Abstract
Objectives
The objective of this study was to compare the early migration characteristics and functional outcome of the Triathlon cemented knee prosthesis with its predecessor, the Duracon cemented knee prosthesis (both Stryker).
Methods
A total 60 patients were prospectively randomised and tibial component migration was measured by radiostereometric analysis (RSA) at three months, one year and two years; clinical outcome was measured by the American Knee Society score and the Knee Osteoarthritis and Injury Outcome Score.
Results
There were no statistically significant differences in rotation or translation around or along the three coordinal axes, or in the maximum total point motion (MTPM) during the two-year follow-up.
Conclusions
The Triathlon cemented knee prosthesis has similar early stability and is likely to perform at least as well as the Duracon cemented knee prosthesis over the longer term.
Article focus
Safety study for a new design of total knee replacement (TKR)
Comparison of the single-radius Triathlon TKR with its predecessor, the multi-radius Duracon knee system, with regard to fixation
Key messages
The fixation of the Triathlon TKR, as measured by radiostereometric analysis (RSA) is equivalent to its predecessor, the Duracon TKR
Strengths and limitations
RSA was used as an objective measurement tool to assess the three-dimensional stability of the implants in this prospectively randomised study
A limitation of the study is the small sample size
Introduction
The current generation of patients undergoing total knee replacement (TKR) are heavier, younger and more active than previously.1 When these demographic changes are coupled with the high expectations that many patients now have regarding their functional outcome following surgery, the result is an increased demand on the mechanical performance of prostheses. In particular, patients require a greater magnitude of and ease of moving into deep knee flexion.
The Triathlon cemented knee prosthesis (Stryker, Mahwah, New Jersey) is a recent introduction to the market and has evolved with modifications from its predecessor, the Duracon cemented knee prosthesis (Stryker). The Duracon prosthesis has been in clinical use for over 20 years and has good mid- to long-term survivorship.2,3 The main difference between the Duracon and Triathlon total knee prostheses is that the Triathlon femoral component has a single axis of rotation (single radius). The single radius is centred about the transepicondylar axis, which provides ligament isometry and a substantial contact area throughout the entire range of movement.4,5 Furthermore, it provides more uniform movement, lower contact stresses on the insert, better mid-flexion stability and more efficient muscle activity.4-6 The Triathlon prosthesis also has an improved flared femoral posterior condyles and rotary arc insert, which work together to provide the possibility for, and help the freedom of femorotibial motion with up to 20° of internal-external rotation during deep knee flexion. With additional improvements to the anatomical fit, the Triathlon prosthesis is intended to allow normal movement of the knee and stability through ≥ 150° of flexion.
A concern that arises with any new prosthesis is whether it will achieve satisfactory long-term implant stability. During the last decades, radiostereometric analysis (RSA) has emerged as a way to assess prosthetic fixation.7-9 The method has been used extensively in both hip and knee arthroplasty,10-25 with data from early post-operative time-points serving as a predictor of late mechanical loosening.10,11,15,18,26 The major benefit of early detection of instability is that the exposure of patients to potentially unstable prostheses can be limited. RSA is therefore an ideal tool for comparing new design concepts with a ‘standardised’ model,27 from which long-term clinical data are readily available.26,28
This study is novel in that the new prosthesis was assessed using RSA alongside its predecessor. This therefore allows the tibial component migration of the new prosthesis to be considered in context with a prosthesis that has over 20 years of real clinical outcomes, rather than using yardstick values provided from historical studies.
Patients and Methods
This study was a randomised, parallel, single-blind study of patients receiving a TKR for treatment of osteoarthritis of the knee. The cohort were a subgroup of patients enrolled in a larger randomised controlled trial conducted in Sweden (ClinicalTrials.gov Identifier: NCT00436982) to evaluate a number of different aspects of a new TKR system. Patients were recruited from a single centre and were prospectively randomised to receive either a cemented Triathlon total knee system or a Duracon total knee system (both Stryker). Randomisation was achieved using a sealed envelope technique, with 30 patients allocated to each group. Two surgeons (MM and STL) were involved in both the selection and operation of the patients. Patients were blinded to the treatment allocated. Ethics Committee approval was obtained from the local medical ethics committee before initiation of the study. Patients were considered for enrolment according to their clinical findings and subject to gaining their written informed consent according to International Conference on Harmonisation Good Clinical Practice (ICH GCP) requirements.29 The inclusion criteria for selection to participate in the study are provided in Table I. The exclusion criteria are provided in Table II.
Table I
Inclusion criteria
| Inclusion criteria | |
|---|---|
| 1 | Exclusive indication of osteoarthritis (Ahlbäck stage II to V41) |
| 2 | Choice of either Duracon or Triathlon system suitable for the patient |
| 3 | Patient understanding the conditions of the study and being willing and able to comply with the scheduled post-operative clinical and radiological evaluations and the prescribed rehabilitation |
| 4 | Patient having signed the Ethics Committee approved Informed Consent Form before surgery |
Table II
Exclusion criteria
| Exclusion criteria | |
|---|---|
| 1 | Previous major knee surgery |
| 2 | Significant disabling problems from the musculoskeletal system other than in the knees |
| 3 | Obesity severe enough to affect subject’s ability to perform activities of daily living (body mass index ≥ 35 kg/m2) |
| 4 | Patients with active or suspected infection |
| 5 | Patients with malignancy – active malignancy |
| 6 | Patients with severe osteoporosis, Paget’s disease, renal osteodystrophy |
| 7 | Patients immunologically suppressed, or receiving steroids in excess of physiologic dose requirements |
| 8 | Patients with a neuromuscular or neurosensory deficit that would limit their ability to assess the performance of the device or that interferes with the patient’s ability to limit weightbearing or places an extreme load on the implant during the healing period |
| 9 | Female patients planning a pregnancy during the course of the study |
| 10 | Patients with systemic or metabolic disorders leading to progressive bone deterioration |
| 11 | Patients who, as judged by the surgeon, are mentally incompetent or unlikely to be compliant with the prescribed post-operative routine and follow-up evaluation schedule |
| 12 | Patients with other severe concurrent joint involvements that can affect their outcome |
| 13 | Patients with other concurrent illnesses, which are likely to affect their outcome such as sickle cell anaemia, systemic lupus erythematosus or renal disease requiring dialysis |
| 14 | Patients under the protection of law (e.g. guardianship) |
Prosthesis
All patients received a chrome-cobalt femoral component. Both the Triathlon and Duracon total knee systems had cemented chrome-cobalt tibial components, a cruciate retaining design and relatively unconstrained polyethylene inserts. The Triathlon tibial tray had a delta-shaped stem and the Duracon tibial tray had a central, round stem with delta shaped wings. The cement used was Refobacin Bone Cement R (Biomet Inc., Warsaw, Indiana). No patella components were used in either group.
Operative technique
Each patient was given pre-operative antibiotics (2 g cloxacillin i.v. 15 to 45 minutes before surgery) and tranexamic acid (100 mg per kg administered as preparing for cementation of components). The surgeries were performed via a ventral incision with a parapatellar medial entrance to the joint using appropriate guide instruments and according to the surgical-technique manual supplied with each knee system. In both systems an extramedullary jig was used for the tibial cuts with 3° of posterior slope built into the cutting blocks. At the time of surgery eight tantalum markers (0.8 mm diameter; RSA Biomedical, Umeå, Sweden) were inserted into the proximal tibial metaphysis and five markers were inserted in the polyethylene tibial insert.7,8,30,31 Post-operatively low-molecular-weight heparin was used for thromboembolic prophylaxis (enoxaparin 100 mg/ml, 0.4 ml sc for ten days, starting at six hours (four to eight) post-operatively).32 Mobilisation was similar for both groups and included full weight-bearing.
