Abstract
Pathological fractures in children can occur as a result of a variety of conditions, ranging from metabolic diseases and infection to tumours. Fractures through benign and malignant bone tumours should be recognised and managed appropriately by the treating orthopaedic surgeon. The most common benign bone tumours that cause pathological fractures in children are unicameral bone cysts, aneurysmal bone cysts, non-ossifying fibromas and fibrous dysplasia. Although pathological fractures through a primary bone malignancy are rare, these should be recognised quickly in order to achieve better outcomes. A thorough history, physical examination and review of plain radiographs are crucial to determine the cause and guide treatment. In most benign cases the fracture will heal and the lesion can be addressed at the time of the fracture, or after the fracture is healed. A step-wise and multidisciplinary approach is necessary in caring for paediatric patients with malignancies. Pathological fractures do not have to be treated by amputation; these fractures can heal and limb salvage can be performed when indicated.
Introduction
A pathological fracture should be suspected in a paediatric patient when there is a fracture associated with minimal trauma, when the location of the fracture is unusual or when an abnormal process in the bone is seen in the radiographs. Intrinsic processes, such as changes in the mineral density of the bone from bone tumours (both benign and malignant), diseases like osteogenesis imperfecta, or infection; and extrinsic processes, such as internal fixation, biopsy tracts and radiation, can cause changes to the normal biomechanics of bone.1 The altered strength of the bone and the load applied are the factors that will determine the risk of a pathological fracture.2 Pathological fractures are often associated with pain and deformity and can be differentiated into micro- or macrofractures. Microfractures most commonly occur in trabecular bone in the metaphysis or vertebral bodies and are typically non-displaced. Many of these go unrecognised.1
Characteristics such as pain, size of the lesion (> 2.5 cm in width or > 3.5 cm in length) and cortical destruction (≥ 50%) have been found to not be independently predictive of fracture risk.2-4 Snyder et al2 used CT with ratios of bending and torsional rigidities in order to predict fractures in benign skeletal lesions, by analysing changes in both the material properties of the bone and the cross-sectional geometry of the lesion, and comparing with the contralateral unaffected bone. This allows quantification of the mechanical proprieties of the bone at the lesion site and determination of the reduction in the load-carrying capacity of the bone. Fracture-risk indices based on lesion size alone also do not take into account the compensatory remodeling of the host bone.2 The downside is additional radiation exposure by the CT scan of the lesion and its contralateral bone for comparison. When used in conditions that affect several bones, such as osteogenesis imperfecta, Ollier disease or polyostotic fibrous dysplasia, the prediction will not be as reliable as it is in normal bone. Leong et al5 prospectively analysed the protocol described by Snyder et al2 and, although they were unable to evaluate the sensitivity of the quantitative CT-based predictions of fracture risk, they were able to evaluate prospectively the specificity of the protocol as compared with the criteria predicated on the size of the lesion as measured on plain radiographs. This avoided surgery that would most likely have been unnecessary in 30 patients predicted to be at high risk for fracture on the basis of the lesion size, but not on the CT protocol. They suggested that the CT method should be used as an objective tool that can help patients and physicians to come to the best decision, which also depends on the patient’s activity level and individual preferences.5 Pireau et al6 found the bone cyst index (BCI), measured on T1-weighted MRI, to be a reliable intra- and interobserver tool to predict pathological fractures in unicameral bone cysts (UBCs), when compared with the bone cyst diameter and the cortical thickness. The BCI is obtained by dividing the cyst area by the diameter of the diaphysis squared; a value > 4 for the humerus and > 3.5 for the femur are considered to be high risk for fracture.6
Initial evaluation
In children, most pathological fractures are due to benign bone tumour or tumour-like conditions, metabolic diseases and infection. A malignancy, however, can sometimes be the cause and should always be kept in mind.
