Abstract
Objectives
Pseudotumours (abnormal peri-prosthetic soft-tissue reactions) following metal-on-metal hip resurfacing arthroplasty (MoMHRA) have been associated with elevated metal ion levels, suggesting that excessive wear may occur due to edge-loading of these MoM implants. This study aimed to quantify in vivo edge-loading in MoMHRA patients with and without pseudotumours during functional activities.
Methods
The duration and magnitude of edge-loading in vivo was quantified during functional activities by combining the dynamic hip joint segment contact force calculated from the three-dimensional (3D) motion analysis system with the 3D reconstruction of orientation of the acetabular component and each patient’s specific hip joint centre, based on CT scans.
Results
Edge-loading in the hips with pseudotumours occurred with a four-fold increase in duration and magnitude of force compared with the hips without pseudotumours (p = 0.02).
Conclusions
The study provides the first in vivo evidence to support that edge-loading is an important mechanism that leads to localised excessive wear (edge-wear), with subsequent elevation of metal ion levels in MoMHRA patients with pseudotumours.
Article focus
Quantification of in vivo edge-loading in MoMHRA patients with and without pseudo-tumours during functional activities
Key messages
Edge-loading is an important in vivo mechanism that leads to localised excessive wear with subsequent elevation of metal ion levels in MoMHRA patients with pseudotumours
Although orientation of the acetabular component appears to be an important factor in edge-loading, the aetiology of edge-loading is likely to be multifactorial
Strengths and limitations
This is the first study to provide in vivo evidence of edge-loading in MoMHRA patients
Generalisation of the current study findings to MoMHRA with larger femoral component sizes may be limited as the femoral component sizes in the current study are ≤ 50 mm
Introduction
Despite the encouraging short-term clinical follow-up studies of metal-on-metal hip resurfacing arthroplasty (MoMHRA),1-3 recent reports of abnormal peri-prosthetic soft-tissue reactions are causing concerns.4-10 These so-called soft-tissue pseudotumours,9 defined as non-infected solid and/or cystic soft-tissue mass in patients with MoMHRA,11 have been associated with elevated serum and hip aspirate levels of cobalt (Co) and chromium (Cr), the principal elements in the CoCr alloy used in MoMHRA implants.9,12,13 As metal-on-metal (MoM) implant wear has been reported to be positively correlated with the elevation of metal ion concentrations in vivo,14 this suggests that pseudo-tumours in patients with contemporary MoMHRA implants are associated with increased wear at the MoM articulation.
Recently, the maximum wear of the MoMHRA acetabular components revised due to pseudotumour has been observed to occur near the edge of the implant, indicating edge-loading.15 Although retrieval studies provide valuable information, these studies are limited by the fact that the implants were retrieved as a result of failure, with inevitable selection bias. This selection bias excludes examination of well-functioning implants or implants prior to failure. The extrapolation of in vitro retrieval study results to in vivo conclusions may therefore not always be appropriate.
In order to determine if there is edge-loading in vivo, it is necessary to determine where the hip joint reaction force passes through the acetabular component. If the resultant hip joint force were directed near the edge of the acetabular component, edge-loading would be indicated. Measuring in vivo hip joint forces in patients with implants in situ during functional activities, however, presents numerous difficulties. Researchers have utilised both instrumented total hip prostheses with in-built force transducers16-19 and mathematical modelling20-23 in order to measure or predict in vivo resultant forces across the hip joint. Video fluoroscopy has been used to determine the in vivo kinematics of the hip joint.24,25 More recently, a combination of the computed tomography (CT) and three-dimensional (3D) lower limb motion capture data has been used to determine the in vivo hip contact force vector with the acetabular component in MoMHRA patients during functional activities such as walking and stair descent.26 However, the incidence, duration and magnitude of edge-loading during functional activities have not been previously quantified.
The aim of this study was to assess whether edge--loading occurs in MoMHRA patients with and without pseudo-tumours by quantifying the in vivo resultant hip joint segment force relative to the acetabular component (force path) during functional activities.
Patients and Methods
The duration and magnitude of edge-loading in vivo was quantified during functional activities by integrating the dynamic hip joint segment contact force calculated from the 3D motion analysis system with the 3D reconstruction of orientation of the acetabular component based on the CT scan, as recently described by Mellon et al.26
Patient selection
A total of 21 patients (33 hips) were investigated in this study, which received local research ethics committee approval. The patients were divided into two groups (Table I): Group 1 – MoMHRA patients with pseudotumours (abnormal soft-tissue reactions with or without cystic elements) confirmed with MRI (six patients (nine hips)); the three patients with bilateral implants in this group were found to have pseudo-tumours in both hips (Fig. 1); and Group 2 (control) – patients with well-functioning MoMHRA implants without pseudotumours (15 patients (24 hips)). These patients were recruited from a population of patients who had participated in a MoMHRA surveillance study at the authors’ institution,13 as screening hip ultrasound/MRI scans had been performed in these patients to detect the presence of pseudotumours. Those patients with pseudotumours were invited first, and the invitation to other patients among the total population was subsequently based on matching approximately two -individuals from the control group to one from the pseudotumour group with respect to gender, age, size of the femoral component and time since surgery. Exclusion criteria included the presence of significant pain or impairment which would restrict walking, stair climbing, or sitting onto or rising from a chair. This was to ensure that patients in the study were able to perform these functional activities for the motion analysis study.
Table I
Summary of study patient groups
| Characteristic | Pseudotumour | Non-pseudotumour |
|---|---|---|
| Patients | 6 | 15 |
| Hips | 9 | 24 |
| Male | 1 (1 bilateral) | 3 (3 bilateral) |
| Female | 5 (2 bilateral) | 12 (6 bilateral) |
| Mean age (yrs) (range) | 55 (44 to 58) | 56 (46 to 60) |
| Implant type* | ||
| BHR | 7 | 18 |
| Conserve Plus | 2 | 6 |
| Median femoral component size (mm) (range) | 45 (44 to 50) | 46 (46 to 50) |
| Mean time since surgery (mths) (range) | 60 (36 to 79) | 64 (32 to 88) |
| Surgical approach | Posterior | Posterior |
-
* BHR, Birmingham Hip Resurfacing (Smith & Nephew); Conserve Plus (Wright Medical Technology)
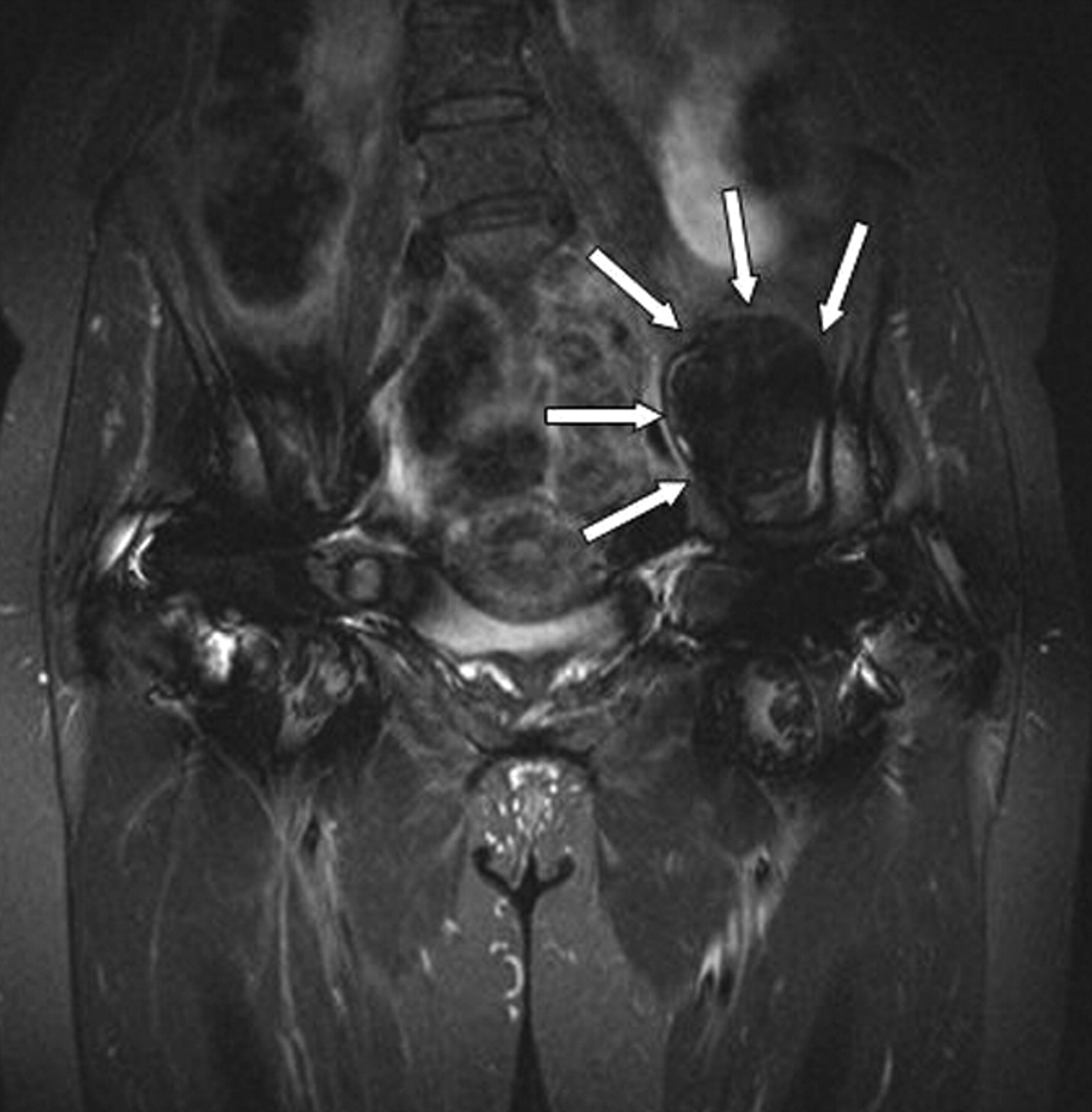
Fig. 1
Coronal Short TI Inversion Recovery (STIR) MRI image of a typical example of predominantly solid pseudotumour with low signal intensity (arrows).