RSA and radiological analysis
Migration of the tibial component was measured using RSA. The first RSA investigation was performed within four days of the operation, after weightbearing had been achieved, and then at three months and one and two years post-operatively. RSA was performed with the patient in a supine position, with the knee of interest inside a calibration cage (Cage 10; RSA Biomedical). The three-dimensional (3D) migration of the tibial component was measured using UmRSA software v6.0 (RSA Biomedical).33
The migration was described as segment motion (translation and rotation) of the geometric centre of the prosthetic markers, compared with the geometric centre of the bone markers, and as the maximum total point motion (MTPM). The 3D motion of the prosthetic marker moving the most was used as a simplistic way to denote the magnitude of the micromotion, and enabled the micromotion between the tibial insert and the tibial bone to be described. Positive directions for translations along the orthogonal axes were: transverse (medial to lateral), longitudinal (caudal to cranial), and sagittal (posterior to anterior). Positive directions for rotations about the coordinate axes were anterior tilt (transverse axis), internal rotation (longitudinal axis), and varus (sagittal axis). An increase in MTPM of > 0.2 mm between the first- and second-year follow-up was considered as continuous migration26 and these patients were classified as ‘at risk’ of future implant loosening. In order to ensure accuracy of the measurements, stable fixation of the tantalum markers within the bone was essential. The upper limit for mean error (ME) of rigid body fitting (a measure of marker stability) was 0.2 mm, and the upper limit for condition number was 100. The upper limit for ME of rigid body fitting and condition number is generally proposed to be 0.35 mm and 150 respectively.25
The precision of the measurements was determined using double examinations performed on the first 20 patients enrolled. Each double examination was made in the same session as the one planned for follow-up. The patient left the calibration cage and walked around before the double examination. The precision of this investigation is described as 2×sd (95% CI) for all rotations and translations respectively.
Plain radiographs were obtained pre-operatively (for classification of disease), before discharge, and at one and two years post-operatively for assessment of component position and the presence of wear, radiolucent lines and stress resorption. Plain radiographs were made but not analysed further as a result of the too short follow-up. Hip-knee-ankle measurements were made at the three-month follow-up.34
Clinical assessment
Clinical evaluation took place pre-operatively and at three months, one year and two years post-operatively and comprised the American Knee Society score (AKSS)35 and the Knee Injury and Osteoarthritis Outcome Score (KOOS) questionnaires.36-40 The severity of osteoarthritis was graded according to the Ahlbäck classification.41
Statistical analysis
Depending on the nature of the study variables, frequencies (for qualitative variables) or descriptive (for continuous variables) have been presented. Descriptive statistics include the mean value with 95% confidence intervals (CI). The chi-squared test was used for qualitative variables, and the independent t-test or Wilcoxon rank test were used for continuous variables after evaluation of the Gaussian distribution. Significance was assumed at a p-value ≤ 0.05.
Results
During the period of this trial 118 TKRs were performed in 118 patients using either Triathlon or Duracon components; of these, 58 patients were excluded from the study because of long travelling time for follow-up or not having met the inclusion criteria. Therefore, 60 patients (21 men and 39 women) were included in the study, with 30 patients randomised to each group (Fig. 1). The two groups were similar in terms of patient demographics and severity of osteoarthritis (Table III).
Table III
Demographics
| Demographic | Duracon | Triathlon | p-value |
|---|---|---|---|
| Mean age (yrs) (range) | 66 (47 to 84) | 69 (47 to 86) | 0.121* |
| Female:male | 17:13 | 22:8 | 0.279* |
| Mean body mass index (kg/m2) (range) | 28.9 (20.5 to 38.3) | 28.7 (19.7 to 39.0) | 0.809* |
| Left:right | 15:15 | 18:12 | 0.604* |
| Primary diagnosis (n, %) | |||
| Osteoarthritis | 29 (97) | 29 (97) | 1.000* |
| Avascular necrosis | 1 (3) | 1 (3) | 1.000* |
| Ahlbäck grade (n, %) | 0.012† | ||
| II | 7 (23) | 1 (3) | |
| III | 20 (67) | 29 (97) | |
| IV | 3 (10) | 0 (0) | |
| V | 0 (0) | 0 (0) | |
| Mean operating time (mins) (range) | 64 (50 to 105) | 66 (50 to 90) | 0.452* |
| Mean hospital stay (days) (range) | 4.8 (4 to 7) | 5.2 (1 to 16) | 0.437* |
-
* t-test † Pearson chi-squared test
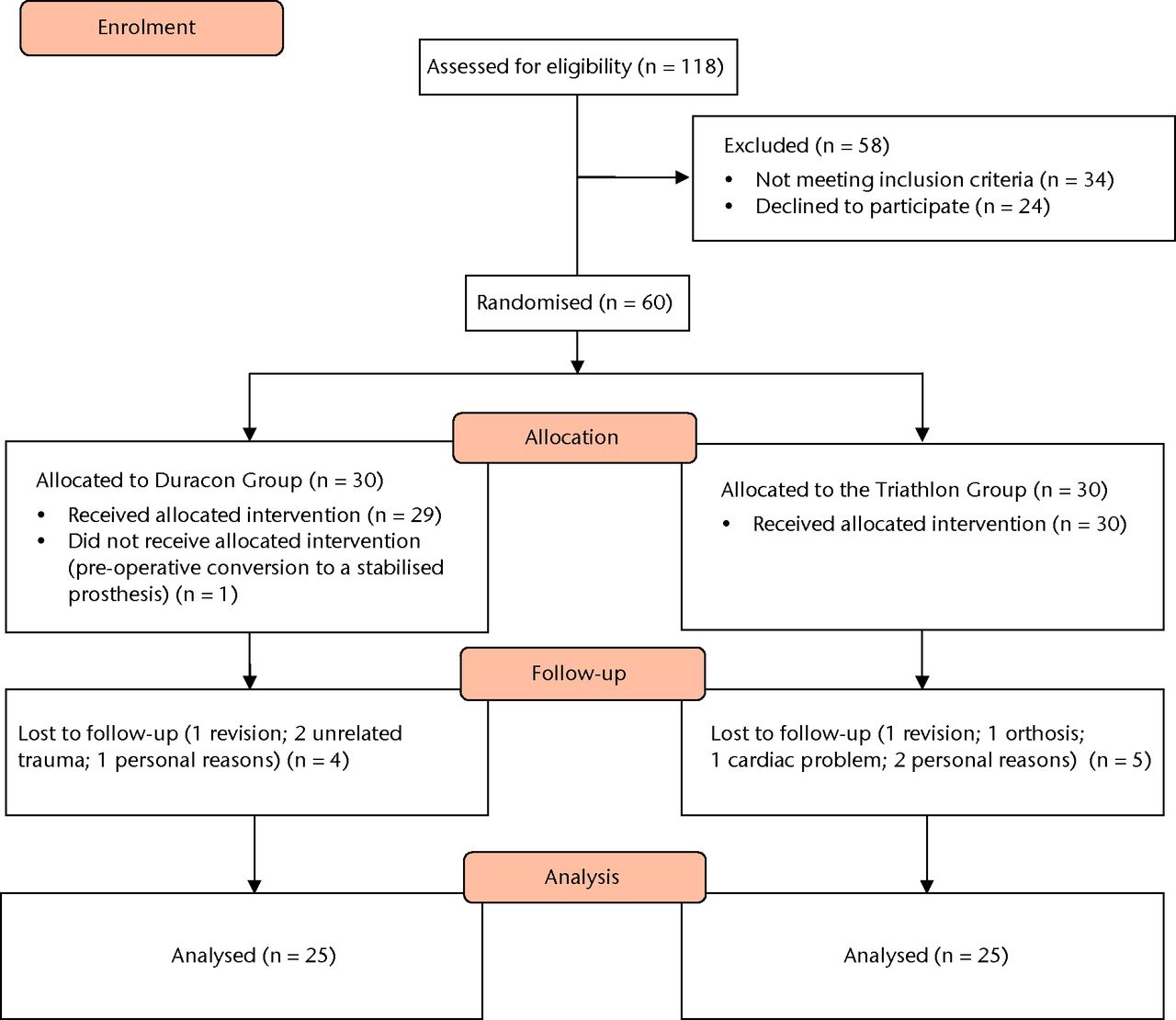
Fig. 1
CONSORT recruitment and follow-up chart.