A detailed history and thorough physical exam is paramount in the evaluation of any patient with a pathological fracture. Certain bone tumours are more common at specific ages (Table I).7 A detailed pain profile of symptoms prior to the fracture should also be obtained in order to further characterise the lesion. Plain radiographs are the initial imaging modality of choice. At least two orthogonal views of the affected bone should be obtained. The location of the lesion in the bone, its size, growth pattern, matrix, periosteal reaction and the number of lesions are all important factors in the differential diagnosis of bone lesions.
Table I
Common predisposing benign and malignant lesions by age (adapted with permission from Arkader A, Dormans JP. Pathologic fractures associated with tumors and unique conditions of the musculoskeletal system. In: Beaty JH, Skaggs DL, Flynn JM, Waters K, eds. Rockwood and Wilkins’ fractures in children. Seventh ed. Philadelphia: Lippincott Williams & Wilkins, 2010:120–191)
| Age (yrs) | Benign lesions | Malignant lesions |
|---|---|---|
| 0 to 5 | Osteomyelitis | Metastatic tumours (neuroblastoma, Wilm’s tumour) |
| Eosinophilic granuloma | Leukaemia | |
| Hand-Schuller-Christian disease | Ewing sarcoma | |
| Fibrosarcoma | ||
| Eosinophilic granuloma/ Letterer-Siwe disease | ||
| 5 to 10 | Unicameral bone cyst (UBC) | Leukaemia |
| Aneurysmal bone cyst (ABC) | Osteogenic sarcoma | |
| Nonossifying fibroma (NOF) | Ewing sarcoma | |
| Osteochondroma | ||
| Fibrous dysplasia | ||
| Enchondroma/Ollier disease | ||
| Neurofibromatosis/Congenital pseudarthrosis of the tibia | ||
| 10 to 20 | UBC | Leukaemia |
| ABC | Lymphoma | |
| NOF | Osteogenic sarcoma | |
| Osteochondroma | Ewing sarcoma | |
| Fibrous dysplasia | ||
| Chondroblastoma | ||
| Giant cell tumour | ||
| Osteoid osteoma |
Almost all lesions will require biopsy at some point. Determining the best time to biopsy can sometimes be a difficult task, and it will be specific to different lesions, as some can wait until the fracture is healed, some can be simultaneous with fracture care and others are required for diagnostic purposes. When a lesion appears malignant on radiographs, the biopsy should not be delayed and should be performed by the treating surgeon, with proper training in orthopaedic oncology and who will be responsible for the final care of the patient, since a poorly performed biopsy can lead to catastrophic consequences.8 Cultures should always be obtained along with the biopsy to rule out an infectious process.
Unicameral bone cyst
UBC represents about 3% of all primary benign bone tumours. It is estimated that approximately 85% are found in patients aged < 20 years.9 Patients will usually present with local pain from a pathological fracture. The radiological appearance of a UBC is of a well-circumscribed, centrally-located meta-diaphyseal radiolucent lesion, with or without medullary expansion. The “fallen leaf” sign can be seen on radiographs, CT scans and MRI, demonstrating the true cystic component of the lesion, with a cortical fragment from a fracture in the central aspect of the cyst.10 The natural history of a UBC is to stabilise in size, and, with the natural growth of the bone, drift away from the physis.11 Although some can heal spontaneously, the majority will become inactive or latent and persist after 12 years of age.11 Approximately 75% of patients with a UBC present with a pathological fracture. A UBC is usually the most common cause of pathological fracture in children with a bone lesion.12,13 The most common sites of pathological fractures due to UBC are the proximal humerus followed by the proximal femur.13,14
The priority for treatment of a UBC is to treat the fracture first and then the lesion. Fracture treatment is achieved with simple immobilisation of the extremity for between four and six weeks (Fig. 1). This applies to fractures in non-weight-bearing areas that are minimally displaced and stable. The majority of the fractures heal, but the UBC will persist in between 20% and 50% of cases.13 When the fracture is unstable or is in a weight-bearing bone, there may be need to proceed to surgery sooner, for both fracture fixation and treatment of the cyst.
