The pathogenesis of rotator cuff disease (RCD) is complex and not fully understood. This systematic review set out to summarise the histological and molecular changes that occur throughout the spectrum of RCD.
Methods
We conducted a systematic review of the scientific literature with specific inclusion and exclusion criteria.
Results
A total of 101 studies met the inclusion criteria: 92 studies used human subjects exclusively, seven used animal overuse models, and the remaining two studies involved both humans and an animal overuse model. A total of 58 studies analysed supraspinatus tendon exclusively, 16 analysed subacromial bursal tissue exclusively, while the other studies analysed other tissue or varying combinations of tissue types including joint fluid and muscle. The molecular biomarkers that were altered in RCD included matrix substances, growth factors, enzymes and other proteins including certain neuropeptides.
Conclusions
The pathogenesis of RCD is being slowly unravelled as a result of the significant recent advances in molecular medicine. Future research aimed at further unlocking these key molecular processes will be pivotal in developing new surgical interventions both in terms of the diagnosis and treatment of RCD.
Article focus
To determine the key histological and molecular changes in rotator cuff disease (RCD) by systematically reviewing the scientific literature
Key messages
The pathogenesis of RCD is complex and multifactorial
The progressive histological changes in RCD are of a characteristic pattern
The levels of several molecular biomarkers are altered in RCD
Strengths and limitations
The studies of RCD are heterogeneous in several ways, including subject type and disease characteristics
The supraspinatus tendon is highly variable morphologically in terms of loading patterns, and this has a consequent effect on the local molecular biomarker levels
Understanding the changes in molecular biomarkers is paramount in guiding the future research and treatment of RCD
Introduction
Rotator cuff disease (RCD) involves a spectrum of shoulder conditions from early tendinopathy to full thickness tears. The natural history and molecular pathophysiology of cuff disease is far from being fully understood. Historically the idea of mechanisms both intrinsic and extrinsic to the tendon have been researched and argued. Codman and Akerson1 initially proposed in 1934 that degeneration within the tendon was the ‘intrinsic’ primary cause of cuff tears. The ‘extrinsic’ theory relating to tendon damage secondary to attrition by surrounding structures was popularised by Neer in 1972,2 and the term ‘impingement’ was coined. The pathogenesis of cuff disease is multifactorial and likely results from a combination of intrinsic, extrinsic and environmental factors.3
The rotator cuff insertion onto the humeral tuberosities is broad, continuous, multilayered and interwoven.4 The supraspinatus and infraspinatus tendons fuse 1.5 cm proximal to their insertions. Tears in the supraspinatus tendon (SST) are the most common and they are most frequently found near to the tendon’s bony insertion; as SST tears become larger they are more likely to involve infraspinatus due to their common insertion. In this context the anatomy of the SST’s insertion is of key relevance in terms of its extracellular matrix composition and has been categorised into four transition zones.5 The first zone is proper tendon, made up of largely type I collagen and small amounts of decorin. The second zone is fibrocartilage and consists of largely types II and III collagen, with small amounts of types I, IX and X collagen. The third zone is mineralised fibrocartilage and consists of type II collagen, with significant amounts of type X collagen and aggrecan. The fourth zone is bone and is largely type I collagen with a high mineral content. This effective bone-tendon attachment is achieved through a functional grading in mineral content and collagen fibre orientation. The SST enthesis is a highly specialised inhomogeneous structure that is subjected to both tensile and compressive forces; this appears important in both the development and propagation of cuff tears.
Tendon homeostasis and its failure in degenerative disease is a complex process that involves the interplay between a variety of cells, matrix components, enzymes, cytokines, growth factors and proteins. The roles of the different anatomical structures involved (the SST itself, the subacromial bursa (SAB) and the glenohumeral joint capsule (GHC)) are yet to be fully determined. The purpose of this systematic review was to summarise the cellular and molecular changes in rotator cuff disease and explain their possible significance in terms of the disease pathogenesis and future research.
Materials and Methods
This systematic review used the PRISMA-Statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) as a guideline in the development of the study protocol and the report of the current study.6 The inclusion criteria and methods of analysis were specified in advance and documented in a protocol.
Information sources and search strategy
Studies were identified by searching the PubMed and Cochrane electronic databases. The search was undertaken in April 2012. The following search terms were used in PubMed: shoulder nerve growth factor, shoulder NGF, shoulder neuronal regulation, shoulder neuropeptide Y, shoulder NPY, shoulder noradrenaline, shoulder VIP, shoulder Acetycholine, shoulder substance P, shoulder TGF, shoulder CGRP, shoulder IB4, shoulder galanin, shoulder recept shoulder opio*, shoulder histological, shoulder molecul*, shoulder somatostatin, shoulder encephalin, shoulder endorphin, shoulder neurokinin, shoulder histamine, shoulder prostagland*, shoulder NMDA, shoulder AMPA, shoulder glutam*, shoulder collagen, shoulder matrix, shoulder GAG and Glycosamino*, shoulder proteoglycan, shoulder apoptosis, shoulder cytokines, shoulder chemokines, shoulder growth factor*, shoulder VEGF, shoulder Interleuk*, shoulder TIMP, shoulder metalloprot*, shoulder MMP, shoulder ADAMT, shoulder TNF, shoulder tendinopathy*, shoulder degenerative disease.
All searches were repeated with the word ‘shoulder’ being substituted by ‘rotator cuff’. Additional studies were located by searching reference lists of short listed articles. Hand searches were undertaken on the British and American editions of the Journal of Bone and Joint Surgery and the Journal of Shoulder and Elbow Surgery.
Study selection
The citations identified from the searches were combined and duplicates excluded. All citations for papers clearly referring to a topic other than the shoulder were excluded, as were others whose title clearly showed that the paper was not relevant to the current study.
Full copies of the remaining papers were obtained and assessed. Papers concerning the cellular or molecular changes in degenerative RCD were included. Degenerative RCD included patients with asymptomatic age-related degeneration (ARD) and symptomatic patients including the diagnoses of subacromial bursitis, impingement syndrome (IS), rotator cuff tendinopathy, rotator cuff tear (RCT), calcifying tendinopathy (CT), long head of biceps (LHB) tendinopathy and cuff tear arthropathy.
Papers that studied cuff tear models and in vitro studies were excluded. Papers relating to animal overuse models and animal impingement models were included. Papers relating to non-degenerative conditions (such as frozen shoulder) were excluded unless the results for the patients with degenerative disease could be separated. Papers describing solely macroscopic changes, molecular changes that had no control groups for comparison, or relating to studies of any tissue or fluid not located in the region of the shoulder were excluded.
Data collection process
The descriptive histological results and molecular changes were recorded and have been summarised in Tables I, II and III; where the molecular change was within different RCD subgroups this was documented in the results.