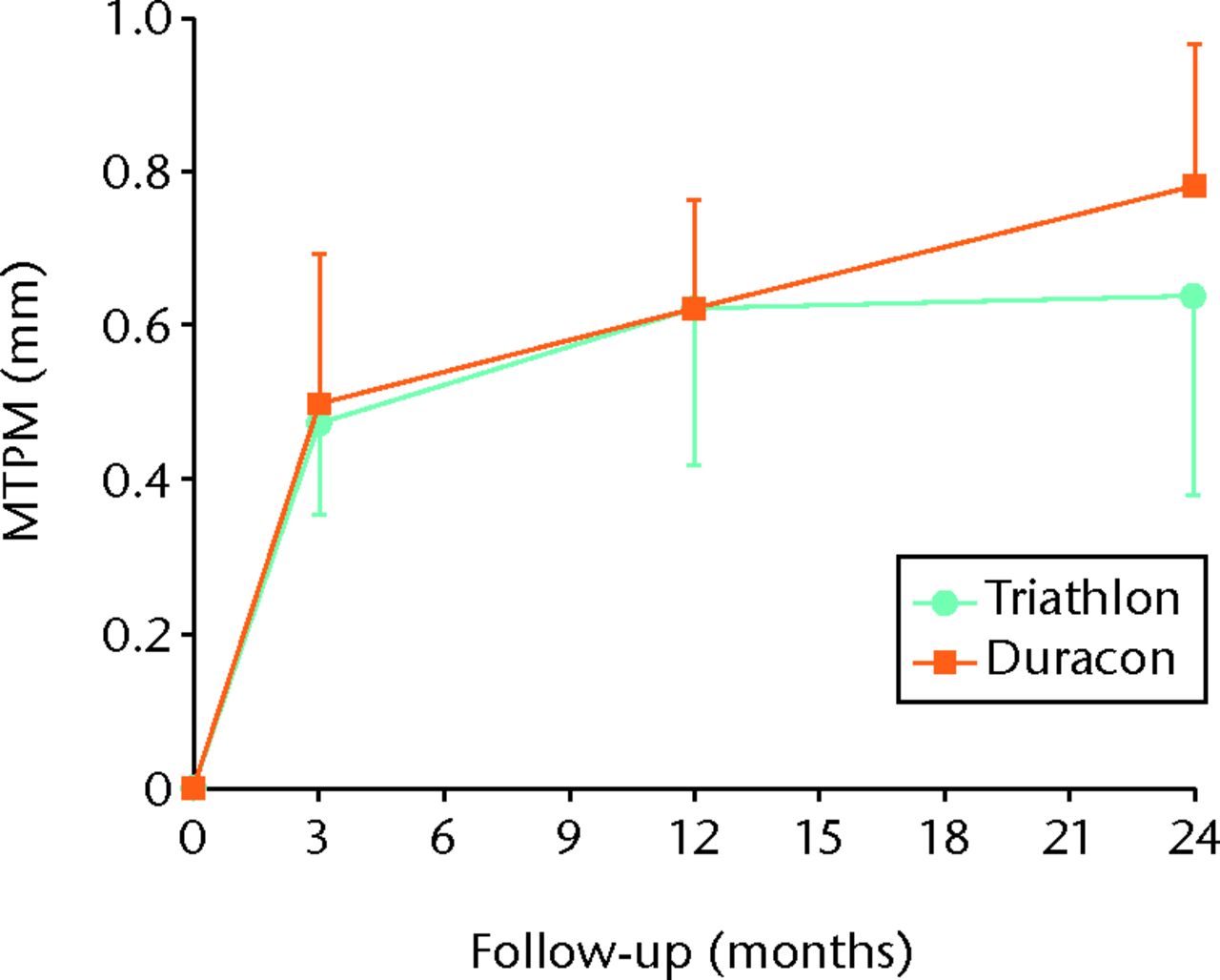
Fig. 2
Graph showing the mean maximum total point motion (MTPM) for both the Triathlon and Duracon groups over the two-year follow-up. Error bars denote the 95% confidence interval.
At two years, 25 patients were available for follow-up in each group. In the Duracon group, one patient left the study because of pre-operative conversion to a stabilised prosthesis, another left the study because of personal reasons, two patients suffered trauma preventing further participation and one patient needed a revision. In the Triathlon group, one patient required revision because of deep infection, two patients left the study because of personal reasons, one patient needed an orthosis because of instability of the knee and one patient had cardiac problems. No patients were lost to follow-up.
Radiological analysis and RSA
Routine radiographs of the knee revealed no radiolucent or sclerotic lines. The mean hip-knee-ankle (HKA) angle was similar between the groups, with 179° (median 178.4°; 172° to 185°) and 179° (median 178.0; 174° to 188°) for the Duracon and Triathlon groups, respectively (p = 0.849, t-test).