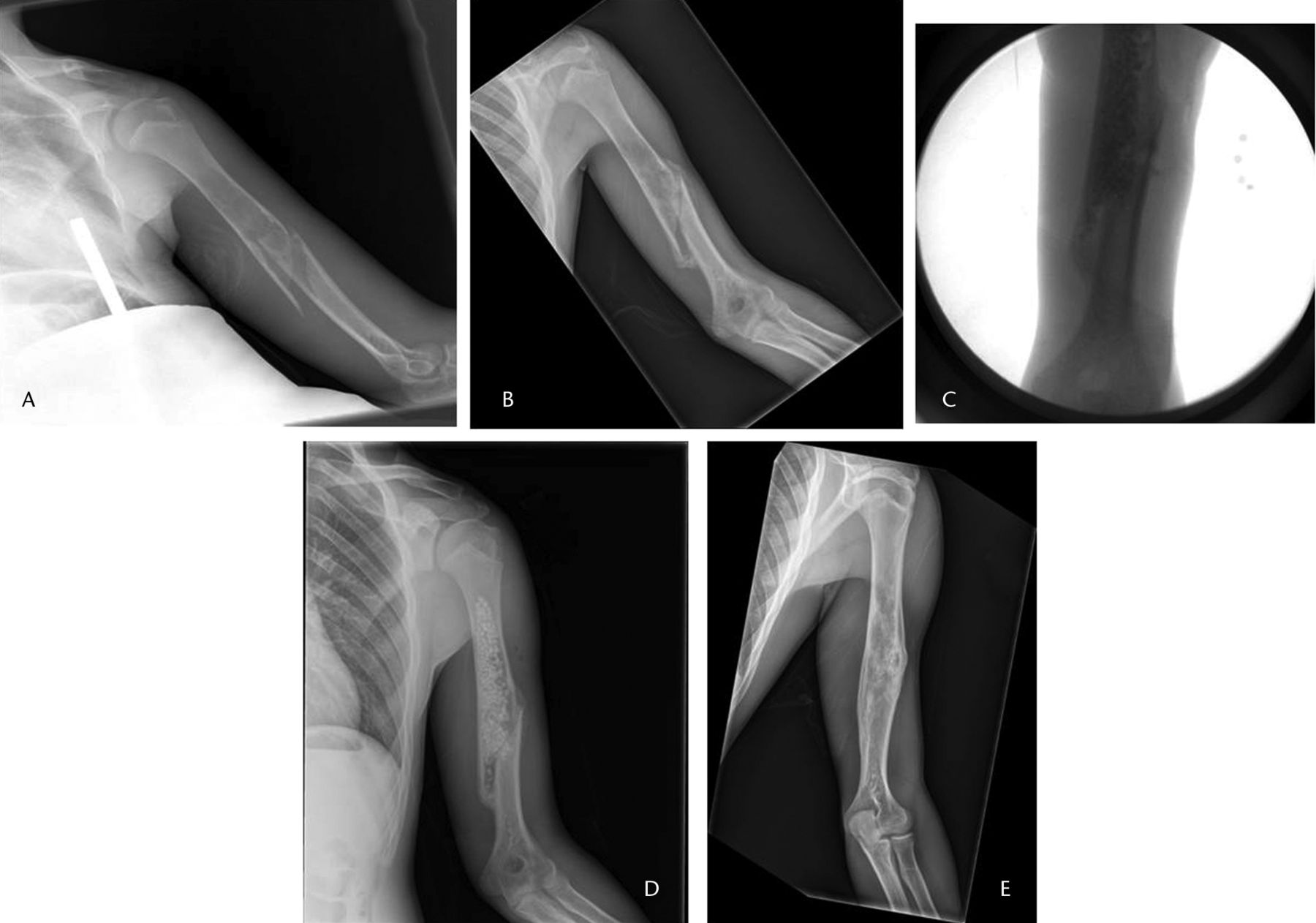
Fig. 1
Radiographs of a 16-year-old male patient, a) at presentation, showing a pathological fracture of the left humerus through a unicameral bone cyst, b) at two months after treatment in a sling with interval healing, c) and d) after percutaneous curettage and bone grafting, respectively, and e) at two years post-operatively, showing a healed cyst.