3D lower limb motion analysis
Markers were attached on specific anatomical landmarks in accordance with the Plug-In-Gait lower body marker set.27 Data was captured using a 12-camera Vicon Nexus Motion Analysis System (Oxford Metrics Ltd, Oxford, United Kingdom) operating at a video capture rate of 100 Hz and two floor-embedded force platforms (Advanced Medical Technology Inc., Watertown, Massachusetts) with an analogue data capture rate of 1000 Hz, while patients performed functional activities (level walking, rising from and sitting down onto a chair, and stair climbing). Data processing was performed with commercially available software (BodyBuilder) (Vicon Motion Systems; Oxford Metrics., Oxford, United -Kingdom) using the Plug-In-Gait model (Version 1.9) with customisation of the model on a patient-specific basis to account for individual hip joint centre location determined using CT data. Inter-segmental hip joint force and moments were calculated in a sequential process using a well-established inverse dynamics method.27
CT scans
CT scans of the patients’ pelvis and lower limbs were obtained using a high-resolution 64-slice CT scanner (Siemens Somatom; Siemens Medical Solutions USA Inc., New York, New York) with metal artifact reduction sequence. In order to ensure that the pelvic coordinate system in the motion analysis system (defined by three pelvic markers) could also be defined within CT data, the pelvic motion analysis markers were replaced with radio-opaque markers at the end of the motion analysis, prior to CT scanning. The medical imaging software package -SliceOmatic v.4.2 (Virtual Magic Inc., Montreal, Canada) was used to locate the coordinates of multi-modality markers, anatomical pelvic landmarks and to determine orientation of MoMHRA acetabular components (relative to the anterior pelvic plane defined by anterior superior iliac spines and pubic tubercle) within the CT images. The location of the patient-specific hip joint centre in each resurfaced hip was determined by extracting the 3D co-ordinates of a minimum of 30 points distributed over the head of the femoral and the acetabular components.
Definition of edge-loading
Edge-loading was defined to occur when the ‘force path’ (the locus of the force vector intersection with the acetabular component throughout the load bearing) was within a distance ≤ 10% of the radius from the edge of the component (i.e. the zone at the edge of the acetabular component designated as zone 1) (Fig. 2). The figure of 10% was based on observations of edge-wear scars from a previously reported MoMHRA retrieval study.15
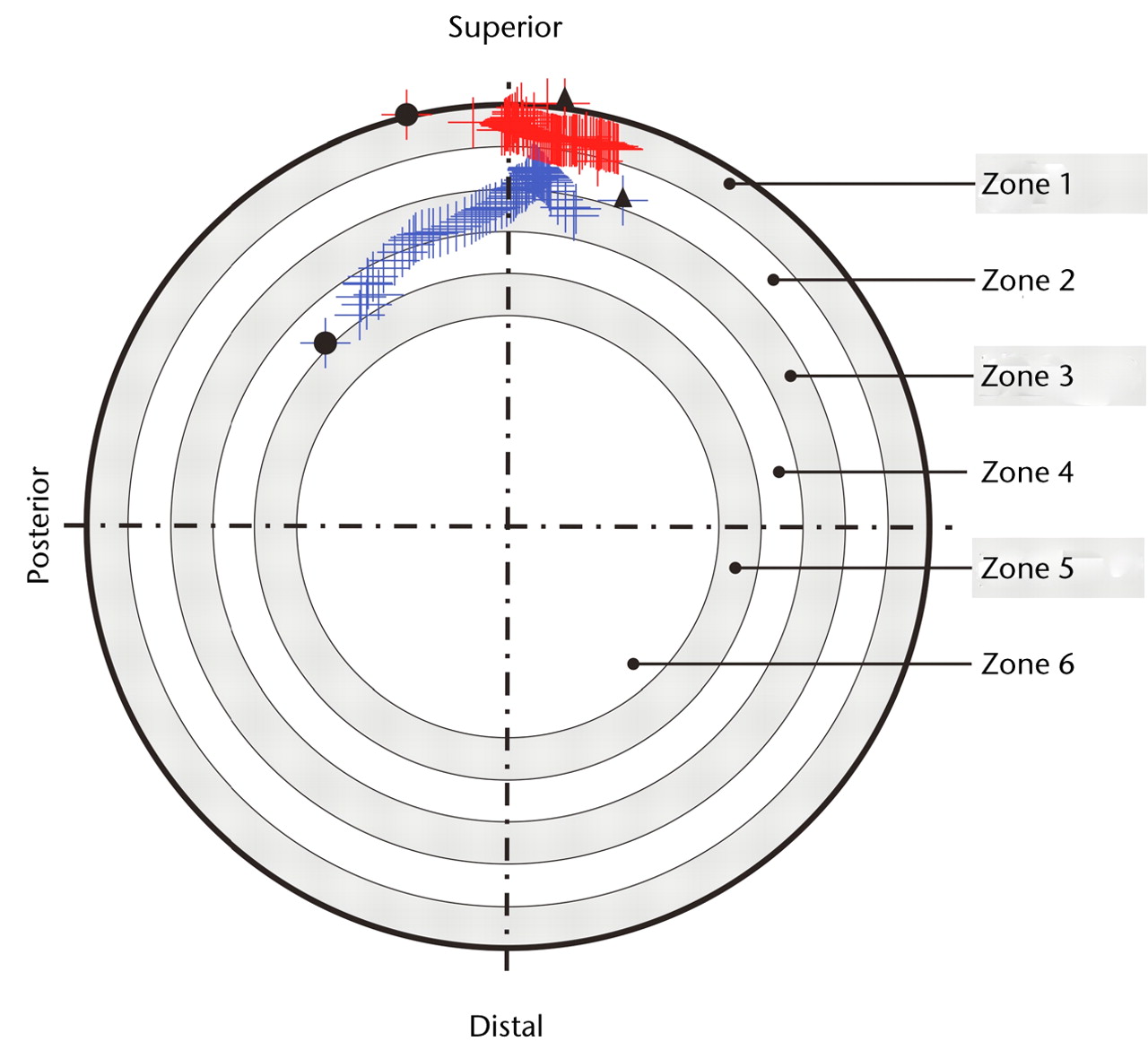
Fig. 2
Diagram showing force paths projected on the acetabular component viewed in the direction through the centre of the component. The inner bearing surface was divided into concentric zones defined in 10% increments of the component face radius, with the zone at the edge designated as zone 1. In hip A (in blue), the force path does not enter the outer most radial zone (zone 1), thus no edge-loading is observed. In hip B (in red), during walking, the force path enters the outer most radial zone (zone 1), indicating edge-loading. The black circles (●) indicate force path at heelstrike and the black triangles (p) indicate force path locus at toe-off.
Serum metal ion analysis
The venous blood samples were collected from all patients in the study in accordance with the consensus protocol.28 Serum Co and Cr levels were analysed in a blinded fashion using Inductively-Coupled Plasma Mass Spectroscopy (ICP-MS). The detection limit of Co and Cr in serum was 0.25 µg/l.