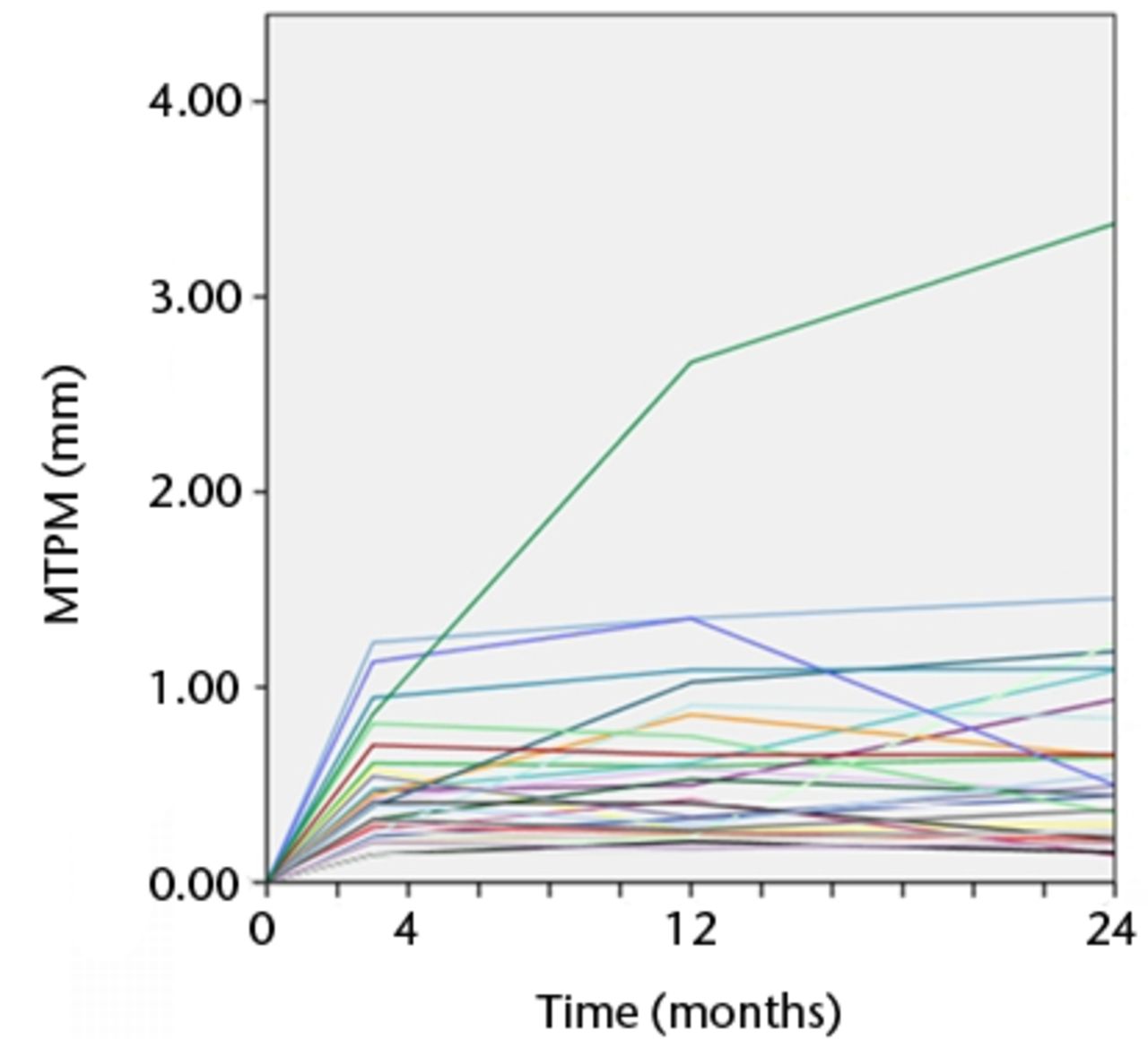
The precision of the RSA system was 0.12 mm, 0.21 mm, and 0.14 mm for x-, y-, and z-translations, respectively, and 0.12°, 0.11°, and 0.09° for x-, y-, and z- rotations, respectively. The migration of the tibial components is presented for each timepoint in Table IV. Graphs showing maximal total point motion and subsidence are presented in Figures 2 to 4. There were no statistically significant differences in rotation or translation around or along the three coordinal axes after two-year follow-up (all p > 0.056) (Table IV). There were no significant differences in MTPM or subsidence between the two groups at any timepoint (Table IV, Fig. 4). For both groups, the MTPM had stabilised sometime within the first three months. One patient in the Triathlon group was considered an extreme outlier with regard to MTPM (Fig. 3).
Table IV
The mean translation and rotation of the tibial component measured by radiostereometric analysis (RSA) at three months, one year and two years (CI, confidence interval; MTPM, maximal total point motion)
| 3 months | 1 year | 2 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RSA assessment | Duracon | Triathlon | p-value | Duracon | Triathlon | p-value | Duracon | Triathlon | p-value | ||
| Mean (95% CI) translation (mm) | |||||||||||