Table I
Histological changes in rotator cuff disease (RCD)
| Age-related changes (ARD)* | Rotator cuff tendinopathy/ Impingement syndrome (IS)/ Calcific tendinopathy (CT)† | Rotator cuff tears (RCTs)‡ | |
|---|---|---|---|
| Cellular changes | Rounding of tenocyte nuclei16,71,101 | Rounding of tenocytes18,86 | Rounding of tenocyte nuclei12,52,65,69,71 |
| Increased cellularity86,94 | Plump mesenchymal cells present43 | ||
| Increased apoptosis12,94 | Increased cellular proliferation43,54 | ||
| Bursal inflammatory cell infiltrate12,55,76 | More variable cellularity52,54,65,69,85 | ||
| No bursal inflammatory cell infiltrate78 | Increased cellularity65 (in small RCT vs larger RCT56,82) | ||
| Macrophages and multinucleate cells located around areas of resorption in CT8,95,96 | Increased apoptosis 12,54,58,106 (α increasing degeneration102) | ||
| Chondrocyte type cells8,95,96 | Inflammatory cell infiltrate,27,82 no inflammatory cell infiltrate102 | ||
| Bursal inflammatory cell infiltrate12,27 (small > large RCT58) (ftRCT > ptRCT82) | |||
| Lymphocyte infiltrate31 | |||
| Scanty T/B cells 12,27 | |||
| Extracellular matrix changes | Loss of matrix organisation49,71,101 | Loss of matrix organisation8,77,86 | Loss of matrix organisation23,30,33,52-54,56,65,71,77,85,102 (α tear size82) |
| Fibrocartilagenous change1,16,20 | Increased mechanoreceptor numbers18 | Thinning of collagen fibres53,92 | |
| Mucoid/myxoid degeneration15,19 | Fatty degeneration/infiltration78 | Fatty degeneration/infiltration23,30,31,33,43,62,78 | |
| Fatty degeneration/infiltration15 | Fibrocartilagenous change around areas of calcification 78,95,98 | Focal areas of tissue necrosis23,30,85,92 | |
| Increased GAG16 | Mucoid/myxoid degeneration 31,33,57,58,60,106 | ||
| Calcified deposits1,16,20 | Calcified deposits30,31,33 | ||
| Procollagen type I at tear margins32 | |||
| Amyloid deposition17 | |||
| Vascularity | Increased vascularity20 | Increased vascularity94 | Focal areas of increased vascularity22,69,85 (α increasing degeneration 102) |
| Bursal vascular proliferation55 | Increased vascularity45,52,65 | ||
| Vascular proliferation33,43,54 | |||
| Unchanged overall vascularity24 | |||
| Increased bursal vascularity (RCT vs non-RCT36) (ftRCT vs ptRCT82) | |||
| Increased LHB vascularity in RCT46 | |||
| Overall change | Increased general degeneration34,49,71,72,101 | Increased general degeneration94 | Increased general degeneration 54,57-60,71,106 |
| Chondroid metaplasia15,19 | Chondroid metaplasia12 | Chondroid metaplasia 12,22,23,33,53,56-58,60 | |
| Hyaline degeneration71 | Increased bursal fibrosis35,55,70 | Hyaline degeneration33,52,65,71 | |
| Reduced cellularity20 | Increased LHB degeneration39 | Fibrocartilagenous/granulation tissue at tear edges22,23,27,30,43,56,92 | |
| Failed tendon healing1 | Increased bursal reaction55,78,99,104 | Increased bursal fibrosis35 | |
| Glenohumeral joint degeneration increased with RCTs20,34 | Degeneration of acromion under surface/CAL77,95 | Increased bursal reaction56,78,99,104 (ftRCT > ptRCT36) | |
| Degeneration of acromion under surface/CAL62,67,81 | Lower number of axons innervated LHBT84 | Glenohumeral joint synovial inflammation28 | |
| Increased subscapularis tendon degeneration60 | |||
| Degeneration of acromion under surface/CAL77,95 (RCT > non-RCT67,90) | |||
| Supraspinatus muscle fatty infiltration/degeneration87 | |||
-
* GAG, glycosaminoglycan; CAL, coracoacromial ligament † LHB, long head of biceps; LHBT, long head of biceps tendinopathy ‡ ft, full-thickness; pt, partial thickness
Results
Study selection and characteristics
The search strategy revealed a total of 6145 results (Fig. 1). After removal of duplicate entries, 3956 unique papers remained. Screening of the titles and abstracts revealed 190 papers eligible for inclusion. Further assessment of eligibility, based on full-text papers, led to the exclusion of 89 papers. This left 101 papers meeting our criteria for inclusion.1,7-106
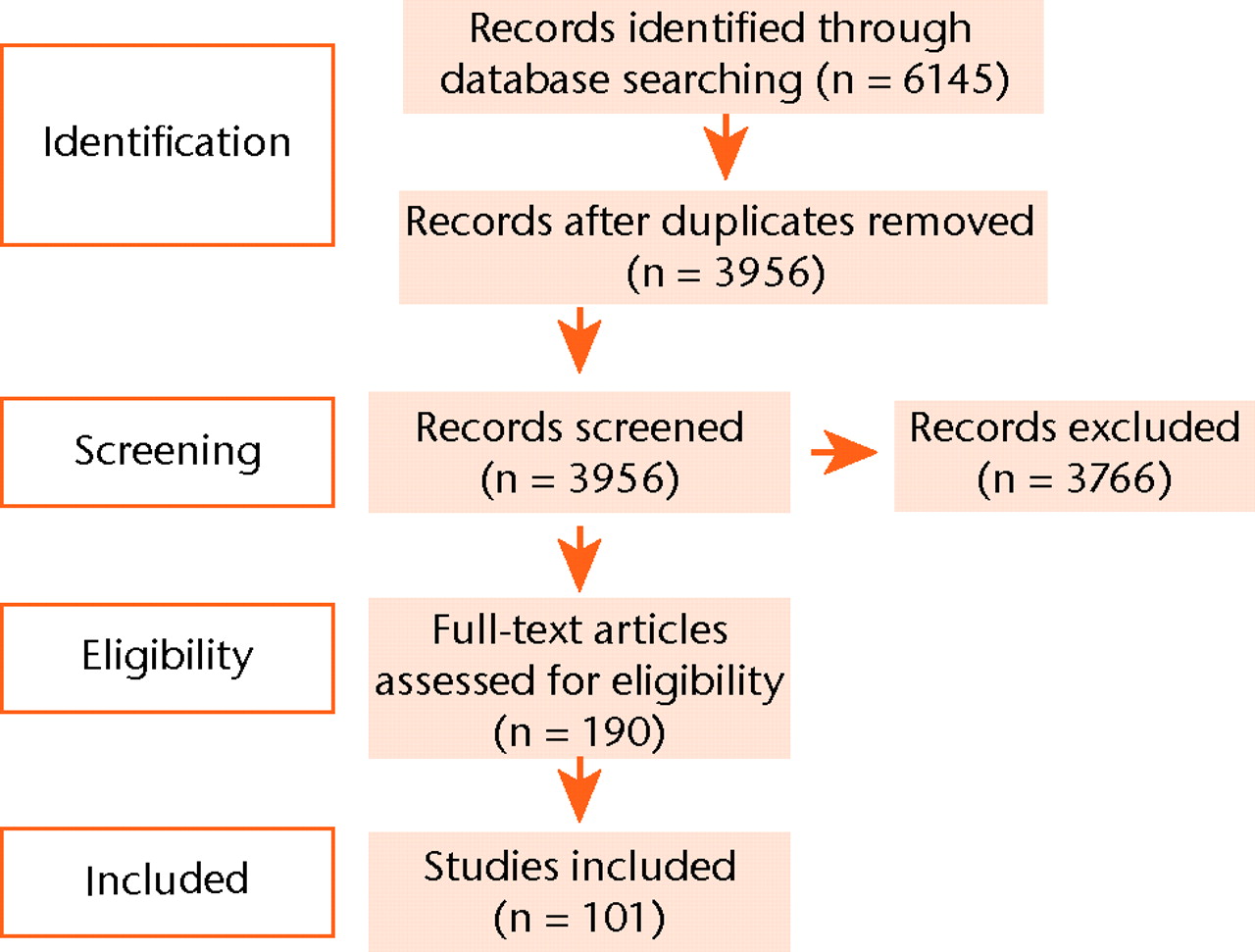
Fig. 1
Flow chart of systematic review protocol.
