Abstract
Objectives
To study the vascularity and bone metabolism of the femoral head/neck following hip resurfacing arthroplasty, and to use these results to compare the posterior and the trochanteric-flip approaches.
Methods
In our previous work, we reported changes to intra-operative blood flow during hip resurfacing arthroplasty comparing two surgical approaches. In this study, we report the vascularity and the metabolic bone function in the proximal femur in these same patients at one year after the surgery. Vascularity and bone function was assessed using scintigraphic techniques. Of the 13 patients who agreed to take part, eight had their arthroplasty through a posterior approach and five through a trochanteric-flip approach.
Results
One year after surgery, we found no difference in the vascularity (vascular phase) and metabolic bone function (delayed phase) at the junction of the femoral head/neck between the two groups of patients. Higher radiopharmaceutical uptake was found in the region of the greater trochanter in the trochanteric-flip group, related to the healing osteotomy.
Conclusions
Our findings using scintigraphic techniques suggest that the greater intra-operative reduction in blood flow to the junction of the femoral head/neck, which is seen with the posterior approach compared with trochanteric flip, does not result in any difference in vascularity or metabolic bone function one year after surgery.
Article focus
Does a drop in intra-operative blood flow to the femoral head during the posterior approach influence long-term vascularity or metabolic bone function?
Key messages
One year after surgery, scintigraphic imaging could not demonstrate any residual difference in vascularity or metabolic bone function to the femoral head/neck region
Strengths and limitations
Bone scintigraphy is a functional imaging tool that enables vascularity and metabolic bone function to be quantitatively assessed, and this can be performed in the presence of metal implants
Bone scintigraphy is limited by the sensitivity and spatial resolution of the imaging equipment
The study is limited by the small number of patients investigated
Introduction
The functional anatomy of the blood supply to the head and neck of the femur has been a point of debate for many years. However, since the femoral head and neck are removed during total hip replacement, the issue was mostly of academic interest to arthroplasty surgeons. The use of resurfacing arthroplasty, where the head and neck are preserved, has recently brought this debate back into focus.
Traditionally, the posterior approach was favoured by the majority of surgeons performing resurfacing arthroplasty of the hip, but this approach has been associated with damage to vessels supplying the femoral head and neck.1,2 Other surgical approaches may avoid such injury; including the trochanteric flip approach described by Ganz et al.3 Several studies have subsequently confirmed that there is a significant difference in intra-operative blood flow between the two approaches.4-7 However, most patients undergoing resurfacing through a posterior approach do not suffer avascular necrosis.8 Our hypothesis was that this may be because the reduction in blood flow associated with a posterior approach is only transient, and recovers in the post-operative period.
We studied the same group of patients who had taken part in our previous intra-operative study.1 The aim of this study was to compare the vascularity and metabolic bone function at the proximal femur between posterior and trochanteric flip groups at one year after hip resurfacing. Metabolic bone function is influenced by bone vascularity and osteoblastic activity and was assessed by a scintigraphic technique, using the relative uptake of a bone-seeking radiopharmaceutical.
Patients and Methods
The Local Research Ethics Committee approved the study and a licence was obtained from the Administration of Radioactive Substances Advisory Committee (ARSAC).
All 24 patients who took part in the intra-operative study were considered for inclusion. Of these, 13 patients agreed to take part. A summary of patient details is given in Table I.
Table I
Patient details
| Surgical approach | ||
|---|---|---|
| Characteristic | Trochanteric flip (n = 5) | Posterior (n = 8) |
| Male:female | 4:1 | 3:5 |
| Mean age (yrs) (range) | 62.8 (60 to 71) | 51.3 (32 to 60) |
| Side (right:left) | 1:4 | 6:2 |
| Mean time since surgery (mths) (range) | 10.6 (10 to 12) | 11.1 (10 to 12) |
Planar and Single Photon Emission Computed Tomography (SPECT/CT) scans
Each patient had an injection of 600 MBq Tc-99m-oxidronate (Technoscan; HDP Covidien, Fareham, United Kingdom) through an intravenous cannula. An initial series of anterior planar dynamic images (5 seconds per frame) were acquired for 120 seconds starting at the time of injection (arterial phase). An anterior planar static image was subsequently acquired at 300 seconds post-injection (venous phase). An anterior planar static image and a SPECT acquisition, with an accompanying low-dose CT, were performed at three hours after the injection using a dual headed SPECT/CT system (Hawkeye; GE Healthcare, Amersham, United Kingdom) fitted with low energy high-resolution collimators. SPECT images were acquired over 360° and consisted of 120 projections (60 per head), each of 30 seconds duration; total emission image acquisition time was 30 minutes. Immediately following emission image acquisition, a low dose CT acquisition (120 kV, 2.5 mA, 10 mm slice thickness) was acquired without the patient moving from the same bed position. Tomographic images were obtained by an iterative reconstruction technique incorporating a measured attenuation correction map. Reconstruction filtration consisted of a Butterworth low-pass filter with an order 15 and cut-off of 0.35 cycles/pixel. The data was saved in coronal, transverse and sagittal slices. Total radiation exposure to the patient was 4 mSv.
Image analysis
Images were analysed using a GE Xeleris functional imaging workstation (GE Healthcare, Hatfield, United Kingdom). The images were studied in two phases; 1) the early phase images taken within 5 minutes of isotope injection, and 2) delayed phase images taken three hours after injection. The early phase was sub-divided into arterial and venous phases. The dynamic arterial phase images showed tracer in only major arterial trunks; these images were not assessed further. The venous images were assessed using defined regions of interest (ROIs) derived from the delayed planar image. The planar vascular and delayed images were aligned and the three ROIs defined on the delayed image were applied to both the vascular and delayed images (Fig. 1).