| Medial–lateral | 0.20 (0.06) | -0.10 (0.09) | 0.04 | 0.01 (0.09) | -0.11 (0.10) | 0.09 | 0.02 (0.10) | -0.09 (0.10) | 0.165 | ||
| Caudal–cranial | -0.01 (0.07) | -0.06 (0.09) | 0.426 | -0.13 (0.13) | -0.15 (0.17) | 0.867 | -0.10 (0.16) | -0.19 (0.22) | 0.541 | ||
| Posterior–anterior | 0.17 (0.09) | -0.02 (0.09) | 0.004 | 0.13 (0.09) | -0.01 (0.11) | 0.045 | 0.17 (0.13) | -0.07 (0.13) | 0.058 | ||
| Mean (95% CI) rotation (°) | |||||||||||
| Anterior tilt | 0.13 (0.16) | -0.02 (0.11) | 0.127 | -0.03 (0.24) | -0.13 (0.16) | 0.511 | -0.09 (0.28) | -0.30 (0.20) | 0.236 | ||
| Internal rotation | 0.15 (0.31) | -0.03 (0.10) | 0.288 | 0.00 (0.12) | 0.00 (0.08) | 0.968 | 0.00 (0.14) | 0.01 (0.11) | 0.947 | ||
| Varus | 0.06 (0.09) | 0.12 (0.10) | 0.316 | -0.01 (0.14) | 0.18 (0.15) | 0.069 | -0.69 (0.20) | 0.19 (0.15) | 0.056 | ||
| Mean (95% CI) MTPM (mm) | 0.50 (0.19) | 0.45 (0.12) | 0.649 | 0.62 (0.14) | 0.60 (0.21) | 0.862 | 0.76 (0.18) | 0.63 (0.26) | 0.462 | ||
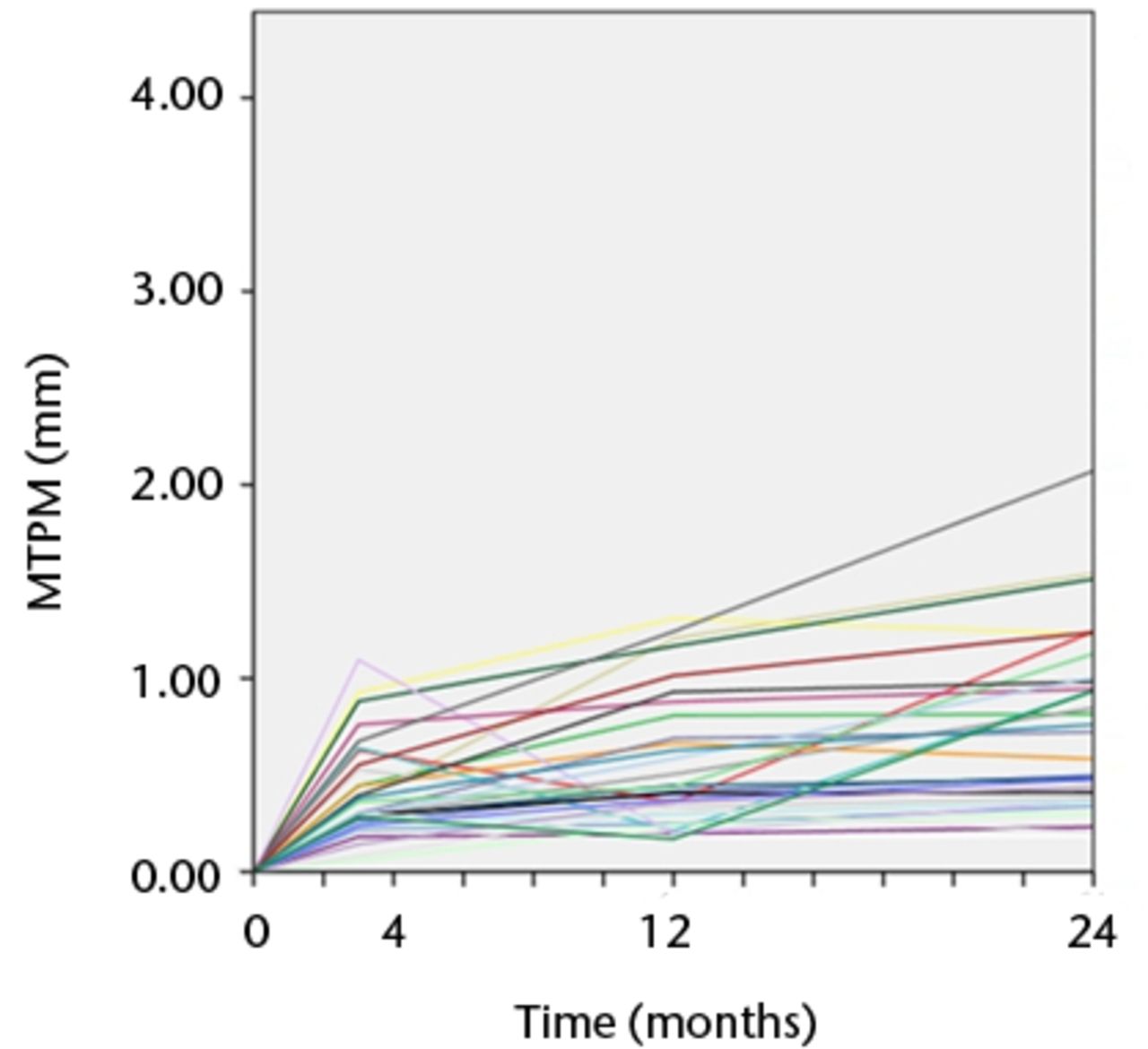
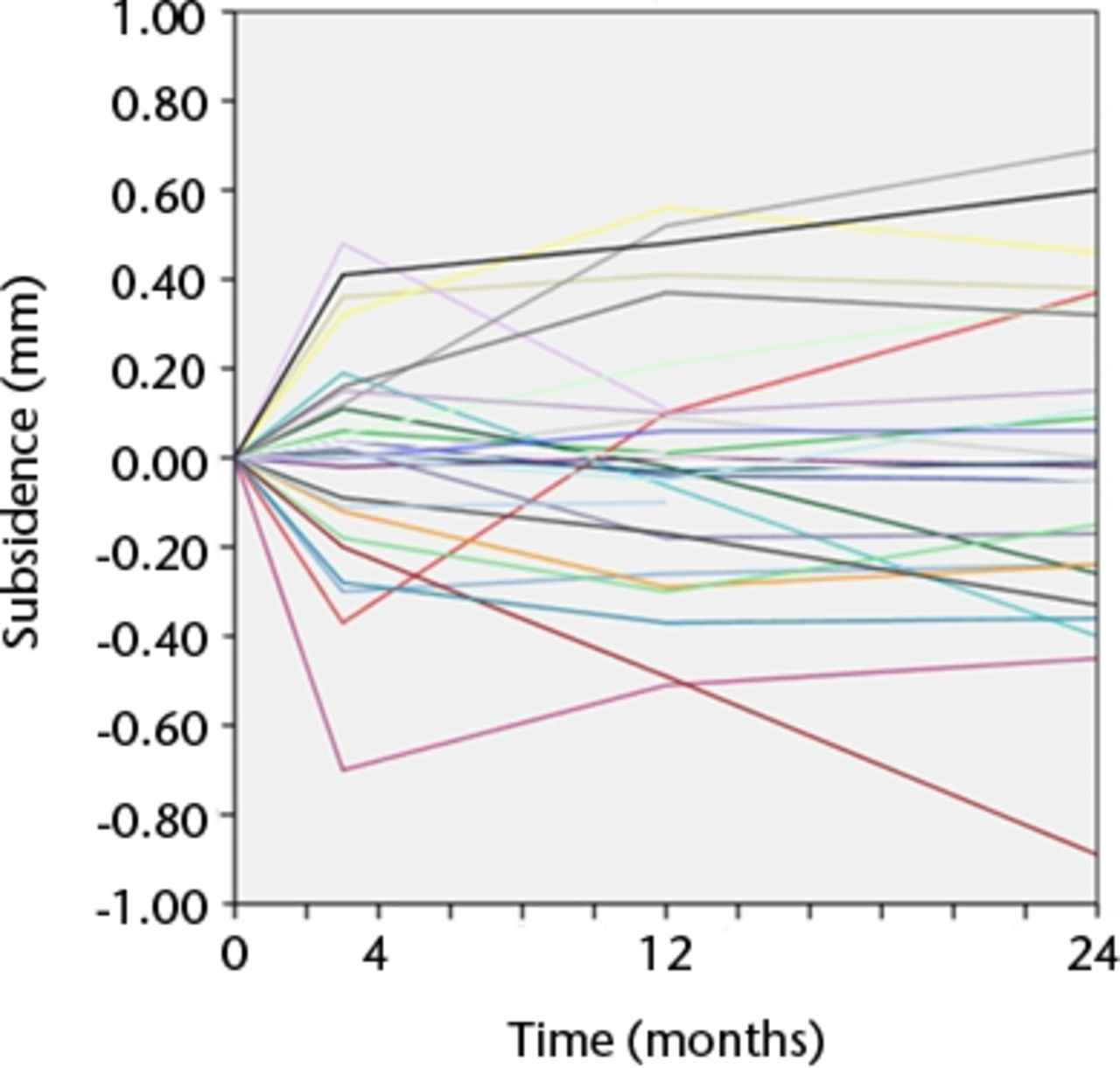
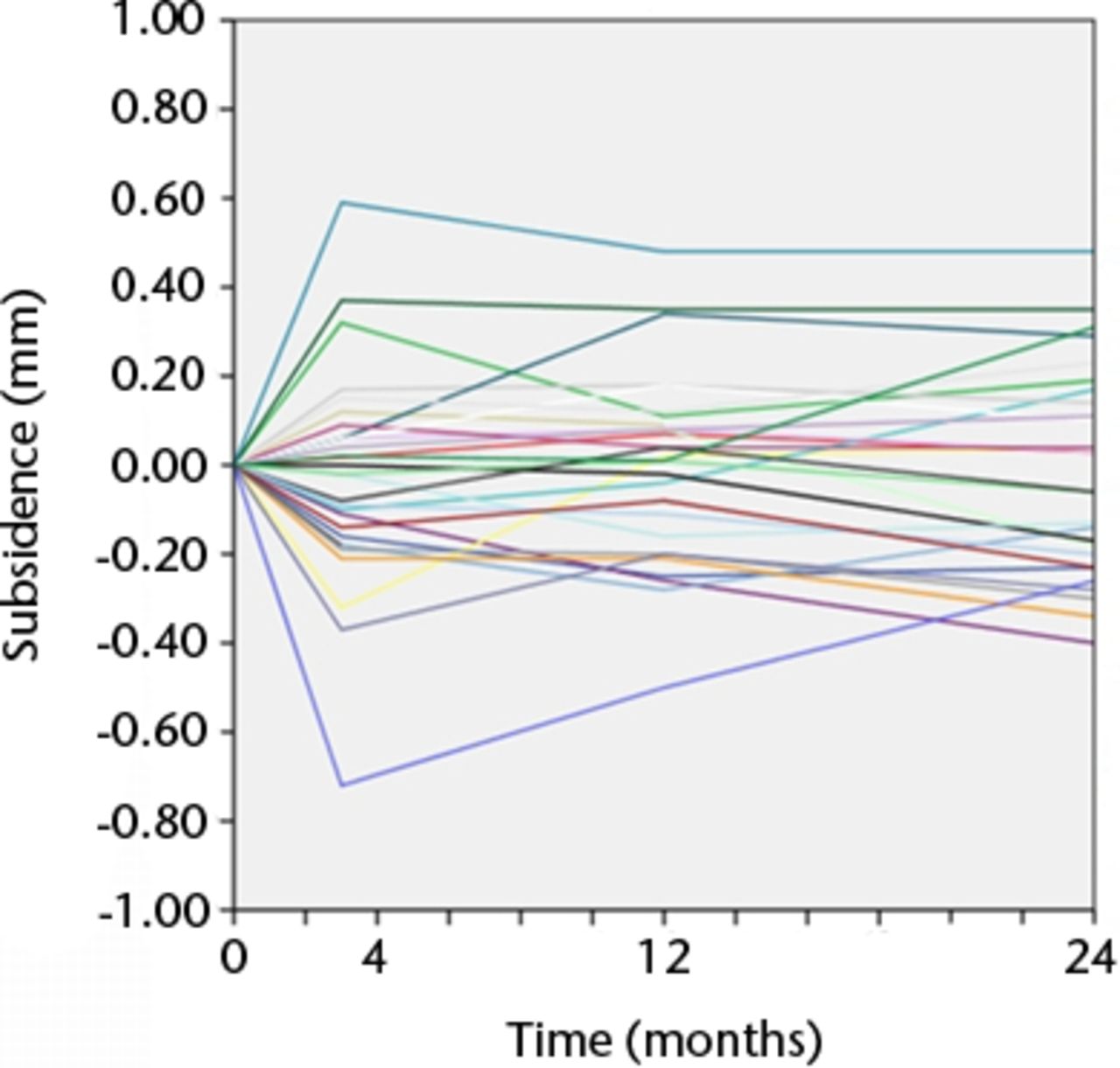
Figs. 3a - 3b
Graphs showing the individual subsidence for each knee in a) the Duracon and b) the Triathlon group. Each line represents the migration pattern for an individual patient.