A total of 92 studies used exclusively human subjects, 12 of which used cadavers. Seven studies used exclusively animal overuse models (six rat and one dog model), and two studies used both human subjects and a model of animal overuse. The SST alone was analysed in 58 studies, SAB tissue alone in 16, while the other studies analysed other tissue or varying combinations of tissue types. All studies analysed RCD in humans or the effects of overuse in an animal model. A total of 43 studies were exclusively histological, 36 studies exclusively molecular, while the remaining 22 analysed both histological and molecular changes. All studies on molecular changes had control groups or performed sub-group analyses based on specific subject characteristics. The types of control included those undergoing surgery for other reasons (instability/trauma), cadaveric specimens and subscapularis samples. A wide variety of techniques were used, including immunohistochemistry (IHC), reverse transcription polymerase chain reaction (RTPCR) and enzyme linked immunosorbent assays (ELISAs).
The histological changes in RCD are summarised in Table I. The significant molecular changes are summarised in Tables II and III.
Table II
The changes to extracellular matrix (ECM) components and enzymes in rotator cuff disease (RCD) (also includes changes to other enzymes and transcription factors) (↑, increased; ↓, decreased)
| Matrix components | Matrix enzymes |
|---|---|
| Type I collagen ↑50 | MMP-1 ↑14,27,40,45,47,66,99 ↓48 |
| Type II collagen ↑23,67 | MMP-2 ↑66 ↑ (ftRCT vs ptRCT89) |
| Type III collagen ↑,10,39,43,50,72,74 ↑ (RCT vs non-RCT36) | MMP-3 ↑39,66 ↓45,47,48,51 ↑ (ftRCT vs ptRCT105 |
| Type X collagen ↑67 | MMP-9 ↑14,45,47,82,99 ↑ (ftRCT vs ptRCT89) |
| Type I collagen α1 ↓9 ↑ (ftRCT vs ptRCT82) | MMP-13 ↑37,48,51,66,82 |
| Type I collagen α2 ↓7,38 | TIMP-1 ↓51 |
| Type II collagen α1 ↑7,9,38 | TIMP-2 ↓51 |
| Type III collagen α1 ↑9,93 ↓7 | TIMP3 ↓7,38 |
| Type VI collagen ↑8 α1 ↑9 | ADAM10 ↓7 |
| Collagen crosslinking ↑10 | Transglutaminase 2 ↓65 |
| Total collagen content ↓10,72,74 | |
| Calcium phosphate ↑72 | |
| Aggrecan ↑7,9,38,50 | Other enzymes |
| Biglycan ↑9 | COX-1 ↑14,99 |
| Decorin ↑9 ↓7,50 | COX-2 ↑14,68,82,99 |
| Clusterin ↑7,58 | Cathepsin D ↑27 |
| Elastin ↓7 | iNOS ↑82,88 |
| Fibronectin ↑(RCT vs non-RCT92) | eNOS ↑88 |
| Osteopontin ↑91 | |
| Tenascin-C ↑23,35 | |
| Versican ↑9 | Transcription factors |
| GAG content ↑9,72 | SOX9 ↑7,9 |
| Chondroitin sulphate ↑8,23,72,73 | FOXO1A ↑ (massive tears 80) |
| Dermatan sulphate ↑8,72,73 | FOXO3A ↑ (in tears greater than one-third80) |
| Hyalauronan ↑73 | |
| Hyaluronic acid ↑73 | |
| α-skeletal muscle actin and of myosin heavy polypeptide1 ↑21 |
Table III
The changes to cytokines, growth factors, neuronal factors, apoptosis/cell cyle related factors and other factors in rotator cuff disease (RCD) (↑, increased; ↓, decreased)
| Cytokines/growth factors | Apoptosis/cell cycle related |
|---|---|
| IL-1α ↑14,99 | HIF-1α ↑11,45,58 ↓59,60,61 |
| IL-1ra ↑26-28 | BNIP-3 ↑11 |
| IL-1β ↑13,14,26-28,42,75,82,99 | BCL-2 ↑58 |
| IL-2 ↓60,61 | Caspase 3 ↑59,60 |
| Il-6 ↑13,14,42,60,82,99 | Caspase 8 ↑59-61 |
| IL-11 ↑60,61 | Heat shock protein 27 ↑59-61 |
| Il-15 ↑60 | Heat shock protein 70 ↑59-61 |
| Il-18 ↑60 | poly(ADP-ribose) polymerase ↑60,61 |
| Stromal derived factor-1α (SDF-1α) ↑13,41) | type-2 angiotensin II receptor ↓60,61 |
| TNFα ↑14,42,75,82,99 | cFLIP ↑59 |
| VEGF ↑45,46,58,68,82 ↑ (associated with motion pain104) | cFLIP receptor ↑60 |
| IGF-1 ↓7,38 | p-53 induced gene I, cell division cycle 25A, Max protein, meiotic recombination 11 homolog A ↑61 |
| TGF-β ↑67,75 | Peroxiredoxin 5 ↑100 |
| bFGF ↑67,75 | P53 ↑54,61 |
| FGF 18 ↑61 | P53 inhibitors ↓54 |
| BMP2 and BMP7 ↑67 | NF-κB ↓54 |
| Small inducible cytokines ↑14 | Receptor activator of NF-κB ↑60 |
| Macrophage inhibitory factor (MIF) ↑60 | |
| Heparin affinity regulatory peptide (HARP) ↑9 | |
| Five-lipoxygenase activating protein ↑68 | |
| Hepatocyte growth factor ↓61 | |
| Neuronal factors | Others |
| Substance P ↑40 (higher in non-perforated RCTs vs perforated25) | Ubiquitin proteasome pathway UBE2A and UBE3A ↑ (massive tears vs small/controls80) |
| β-endorphin ↑40 | Calpain (CAPN1) and CTSB (lysosomal enzyme) ↑ (massive tears vs small/controls80) |
| Anti-NGF30 ↑60,61 | vWF ↑68 |
| PGP9.5, GAP43 ↑103 | T-cell receptor variable βchain ↑60 |
| glutamate receptor 5, glutamate receptor metabotropic 6, glutamate receptor inotropic 3A, GABA receptor α1 ↑61 | Ig heavy chain, T cell receptor α chain ↓60 |
| AMPA1, glutamate receptor interacting protein 1/2 ↓61 | GATA binding protein, PAF acetylhydrolase, Attractin, IgG-2b chain ↑60,61 |
| Insulin induced gene 1, FGFr1, nuclear receptor coactivator 2, G protein coupled receptor 54, Ephrin A1, Thyrotroph embryonic factor, Odd Oz/ten-m homolog 2, POU domain, TNF 11, TGF-β binding protein 3, T cell receptor β chain, cytochrome b-245, CD3 γ chain, polyprotein 1-microglobulin, Fc receptor IgE, solute carrier family 2, adenosine deaminsae, integrin-linked kinase ↑61 | |
| Dynein, nuclear receptor subfamily 2 group F member 1, Homeobox A1, FGF receptor 3, MHC class I-like sequence, T-cell receptor β chain, killer cell lectin-like receptor, strain T-cell receptor ↓61 | |
| T-cell receptor ↓60,61 |
A reduction in overall collagen content and were seen in RCD; type II and III collagen content were increased in multiple studies. Overall glycosaminoglycan (GAG) levels were increased, while certain proteoglycans levels were increased (tenascin-C, fibronectin, aggrecan, and biglycan) and others reduced (elastin). The general in RCD was towards a fibrocartilagenous phenotype.