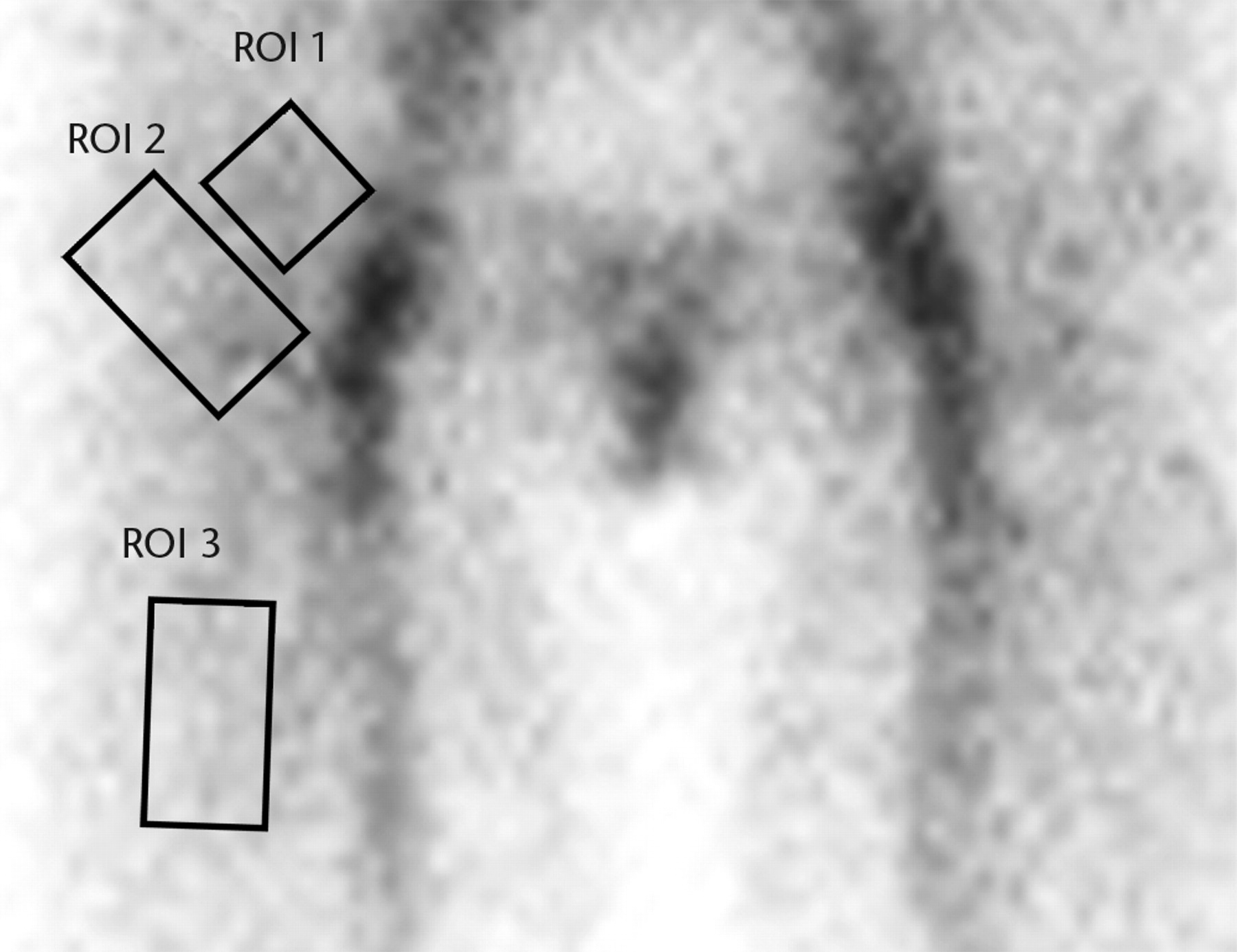
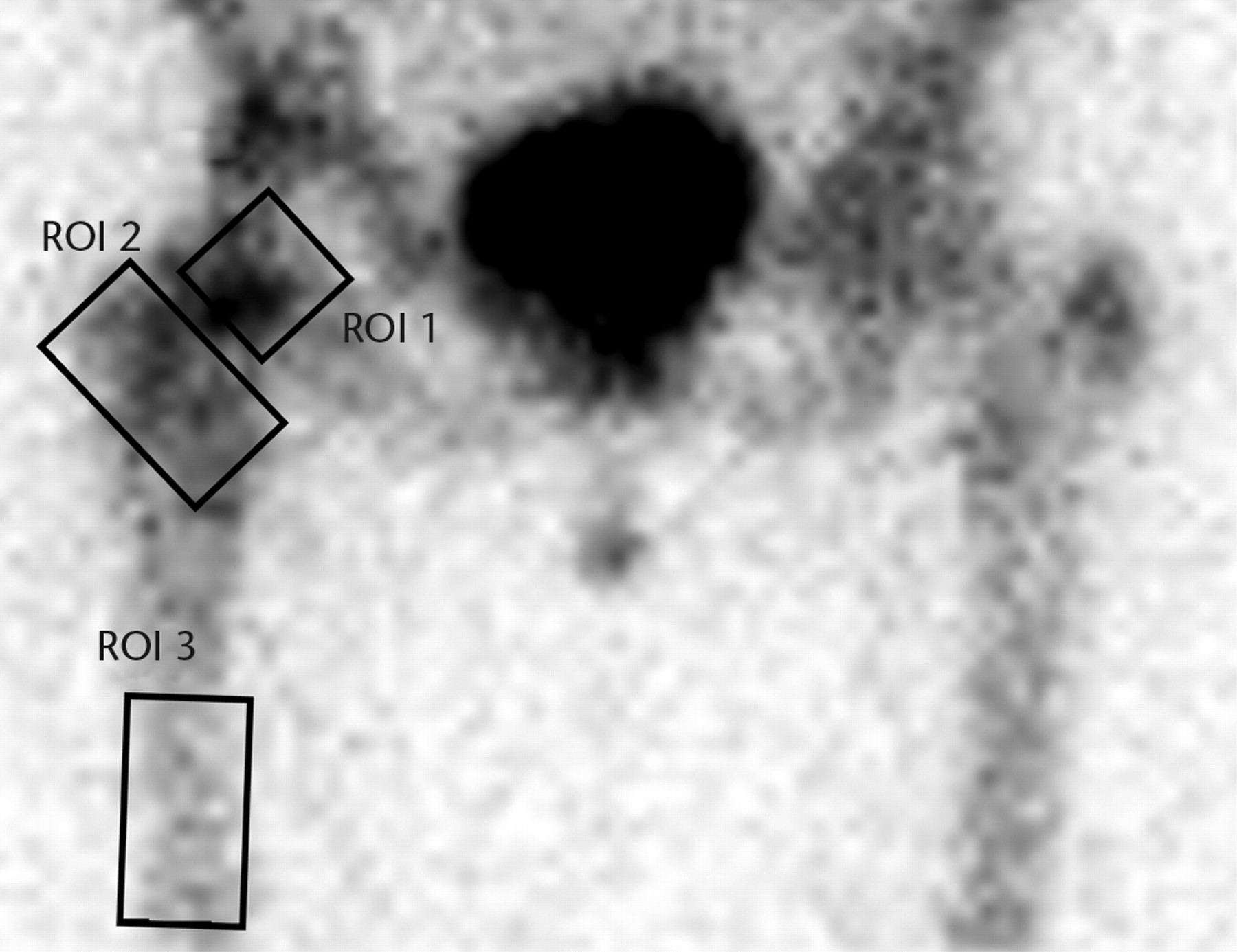
Figs. 1a - 1b
Planar images showing the three regions of interest (ROIs) in a) the early (vascular) phase and b) the late phase.