Figs. 4a - 4b
Graphs showing the individual maximum total point motion (MTPM) for each knee in a) the Duracon and b) the Triathlon group. Each line represents the migration pattern for an individual patient.
There were seven tibial trays in the Duracon group and five tibial trays in the Triathlon group that demonstrated continuous migration (increase in MTPM > 0.2 mm) between the first- and second-year follow-up.
Clinical assessment
Both groups showed an improvement in clinical outcome score post-operatively. The Triathlon group scored significantly better for the ADL KOOS sub score at the one- and two-year evaluations (p = 0.03 and p = 0.025, respectively). There was no significant difference in the clinical outcome scores between the two groups at any other time point (Fig. 5, Table V).
Table V
Clinical outcome scores according to the Knee Injury and Osteoarthritis Outcome Score (KOOS) (CI, confidence interval)
| Pre-operative | 3 months | 1 year | 2 years | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) KOOS subscore | Duracon | Triathlon | p-value | Duracon | Triathlon | p-value | Duracon | Triathlon | p-value | Duracon | Triathlon | p-value | |||
| Pain | 44 (5) | 41 (6) | 0.455 | 70 (9) | 71 (8) | 0.838 | 81 (7) | 74 (7) | 0.175 | 86 (8) | 78 (8) | 0.216 | |||
| Symptom | 53 (6) | 50 (7) | 0.478 | 68 (6) | 67 (7) | 0.826 | 78 (6) | 75 (7) | 0.514 | 85 (5) | 79 (6) | 0.196 | |||
| Activities of daily living | 50 (6) | 46 (5) | 0.346 | 72 (9) | 74 (7) | 0.821 | 84 (6) | 73 (8) | 0.03 | 92 (5) | 77 (9) | 0.025 | |||
| Sports/recreation | 10 (5) | 7 (3) | 0.247 | 27 (7) | 20 (7) | 0.225 | 39 (11) | 30 (12) | 0.245 | 43 (12) | 30 (9) | 0.111 | |||
| Quality of life | 24 (6) | 24 (5) | 0.995 | 56 (9) | 52 (8) | 0.459 | 66 (8) | 57 (10) | 0.14 | 71 (10) | 62 (10) | 0.211 | |||
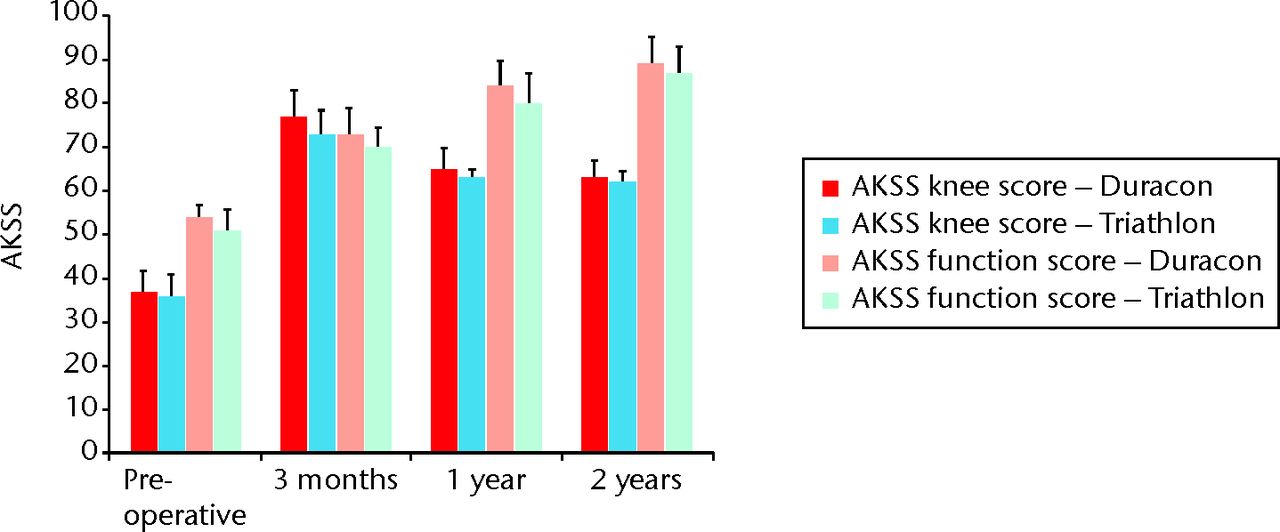
Fig. 5
Bar chart showing the American Knee Society scores (AKSS; both knee score and function score) for both the Duracon and Triathlon groups. Error bars denote the 95% confidence interval.
Adverse events
In the Duracon group three patients had deep-vein thrombosis (DVT) confirmed on ultrasound; one patient had a superficial infection; one patient had a urinary tract infection; one patient developed a dropped foot; one patient had patella misalignment; and one patient sustained a hip fracture five months post-operatively.
In the Triathlon group three patients had DVT; one patient developed synovitis; one patient had a superficial infection; one patient developed depression; one patient had a stroke; one patient had knee instability; one patient had a cardiac event; one patient sustained a fracture of the proximal humerus three weeks post-operatively; and one patient sustained a fracture of the proximal tibia at ten weeks post-operatively, which was treated conservatively. A record of recovery was available for all patients except for the patient with the dropped foot.
Discussion
The modification of arthroplasty components introduces a risk of altering the long-term stability for the modified components. The early detection of implants that are likely to have compromised long term stability is essential for reducing the exposure of patients to potentially unsafe components.27,42 RSA is one method that can be used as a screening tool to identify such components. In this study, screening of the Triathlon cemented knee prosthesis indicated that the long term stability for this prosthesis is likely to be similar to that of its predecessor, the Duracon cemented knee prosthesis. This is based on similarity in the magnitude and pattern of component migration and on the proportion of knees that were classified as being “at risk” of loosening.
An increase in MTPM of > 0.2 mm between the first and second year has been proposed to indicate that patients are at risk of loosening at ten years.26 The predictive power for identifying prostheses that are at risk of loosening at 10 years is reported by Ryd et al26 to be 85%. This predictive power comprises a relatively poor sensitivity, but a specificity of 97%. Given this very high specificity, patients classified as being “at risk” (> 0.2 mm of migration between one and two years) are highly likely to suffer from clinical loosening at ten years. It is therefore interesting that 23% of the Duracon components in the present study were classified as being ‘at risk’, as this is at odds with data from a number of joint replacement registries that report superior long-term stability for the Duracon prosthesis.2,3 The Swedish Knee Registry reports that the Duracon prosthesis has a relative risk of revision of 1.01 (95% CI 0.86 to 1.2) when compared with the AGC prosthesis (Biomet), which is considered to be the “gold standard” by this registry,3 and the Australian Joint Replacement Registry reports a cumulative revision rate of 5.3% at ten years for cemented Duracon TKRs.2 Using these same RSA criteria, a high proportion of ‘at risk’ components have also been reported for cementless TKRs using the Duracon prosthesis. Hansson et al13 reported that 56% of porous-coated components had continuous migration (according to the classification of Ryd et al26), while 33% of hydroxyapatite-coated components had continuous migration, whereas registry data reports a cumulative revision rate for Duracon with cemented fixation of only 4.2% at ten years.3 Furthermore, while the proportion of Triathlon components classified as being ‘at risk’ in the present study was lower (17%) than for Duracon, based on a cumulative revision rate of 2.5% at five years given by the Australian registry,2 this is also considerably higher than should be expected. This raises a question as to whether classification according to the threshold of 0.2 mm introduced by Ryd et al26 in 1995 requires re-evaluation.