Although cysts located in the proximal femur are more likely to heal with a fracture than cysts in the proximal humerus,13 UBCs of the proximal femur can cause varus deformity and even avascular necrosis if not treated properly. Dormans and Pill1 described a classification system to guide treatment of lytic lesions of the femoral neck depending on the location and size of the lesion. This classification can be used for UBCs, as well as for other benign lytic lesions. We have updated this classification to include lesions of the proximal femur that extend into the diaphysis, where we recommend diaphyseal fixation (Fig. 2).1
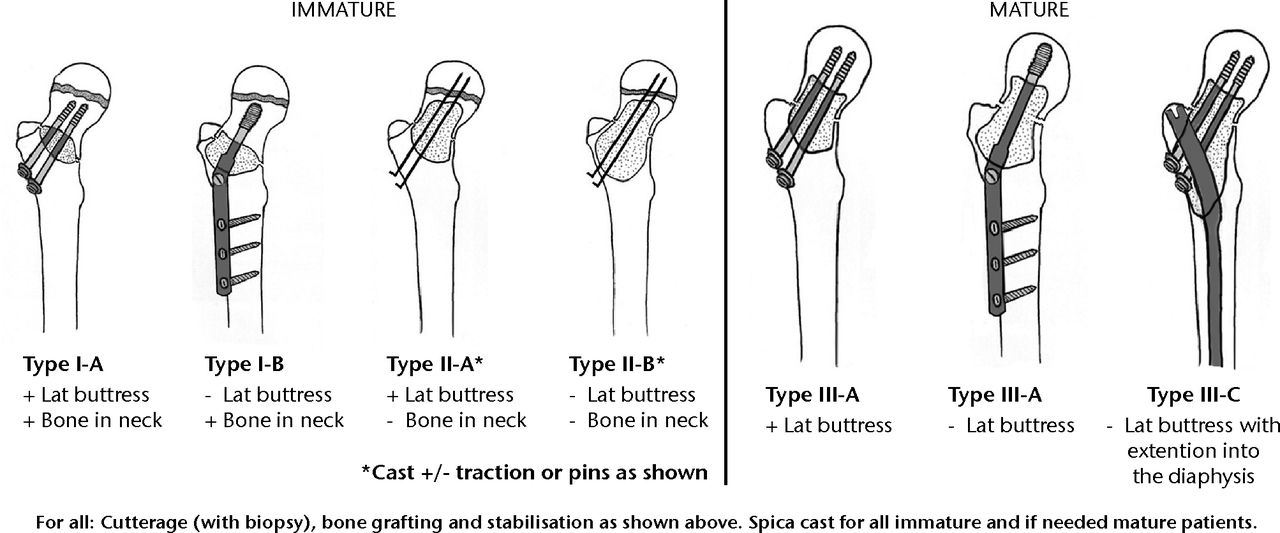
Fig. 2
Diagrams showing the classification system for the treatment of pathological fractures of the proximal femur associated with bone cysts in children (adapted with permission from Dormans JP, Pill SG. Fractures through bone cysts: unicameral bone cysts, aneurysmal bone cysts, fibrous cortical defects, and nonossifying fibromas. Instr Course Lect 2002;51:457–467).