Statistical analysis
The data set was assessed for normality. Inter-group comparisons of the zone duration and the force impulse variables between the hips in groups 1 and 2 were performed using the Student’s t-test. Inter-group comparisons of the angles of the acetabular components and serum metal ion levels were performed using the Mann-Whitney non-parametric tests. Differences at p < 0.05 were considered to be significant. SPSS statistical software release 13.0 (SPSS Inc., Chicago, Illinois) was used to perform the statistical analyses.
Results
Of the 21 MoMHRA patients analysed, data from two patients (three hips) in the control group were excluded due to loss of the sacrum marker during motion analysis in one patient and movement artefact in the CT images in the other. Thus, complete data from 30 hips (nine hips with pseudotumours in six patients in group 1; and 21 hips without pseudotumours in 13 patients in group 2) were available for analysis.
Edge-loading
During walking, edge-loading (predominantly superiorly at the 12 o’clock position) was observed in all nine hips (100%) in the pseudotumour group compared with five of 21 hips (24%) in the group without pseudotumours. However, during more strenuous activities of daily living, such as rising from a chair and stair climbing, edge-loading (predominantly posteriorly at the 10 o’clock position) was observed in all hips in both groups. Representative force path plots for non-edge-loading and edge-loading hips during walking are shown in Figure 2.
Zone duration of force paths
Although edge-loading occurred in all hips in both groups, the distribution of the time spent by the force path in each zone differed significantly. During the stance phase of walking, the mean duration of force path in zone 1 (edge-loading zone) was greater in the hips with pseudotumours (39% of the stance phase) compared with those without pseudo-tumours (39% versus 15% of the stance phase; p = 0.05).