A small amount of continuous migration without subsequent stabilisation has been reported for cemented tibial components as a result of continuous remodelling of the interface between the proximal tibia and the cement mantle.43,44 It is perhaps unsurprising therefore that a large proportion of components showed continuous migration in this study. Certainly the small positive difference seen with the Triathlon component in comparison with the Duracon component mirrors the medium term data available for these components in the registries; for example, the five-year revision rate for the Triathlon prosthesis ranges from 1.56% to 2.5%, compared with the five-year revision rate of 3.2% for Duracon.2,45 It must, however, be noted that the amount of migration measured in this study is a combination of both the migration of the tibial component relative to the bone and the migration of the relatively unconstrained polyethylene insert relative to the tibial component. The migration of the tibial insert relative to the tibial component may also have contributed to the proportion of components that showed continuous migration in this study.
It was not possible to report a significant decrease in migration demonstrated by the Triathlon prosthesis in this study, despite the difference in the means observed. This is likely a consequence of the small sample size, and the expected small difference and supporting evidence noted in the registry data would likely be a consequence of the improved kinematics observed for single radius prostheses.4 Single radius prostheses provide functional benefits over multi radius prostheses in that patients require less compensatory adaptation following single radius knee replacement and less relative hamstring co-activation to maintain joint stability.6 The single radius prosthesis provides better support during knee flexion and extension as well as a more physiological quadriceps force and more uniform movement.5,46,47 The combination of these benefits is likely to result in lower mechanical forces being experienced by single radius prostheses that in turn should have a positive effect on migration and increase their mechanical stability over time. However, longer term follow-up of the Triathlon prosthesis is needed to confirm this.
A limitation of this study is the small sample size in each group. A sample size of 25 to 30 patients is reportedly sufficient for the screening of implants using RSA10,25,26; however, the more ‘at risk’ events that are reported, the greater the sample size that is required to be confident that the results obtained from the study groups are generalisable to the population.48 However, the study is strengthened in that both the modified implant and its predecessor were screened under the same conditions in a randomised controlled trial, which may mitigate the problem of having a small sample size to some degree. Furthermore, some confidence in the validity of the RSA data from this study can be gained because of the similarities in migration, particularly MTPM, with other cemented tibial components.43,44,49,50 A further limitation is that despite the proposed benefits to knee motion and stability – due to changing from a multiradius femoral component in the Duracon prosthesis to a single radius design with the Triathlon prosthesis – no kinematic assessments or evaluation of the migration characteristics of the femoral component were made.
The results of this study demonstrate that the tibial component of the Triathlon knee replacement system has migration characteristics that are similar to its ‘elderly’ predecessor, the Duracon knee replacement system, which would indicate that the long-term mechanical stability of the tibial component of the Triathlon system will be similar to that of the Duracon system over the longer term. The Triathlon prosthesis provides benefits of more uniform movement, lower contact stresses on the tibial insert and greater stability during knee flexion, however, further research is required to confirm these benefits in a clinical setting and more specifically to determine how the new design features of the Triathlon prosthesis will affect femoral component stability and mechanical performance of the tibial insert.
The authors would like to thank the study coordinator M. Davidsson for her excellent help in monitoring the patients and the files, and A. Pearce for assistance with the preparation of the manuscript.
1 Greene KA , HarwinSF. Maximizing patient satisfaction and functional results after total knee arthroplasty. J Knee Surg2011;24:19–24.CrossrefPubMed Google Scholar
2 No authors listed. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report AOA 2011. http://www.dmac.adelaide.edu.au/aoanjrr/publications.jsp (date last accessed 20 September 2012). Google Scholar
3 No authors listed. The Swedish Knee Arthroplasty Register. Annual report, 2011. http://www.knee.nko.se/english/online/thePages/publication.php (date last accessed 20 September 2012). Google Scholar
4 Kessler O , DürselenL, BanksS, MannelH, MarinF. Sagittal curvature of total knee replacements predicts in vivo kinematics. Clin Biomech (Bristol, Avon)2007;22:52–58.CrossrefPubMed Google Scholar
5 Wolterbeek N , GarlingEH, MertensBJ, NelissenRG, ValstarER. Kinematics and early migration in single-radius mobile- and fixed-bearing total knee prostheses. Clin Biomech (Bristol, Avon)2012;27:398–402.CrossrefPubMed Google Scholar
6 Wang H , SimpsonKJ, FerraraMS, et al.Biomechanical differences exhibited during sit-to-stand between total knee arthroplasty designs of varying radii. J Arthroplasty2006;21:1193–1199.CrossrefPubMed Google Scholar
7 Aronson AS , HoistL, SelvikG. An instrument for insertion of radiopaque bone markers. Radiology1974;113:733–734.CrossrefPubMed Google Scholar
8 Selvik G . Roentgen stereophotogrammetry: a method for the study of the kinematics of the skeletal system. Acta Orthop Scand Suppl1989;232:1–51. Google Scholar
9 Selvik G , AlberiusP, AronsonAS. A roentgen stereophotogrammetric system: construction, calibration and technical accuracy. Acta Radiol Diagn (Stockh)1983;24:343–352. Google Scholar
10 Kärrholm J , BorssénB, LöwenhielmG, SnorrasonF. Does early micromotion of femoral stem prostheses matter?: 4-7-year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg [Br]1994;76-B:912–917. Google Scholar
11 Mjoberg B , FranzenH, SelvikG. Early detection of prosthetic-hip loosening: comparison of low- and high-viscosity bone cement. Acta Orthop Scand1990;61:273–274. Google Scholar
12 Garling EH , ValstarER, NelissenRG. Comparison of micromotion in mobile bearing and posterior stabilized total knee prostheses: a randomized RSA study of 40 knees followed for 2 years. Acta Orthop2005;76:353–361.PubMed Google Scholar
13 Hansson U , RydL, Toksvig-LarsenS. A randomised RSA study of Peri-Apatite HA coating of a total knee prosthesis. Knee2008;15:211–216.CrossrefPubMed Google Scholar
14 Hansson U , Toksvig-LarsenS, JornLP, RydL. Mobile vs. fixed meniscal bearing in total knee replacement: a randomised radiostereometric study. Knee2005;12:414–418.CrossrefPubMed Google Scholar
15 Hauptfleisch J , Glyn-JonesS, BeardDJ, GillHS, MurrayDW. The premature failure of the Charnley Elite-Plus stem: a confirmation of RSA predictions. J Bone Joint Surg [Br]2006;88-B:179–183.CrossrefPubMed Google Scholar