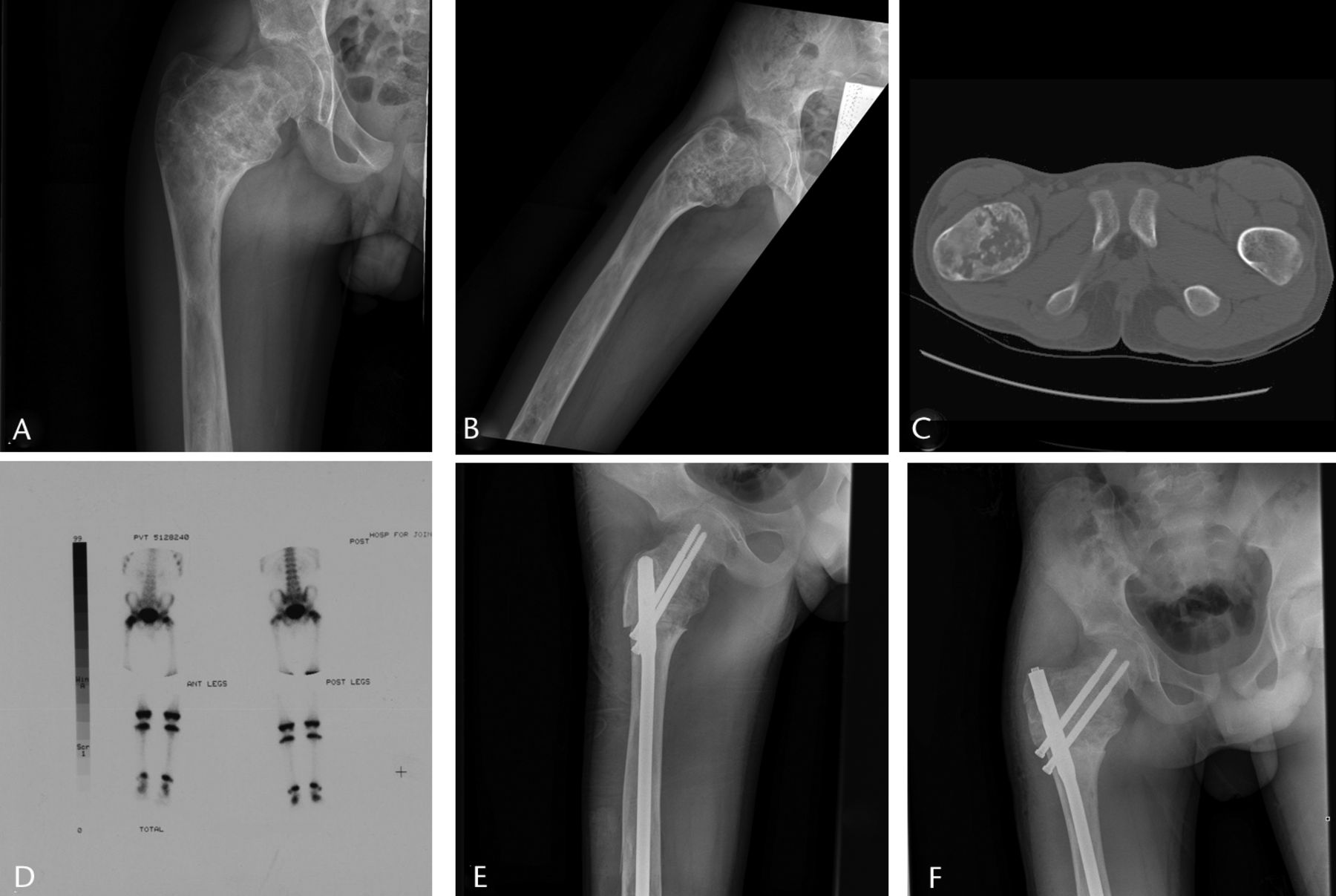
Fig. 3
Imaging in a 14-year-old male patient with a sudden onset of right hip pain, a) and b) radiographs at presentation, showing a Shepherd deformity and pathological fracture of the proximal femur, c) an axial CT scan showing the fracture, d) a bone scan, used to rule out polyostotic fibrous dysplasia, showing that it was confined to the right proximal femur, e) after proximal femoral osteotomy to correct the deformity and fixation with intramedullary nail, and f) at two months post-operatively showing healing of the fracture and osteotomies, at which point the patient had no pain.