The differences in zone duration between the groups were activity-dependent (Fig. 3). During stair climbing, the differences were accentuated. There was a statistically significant four-fold increase in the mean edge-loading zone duration in the hips in the pseudotumour group compared with those in the non-pseudotumour group (51% versus 11% of the stance phase; p = 0.02). This indicated that the edge-loading lasted for five times as long in the hips with pseudotumours compared with those without during stair climbing activity. The overall trend in zone duration between stair ascent and descent was similar in both groups.
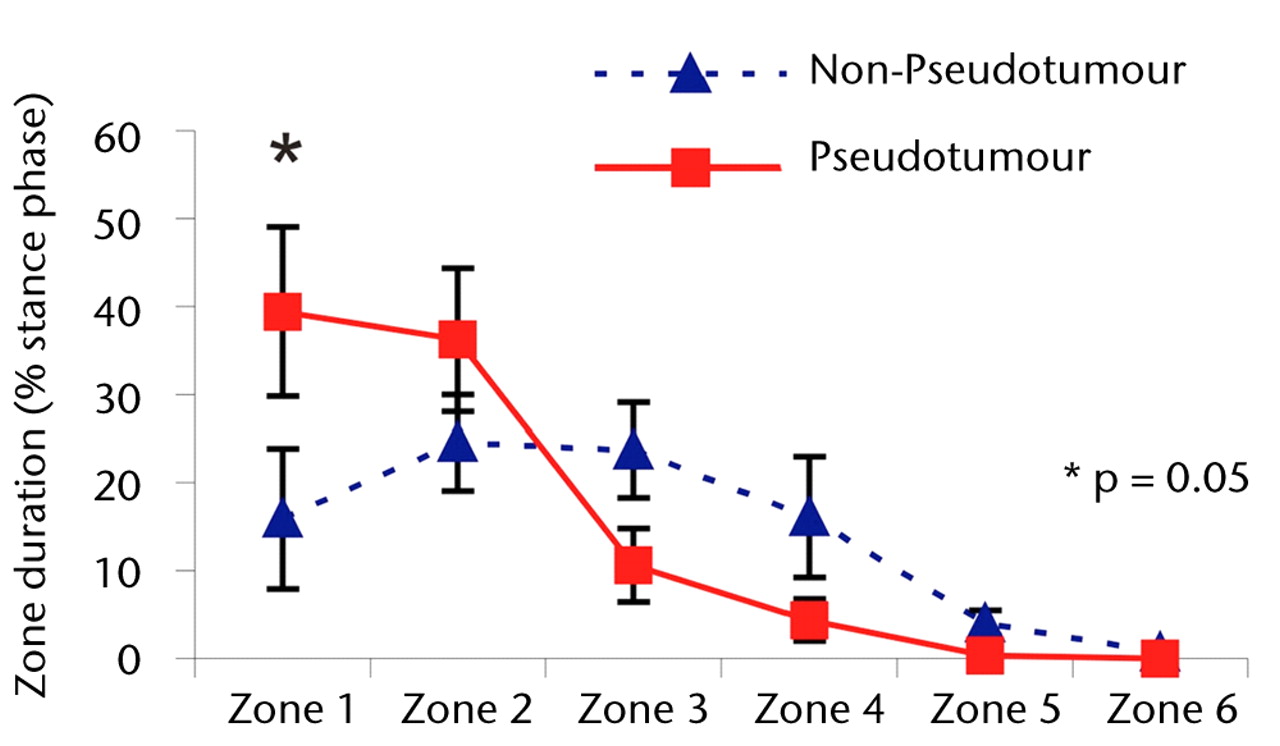
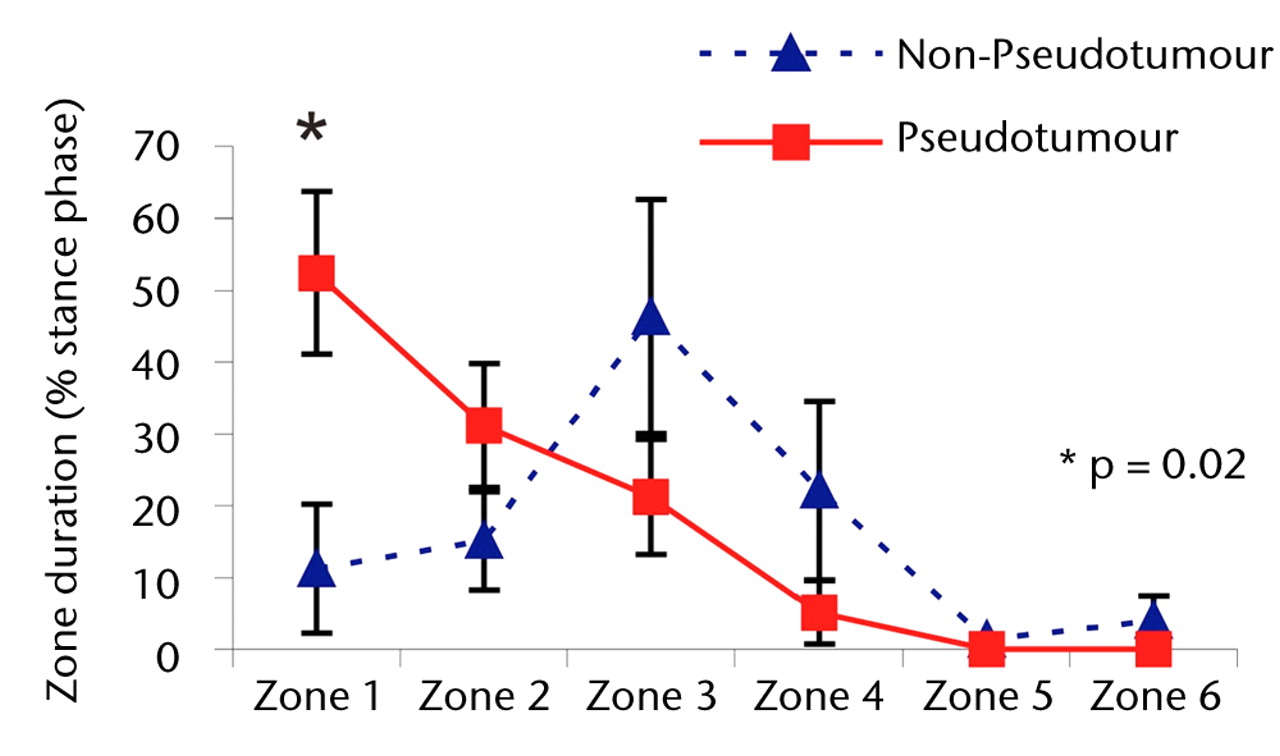
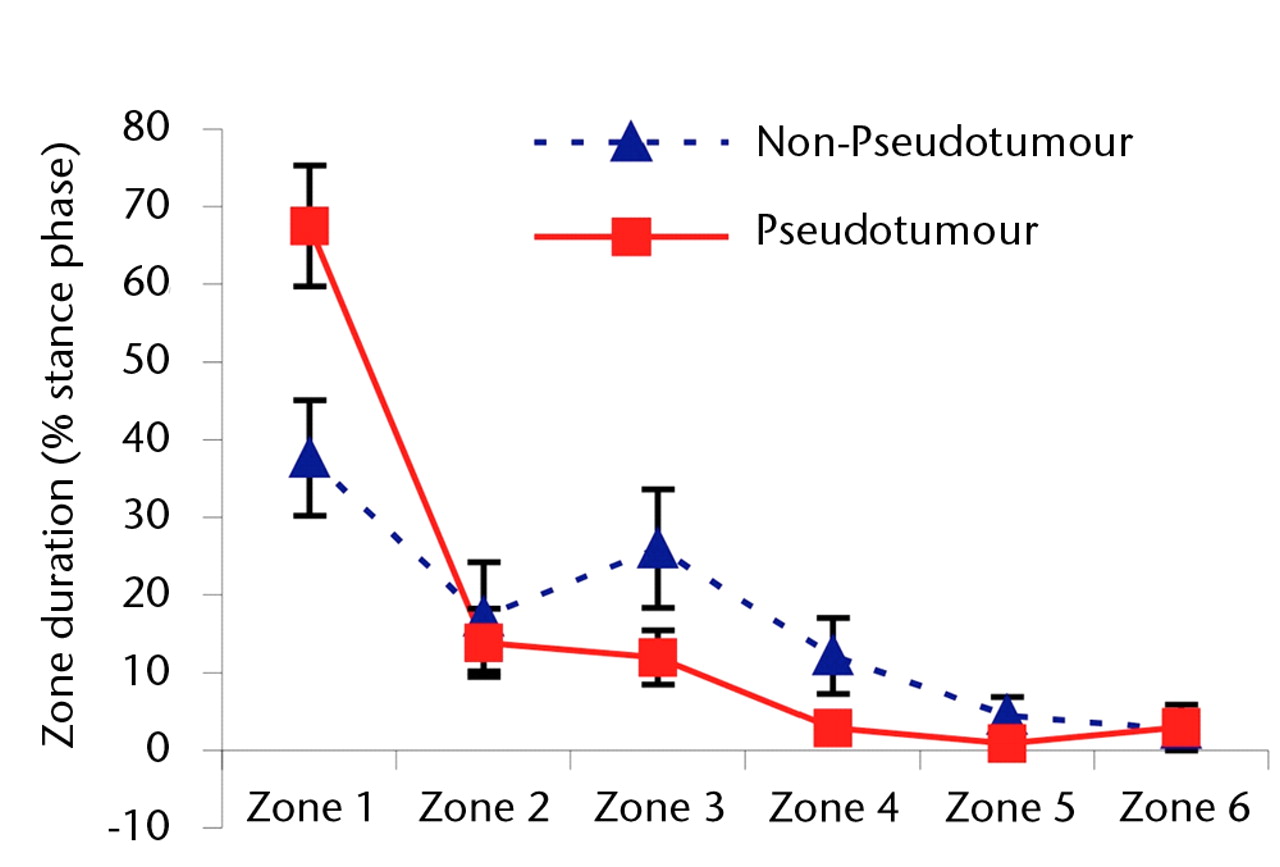
Figs. 3a - 3c
Graphs showing the distribution of ‘zone duration’ (the percentage of total stance time spent by the force path in each zone) during a) walking, b) stair climbing and c) rising from a chair. Zone 1 is defined as the edge-loading zone. The error bars represent standard errors of mean. An asterisk (*) indicates significant difference between the two MoMHRA patient groups.
The differences between the two groups were less pronounced during rising from and sitting down to a chair (Fig. 3). In fact, the highest zone duration occurred in the edge-loading zone for both groups, suggesting that, in terms of edge-loading, rising from and sitting down to a chair was the most provocative activity in the hips without pseudotumours. There was no significant difference in time duration spent in the edge-loading zone between the hips with and without pseudotumours during this activity (p = 0.15).
Force impulse distribution of force paths