The regions of interest were defined as:
ROI 1: the femoral head-neck bone, extending from the margin of the femoral implant into the inter-trochanteric region. This includes the head-neck junction and the neck region. This corresponds to the bone studied intra-operatively using the LASER Doppler flow meter in our previous study.1
ROI 2: the inter-trochanteric region of bone between the neck of the femur and the inter-trochanteric line. This also includes the greater and lesser trochanters. In the trochanteric flip group this included the osteotomy site.
ROI 3: the upper shaft to midshaft of femur. This region was used as a control.
The inter-trochanteric region (ROI 2) was adjusted, when necessary, to avoid overlying any major blood vessels on the venous images. For both the venous and delayed planar images the mean count in the femoral neck and inter-trochanteric regions (ROI 1 and 2) were calculated and expressed as a ratio to the mean count in the corresponding femoral region (ROI 3), which was used as a control.
For the delayed SPECT/CT images all coronal slices were summed to allow regional quantification of tracer uptake. The regions of interest were defined as for the planar images but with two additional ROIs to quantify background (non-bone) activity in the region of the prosthesis (ROI 4) and mid-femur (ROI 5) (Fig. 2).
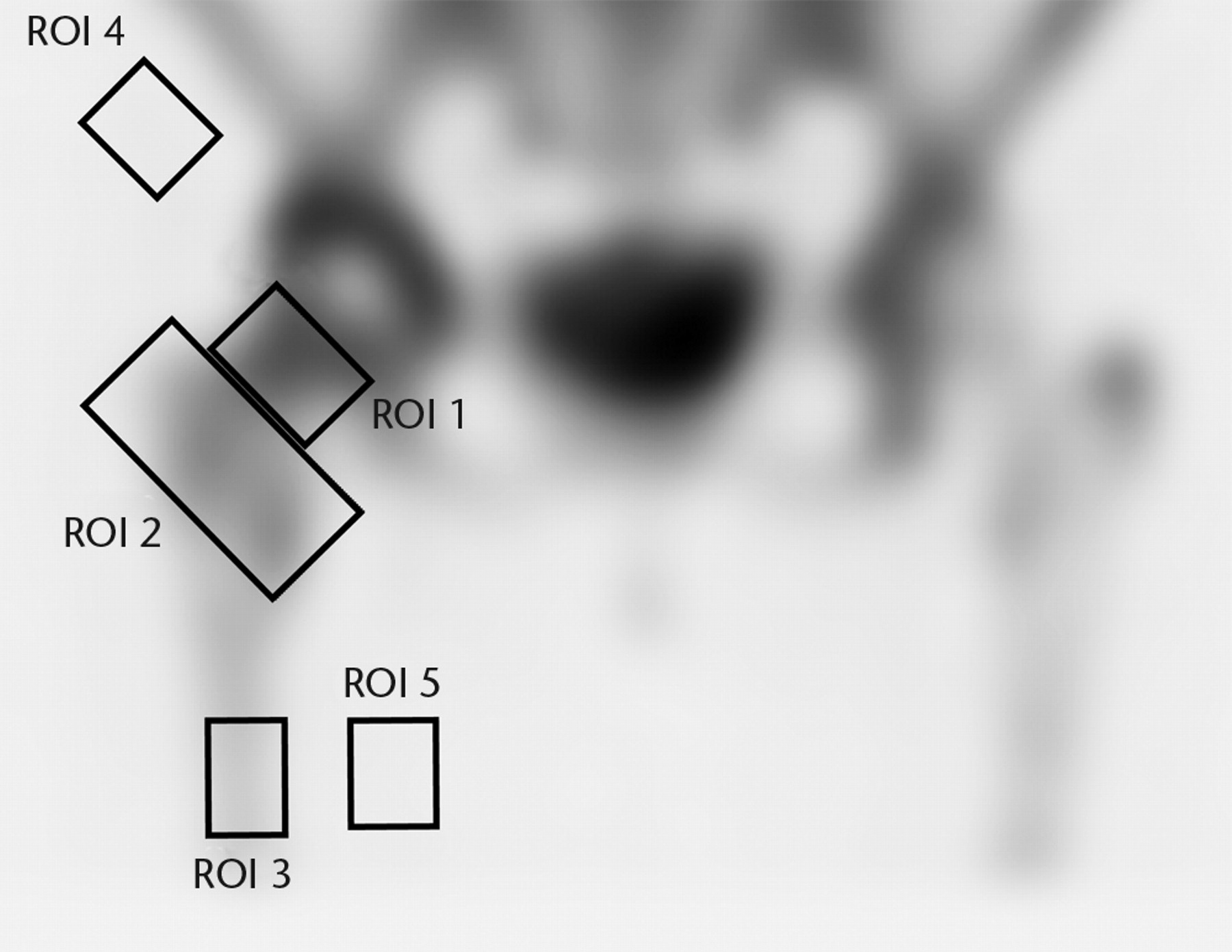
Fig. 2
A single photon emission computed tomography (SPECT-CT) image showing the five regions of interest (ROIs).
Statistical analysis
Average background corrected counts derived from the femoral neck and inter-trochanteric ROIs were calculated and expressed as a ratio to the background corrected counts from the mid femur region. Differences in radionuclide uptake between approaches (Trochanteric flip and Posterior) were displayed graphically using box and whisker plots and formally tested using Mann-Whitney tests, with significance set at the 5% level.
In order to assess the reliability of the definition of the ROIs for the SPECT/CT image data, three experienced assessors assessed images and independently determined appropriate ROIs. Intraclass correlation coefficients (ICC) were calculated for each ROI and used to assess interobserver variation using the ratings suggested by Landis and Koch9,10 to assess agreement: 0 to 0.2 poor, 0.2 to 0.4 fair, 0.4 to 0.6 moderate, 0.6 to 0.8 substantial, and 0.8 to 1.0 almost perfect.
Results
The dynamic arterial phase images were visually assessed as showing no increased activity around the prosthesis for any patient; therefore these data were not analysed further.