The objective of treatment is to eradicate the cyst and prevent future fractures and deformities, especially in active cysts adjacent to the physis. Current treatment options for UBCs typically include a combination of some of the following: decompression or mechanical disruption of cyst wall, injection (steroids, bone marrow aspirate, demineralised bone matrix or bone-substitutes) and fixation for structural support when in a weight-bearing bone.15
There is a current trend towards agreement that percutaneous techniques that create mechanical disruption of the cyst membrane, concomitant migration of bone marrow cells for osteoinduction, and the use of calcium sulfate substitute for osteoconduction yield better outcomes when compared with other treatments.15,16 The technique consisting of percutaneous intramedullary decompression, curettage and grafting with calcium sulphate pellets, as described by Dormans et al,17 yielded higher rates of healing when compared with previously published data. Hou et al16 described patients treated with minimally invasive curettage, ethanol cauterisation, disruption of the cystic boundary, insertion of a synthetic calcium sulphate bone-graft substitute, and placement of a cannulated screw to provide drainage, and reported that these patients had the highest rate of healing and shortest time to union when compared with three other techniques. Canavese et al18 compared patients treated with one of autologous bone marrow injection, methylprednisolone injection or percutaneous curettage alone for treatment of UBC, and described that percutaneous curettage alone had better results (healing in 70%) when compared with the other two treatment types (41% and 21% for methylprednisolone and bone marrow aspirate, respectively). Wright et al19 performed a randomised clinical trial comparing rates of healing in UBC between those treated with intralesional injections of bone marrow aspirate and those treated with methylprednisolone. They reported that 16 of 38 UBCs (42%) treated with methylprednisolone healed, compared with nine of 39 (23%) of those treated with bone marrow aspirate only19; these rates lower than previously published studies on both treatments (100% by Lokiec et al,20 67% by Yandow et al21 and 60% by Scaglietti et al22). Although scraping the cyst was part of the trial protocol, the completeness of this step was not reported. A recent report on the long-term (seven-year) follow-up of 23 of the patients in the study by Wright et al19 demonstrated that although the distance from physeal scar had increased (p < 0.0001), reduction of the cyst area and overall cyst healing had not changed since completion of the trial (p = 0.06 and p = 0.5, respectively).23 Although 78% (18 of 23) of the growth plates were closed, none of the cysts had completely healed.
While there is an apparent high healing rate, there is still a rate of recurrence/persistence that ranges from 8% to 30% with these percutaneous techniques.16,18,24 These patients have a slightly higher chance of pathological fracture and are typically submitted to a repeat procedure until healing. The success rate increased to 94% after a repeat surgery, reaching a 100% healing rate in patients undergoing more than two repeated surgeries.24
Non-ossifying fibroma
Non-ossifying fibromas (NOFs), also known as fibrous cortical defects or nonosteogenic fibromas, are the most common benign bone lesion in children. It is estimated that between 30% and 40% of people aged < 20 years have a NOF, and these are mostly asymptomatic.25,26 The radiological appearance of an NOF is a lytic, well-defined lobulated lesion, located eccentrically in the metaphysis. Multi-locular appearance, ridges in the cortical wall, sclerotic scalloped borders and erosion of the cortex are frequent findings.25,27 There is no periosteal reaction in the absence of a pathological fracture. Most are in the distal ends of long bones. A frequent location is the distal femur at the origin of the medial head of the gastrocnemius. The natural history of NOF is to gradually disappear with age; they are expected to start regressing within years after its recognition, and usually the disappearance may take several years.28 In a few cases a NOF can cause pain and swelling, especially associated with athletic activity.