The collagenases matrix metalloproteinase (MMP)-1 and -13 were increased in RCD, while the gelatinases MMP-2 and MMP-9 were also increased in RCD. MMP-3 levels were altered in seven studies, being increased in three and decreased in four. Tissue inhibitor of metalloproteinases (TIMP)-1, -2 and -3 have all been shown to be decreased in RCD, while no change in TIMP-4 was demonstrated. Overall there is a clear catabolic trend in RCD.
The changes in terms of cytokines were generally pro-inflammatory. Several members of the Interleukin family were increased in RCD (Interleukin-1α, 1β, -6, 11, 15, 18 and IL1-receptor antagonist). Tumour necrosis factor (TNF)-α, stromal derived factor-1α and the small inducible cytokines were all increased. The cyclo-oxygenases (1 and 2), Cathepsin D and nitric oxide synthase were all increased in RCD. Several growth factors were increased in RCD including vascular endothelial growth factor (VEGF), transforming growth factor (TGF)-β, fibroblast growth factor (FGF), bone morphogenetic protein (BMP) 2 and BMP 7. Insulin-like growth factor (IGF)-1 was decreased in RCD.
Several proteins associated with apoptosis are increased in RCD, including p53, poly(ADP-ribose) polymerase, caspases 3/8, B-cell lymphoma (BCL)-2, BNIP3, type II angiotensin receptor, cFLIP and cFLIP receptor. Peroxiredoxin 5 and the heat shock proteins 27/70 were increased, while there was no obvious trend in hypoxia-inducible factor (HIF)-1α levels; these substances may all play a protective role for cells at times of high stress. In terms of neuropeptides, increases in substance P and β-endorphin were seen in RCD. The increase in PGP9.5 and GAP43 is likely to represent neoinnervation in RCD.
Discussion
The results of this systematic review must be seen in the context of the heterogeneity of the studies and of RCD in general. RCD includes a whole spectrum of changes to the histological and molecular characteristics of the tissue. Different studies analysed the tissue of patient groups that were highly variable in terms of disease stage, symptomatology and patient demographics. It has been shown that significant molecular differences are found depending on if the sampled tendon is from an over-stressed or stress-shielded region.107 Several studies used animal overuse models which are hypothesised to mimic the pathophysiology of RCD, however there are likely to be some significant differences between the molecular changes found in these models and RCD in humans. There was also significant diversity between studies in terms of what was measured. Some studies measured molecular biomarker levels directly, some measured the gene expression of molecular biomarkers using mRNA and some used both of these techniques. All these factors may account for some of the apparent discrepancies in the study findings.
The cellular changes that occur as cuff disease progresses have been well described.12,56 Small tears of the rotator cuff show features consistent with an attempt to heal such as increased fibroblast cellularity, blood vessel proliferation and the presence of a significant inflammatory component. These features of attempting healing diminish as the size of the tear and the amount of tendon degeneration increase. The progressive tendon degeneration is characterised by thinning of the collagen fibres, a loss of collagen structure, myxoid degeneration, hyaline degeneration, chrondroid metaplasia and fatty infiltration.33 The overall picture is one of pathological chondroplasia in which tissue which normally exhibits a tensional morphology is replaced by tissue of a fibrocartilage-like phenotype.23
The regulation of matrix turnover involves several cell types and numerous cytokines. The tenocyte is the resident fibroblast present in tendon and is arguably the most pivotal cell type; other cells involved include extrinsic fibroblasts and inflammatory cells such as the macrophage. Tenocytes have been shown to produce a number of cytokines in response to increased strain such as Interleukin-1β (IL-1β), Interleukin-6 (IL-6), VEGF, HIF-1α, TGF-β and prostaglandin-E2 (PG-E2).108-111 Our results show that numerous cytokines and growth factors including IL-1β, IL-6, VEGF, bFGF, TGF-β, TNF-α, HIF-1α, cyclooxygenase (COX)-1, COX-2 and nitric oxide synthase (NOS) are all increased in RCD. VEGF, IGF-1, TGF-β and FGF are all increased during normal tendon healing112and their increase in RCD is demonstrative of tendon attempting to heal. An increased IL-1β production has been hypothesised to promote cell survival in a high stress environment.109 Numerous complex interactions occur between these cytokines, the extracellular matrix synthesis, catabolic mediators and cytoskeleton assembly.113 Pro-inflammatory cytokines affect extracellular matrix homeostasis, accelerate remodelling, amplify biomechanical adaptivity and promote tenocyte apoptosis. The trend towards a pro-inflammatory state in RCD is indicative of the imbalance that occurs between the catabolic and anabolic systems, the cytokines being key regulatory factors of these. As RCD progresses and cuff tears become increasingly sizeable there is a clear increase in apoptosis, as evidenced by increases in several apoptosis related markers including BNIP-3, BCL-2, the caspases and the heat shock proteins. The increase in p53 activity in RCD may also be important in promoting apoptosis.
Tendon is highly mechanically adaptive and a characteristic feature of RCD is the progressive mechanical failure of tendon to meet the physical demands placed upon it.112 Total collagen content decreases, while there is a significant increase in the proportion of type II and III collagen relative to type I collagen. This change in collagen makeup goes hand in hand with a transformation of the matrix from larger organised fibrils to smaller disorganised fibrils with decreasing mechanical properties. The mature and hydroxylysine cross-links are significantly increased which may be a feature of the incomplete remodelling found in scar tissue.10 The increase in the glycoproteins tenascin-C and fibronectin is consistent with a wound healing process occurring in degenerate tendon. The changes to several different proteoglycans in RCD are varied but the overall picture appears to be of fibrocartilagenous change; this is characterised by increased aggrecan and biglycan, with decreased decorin. Therefore overall the matrix changes are consistent with the degenerate tendon attempting to heal, with a progressively mechanically weak scar tissue being laid down as part of this failing remodelling process.