For both the venous and delayed planar images the mean count in the femoral neck and inter-trochanteric regions (ROIs 1 and 2) were calculated and expressed as a percentage of the mean count in the corresponding femoral region (ROI 3). Figure 3 shows boxplots of these data for ROI 1 and ROI 2 for both early and late data. The median radionuclide uptake and range for ROI 1 in the trochanteric-flip and posterior approach groups were 1.20% (1.00% to 1.33%) and 1.32% (1.14% to 1.43%) for early data and 2.75% (1.97% to 3.50%) and 2.97% (2.24% to 3.83%) for the late data. Equivalent figures for ROI 2 were 1.18% (1.03% to 1.43%) and 1.17% (0.95% to 1.28%) for early data and 3.02% (2.59% to 3.41%) and 2.11% (1.52% to 2.80%) for the late data. There was no statistically significant difference in radionuclide uptake between the posterior approach group and the trochanteric flip approach group in the head/neck region (ROI 1) for either early or late data (p = 0.127 and p = 0.683, respectively; Mann-Whitney test). However, for the inter-trochanteric region (ROI 2), patients who underwent the trochanteric flip approach showed a significant increase in uptake compared with the posterior approach for late phase (p = 0.028; Mann-Whitney test), but not for the early phase (p = 0.943).
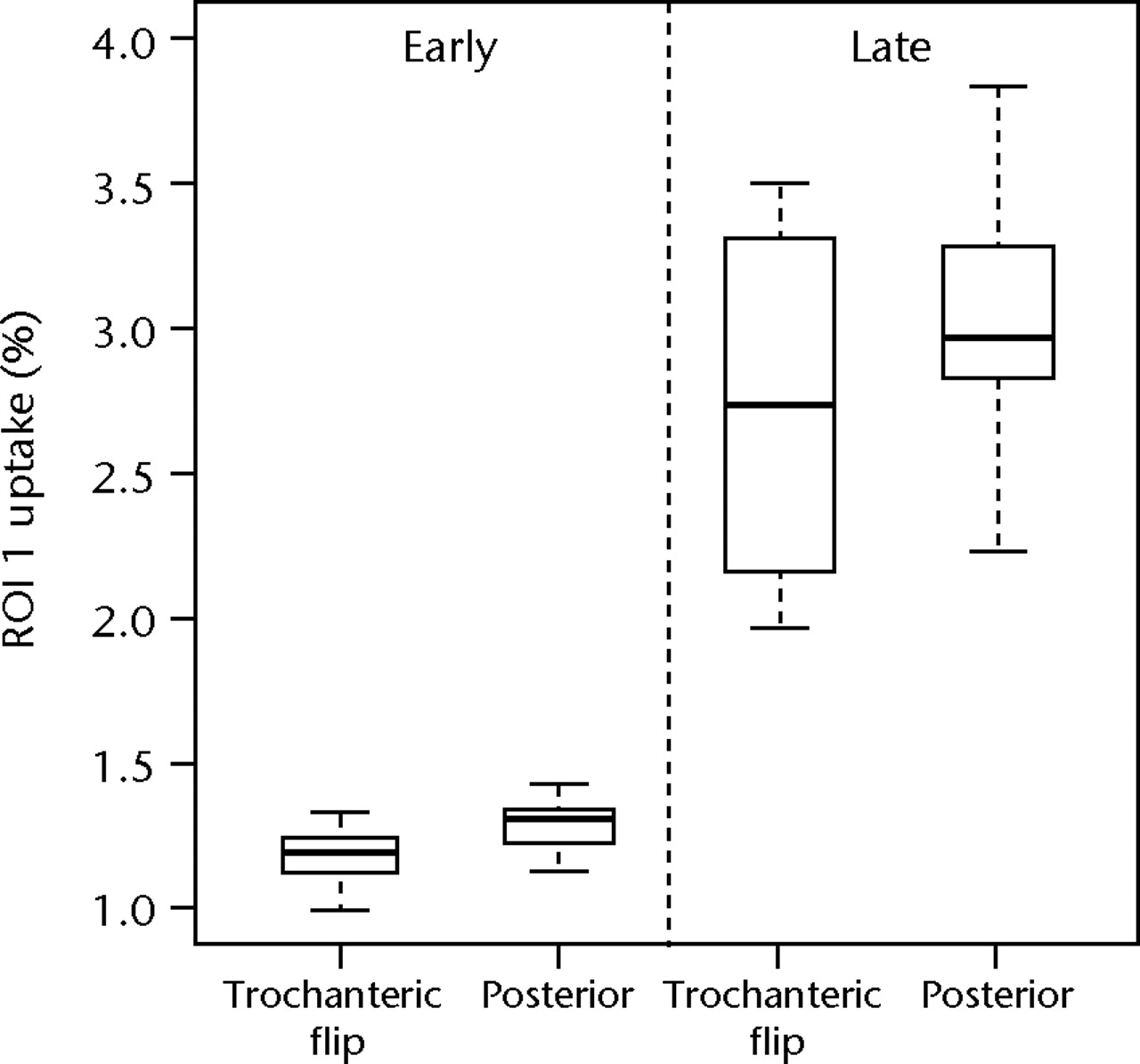
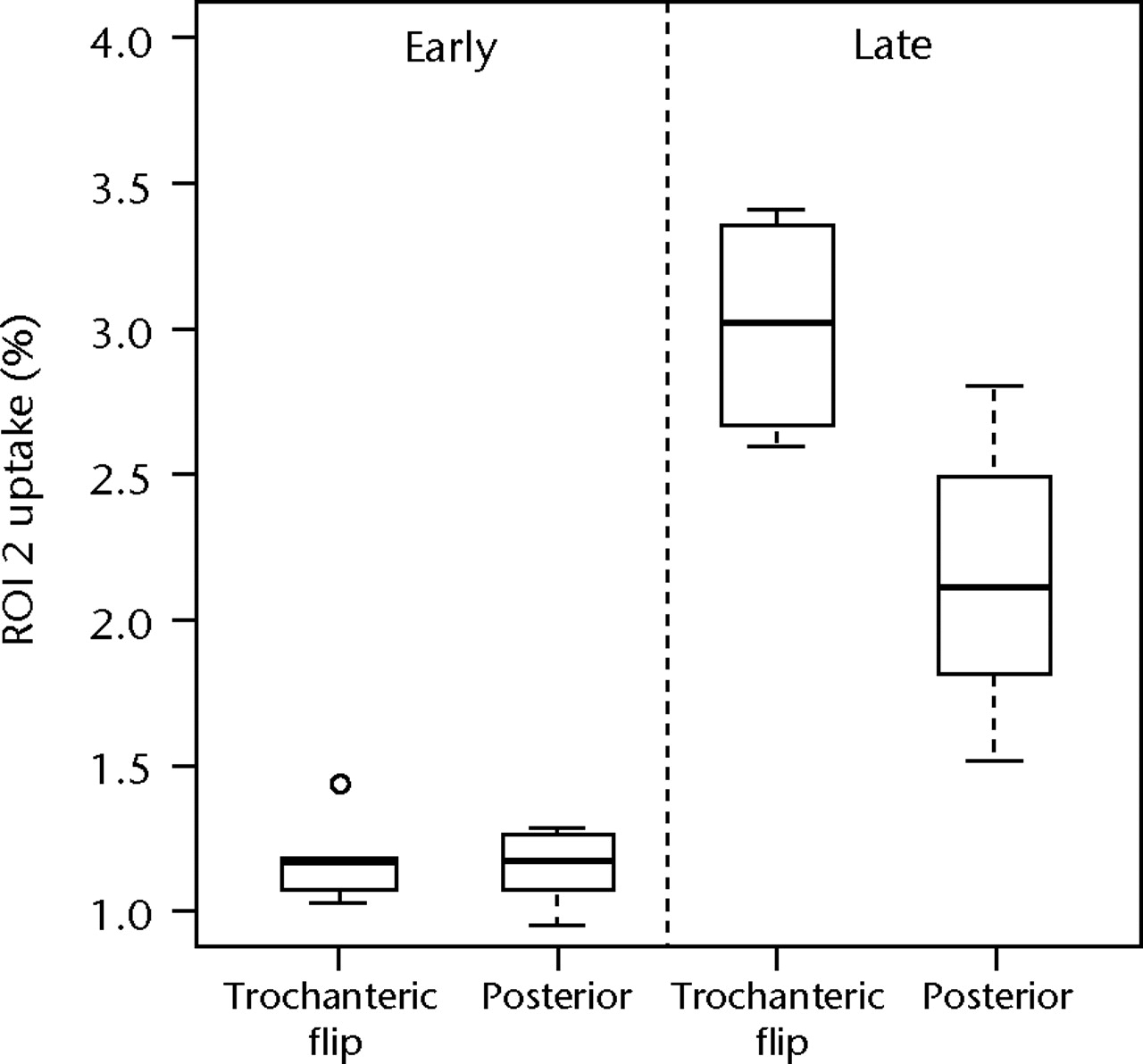
Figs. 3a - 3b
Boxplots of radionuclide uptake for a) region of interest (ROI) 1 and b) ROI 2 for planar image data, both expressed as a percentage of uptake in ROI 3, for each surgical approach (trochanteric flip and posterior) for late and early data. The boxes represent the median and interquartile range (IQR), the whiskers denote 1.5×IQR and ° denotes outliers.