Overall, a similar trend was observed with force impulse distribution of force paths as seen with zone duration (Fig. 4). During stair climbing, there was a significant four-fold increase in the value of normalised force impulse located in the edge-loading zone in the hips with pseudotumours compared with the hips without (p = 0.04). This indicated that edge-loading occurred with four times greater force impulse in the hips with pseudotumours than the hips without for each stair climbing cycle.
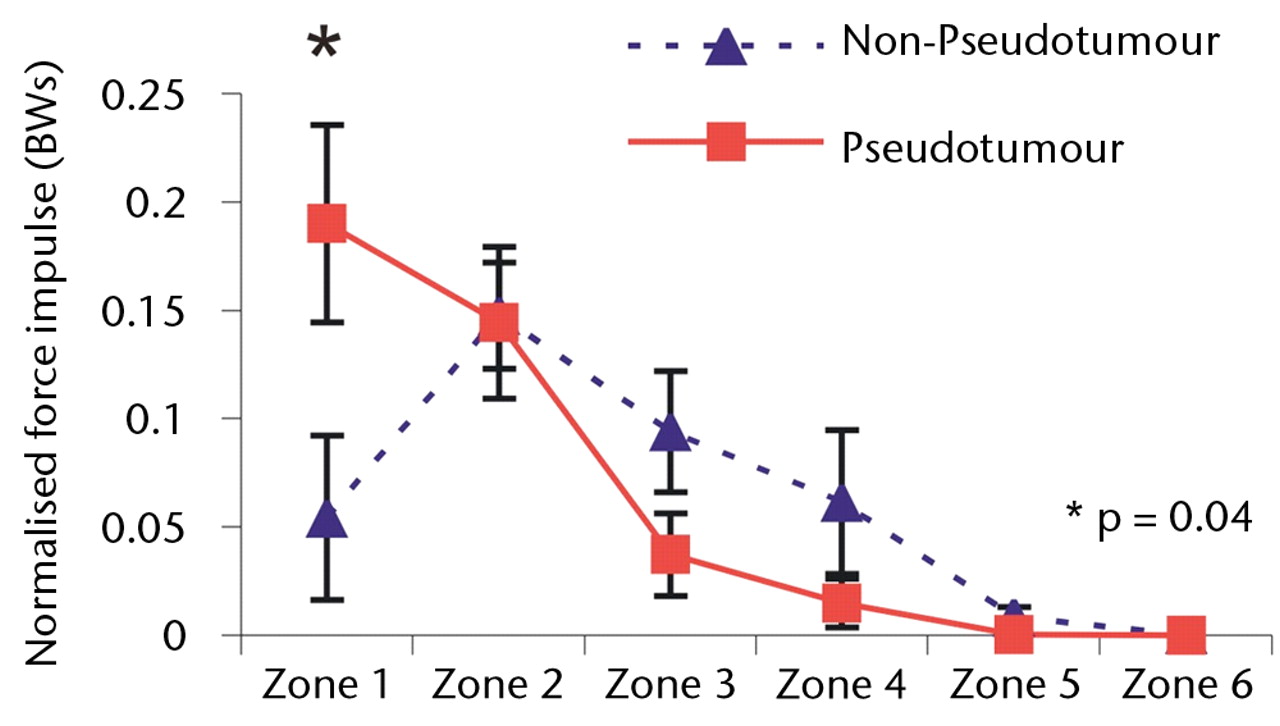
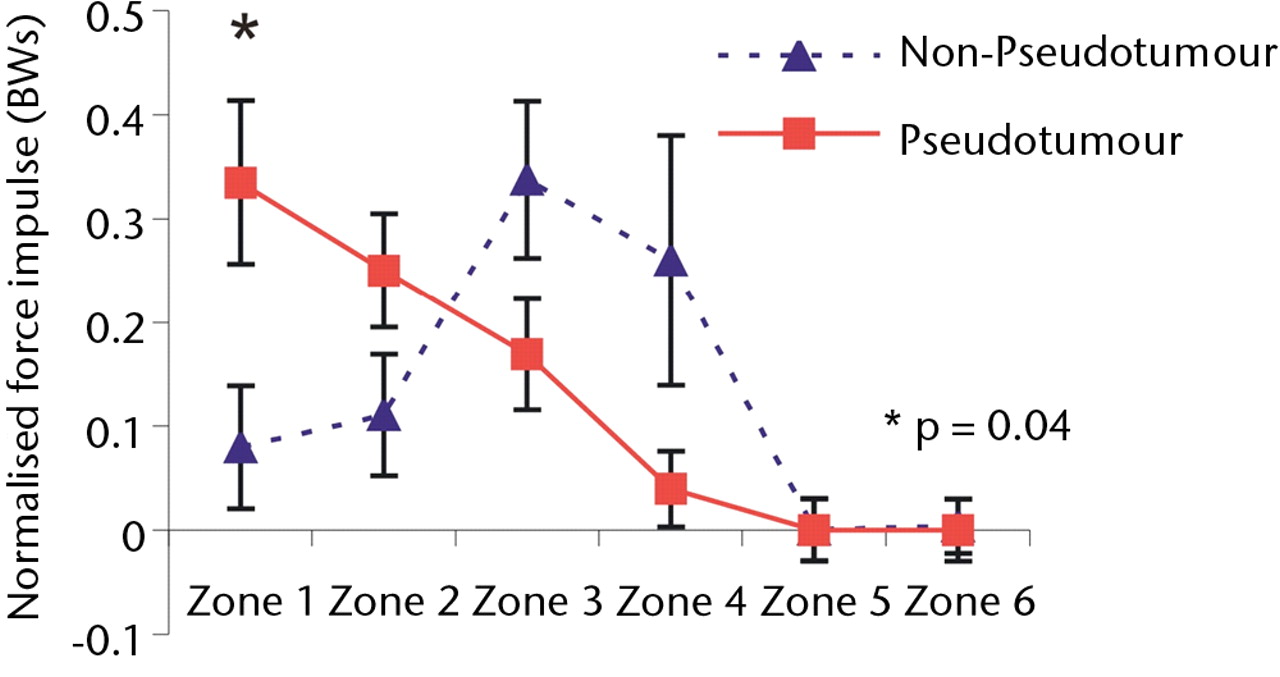
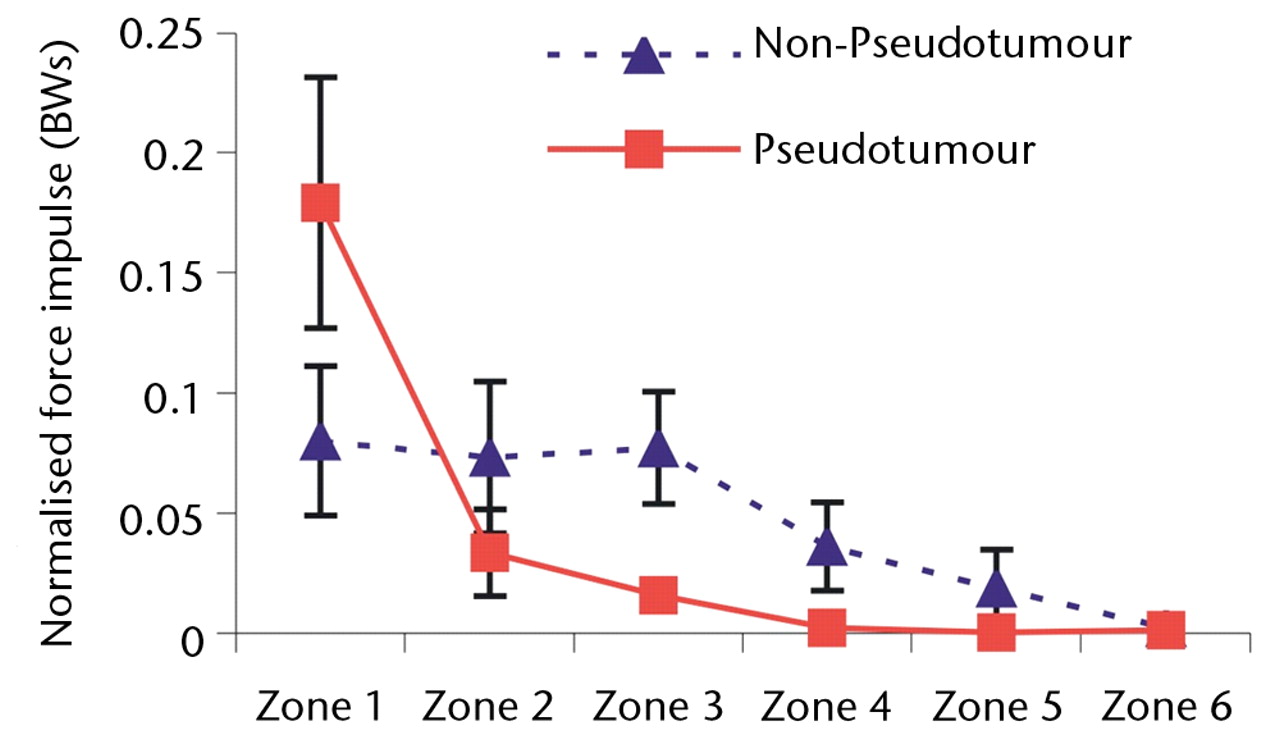
Figs. 4a - 4c
Graphs showing the distribution of normalised hip joint ‘force impulse’ (the cumulative magnitude of the segment force throughout activity over time estimated by calculating the area under the force/time curve normalised to patient body weight) in each zone during a) walking, b) stair climbing and c) rising from a chair. Zone 1 is defined as the edge-loading zone. The error bars represent standard errors of mean. An asterisk (*) indicates significant difference between the two MoMHRA patient groups.
Orientation of the acetabular component
The hips with pseudotumours were associated with a higher median inclination angle of the acetabular component compared with those without (52.0° (35.5° to 60.7°) -versus 45.1° (27.5° to 62.0°), p = 0.10), higher anteversion angle (21.2° (8.8° to 47.0°) versus 16.0° (3.4° to 35.0°, p = 0.18) and significantly higher median combined inclination and anteversion angle (75.6° (58.4° to 100.0°) -versus 58.75° (30.1° to 90.0°), p = 0.04).
Of the 21 hips without pseudotumours, 16 (76%) had inclination and anteversion angles within the safe zone of Lewinnek29 (Fig. 5). In comparison, only three of the nine hips with pseudotumours (33%) had their orientation angles within the safe zone.
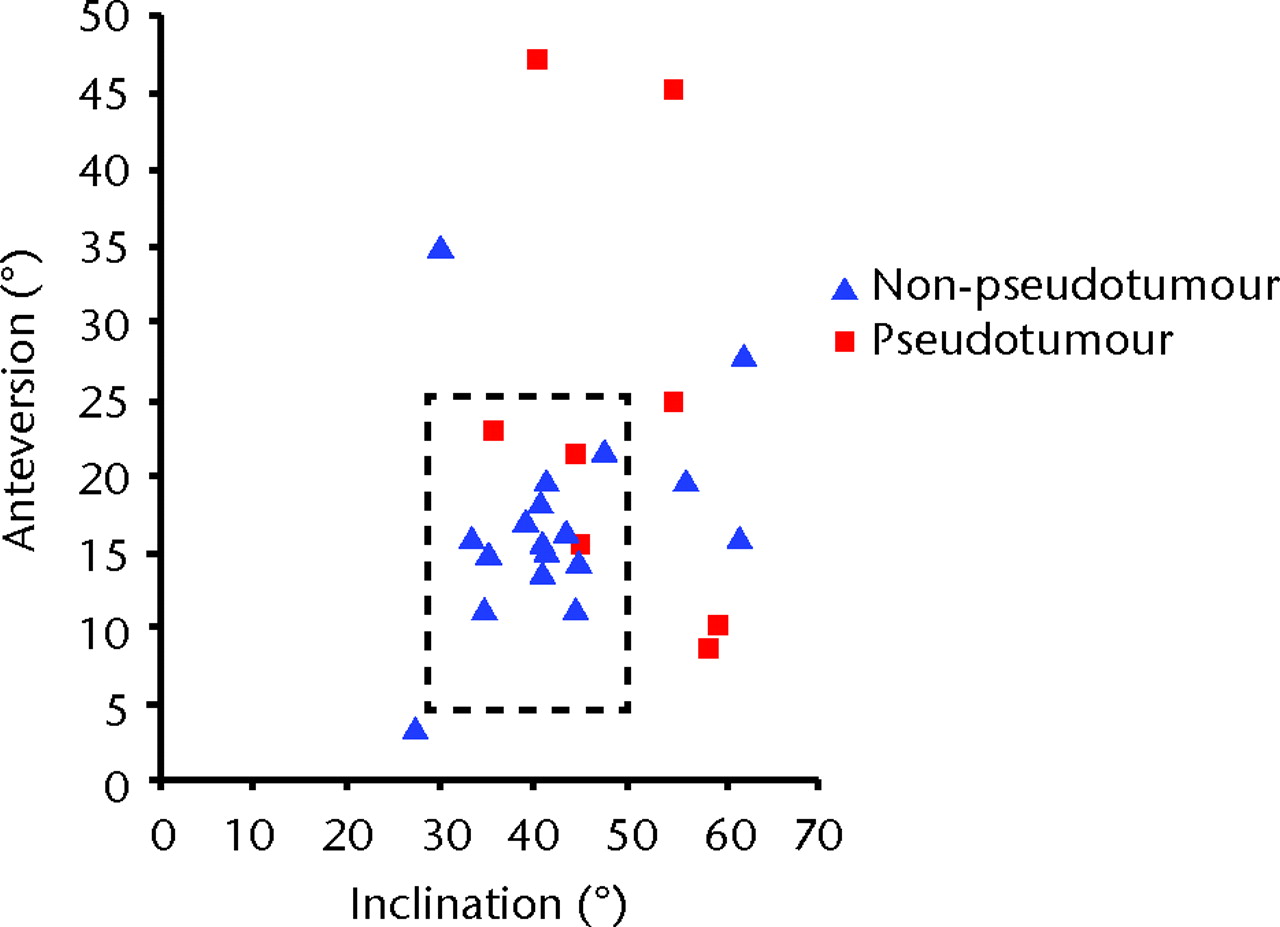
Fig. 5
Scatter graph showing the angles of inclination and anteversion of the acetabular component for the pseudotumour group (9 hips in 6 patients) and the non-pseudotumour group (21 hips in 13 patients). Lewinnek’s safe zone29 is outlined by the dotted rectangle.