Higher levels of matrix remodelling and turnover have been linked with RCD114 and the tissue-degrading enzymes of the metalloproteinase family are important in this process. The family includes the MMPs, their close relatives ‘a disintegrin and metalloproteinase’ (ADAMs) and ‘a disintegrin and metalloproteinase with thrombospondin motifs’ (ADAMTS). The MMP family consists of 24 known MMPs including the collagenases (MMP1, -8 and -13), the gelatinases (MMP-2 and -9), membrane-type MMPs (MT-MMPs), the stromelysins (MMP-3 and -10 and the matrilysins (MMP-7 and -26).115 The collagenases, as well as MMP-2 and -14, have important collagenolytic activity. The ADAMTS are divided into four groups, of which ADAMTS -1,-4,-5,-8,-9,-15,-20 are the aggrecanases and ADAMTS -2,-3,-14 are the procollagen N-proteinases. The TIMPs are endogenous inhibitors of the metalloproteinases and there are four in humans; they reversibly inhibit all MMPs by a 1:1 interaction with the zinc binding site. The MMPs do not solely degrade tissue; they may have anti-inflammatory actions by processing certain cytokines and chemokines.114
The increased collagen turnover in RCD is consistent with the increase in two collagenases (MMP-1 and -13) and two gelatinases (MMP-2 and -9). MMP-3 is thought to be important in the regulation of matrix turnover and is reduced in the degenerate SST; this is consistent with tendinopathy resulting from a failure in tendon repair or matrix maintenance. The decreases in TIMP-1,-2, and -3 in RCD are also consistent with this catabolic picture of increased matrix degradation and failing remodelling. The roles of the ADAMs and ADAMTS have yet to be determined in RCD. Older tendon is more susceptible to mechanically induced failure involving MMP activity116 and this may be related to age-related change in tenocytes.117 The role of tendon stem cells (TSCs) remains to be determined but their responses to differing mechanical stimuli hint towards an important role.118-120 TSCs have been shown to proliferate and produce collagen in response to exercise, while they have been shown to differentiate into non-tenocytes if excessively mechanically loaded. A recent report suggested that an extracellular matrix rich niche, organised partly by biglycan and fibromodulin, controls the self-renewal and differentiation of TSCs.121 The self-renewal capacity and differentiation capability of TSCs reduces with increasing age122,123 and this is likely to be important in explaining the age-related nature of RCD.
This review has summarised just how much progress has been made in recent years, particularly in the advent of modern molecular medical techniques. Intrinsic, extrinsic and environmental factors all have an important role to play in the disordered tendon homeostasis of RCD which can lead to progressive mechanical failure. Among the key questions that remain to be answered include why some patients’ tendons degenerate, while others do not, and why some patients experience pain, while others with the same amount of macroscopic tendon degeneration do not. Certainly there is still a great deal to be understood as regards the pathogenesis of RCD and undoubtedly, unlocking these secrets could pave the way for some very exciting new treatments in the future.
Supplementary material
A table giving details of each of the 101 studies included in this review is available with this article on our website www.bjr.boneandjoint.org.uk
1 Codman EA , AkersonIB. The pathology associated with rupture of the supraspinatus tendon. Ann Surg1931;93:348–359.CrossrefPubMed Google Scholar
2 Neer CS 2nd . Impingement lesions. Clin Orthop Relat Res1983;173:70–77. Google Scholar
3 Lewis JS . Rotator cuff tendinopathy. Br J Sports Med2009;43:236–241.CrossrefPubMed Google Scholar
4 Clark JM , HarrymanDT 2nd. Tendons, ligaments, and capsule of the rotator cuff: gross and microscopic anatomy. J Bone Joint Surg [Am]1992;74-A:713–725. Google Scholar
5 Thomopoulos S , GeninGM, GalatzLM. The development and morphogenesis of the tendon-to-bone insertion: what development can teach us about healing. J Musculoskelet Neuronal Interact2010;10:35–45. Google Scholar
6 Moher D , LiberatiA, TetzlaffJ, AltmanDG, PRISMAGroup. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ2009;339:2535.CrossrefPubMed Google Scholar
7 Archambault JM , JelinskySA, LakeSP, et al.Rat supraspinatus tendon expresses cartilage markers with overuse. J Orthop Res2007;25:617–624.CrossrefPubMed Google Scholar
8 Archer RS , BayleyJI, ArcherCW, AliSY. Cell and matrix changes associated with pathological calcification of the human rotator cuff tendons. J Anat1993;182:1–11.PubMed Google Scholar
9 Attia M , ScottA, DuchesnayA, et al.Alterations of overused supraspinatus tendon: a possible role of glycosaminoglycans and HARP/pleiotrophin in early tendon pathology. J Orthop Res2012;30:61–71.CrossrefPubMed Google Scholar
10 Bank RA , TeKoppeleJM, OostinghG, HazlemanBL, RileyGP. Lysylhydroxylation and non-reducible crosslinking of human supraspinatus tendon collagen: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis1999;58:35–41.CrossrefPubMed Google Scholar
11 Benson RT , McDonnellSM, KnowlesHJ, et al.Tendinopathy and tears of the rotator cuff are associated with hypoxia and apoptosis. J Bone Joint Surg [Br]2010;92-B:448–453.CrossrefPubMed Google Scholar
12 Benson RT , McDonnellSM, ReesJL, AthanasouNA, CarrAJ. The morphological and immunocytochemical features of impingement syndrome and partial-thickness rotator-cuff tear in relation to outcome after subacromial decompression. J Bone Joint Surg [Br]2009;91-B:119–123.CrossrefPubMed Google Scholar
13 Blaine TA , CoteMA, ProtoA, et al.Interleukin-1β stimulates stromal-derived factor-1α expression in human subacromial bursa. J Orthop Res2011;29:1695–1699.CrossrefPubMed Google Scholar
14 Blaine TA , KimYS, VoloshinI, et al.The molecular pathophysiology of subacromial bursitis in rotator cuff disease. J Shoulder Elbow Surg2005;14(Suppl):84S–849.CrossrefPubMed Google Scholar
15 Buck FM , GrehnH, HilbeM, et al.Magnetic resonance histologic correlation in rotator cuff tendons. J Magn Reson Imaging2010;32:165–172.CrossrefPubMed Google Scholar
16 Chard MD , CawstonTE, RileyGP, GreshamGA, HazlemanBL. Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis1994;53:30–34.CrossrefPubMed Google Scholar
17 Cole AS , Cordiner-LawrieS, CarrAJ, AthanasouNA. Localised deposition of amyloid in tears of the rotator cuff. J Bone Joint Surg [Br]2001;83-B:561–564.CrossrefPubMed Google Scholar
18 de Castro Pochini A , EjnismanB, de Seixas AlvesMT, et al.Overuse of training increases mechanoreceptors in supraspinatus tendon of rats SHR. J Orthop Res2011;29:1771–1774.CrossrefPubMed Google Scholar
19 Determe D , RongièresM, KanyJ, et al.Anatomic study of the tendinous rotator cuff of the shoulder. Surg Radiol Anat1996;18:195–200.CrossrefPubMed Google Scholar