For SPECT/CT image data, uptake in ROI 1 and ROI 2 was expressed as a percentage of the average count in the corresponding femoral region (ROI 3). The estimated ICCs based on three independent observations of the SPECT/CT images for ROI 1 and 2 were 0.694 (95% bootstrapped confidence interval (CI) 0.388 to 0.827) and 0.666 (95% CI 0.438 to 0.889), indicating that there was substantial agreement between assessors in the definition of the both ROI 1 and ROI 2.
Figure 4 shows boxplots of these data for ROI 1 and ROI 2 data. The median radionuclide uptake and range for ROI 1 in the trochanteric-flip and posterior approach groups were 4.12% (2.76% to 5.06%) and 3.56% (2.59% to 4.62%), respectively, and in ROI 2 were 3.22% (2.85% to 3.72%) and 1.83% (1.45% to 3.37%), respectively. There was no statistically significant difference in radionuclide uptake between the posterior approach group and the trochanteric flip approach group in the head/neck region (ROI 1) (p = 0.724, Mann-Whitney test). However, for the inter-trochanteric region (ROI 2), patients who underwent the trochanteric flip approach showed a significant increase in uptake compared with the posterior approach (p = 0.011, Mann-Whitney). A replication of the Mann-Whitney tests based on the definitions of the ROIs by the secondary assessors gave p-values of 1.000 and 0.284 for ROI 1, and 0.003 and 0.006 for ROI 2. Therefore, the inferences drawn from these data were not dependent on the individual assessor used to define the ROIs.
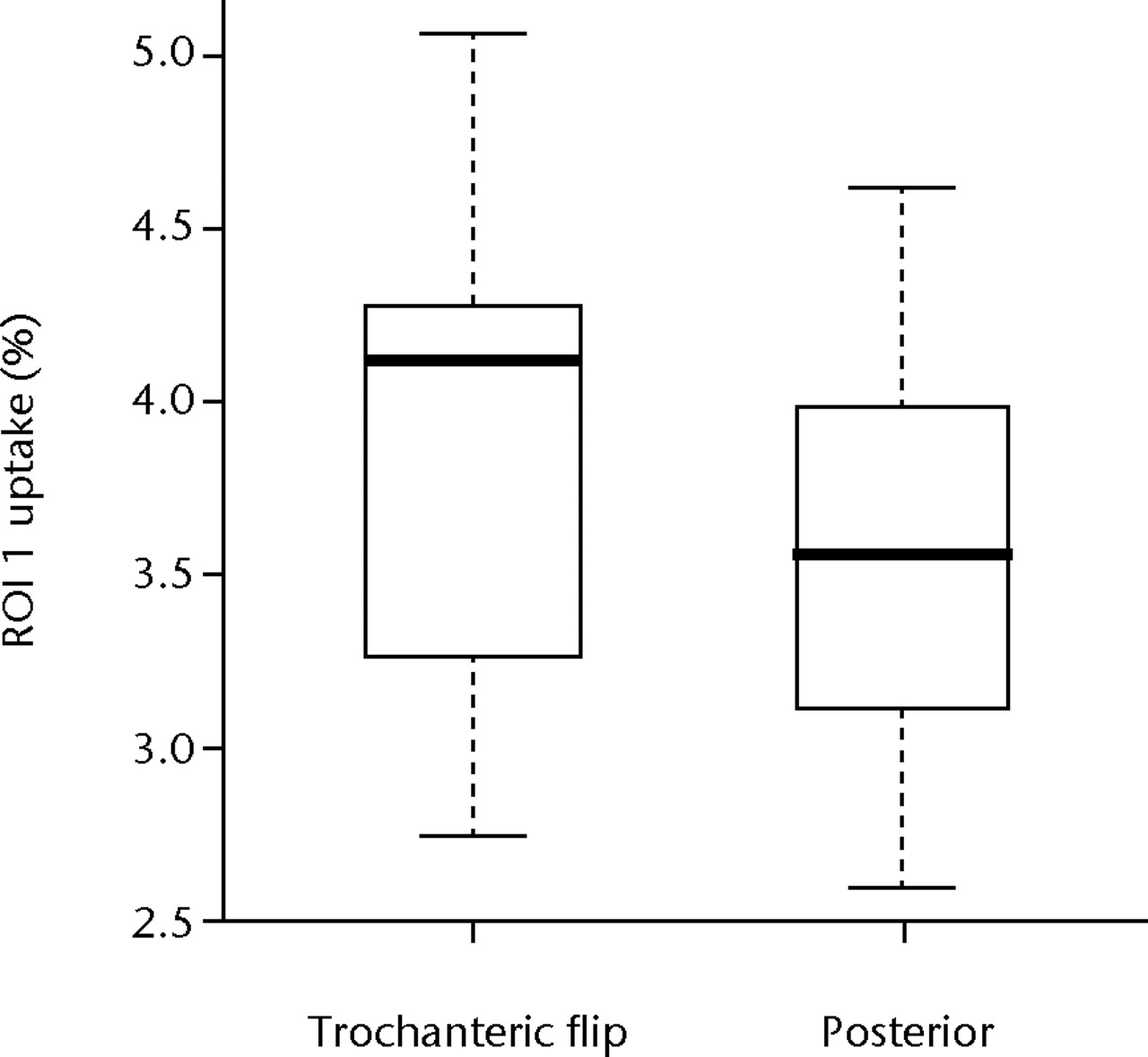
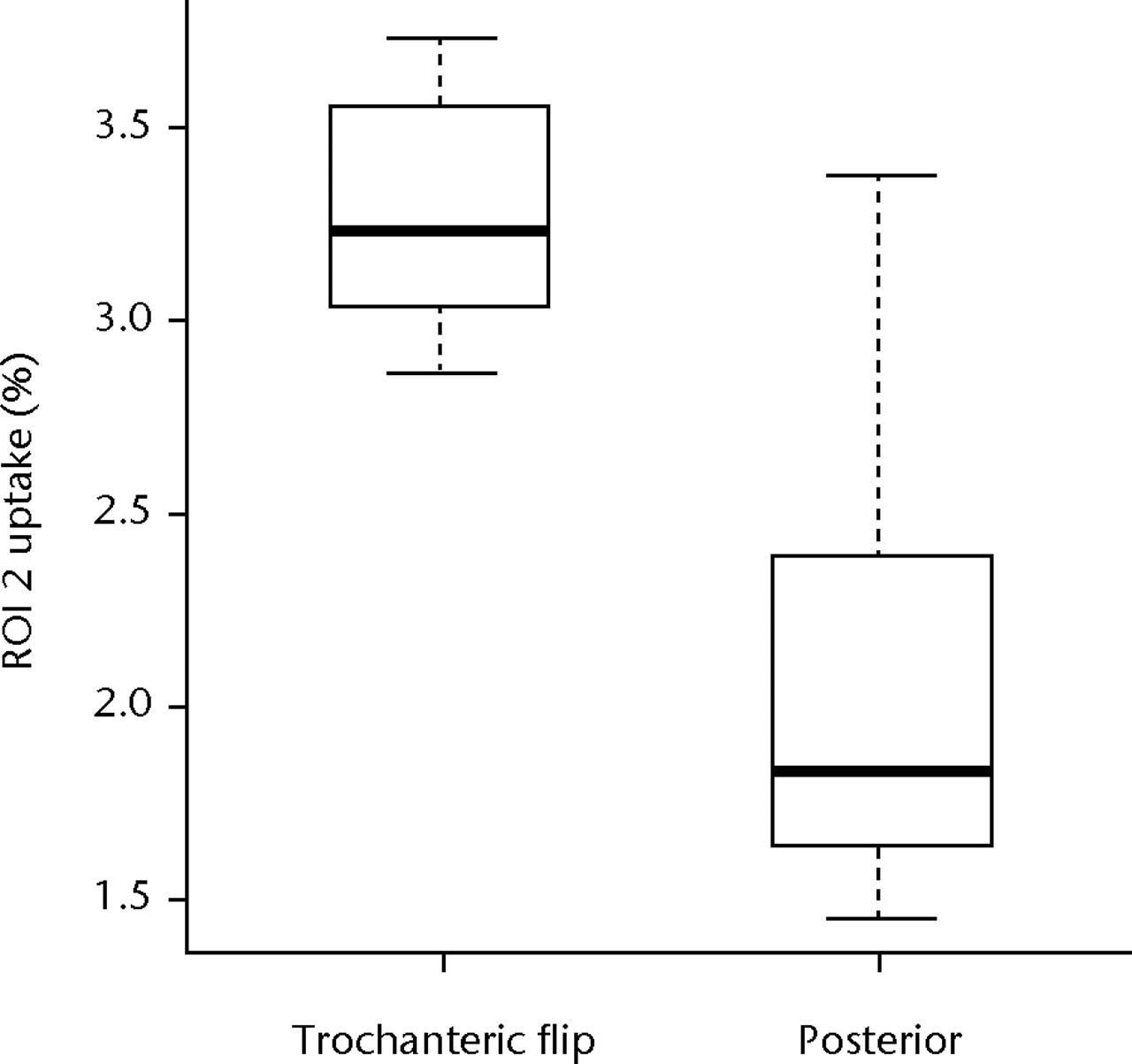
Figs. 4a - 4b
Boxplots of radionuclide uptake for a) region of interest (ROI) 1 and b) ROI 2 for single photon emission computed tomography (SPECT-CT) image data, both expressed as a percentage of uptake in ROI 3, for each surgical approach (trochanteric flip and posterior). The boxes represent the median and interquartile range (IQR), the whiskers denote 1.5×IQR and ° denotes outliers.