NOFs are typically not associated with pathological fractures27,29; however, when fractures do occur, it is usually in larger NOFs and almost always in the lower extremity.13,27 Ortiz et al13 reported that of the 17 patients with pathological fractures through a NOF in his series, 16 of the fractures were in the lower extremity. Arata et al27 reported that if a NOF involved > 50% of the transverse diameter of the bone, or if it measured > 33 mm in length, there was an increased risk of pathological fracture. However, Easley and Kneisl3 showed that 59% of their cases of large NOF exceeded these threshold measurements and also did not fracture. They suggested that the majority of patients with large NOFs can be monitored without intervention, as there is evidence to support spontaneous resolution of the majority of these lesions.3 Small NOFs can also have pathological fractures, or even microfractures and stress fractures. Shimal et al30 described five cases of smaller NOFs associated with fatigue-type stress fractures of the involved bone and lesion, stating that the NOF introduced an element of insufficiency to those stress fractures. They also suggested that the two conditions were inter-related since NOFs are said to occur at the site of insertion of a tendon or ligament exactly where abnormal muscular forces initiating a stress fracture are maximally applied.30
Treatment of a pathological fracture of an NOF generally follows the same principles used in the treatment of UBCs. The order of priority is the fracture first, and then the lesion, if necessary. Fractures in NOFs are known to have the best outcome of all pathological fractures.13 The fracture usually will heal with conservative treatment consisting of closed reduction, if necessary, and casting, since most are in the lower extremity. Fractures will usually heal in four to six weeks, but sometimes can take up to 12 weeks.27,31 The NOF will also heal in almost all cases, although it can take much longer to resolve completely, thus treatment of the lesion is not usually recommended when the fracture is already healed in an asymptomatic patient.13,27,31 Cases that require surgical treatment at the time of fracture are rare, these are usually the ones that cannot be adequately closed, reduced and immobilised.13 In these cases the NOFs should be curetted and bone grafted at the same time an open reduction and internal fixation is performed.27 A period of protected weightbearing is typically warranted until the fracture heals.
Fractures that were treated by conservative means and healed but have persistent pain are also atypical and can be treated with elective biopsy, curettage and bone grafting alone.29 Enlargement of the lesion is not expected after the fracture heals, and a different process should be suspected if this occurs.
Aneurysmal bone cyst
Aneurysmal bone cyst (ABC) is a benign but locally aggressive tumour. The prevalence of primary ABC is about 2% of all benign tumours of bone. A total of 80% of cases will be diagnosed in patients younger than 20 years of age.32 Usually patients will present with local pain that has been present for several weeks or months, or a pathological fracture. Rapid growth of the lesion can occur, mimicking a malignancy. On radiographs, an ABC is an expansile, lytic lesion that elevates the periosteum, contained by a thin shell of cortical bone.33 It can have well-defined margins or a permeative appearance, resembling a malignancy.33 Common locations include the femur, tibia, fibula, humerus and the spine.32,34 MRI, typically, will show a fluid-fluid level, depicting the multi-loculated cavities filled with fluid.34 ABCs can sometimes be secondary to other primary benign lesions or malignant bone tumours.
The natural history of ABCs was described by Dabska and Buraczewski33 based on radiology. They divided the lesions into four phases: 1) the initial phase, described as osteolysis of the marginal part of the bone; 2) the growth phase, characterised by the progressive destruction of bone; 3) the stabilisation phase, defined by the classic ABC appearance: an expansile lesion with a distinct bony shell and osseous septations; and 4) the healing phase, where progressive ossification of the lesion is obvious and results in a bony mass with a somewhat irregular structure.33 The real incidence of spontaneous healing is difficult to assess as there are no series evaluating the natural history of ABCs without treatment.32 In most cases, when an ABC is diagnosed, surgical treatment is recommended.