16 Lindstrand A , StenströmA, RydL, Toksvig-LarsenS. The introduction period of unicompartmental knee arthroplasty is critical: a clinical, clinical multicentered, and radiostereometric study of 251 Duracon unicompartmental knee arthroplasties. J Arthroplasty2000;15:608–616.CrossrefPubMed Google Scholar
17 Nilsson KG , DalenT. Inferior performance of Boneloc bone cement in total knee arthroplasty: a prospective randomized study comparing Boneloc with Palacos using radiostereometry (RSA) in 19 patients. Acta Orthop Scand1998;69:479–483.CrossrefPubMed Google Scholar
18 Nilsson KG , KärrholmJ. RSA in the assessment of aseptic loosening. J Bone Joint Surg [Br]1996;78-B:1–3.PubMed Google Scholar
19 Ornstein E . Hip revisions with impacted morselized allograft bone and cement: patient outcome, prosthetic fixation and risks. Acta Orthop Scand Suppl2002;73:1–66. Google Scholar
20 Ryd L . Roentgen stereophotogrammetric analysis of prosthetic fixation in the hip and knee joint. Clin Orthop Relat Res1992;276:56–65.PubMed Google Scholar
21 Toksvig-Larsen S , RydL, LindstrandA. Fixation of the tibial component in knee arthroplasty after six weeks. Int Orthop1995;19:89–93.CrossrefPubMed Google Scholar
22 Trozzi C , KapteinBL, GarlingEH, et al.Precision assessment of model-based RSA for a total knee prosthesis in a biplanar set-up. Knee2008;15:396–402.CrossrefPubMed Google Scholar
23 van der Linde MJ , GarlingEH, ValstarER, ToninoAJ, NelissenRG. Periapatite may not improve micromotion of knee prostheses in rheumatoid arthritis. Clin Orthop Relat Res 2006;448:122–128. Google Scholar
24 Zhou ZK , LiMG, BorlinN, WoodDJ, NivbrantB. No increased migration in cups with ceramic-on-ceramic bearing: an RSA study. Clin Orthop Relat Res2006;448:39–45.CrossrefPubMed Google Scholar
25 Valstar ER , GillR, RydL, et al.Guidelines for standardization of radiostereometry (RSA) of implants. Acta Orthop2005;76:563–572.CrossrefPubMed Google Scholar
26 Ryd L , AlbrektssonBE, CarlssonL, et al.Roentgen stereophotogrammetric analysis as a predictor of mechanical loosening of knee prostheses. J Bone Joint Surg [Br]1995;77-B:377–383.PubMed Google Scholar
27 Malchau H. On the importance of stepwise introduction of new implant technology: assesment of total hip replacement using clinical evaluation, radiostereometry, digitised radiography and a national hip registry. PhD Dissertation. Google Scholar
28 Karrholm J . Roentgen stereophotogrammetry: review of orthopedic applications. Acta Orthop Scand1989;60:491–503. Google Scholar
29 World Medical Association. Declaration of Helsinki: ethical principles for medical research involving human subjects. 59th WMA General Assembly, Seoul, October 2008. Google Scholar
30 Ryd L . Micromotion in knee arthroplasty: a roentgen stereophotogrammetric analysis of tibial component fixation. Acta Orthop Scand Suppl1986;220:1–80. Google Scholar
31 Ryd L , LindstrandA, RosenquistR, SelvikG. Tibial component fixation in knee arthroplasty. Clin Orthop Relat Res1986;213:141–149. Google Scholar
32 Benoni G , FredinH. Fibrinolytic inhibition with tranexamic acid reduces blood loss and blood transfusion after knee arthroplasty: a prospective, randomised, double-blind study of 86 patients. J Bone Joint Surg [Br]1996;78-B:434–440.PubMed Google Scholar
33 Börlin N , ThienT, KärrholmJ. The precision of radiostereometric measurements: manual vs digital measurements. J Biomech2002;35:69–79. Google Scholar
34 Odenbring S , BerggrenAM, PeilL. Roentgenographic assessment of the hip-knee-ankle axis in medial gonarthrosis: a study of reproducibility. Clin Orthop Relat Res1993;289:195–196. Google Scholar
35 Insall JN , DorrLD, ScottRD, ScottWN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res1989;248:13–14.PubMed Google Scholar
36 Bellamy N . Outcome measurement in osteoarthritis clinical trials. J Rheumatol Suppl1995;43:49–51.PubMed Google Scholar
37 Roos EM , KlässboM, LohmanderLS. WOMAC osteoarthritis index: reliability, validity, and responsiveness in patients with arthroscopically assessed osteoarthritis: Western Ontario and MacMaster Universities. Scand J Rheumatol1999;28:210–215. Google Scholar
38 Roos EM , RoosHP, EkdahlC, LohmanderLS. Knee injury and Osteoarthritis Outcome Score (KOOS): validation of a Swedish version. Scand J Med Sci Sports1998;8:439–448. Google Scholar
39 Roos EM , RoosHP, LohmanderLS. WOMAC Osteoarthritis Index: additional dimensions for use in subjects with post-traumatic osteoarthritis of the knee: Western Ontario and MacMaster Universities. Osteoarthritis Cartilage1999;7:216–221. Google Scholar
40 Roos EM , RoosHP, LohmanderLS, EkdahlC, BeynnonBD. Knee Injury and Osteoarthritis Outcome Score (KOOS): development of a self-administered outcome measure. J Orthop Sports Phys Ther1998;28:88–96. Google Scholar
41 Ahlbäck S . Osteoarthrosis of the knee: a radiographic investigation. Acta Radiol Diagn (Stockh)1968;Suppl 277:7–72. Google Scholar
42 Malchau H . Introducing new technology: a stepwise algorithm. Spine (Phila Pa 1976)2000;25:285.CrossrefPubMed Google Scholar
43 Hilding M , RydL, Toksvig-LarsenS, AspenbergP. Clodronate prevents prosthetic migration: a randomized radiostereometric study of 50 total knee patients. Acta Orthop Scand2000;71:553–557.CrossrefPubMed Google Scholar
44 Nilsson KG , KärrholmJ, CarlssonL, DalénT. Hydroxyapatite coating versus cemented fixation of the tibial component in total knee arthroplasty: prospective randomized comparison of hydroxyapatite-coated and cemented tibial components with 5-year follow-up using radiostereometry. J Arthroplasty1999;14:9–20.CrossrefPubMed Google Scholar
45 No authors listed. National Joint Registry for England and Wales. 8th Annual Report, 2011. http://www.njrcentre.org.uk (date last accessed 20 September 2012). Google Scholar
46 Gómez-Barrena E , Fernandez-GarcíaC, Fernandez-BravoA, Cutillas-RuizR, Bermejo-FernandezG. Functional performance with a single-radius femoral design total knee arthroplasty. Clin Orthop Relat Res2010;468:1214–1220.CrossrefPubMed Google Scholar
47 Ostermeier S , Stukenborg-ColsmanC. Quadriceps force after TKA with femoral single radius. Acta Orthop2011;82:339–343.CrossrefPubMed Google Scholar
48 Derbyshire B , PrescottRJ, PorterML. Notes on the use and interpretation of radiostereometric analysis. Acta Orthop2009;80:124–130.CrossrefPubMed Google Scholar
49 Henricson A , DalénT, NilssonKG. Mobile bearings do not improve fixation in cemented total knee arthroplasty. Clin Orthop Relat Res2006;448:114–121. Google Scholar
50 Onsten I , NordqvistA, CarlssonAS, BesjakovJ, ShottS. Hydroxyapatite augmentation of the porous coating improves fixation of tibial components: a randomised RSA study in 116 patients. J Bone Joint Surg [Br]1998;80-B:417–425. Google Scholar
Funding statement:
This work was supported by Stryker SA.
Author contributions:
M. Molt: Writing the paper, Data analysis, Data collection, Performed surgeries
P. Ljung: Control of manuscript
S. Toksvig-Larsen: Study protocol, Data analysis, Data collection, Statistical analysis, Performed surgeries, Control of manuscript
ICMJE Conflict of Interest:
The authors have no other conflicts of interest. The study sponsor had no involvement in the analysis and interpretation of the results
©2012 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