Serum metal ion levels
The patients with a pseudo-tumour had significantly higher median serum Co levels compared with the patients without (14.3 µg/l (10.6 to 64.1) versus 1.9 µg/l (1.2 to 5.0), p < 0.001) and Cr levels (21.2 µg/l (13.8 to 45.2) versus 1.8 µg/l (0.7 to 7.6), p < 0.001) (Fig. 6).
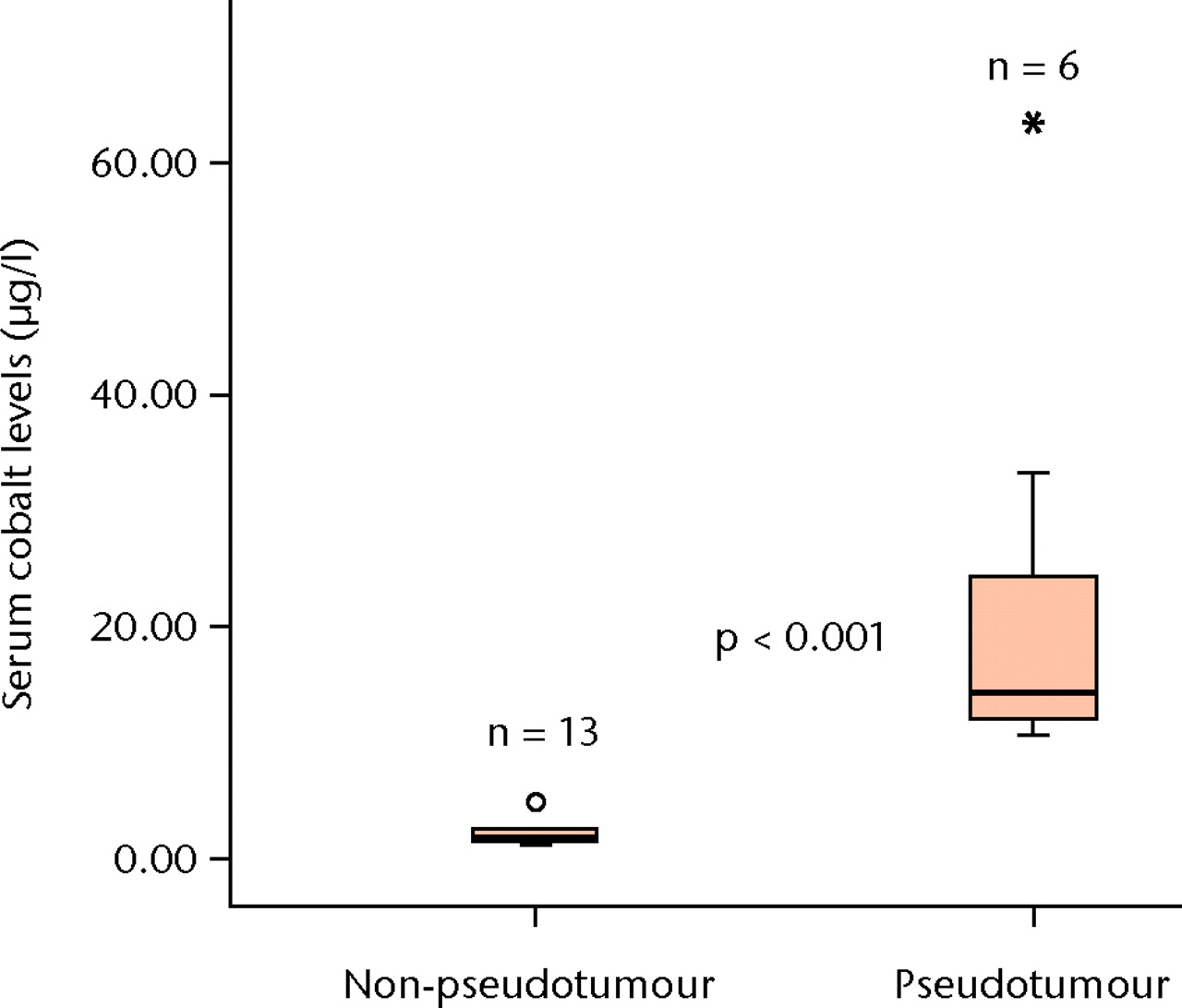
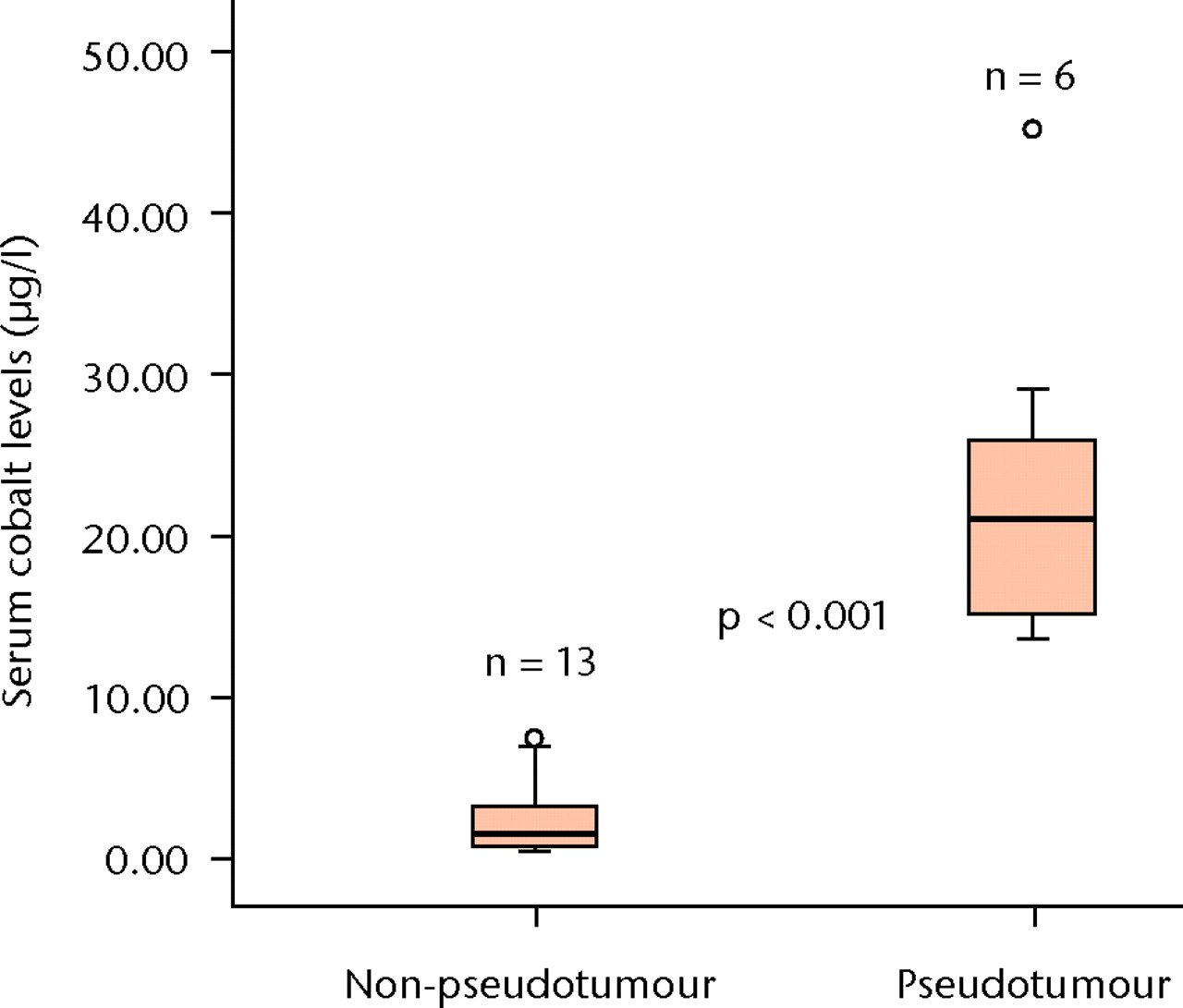
Figs. 6a - 6b
Boxplots showing the median serum cobalt (Co) (a) and chromium (Cr) (b) level measurements in the six patients with psuedotumour and the 13 patients without. The boxes represent the median and interquartile range, and the whiskers denote the range of data excluding outliers (°, between 1.5 and 3×IQR) and extremes (*, > 3×IQR).