20 Feeney MS , O'DowdJ, KayEW, ColvilleJ. Glenohumeral articular cartilage changes in rotator cuff disease. J Shoulder Elbow Surg2003;12:20–23.CrossrefPubMed Google Scholar
21 Fuchs B , ZumsteinM, RegenfelderF, et al.Upregulation of alpha-skeletal muscle actin and myosin heavy polypeptide gene products in degenerating rotator cuff muscles. J Orthop Res2008;26:1007–1011.CrossrefPubMed Google Scholar
22 Fukuda H , HamadaK, YamanakaK. Pathology and pathogenesis of bursal-side rotator cuff tears viewed from en bloc histologic sections. Clin Orthop Relat Res1990;254:75–80.PubMed Google Scholar
23 Gigante A , MarinelliM, ChillemiC, GrecoF. Fibrous cartilage in the rotator cuff: a pathogenetic mechanism of tendon tear?J Shoulder Elbow Surg2004;13:328–332.CrossrefPubMed Google Scholar
24 Goodmurphy CW , OsbornJ, AkessonEJ, et al.An immunocytochemical analysis of torn rotator cuff tendon taken at the time of repair. J Shoulder Elbow Surg2003;12:368–374.CrossrefPubMed Google Scholar
25 Gotoh M , HamadaK, YamakawaH, InoueA, FukudaH. Increased substance P in subacromial bursa and shoulder pain in rotator cuff diseases. J Orthop Res1998;16:618–621.CrossrefPubMed Google Scholar
26 Gotoh M , HamadaK, YamakawaH, et al.Perforation of rotator cuff increases interleukin 1beta production in the synovium of glenohumeral joint in rotator cuff diseases. J Rheumatol2000;27:2886–2892.PubMed Google Scholar
27 Gotoh M , HamadaK, YamakawaH, et al.Significance of granulation tissue in torn supraspinatus insertions: an immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. J Orthop Res1997;15:33–39.CrossrefPubMed Google Scholar
28 Gotoh M , HamadaK, YamakawaH, et al.Interleukin-1-induced glenohumeral synovitis and shoulder pain in rotator cuff diseases. J Orthop Res2002;20:1365–1371.CrossrefPubMed Google Scholar
29 Gotoh M , HamadaK, YamakawaH, et al.Interleukin-1-induced subacromial synovitis and shoulder pain in rotator cuff diseases. Rheumatology (Oxford)2001;40:995–1001.CrossrefPubMed Google Scholar
30 Goutallier D, Postel JM, Van Driessche S, Voisin MC. Histological lesions of supraspinatus tendons in full thickness tears of the rotator cuff. Rev Chir Orthop Reparatrice Appar Mot 2005;91:109–113 (in French). Google Scholar
31 Gumina S , Di GiorgioG, BertinoA, et al.Inflammatory infiltrate of the edges of a torn rotator cuff. Int Orthop2006;30:371–374.CrossrefPubMed Google Scholar
32 Hamada K , OkawaraY, FryerJN, TomonagaA, FukudaH. Localization of mRNA of procollagen alpha 1 type I in torn supraspinatus tendons: in situ hybridization using digoxigenin labeled oligonucleotide probe. Clin Orthop Relat Res1994;304:18–21. Google Scholar
33 Hashimoto T , NobuharaK, HamadaT. Pathologic evidence of degeneration as a primary cause of rotator cuff tear. Clin Orthop Relat Res2003;415:111–120.CrossrefPubMed Google Scholar
34 Hijioka A , SuzukiK, NakamuraT, HojoT. Degenerative change and rotator cuff tears: an anatomical study in 160 shoulders of 80 cadavers. Arch Orthop Trauma Surg1993;112:61–64. Google Scholar
35 Hyvönen P , MelkkoJ, LehtoVP, JalovaaraP. Involvement of the subacromial bursa in impingement syndrome of the shoulder as judged by expression of tenascin-C and histopathology. J Bone Joint Surg [Br]2003;85-B:299–305.CrossrefPubMed Google Scholar
36 Ishii H , BrunetJA, WelshRP, UhthoffHK. “Bursal reactions” in rotator cuff tearing, the impingement syndrome, and calcifying tendinitis. J Shoulder Elbow Surg1997;6:131–136. Google Scholar
37 Jacob J , EisemonE, Sheibani-RadS, et al.Matrix metalloproteinase levels as a marker for rotator cuff tears. Orthopedics2012;35:474–478.CrossrefPubMed Google Scholar
38 Jelinsky SA , LakeSP, ArchambaultJM, SoslowskyLJ. Gene expression in rat supraspinatus tendon recovers from overuse with rest. Clin Orthop Relat Res2008;466:1612–1617.CrossrefPubMed Google Scholar
39 Joseph M , MareshCM, McCarthyMB, et al.Histological and molecular analysis of the biceps tendon long head post-tenotomy. J Orthop Res2009;27:1379–1385.CrossrefPubMed Google Scholar
40 Karahan S , KincaidSA, BairdAN, KammermannJR. Distribution of beta-endorphin and substance P in the shoulder joint of the dog before and after a low impact exercise programme. Anat Histol Embryol2002;31:72–77.CrossrefPubMed Google Scholar
41 Kim YS , BiglianiLU, FujisawaM, et al.Stromal cell-derived factor 1 (SDF-1, CXCL12) is increased in subacromial bursitis and downregulated by steroid and nonsteroidal anti-inflammatory agents. J Orthop Res2006;24:1756–1764.CrossrefPubMed Google Scholar
42 Ko JY , WangFS, HuangHY, et al.Increased IL-1beta expression and myofibroblast recruitment in subacromial bursa is associated with rotator cuff lesions with shoulder stiffness. J Orthop Res2008;26:1090–1097.CrossrefPubMed Google Scholar
43 Kumagai J , SarkarK, UhthoffHK. The collagen types in the attachment zone of rotator cuff tendons in the elderly: an immunohistochemical study. J Rheumatol1994;21:2096–2100.PubMed Google Scholar
44 Lakemeier S , BraunJ, EfeT, et al.Expression of matrix metalloproteinases 1, 3, and 9 in differing extents of tendon retraction in the torn rotator cuff. Knee Surg Sports Traumatol Arthrosc2011;19:1760–1765.CrossrefPubMed Google Scholar
45 Lakemeier S , ReicheltJJ, PatzerT, et al.The association between retraction of the torn rotator cuff and increasing expression of hypoxia inducible factor 1alpha and vascular endothelial growth factor expression: an immunohistological study. BMC Musculoskelet Disord2010;11:230. Google Scholar
46 Lakemeier S , ReicheltJJ, TimmesfeldN, et al.The relevance of long head biceps degeneration in the presence of rotator cuff tears. BMC Musculoskelet Disord2010;11:191.CrossrefPubMed Google Scholar
47 Lakemeier S , SchwuchowSA, PeterleinCD, et al.Expression of matrix metalloproteinases 1, 3, and 9 in degenerated long head biceps tendon in the presence of rotator cuff tears: an immunohistological study. BMC Musculoskelet Disord2010;11:271.CrossrefPubMed Google Scholar
48 Lehmann LJ , SchollmeyerA, StoeveJ, ScharfHP. Biochemical analysis of the synovial fluid of the shoulder joint in patients with and without rotator cuff tears. Z Orthop Unfall2010;148:90–94 (in German). Google Scholar
49 Lindblom K . Arthrography and roentgenography in ruptures of tendons of the shoulder joint. Acta Radiol1939;20:548–562. Google Scholar
50 Lo IK , BoormanR, MarchukL, et al.Matrix molecule mRNA levels in the bursa and rotator cuff of patients with full-thickness rotator cuff tears. Arthroscopy2005;21:645–651.CrossrefPubMed Google Scholar
51 Lo IK , MarchukLL, HollinsheadR, HartDA, FrankCB. Matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase mRNA levels are specifically altered in torn rotator cuff tendons. Am J Sports Med2004;32:1223–1229.CrossrefPubMed Google Scholar