In summary, the results in both delayed and late phases of planar images and the SPECT CT images did not show any statistically significant difference in ROI 1 (the head/neck region) between the groups, but increased uptake was seen in trochanteric flip group in ROI 2 (the inter-trochanteric region) in late and SPECT CT images.
Discussion
Previous studies have shown a clear decrease in the blood supply to the junction of the head and neck during hip surgery. The posterior approach appears to cause a larger decrease than other approaches,1,5-7 so alternatives such as the trochanteric-flip osteotomy have been used in an attempt to preserve the vascularity.3,11 Our intra-operative blood flow study showed a 40% drop in the posterior approach group in comparison with only a 11% drop seen with the trochanteric flip approach.1 However, the clinical importance of this intra-operative reduction in blood flow, and the potential for recovery in the early post-operative period, have not been investigated as extensively.8,12
The post-operative assessment of the femoral head and neck using imaging remains a challenge: especially in the presence of metal implants. Of the modalities that are available, the most suitable would appear to be functional bone imaging with SPECT-CT or PET–CT scans.13,14 F18 sodium fluoride-positron emission tomography (PET) scans have a higher sensitivity but availability is currently limited.15 We decided to use SPECT-CT in this study for several reasons including the wide use of the general imaging technique, the availability of the facility and cost.