Discussion
Although previous retrieval studies have reported edge-loading as a mechanism that leads to increased wear in MoMHRA implants,14,15,30-32 this study integrating dynamic motion analysis with CT data is the first to quantify duration and magnitude of edge-loading in vivo during functional activities in MoMHRA patients. The observation of edge-loading in patients with well--functioning MoMHRA in the current study has not been previously reported; however, similar findings have been reported with the multi-directional in vivo wear paths measured in patients with metal-on-polyethylene total hip replacement.33 In this study by Bennett et al,33the 3D loci of points on the acetabular component over which the femoral component moved, clearly demonstrated that the wear path extended over the edge of the acetabular component during level walking. Furthermore, in vivo studies using video fluoroscopy have reported that edge-loading with heelstrike can occur during normal walking due to microseparation in metal-on-metal hip bearings.24,25
While edge-loading occurred in MoMHRA hips both with and without pseudotumours during functional activities, the distribution of ‘time duration’ and ‘force magnitude’ differed significantly. Edge-loading in the hips with pseudotumours occurred when significantly greater magnitudes of force impulse were experienced near the edge of the acetabular component for a significantly longer period of time. The significant differences in time and force impulse distribution of force paths in the edge-loading zone were activity-dependent, with proportionally greater difference observed during stair climbing. During a 12-hour period, it has been reported that patients with total hip arthroplasty perform 42 cycles of stair climbing, with estimates of 15 000 cycles per year.34,35 These significant increases in time and force impulse distribution of force paths in the edge-loading zone during more strenuous activities of daily living may be important factors responsible for increased wear in MoMHRA patients with pseudotumours. It may be hypothesised that there is a cumulative threshold of contact stress that must be exceeded by repeated episodes of edge-loading before resulting in significant localised excessive wear (edge-wear). Therefore, edge-loading can be a ‘benign’ process that occurs during activities of daily living in patients with well-functioning MoMHRA. However, once the contact stress generated by edge-loading exceeds the tolerance of the MoM bearing due to increase in frequency, duration and/or magnitude of contact force, a vicious cycle of wear may ensue that leads to edge-wear scars reported in retrieval study of MoMHRA implants revised due to pseudotumours.15 Thus, the in vivo observation of edge-loading with significantly increased duration and magnitude of force impulse may, in part, explain significantly elevated serum metal levels in the pseudotumour patient group. In fact, no patient in the pseudotumour group had ‘normal’ median serum and aspirate levels of Co and Cr ions reported in well-functioning MoMHRA.36-39
Retrieval studies of MoM prostheses have reported that acetabular components with high inclination angles demonstrate increased wear secondary to edge loading.31,40 In addition, increased risk of pseudotumour formation has been correlated with steep positioning of the acetabular component in MoMHRA patients.30,41 In the current study, there was a trend towards patients in the pseudotumour group having a steeper acetabular component, although the difference was not statistically significant (p = 0.10). Increased anteversion combined with increased inclination has also been reported to be associated with elevated concentration of metal ions.42 Increases in anteversion and inclination have been suggested to decrease cover of the femoral component by the acetabular component, thus decreasing the area for generation of fluid-film lubrication. This is consistent with the current study’s finding that the patients with pseudotumours, who had significantly elevated metal ion levels compared with the patients without pseeudotumours, were found to have a significantly greater median combined inclination and anteversion angle (75.6° versus 58.75°, p = 0.04), with 67% (six of nine) of acetabular components found outside the safe zone of Lewinnek.29
Although explained in part by the orientation of the acetabular component, the aetiology of edge-loading is likely to be multifactorial. Edge-loading was observed in all hips, including those with acetabular components within the safe zone of Lewinnek.29 This indicates that there may be other important causative factors. These may include implant, patient and surgical factors. Different implant designs may influence safe inclination and anteversion angles of the component. Implants with a greater angle subtended by the acetabular component would shift the loci of hip joint force vector away from the edge for any given orientation by providing more cover. Although the implants in the pseudotumour group were predominantly Birmingham Hip Resurfacing systems (BHR; Smith & Nephew, Memphis, Tennessee) (seven of nine, 78%), which have a lower included angle than the Conserve Plus (Wright Medical Technology, Memphis, Tennessee) implants, the number of implants in this study was too small to detect any difference. Moreover, the optimal implant orientation may also be different for each individual patient due to variables such as bony anatomy.
The results of this study need to be considered in light of the potential limitations. First, a resultant hip joint segment force measured in this study is a computational quantity derived from combined measurement of body position and simultaneous measurement of ground reaction force, representing the sum of hip joint reaction forces and muscle forces. As such, hip joint segment force does not provide any specific information in terms of muscle activation nor account for the stress on the MoMHRA implants at the articulation. However, the focus of the study was on the comparative differences between the two patient groups. Secondly, the sizes of the femoral component in the well-functioning MoMHRA group in the current study were ≤ 50 mm, which were deliberately matched to the small sizes in the pseudotumour group. Thus, any generalisation of the current study findings to MoMHRA with larger femoral component sizes should be done with caution. Lastly, the power of the study may be low due to small sample sizes. The sample size of the pseudotumour group was limited clinically by the number of patients with MRI confirmed diagnosis from the on-going clinical study. However, the selection of the control group was carefully matched for gender, age, femoral component size and time since index surgery, in order to minimise the potential confounding factors. Despite the small number of patients, the trends observed were consistent and significant differences were found.
In conclusion, edge-loading in MoMHRA hips with pseudotumours occurred in vivo with significantly longer duration and greater magnitude of force compared to the MoMHRA hips without pseudotumours during activities of daily living. The results of this study, therefore, provide the first in vivo evidence to support that edge-loading is an important mechanism that leads to localised excessive wear, with subsequent elevation of metal ion levels in MoMHRA patients with pseudo-tumours. Although orientation of the acetabular component appears to be an important factor in edge-loading, the aetiology of edge-loading is likely to be multi-factorial. Further research is required to elucidate the relative importance of implant, patient, and surgical factors that lead to edge-loading in order to minimise the occurrence of such an adverse clinical outcome and to ensure long-term implant survivorship.