52 Longo UG , FranceschiF, RuzziniL, et al.Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med2008;36:533–538.CrossrefPubMed Google Scholar
53 Longo UG , FranceschiF, RuzziniL, et al.Light microscopic histology of supraspinatus tendon ruptures. Knee Surg Sports Traumatol Arthrosc2007;15:1390–1394.CrossrefPubMed Google Scholar
54 Lundgreen K , LianOB, EngebretsenL, ScottA. Tenocyte apoptosis in the torn rotator cuff: a primary or secondary pathological event?Br J Sports Med2011;45:1035–1039.CrossrefPubMed Google Scholar
55 Matsuzaki S , YonedaM, KobayashiY, FukushimaS, WakitaniS. Dynamic enhanced MRI of the subacromial bursa: correlation with arthroscopic and histological findings. Skeletal Radiol2003;32:510–520.CrossrefPubMed Google Scholar
56 Matthews TJ , HandGC, ReesJL, AthanasouNA, CarrAJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg [Br]2006;88-B:489–495.CrossrefPubMed Google Scholar
57 Millar NL , HueberAJ, ReillyJH, et al.Inflammation is present in early human tendinopathy. Am J Sports Med2010;38:2085–2091.CrossrefPubMed Google Scholar
58 Millar NL , ReillyJH, KerrSC, et al.Hypoxia: a critical regulator of early human tendinopathy. Ann Rheum Dis2012;71:302–310.CrossrefPubMed Google Scholar
59 Millar NL , WeiAQ, MolloyTJ, BonarF, MurrellGA. Heat shock protein and apoptosis in supraspinatus tendinopathy. Clin Orthop Relat Res2008;466:1569–1576.CrossrefPubMed Google Scholar
60 Millar NL , WeiAQ, MolloyTJ, BonarF, MurrellGA. Cytokines and apoptosis in supraspinatus tendinopathy. J Bone Joint Surg [Br]2009;91-B:417–424.CrossrefPubMed Google Scholar
61 Molloy TJ , KempMW, WangY, MurrellGA. Microarray analysis of the tendinopathic rat supraspinatus tendon: glutamate signaling and its potential role in tendon degeneration. J Appl Physiol2006;101:1702–1709.CrossrefPubMed Google Scholar
62 Nakagaki K , OzakiJ, TomitaY, TamaiS. Fatty degeneration in the supraspinatus muscle after rotator cuff tear. J Shoulder Elbow Surg1996;5:194–200.CrossrefPubMed Google Scholar
63 Neuwirth J , FuhrmannRA, VeitA, et al.Expression of bioactive bone morphogenetic proteins in the subacromial bursa of patients with chronic degeneration of the rotator cuff. Arthritis Res Ther2006;8:R92–R99.CrossrefPubMed Google Scholar
64 Ogata S , UhthoffHK. Acromial enthesopathy and rotator cuff tear: a radiologic and histologic postmortem investigation of the coracoacromial arch. Clin Orthop Relat Res1990;254:39–48. Google Scholar
65 Oliva F , ZocchiL, CodispotiA, et al.Transglutaminases expression in human supraspinatus tendon ruptures and in mouse tendons. Biochem Biophys Res Commun2009;379:887–891.CrossrefPubMed Google Scholar
66 Osawa T , ShinozakiT, TakagishiK. Multivariate analysis of biochemical markers in synovial fluid from the shoulder joint for diagnosis of rotator cuff tears. Rheumatol Int2005;25:436–441.CrossrefPubMed Google Scholar
67 Panni AS , MilanoG, LucaniaL, FabbricianiC, LogroscinoCA. Histological analysis of the coracoacromial arch: correlation between age-related changes and rotator cuff tears. Arthroscopy1996;12:531–540.CrossrefPubMed Google Scholar
68 Perry SM , McIlhennySE, HoffmanMC, SoslowskyLJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elbow Surg2005;14(Suppl):79S–83S.CrossrefPubMed Google Scholar
69 Premdas J , TangJB, WarnerJP, MurrayMM, SpectorM. The presence of smooth muscle actin in fibroblasts in the torn human rotator cuff. J Orthop Res2001;19:221–228.CrossrefPubMed Google Scholar
70 Rahme H , NordgrenH, HambergH, WesterbergCE. The subacromial bursa and the impingement syndrome: a clinical and histological study of 30 cases. Acta Orthop Scand1993;64:485–488. Google Scholar
71 Riley GP , GoddardMJ, HazlemanBL. Histopathological assessment and pathological significance of matrix degeneration in supraspinatus tendons. Rheumatology (Oxford)2001;40:229–230.CrossrefPubMed Google Scholar
72 Riley GP , HarrallRL, ConstantCR, CawstonTE, HazlemanBL. Prevalence and possible pathological significance of calcium phosphate salt accumulation in tendon matrix degeneration. Ann Rheum Dis1996;55:109–115.CrossrefPubMed Google Scholar
73 Riley GP , HarrallRL, ConstantCR, et al.Glycosaminoglycans of human rotator cuff tendons: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis1994;53:367–376.CrossrefPubMed Google Scholar
74 Riley GP , HarrallRL, ConstantCR, et al.Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Ann Rheum Dis1994;53:359–366.CrossrefPubMed Google Scholar
75 Sakai H , FujitaK, SakaiY, MizunoK. Immunolocalization of cytokines and growth factors in subacromial bursa of rotator cuff tear patients. Kobe J Med Sci2001;47:25–34.PubMed Google Scholar
76 Santavirta S , KonttinenYT, Antti-PoikaI, NordstromD. Inflammation of the subacromial bursa in chronic shoulder pain. Arch Orthop Trauma Surg1992;111:336–340.CrossrefPubMed Google Scholar
77 Sarkar K , TaineW, UhthoffHK. The ultrastructure of the coracoacromial ligament in patients with chronic impingement syndrome. Clin Orthop Relat Res1990;254:49–54.PubMed Google Scholar
78 Sarkar K , UhthoffHK. Ultrastructural localization of calcium in calcifying tendinitis. Arch Pathol Lab Med1978;102:266–269.PubMed Google Scholar
79 Sarkar K , UhthoffHK. Ultrastructure of the subacromial bursa in painful shoulder syndromes. Virchows Archiv A Pathol Anat Histopathol1983;400:107–117.CrossrefPubMed Google Scholar
80 Schmutz S , FuchsT, RegenfelderF, et al.Expression of atrophy mRNA relates to tendon tear size in supraspinatus muscle. Clin Orthop Relat Res2009;467:457–464.CrossrefPubMed Google Scholar
81 Shah NN , BaylissNC, MalcolmA. Shape of the acromion: congenital or acquired: a macroscopic, radiographic, and microscopic study of acromion. J Shoulder Elbow Surg2001;10:309–316. Google Scholar
82 Shindle MK , ChenCC, RobertsonC, et al.Full-thickness supraspinatus tears are associated with more synovial inflammation and tissue degeneration than partial-thickness tears. J Shoulder Elbow Surg2011;20:917–927.CrossrefPubMed Google Scholar
83 Shirachi I , GotohM, MitsuiY, et al.Collagen production at the edge of ruptured rotator cuff tendon is correlated with postoperative cuff integrity. Arthroscopy2011;27:1173–1179.CrossrefPubMed Google Scholar
84 Singaraju VM , KangRW, YankeAB, et al.Biceps tendinitis in chronic rotator cuff tears: a histologic perspective. J Shoulder Elbow Surg2008;17:898–904.CrossrefPubMed Google Scholar
85 Sonnabend DH , YuY, HowlettCR, HarperGD, WalshWR. Laminated tears of the human rotator cuff: a histologic and immunochemical study. J Shoulder Elbow Surg2001;10:109–115.CrossrefPubMed Google Scholar
86 Soslowsky LJ , ThomopoulosS, TunS, et al.Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg2000;9:79–84.PubMed Google Scholar
87 Steinbacher P , TauberM, KoglerS, et al.Effects of rotator cuff ruptures on the cellular and intracellular composition of the human supraspinatus muscle. Tissue Cell2010;42:37–41.CrossrefPubMed Google Scholar