Using planar data imaging, we found that there was no statistically significant difference of radionuclide uptake in the femoral head/neck region (ROI 1) between the posterior approach and the trochanteric-flip approach one year after a resurfacing arthroplasty of the hip both during early (p = 0.127) and delayed phases (p = 0.683). This indicates that the intra-operative reduction in blood flow, which we identified in our patients having a posterior approach, does not give rise to any compromise in vascularity as assessed by a scintigraphic technique at one year after surgery. The initial loss of blood supply may be transient but appears to recover during the post-operative period. However, we cannot say exactly when it recovers within the period of one year.
It is well known that chronic ischemia stimulates collateral vessel formation in many tissues, including bone. This was shown by Freeman16 and later tested by Whiteside et al17 using a canine model. It is possible that the reduction in blood flow caused during surgery may lead to the development of collateral circulation in the proximal femur. The only statistically significant difference between the two groups occurred in the delayed phase of inter-trochanteric region (ROI 2), where the trochanteric flip group showed increased bone activity compared with the posterior group (p = 0.028). This can be explained by the increased osteoblastic activity in this region caused by the healing of the trochanteric flip osteotomy. This suggests that the osteotomy site is still active even after one year. However in the same region (ROI 2) during the vascular phase there was no difference between the two groups (p = 0.943), supporting our hypothesis that post-operative vascularity is the same in both groups.
Further analysis of the images using SPECT-CT data showed similar findings. SPECT-CT has been used in many clinical scenarios18-20 and of the various methods available to detect post-operative bone function, SPECT seems to be the most appropriate for this group of patients.21 Bone SPECT has a lower false negative rate than plain bone scans in diagnosing impaired or enhanced bone function,22 and therefore this technique has been recommended as a tool to assess post-operative bone activity in orthopaedic patients.23,24 MRI is another option,25 but the metal resurfacing implants interfere with the results, particularly around the head and neck of the femur.23,26
There have been concerns regarding the validity of the attenuation correction technique used to obtain the SPECT-CT images, due to the adjacent metal of the prosthesis. To validate the attenuation correction, we conducted a separate phantom modelling study that confirmed the attenuation correction method works adequately even in the presence of the resurfacing prosthesis. This was done by making phantom models of the hip with point sources of the radionuclide Tc99m, kept under and adjacent to the resurfacing metal implants. SPECT-CT images were acquired with the implants initially and the tests repeated without using the implants. There was no significant difference in corrected uptake between the two groups, indicating that the attenuation correction system functioned correctly even in the presence of a resurfacing implant.27
The main limitation is the relatively small number of subjects available for this study. Our initial study of intra-operative blood flow included a formal sample size calculation.1 However, the nature of the current investigation meant that several of the 24 subjects included in the earlier study had to be excluded or declined to take part. This raises the possibility of both a type II error, that is a lack of statistical power resulting in our false acceptance of the null hypothesis of no difference between the surgical approaches, or a type I error, false rejection of the null hypothesis, caused by the disproportionate influence of a small number of measurements. However, the relatively narrow ranges observed for both planar and SPECT CT image data (Figs 3 and 4) indicate good precision in our data, with no obvious outliers with strong leverage on the analysis. Also, the fact that a highly significant difference was observed for ROI 2 suggests that if there were actually similar differences between the two groups for ROI 1, there was sufficient power to reject the null hypotheses in these cases. For these reasons, and the strong evidence of substantial reliability in definitions of ROIs (ICCs of 0.694 and 0.666 for ROI 1 and ROI 2, respectively), we believe that the results do provide evidence for differences in metabolic bone function in the inter-trochanteric region (ROI 2) and no significant difference at the head/neck junction (ROI 1) despite the small number of patients.
The second limitation is that we have not included direct measurements from the part of the bone directly covered by the metal implant i.e. the head-neck region is a ‘surrogate’ for the activity under the implant itself. However, our previous intra-operative study addressed the blood-flow in this same head/neck region and demonstrated clear differences between the approaches in this area.2
Despite our results, loosening, fractures and avascular necrosis do still occur in the early post-operative period in some patients.28 The cause of such complications is likely to be multi-factorial, with the blood flow to this region of bone playing a role.29 This is clinically extremely relevant, as the failures have raised concerns over the widespread use of resurfacing as an alternative to total hip arthroplasty.30-32 Data from the National Joint Registry for England and Wales has shown a sharp fall in hip resurfacing operations over the last few years.33
In conclusion, this study demonstrates that the decrease in vascularity at the head/neck junction shown during the intra-operative period appears to be transient and vascularity and bone metabolism is the same in both groups at one year after surgery. Secondly, the study shows that the trochanteric osteotomy site still shows metabolic bone activity even one year after surgery. Further studies may be needed to determine when and how this blood flow recovers.
The authors would like to acknowledge the contribution of S. Manketlow and R. Penny, Department of Nuclear Medicine, University Hospitals of Coventry and Warwickshire. This study was performed on behalf of the University Hospital Coventry and Warwickshire Resurfacing Arthroplasty Group. This group consists of Mr S. Krikler, Mr U. Prakash, Mr P. Foguet, Mr R. King, Professor M. Costa and Professor D. Griffin.