1 Hing CB , BackDL, BaileyM, et al.The results of primary Birmingham hip resurfacings at a mean of five years: an independent prospective review of the first 230 hips. J Bone Joint Surg [Br]2007;89-B:1431–1438. Google Scholar
2 Steffen RT , PanditHP, PalanJ, et al.The five-year results of the Birmingham Hip Resurfacing arthroplasty: an independent series. J Bone Joint Surg [Br]2008;90-B:436–441.CrossrefPubMed Google Scholar
3 Treacy RB , McBrydeCW, PynsentPB. Birmingham hip resurfacing arthroplasty: a minimum follow-up of five years. J Bone Joint Surg [Br]2005;87-B:167–170. Google Scholar
4 Mabilleau G , KwonYM, PanditH, MurrayDW, SabokbarA. Metal-on-metal hip resurfacing arthroplasty: a review of periprosthetic biological reactions. Acta Orthop2008;79:734–747.CrossrefPubMed Google Scholar
5 Boardman DR , MiddletonFR, KavanaghTG. A benign psoas mass following metal-on-metal resurfacing of the hip. J Bone Joint Surg [Br]2006;88-B:402–404.CrossrefPubMed Google Scholar
6 Campbell P , ShimminA, WalterL, SolomonM. Metal sensitivity as a cause of groin pain in metal-on-metal hip resurfacing. J Arthroplasty2008;23:1080–1085.CrossrefPubMed Google Scholar
7 Fang CS , HarvieP, GibbonsCL, et al.The imaging spectrum of peri-articular inflammatory masses following metal-on-metal hip resurfacing. Skeletal Radiol2008;37:715–722.CrossrefPubMed Google Scholar
8 Hart A , SabahS, HenckelJ, et al.The painful metal-on-metal hip resurfacing. J Bone Joint Surg [Br]2009;91-B:738–744.CrossrefPubMed Google Scholar
9 Pandit H , Glyn-JonesS, McLardy-SmithP, et al.Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg [Br]2008;90-B:847–851.CrossrefPubMed Google Scholar
10 Langton DJ , JamesonSS, JoyceTJ, et al.Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg [Br]2010;92-B:38–46.CrossrefPubMed Google Scholar
11 Grammatopolous G , PanditH, KwonYM, et al.Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg [Br]2009;91-B:1019–1024.CrossrefPubMed Google Scholar
12 Kwon YM, Ostlere S, McLardy-Smith P, et al. Metal ion levels in pseudotumours associated with metal-on-metal hip resurfacings. Procs 55th Orthopaedic Research Society Annual Meeting, Las Vegas, 2009. Google Scholar
13 Kwon YM , OstlereSJ, McLardy-SmithP, et al.“Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty2011;26:511–518. Google Scholar
14 De Smet K , De HaanR, CalistriA, et al.Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg [Am]2008;90-A(Suppl 4):202–208.CrossrefPubMed Google Scholar
15 Kwon YM , Glyn-JonesS, SimpsonDJ, et al.Analysis of wear of retrieved metal-on-metal hip resurfacing implants revised due to pseudotumours. J Bone Joint Surg [Br]2010;92-B:356–361.CrossrefPubMed Google Scholar
16 Taylor SJ , PerryJS, MeswaniaJM, et al.Telemetry of forces from proximal femoral replacements and relevance to fixation. J Biomech1997;30:225–234.CrossrefPubMed Google Scholar
17 Bergmann G , GraichenF, RohlmannA, LinkeH. Hip joint forces during load carrying. Clin Orthop Relat Res1997;335:190–201.PubMed Google Scholar
18 Bergmann G , GraichenF, RohlmannA. Hip joint loading during walking and running, measured in two patients. J Biomech1993;26:969–990.CrossrefPubMed Google Scholar
19 Davy DT , KotzarGM, BrownRH, et al.Telemetric force measurements across the hip after total arthroplasty. J Bone Joint Surg [Am]1988;70-A:45–50.PubMed Google Scholar
20 Liu F , LeslieI, WilliamsS, FisherJ, JinZ. Development of computational wear simulation of metal-on-metal hip resurfacing replacements. J Biomech2008;41:686–694.CrossrefPubMed Google Scholar
21 Watanabe Y , ShibaN, MatsuoS, et al.Biomechanical study of the resurfacing hip arthroplasty: finite element analysis of the femoral component. J Arthroplasty2000;15:505–511.CrossrefPubMed Google Scholar
22 Brand RA , CrowninshieldRD, WittstockCE, et al.A model of lower extremity muscular anatomy. J Biomech Eng1982;104:304–310.CrossrefPubMed Google Scholar
23 Komistek RD , StiehlJB, DennisDA, PaxsonRD, Soutas-LittleRW. Mathematical model of the lower extremity joint reaction forces using Kane's method of dynamics. J Biomech1998;31:185–189.CrossrefPubMed Google Scholar
24 Dennis DA , KomistekRD, NorthcutEJ, OchoaJA, RitchieA. “In vivo” determination of hip joint separation and the forces generated due to impact loading conditions. J Biomech2001;34:623–629. Google Scholar
25 Komistek RD , DennisDA, OchoaJA, HaasBD, HammillC. In vivo comparison of hip separation after metal-on-metal or metal-on-polyethylene total hip arthroplasty. J Bone Joint Surg [Am]2002;84-A:1836–1841. Google Scholar
26 Mellon SJ , KwonYM, Glyn-JonesS, MurrayDW, GillHS. The effect of motion patterns on edge-loading of metal-on-metal hip resurfacing. Med Eng Phys2011;33:1212–1220.CrossrefPubMed Google Scholar
27 Davis RB , OunpuuS, TyburskiD, GageJR. A gait analysis data collection and reduction technique. Human Movement Science1991;10:575–587. Google Scholar
28 MacDonald SJ , BrodnerW, JacobsJJ. A consensus paper on metal ions in metal-on-metal hip arthroplasties. J Arthroplasty2004;19(Suppl 3):12–16.CrossrefPubMed Google Scholar
29 Lewinnek GE , LewisJL, TarrR, CompereCL, ZimmermanJR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg [Am]1978;60-A:217–220.PubMed Google Scholar
30 De Haan R , PattynC, GillHS, et al.Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg [Br]2008;90-B:1291–1297.CrossrefPubMed Google Scholar
31 Campbell P , BeauléPE, EbramzadehE, et al.The John Charnley Award: a study of implant failure in metal-on-metal surface arthroplasties. Clin Orthop Relat Res2006;453:35–46.CrossrefPubMed Google Scholar
32 Hussain A, Counsell L, Kamali A. Clinical effects of edge loading on metal-on-metal hip resurfacings. Procs British Hip Society, Manchester, 2009. Google Scholar
33 Bennett D , HumphreysL, O'BrienS, et al.Wear paths produced by individual hip-replacement patients: a large-scale, long-term follow-up study. J Biomech2008;41:2474–2482. Google Scholar
34 Morlock M , SchneiderE, BluhmA, et al.Duration and frequency of every day activities in total hip patients. J Biomech2001;34:873–881.CrossrefPubMed Google Scholar
35 Bergmann G , DeuretzbacherG, HellerM, et al.Hip contact forces and gait patterns from routine activities. J Biomech2001;34:859–871.CrossrefPubMed Google Scholar
36 Back DL , YoungDA, ShimminAJ. How do serum cobalt and chromium levels change after metal-on-metal hip resurfacing?Clin Orthop Relat Res2005;438:177–181.CrossrefPubMed Google Scholar
37 Hart AJ , HesterT, SinclairK, et al.The association between metal ions from hip resurfacing and reduced T-cell counts. J Bone Joint Surg [Br]2006;88-B:449–454.CrossrefPubMed Google Scholar
38 Vendittoli PA , GanapathiM, LavigneM. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty. J Bone Joint Surg [Br]2007;89-B:989–990.CrossrefPubMed Google Scholar
39 Daniel J , ZiaeeH, PradhanC, PynsentPB, McMinnDJ. Blood and urine metal ion levels in young and active patients after Birmingham hip resurfacing arthroplasty: four-year results of a prospective longitudinal study. J Bone Joint Surg [Br]2007;89-B:169–173.CrossrefPubMed Google Scholar
40 Bowsher JG , DonaldsonTK, WilliamsPA, ClarkeIC. Surface damage after multiple dislocations of a 38-mm-diameter, metal-on-metal hip prosthesis. J Arthroplasty2008;23:1090–1096.CrossrefPubMed Google Scholar
41 Grammatopoulos G , PanditH, Glyn-JonesS, et al.Optimal acetabular orientation for hip resurfacing. J Bone Joint Surg [Br]2010;92-B:1072–1078.CrossrefPubMed Google Scholar
42 Langton DJ , JamesonSS, JoyceTJ, WebbJ, NargolAV. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg [Br]2008;90-B:1143–1151.CrossrefPubMed Google Scholar
Funding statement:
This work was supported by institutional funding, the NIHR Biomedical Research Unit at the Nuffield Department of Orthopaedic Surgery, Nuffield Orthopaedic Centre. None of the authors have professional or financial affiliations that may be perceived to have biased the presentation.
Author contributions:
Y-M. Kwon: Study design, Data collection, Data analysis, Manuscript writing
S. J. Mellon: Data collection, Data analysis
P. Monk: Data collection
D. W. Murray: Study design, Data analysis, Manuscript writing
H. S. Gill: Study design, Data collection, Data analysis, Manuscript writing
ICMJE Conflict of Interest:
None declared
©2012 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