88 Szomor ZL , AppleyardRC, MurrellGA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res2006;24:80–86.CrossrefPubMed Google Scholar
89 Tajana MS , MurenaL, ValliF, PassiA, GrassiFA. Correlations between biochemical markers in the synovial fluid and severity of rotator cuff disease. Chir Organi Mov2009;93(Suppl):S41–S48.CrossrefPubMed Google Scholar
90 Takase K , YamamotoK. Histological and ultrastructural changes in the undersurface of the acromion with subacromial impingement. Acta Orthop2005;76:386–391.PubMed Google Scholar
91 Takeuchi E , SugamotoK, NakaseT, et al.Localization and expression of osteopontin in the rotator cuff tendons in patients with calcifying tendinitis. Virchows Arch2001;438:612–617.CrossrefPubMed Google Scholar
92 Tillander B , FranzenL, NorlinR. Fibronectin, MMP-1 and histologic changes in rotator cuff disease. J Orthop Res2002;20:1358–1364.CrossrefPubMed Google Scholar
93 Tomonaga A , HamadaK, GotohM, et al.Expression of procollagen alpha 1 type III mRNA in rotator cuff tears. Tokai J Exp Clin Med2000;25:125–134.PubMed Google Scholar
94 Tuoheti Y , ItoiE, PradhanRL, et al.Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elbow Surg2005;14:535–541.CrossrefPubMed Google Scholar
95 Uhthoff HK , SarkarK. Calcifying tendinitis. Int Orthop (SICOT)1978;2:187–193.CrossrefPubMed Google Scholar
96 Uhthoff HK . Calcifying tendinitis, an active cell-mediated calcification. Virchows Arch A Pathol Anat Histol1975;366:51–58.CrossrefPubMed Google Scholar
97 Uhthoff HK , HammondDI, SarkarK, HooperGJ, PapoffWJ. The role of the coracoacromial ligament in the impingement syndrome: a clinical, radiological and histological study. Int Orthop1988;12:97–104. Google Scholar
98 Uhthoff HK , SarkarK, MaynardJA. Calcifying tendinitis: a new concept of its pathogenesis. Clin Orthop Relat Res1976;118:164–168.PubMed Google Scholar
99 Voloshin I , GelinasJ, MaloneyMD, et al.Proinflammatory cytokines and metalloproteases are expressed in the subacromial bursa in patients with rotator cuff disease. Arthroscopy2005;21:1076.CrossrefPubMed Google Scholar
100 Wang MX , WeiA, YuanJ, et al.Antioxidant enzyme peroxiredoxin 5 is upregulated in degenerative human tendon. Biochem Biophys Res Commun2001;284:667–673.CrossrefPubMed Google Scholar
101 Wilson CL , DuffGL. Pathologic study of degeneration and rupture of the supraspinatus tendon. Arch Surg1943;47:121–135. Google Scholar
102 Wu B , ChenJ, Dela RosaT, et al.Cellular response and extracellular matrix breakdown in rotator cuff tendon rupture. Arch Orthop Trauma Surg2011;131:405–411.CrossrefPubMed Google Scholar
103 Xu Y , BonarF, MurrellGA. Neoinnervation in rotator cuff tendinopathy. Sports Med Arthrosc2011;19:354–359.CrossrefPubMed Google Scholar
104 Yanagisawa K , HamadaK, GotohM, et al.Vascular endothelial growth factor (VEGF) expression in the subacromial bursa is increased in patients with impingement syndrome. J Orthop Res2001;19:448–455.CrossrefPubMed Google Scholar
105 Yoshihara Y , HamadaK, NakajimaT, FujikawaK, FukudaH. Biochemical markers in the synovial fluid of glenohumeral joints from patients with rotator cuff tear. J Orthop Res2001;19:573–579.CrossrefPubMed Google Scholar
106 Yuan J , MurrellGA, WeiAQ, WangMX. Apoptosis in rotator cuff tendonopathy. J Orthop Res2002;20:1372–1379.CrossrefPubMed Google Scholar
107 Smith MM , SakuraiG, SmithSM, et al.Modulation of aggrecan and ADAMTS expression in ovine tendinopathy induced by altered strain. Arthritis Rheum2008;58:1055–1066.CrossrefPubMed Google Scholar
108 Legerlotz K, Jones GC, Screen HR, Riley GP. Cyclic loading of tendon fascicles using a novel fatigue loading system increases interleukin-6 expression by tenocytes. Scand J Med Sci Sports 2011:Epub. Google Scholar
109 Qi J , ChiL, BynumD, BanesAJ. Gap junctions in IL-1β-mediated cell survival response to strain. J Appl Physiol2011;110:1425–1431.CrossrefPubMed Google Scholar
110 Yang G , ImHJ, WangJH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene2005;363:166–172.CrossrefPubMed Google Scholar
111 Petersen W , VarogaD, ZantopT, et al.Cyclic strain influences the expression of the vascular endothelial growth factor (VEGF) and the hypoxia inducible factor 1 alpha (HIF-1alpha) in tendon fibroblasts. J Orthop Res2004;22:847–853.CrossrefPubMed Google Scholar
112 Wang JH , IosifidisMI, FuFH. Biomechanical basis for tendinopathy. Clin Orthop Relat Res2006;443:320–332.CrossrefPubMed Google Scholar
113 Schulze-Tanzil G , Al-SadiO, WiegandE, et al.The role of pro-inflammatory and immunoregulatory cytokines in tendon healing and rupture: new insights. Scand J Med Sci Sports2011;21:337–351.CrossrefPubMed Google Scholar
114 Riley G . The pathogenesis of tendinopathy: a molecular perspective. Rheumatology (Oxford)2004;43:131–142. Google Scholar
115 Pasternak B , AspenbergP. Metalloproteinases and their inhibitors-diagnostic and therapeutic opportunities in orthopedics. Acta Orthop2009;80:693–703.CrossrefPubMed Google Scholar
116 Dudhia J , ScottCM, DraperER, et al.Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity. Aging Cell2007;6:547–556.CrossrefPubMed Google Scholar
117 Chang HN , PangJH, ChenCP, et al.The effect of aging on migration, proliferation, and collagen expression of tenocytes in response to ciprofloxacin. J Orthop Res2012;30:764–768.CrossrefPubMed Google Scholar
118 Zhang J , PanT, LiuY, WangJH. Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. J Orthop Res2010;28:1178–1183.CrossrefPubMed Google Scholar
119 Zhang J , WangJH. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res2010;28:639–643.CrossrefPubMed Google Scholar
120 Zhang J , WangJH. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. J Orthop Res2010;28:198–203.CrossrefPubMed Google Scholar
121 Bi Y , EhirchiouD, KiltsTM, et al.Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med2007;13:1219–1227.CrossrefPubMed Google Scholar
122 Zhou Z , AkinbiyiT, XuL, et al.Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell2010;9:911–915.CrossrefPubMed Google Scholar
123 Tsai WC , ChangHN, YuTY, et al.Decreased proliferation of aging tenocytes is associated with down-regulation of cellular senescence-inhibited gene and up-regulation of p27. J Orthop Res2011;29:1598–1603.CrossrefPubMed Google Scholar
Funding statement:
This manuscript was funded by the Musculoskeletal Biomedical Research Unit of the National Institute for Health Research and the Lord Nuffield Scholarship for Orthopaedic Surgery.
Author contributions:
B. J. F. Dean: Study conception and design, Data collection and analysis, Drafting of the article and critical revision of article
S. Franklin: Drafting of article and critical revision of article
A. J. Carr: Study conception and design, Critical revision of article
ICMJE Conflict of Interest:
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
©2012 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