1 Amarasekera HW , CostaML, FoguetP, et al.The blood flow to the femoral head/neck junction during resurfacing arthroplasty: a comparison of two approaches using Laser Doppler flowmetry. J Bone Joint Surg [Br]2008;90-B:442–445.CrossrefPubMed Google Scholar
2 Crock HV . Anatomy of the medial femoral circumflex artery and its surgical implications. J Bone Joint Surg [Br]2001;83-B:149–150.PubMed Google Scholar
3 Ganz R , GillTJ, GautierE, et al.Surgical dislocation of the adult hip: a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg [Br]2001;83-B:1119–1124. Google Scholar
4 Beaulé PE , CampbellP, ShimP. Femoral head blood flow during hip resurfacing. Clin Orthop Relat Res2007;456:148–152.CrossrefPubMed Google Scholar
5 Khan A , YatesP, LoveringA, BannisterGC, SpencerRF. The effect of surgical approach on blood flow to the femoral head during resurfacing. J Bone Joint Surg [Br]2007;89-B:21–25.CrossrefPubMed Google Scholar
6 Steffen RT , SmithSR, UrbanJP, et al.The effect of hip resurfacing on oxygen concentration in the femoral head. J Bone Joint Surg [Br]2005;87-B:1468–1474.CrossrefPubMed Google Scholar
7 Beaulé PE , CampbellP, LuZ, et al.Vascularity of the arthritic femoral head and hip resurfacing. J Bone Joint Surg [Am]2006;88-A(Suppl 4):85–96.CrossrefPubMed Google Scholar
8 McMahon SJ , YoungD, BallokZ, et al.Vascularity of the femoral head after Birmingham hip resurfacing: a technetium Tc 99m bone scan/single photon emission computed tomography study. J Arthroplasty2006;21:514–521. Google Scholar
9 Landis JR , KochGG. The measurement of observer agreement for categorical data. Biometrics1977;33:159–174.PubMed Google Scholar
10 Koch GG , LandisJR, FreemanJL, FreemanDH Jr, LehnenRC. A general methodology for the analysis of experiments with repeated measurement of categorical data. Biometrics1977;33:133–158.PubMed Google Scholar
11 Steffen RT , FernD, NortonM, MurrayDW, GillHS. Femoral oxygenation during hip resurfacing through the trochanteric flip approach. Clin Orthop Relat Res2009;467:934–939.CrossrefPubMed Google Scholar
12 Forrest N , WelchA, MurrayAD, et al.Femoral head viability after Birmingham resurfacing hip arthroplasty: assessment with use of [18F] fluoride positron emission tomography. J Bone Joint Surg [Am]2006;88-A(Suppl 3):84–89.CrossrefPubMed Google Scholar
13 van der Bruggen W , Bleeker-RoversCP, BoermanOC, GotthardtM, OyenWJ. PET and SPECT in osteomyelitis and prosthetic bone and joint infections: a systematic review. Semin Nucl Med2010;40:3–15.CrossrefPubMed Google Scholar
14 Gholamrezanejhad A , MirpourS, MarianiG. Future of nuclear medicine: SPECT versus PET. J Nucl Med2009;50:N16–N18.PubMed Google Scholar
15 Cheng D , WangY, LiuX, et al.Comparison of 18F PET and 99mTc SPECT imaging in phantoms and in tumored mice. Bioconjug Chem2010;21:1565–1570.CrossrefPubMed Google Scholar
16 Freeman MA . Some anatomical and mechanical considerations relevant to the surface replacement of the femoral head. Clin Orthop Relat Res1978;134:19–24.PubMed Google Scholar
17 Whiteside LA , LangeDR, CapelloWR, FraserB. The effects of surgical procedures on the blood supply to the femoral head. J Bone Joint Surg [Am]1983;65-A:1127–1133.PubMed Google Scholar
18 Mariani G , BruselliL, KuwertT, et al.A review on the clinical uses of SPECT/CT. Eur J Nucl Med Mol Imaging2010;37:1959–1985.CrossrefPubMed Google Scholar
19 Scharf S . SPECT/CT imaging in general orthopedic practice. Semin Nucl Med2009;39:293–307.CrossrefPubMed Google Scholar
20 Linke R , KuwertT, UderM, ForstR, WuestW. Skeletal SPECT/CT of the peripheral extremities. AJR Am J Roentgenol2010;194:W329–W335.CrossrefPubMed Google Scholar
21 Collier BD , CarreraGF, JohnsonRP, et al.Detection of femoral head avascular necrosis in adults by SPECT. J Nucl Med1985;26:979–987.PubMed Google Scholar
22 Calder SJ , McCaskieAW, BeltonIP, FinlayDB, HarperWM. Single-photon-emission computerised tomography compared with planar bone scan to assess femoral head vascularity. J Bone Joint Surg [Br]1995;77-B:637–639.PubMed Google Scholar
23 Ryu JS , KimJS, MoonDH, et al.Bone SPECT is more sensitive than MRI in the detection of early osteonecrosis of the femoral head after renal transplantation. J Nucl Med2002;43:1006–1011.PubMed Google Scholar
24 Horger M , BaresR. The role of single-photon emission computed tomography/computed tomography in benign and malignant bone disease. Semin Nucl Med2006;36:286–294.CrossrefPubMed Google Scholar
25 Thickman D , AxelL, KresselHY, et al.Magnetic resonance imaging of avascular necrosis of the femoral head. Skeletal Radiol1986;15:133–140.CrossrefPubMed Google Scholar
26 Fordyce MJ , SolomonL. Early detection of avascular necrosis of the femoral head by MRI. J Bone Joint Surg [Br]1993;75-B:365–367.CrossrefPubMed Google Scholar
27 Amarasekera HW , CostaML, ParsonsN, et al.SPECT/CT bone imaging after hip resurfacing arthroplasty: is it feasible to use CT attenuation correction in the presence of metal implants?Nucl Med Commun2011;32:289–297.CrossrefPubMed Google Scholar
28 Shimmin AJ , BareJ, BackDL. Complications associated with hip resurfacing arthroplasty. Orthop Clin North Am2005;36:187–193.CrossrefPubMed Google Scholar
29 Carrothers AD , GilbertRE, JaiswalA, RichardsonJB. Birmingham hip resurfacing: the prevalence of failure. J Bone Joint Surg [Br]2010;92-B:1344–1350.CrossrefPubMed Google Scholar
30 Bolland BJ , LathamJM, WhitwellD. Hip resurfacing: early failures cause concern. BMJ2010;341:5632. Google Scholar
31 Patterson AH , ColemanJH. Hip resurfacing: a fresh look at an alternative to total joint arthroplasty. JAAPA2010;23:44–47.PubMed Google Scholar
32 Jiang Y , ZhangK, DieJ, et al.A systematic review of modern metal-on-metal total hip resurfacing vs standard total hip arthroplasty in active young patients. J Arthroplasty2011;26:419–426.CrossrefPubMed Google Scholar
33 Prime MS , PalmerJ, KhanWS. The National Joint Registry of England and Wales. Orthopedics2011;34:107–110. Google Scholar
Funding statement:
A grant for this study was received from the British Orthopaedic Association Joint Action Wishbone Walk Fund
Author contributions:
H. W. Amarasekera: Study design, Patient recruitment, Data collection, Data analysis, Writing the paper
M. L. Costa: Study design, Writing the paper
N. Parsons: Data analysis, Writing the paper
J. Achten: Study design
D. R. Griffin: Study design
P. Roberts: Patient recruitment
N. R. Williams: Performing scans, Data collection, Data analysis, Writing the paper
ICMJE Conflict of Interest:
None declared
©2012 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