Approximately 36% of patients will present with a pathological fracture, which are usually in active lesions.34 A pathological fracture due to an ABC is often treated concomitantly with the lesion, as the lesion will not heal with fracture healing. The use of internal fixation will be determined by the location and displacement of the fracture. As stated before, these are locally aggressive lesions and can be associated with other primary lesions, so a biopsy with frozen section is recommended for all lesions.1 Currently, open curettage with adjuvant therapy and bone grafting is the most widely accepted management option. It has a low rate of recurrence, with minimal risk to the function of the affected area.35 Dormans et al34 described 45 patients who underwent a four-step approach comprising biopsy, curettage, cautery of cyst wall, use of high-speed burr and bone grafting, which resulted in healing of the lesion in 37 cases (82%). Mankin et al36 analysed 150 patients treated primarily with curettage and either implantation of allograft chips or polymethyl methacrylate, and reported a rate of recurrence of 20%. Lesions that occur in the proximal femur should be treated more aggressively, partly because of the high rate of local recurrence and the risk of fracture. We recommend the same guidelines as described previously for UBCs of the proximal femur (Fig. 2).
Fibrous dysplasia
Fibrous dysplasia (FD) is a benign non-hereditary condition that causes destruction of normal bone with fibrous tissue and small, woven spicules of bone. It represents between 5% and 7% of benign bone tumours.37 The lesions of FD develop during skeletal formation and growth and have an inconsistent evolution. Clinical presentation may occur at any age, however there is an increase in the incidence around 10 years of age.38 FD can be monostotic or, less commonly, polyostotic. Polyostotic FD can be associated with endocrine dysfunction and café-au-lait spots (McCune-Albright syndrome) or muscular myxomas (Mazabraud syndrome).37 Both of these syndromes are rare. In a very small subset of patients (0.5%) these lesions can undergo malignant change, typically about 15 years after diagnosis.39 The radiological appearance of FD varies from the classical lytic, ground-glass appearance and distinct sharp rim on the inner borders to more mature lesions, where there is increased thickness of the reactive rim and the lesion appears denser. Polyostotic lesions are larger and can be associated with deformity, such as coxa vara, shepherd’s crook deformity of the proximal femur and bowing of the tibia. FD can occur in the epiphysis, metaphysis or diaphysis of the bone, with a predilection for the long bones, ribs and craniofacial bones. Lesions are typically discovered due to local pain and/or swelling at the site of the lesion, incidentally or due to a pathological fracture.37,38 The natural history of these monostotic lesions is of a significant risk of fracture in the face of limited disease in the proximal femur, whereas its tendency to progress is restricted to a minority of cases. Long-term outcome in non-progressive cases is usually satisfactory, regardless of treatment.40 Polyostotic FD has a more reserved prognosis as it is associated with increased deformity and higher number of pathological micro- and macrofractures, even though there is a tendency of stabilisation of the deformities after adolescence.
Pathological fractures can occur in 50% of the patients with monostotic disease, especially in the proximal femur.40 Lesions that are more likely to remain stable present as heterogeneous lesions in the upper third of the femur or in the midshaft of the tibia. These lesions may convey a significant risk for fracture in the femur, but not in the tibia. Conversely, cervico-diaphyseal lesions in the femur, distal meta-diaphyseal lesions in the tibia, and cystic lesions at any site, may correspond to lesions with a greater tendency to extend and cause secondary events beyond fracture, including deformity, limb shortening, and development of aneurysmal bone cysts.40 Pathological fractures in FD can be treated conservatively in most monostotic cases, especially if the location is not in the lower limbs and there is no deformity in the affected bone. Femoral diaphysis fractures in younger patients can be treated with traction and subsequent casting. FD will persist after the fracture. The lesion can be addressed later, but a whole body bone scan is recommended to exclude the possibility of polyostotic FD. Lesions that are characteristic and pose no risk of further deformity, especially in the upper extremities can be treated with close observation, but a biopsy to confirm the diagnosis is advised.12,37 Polyostotic FD patients should see an endocrinologist to rule out associated syndromes. In fractures that require surgical treatment: biopsy, curettage and bone grafting with cortical graft should be performed, as well as internal fixation when warranted. If the lesion of the proximal femur is small it can be treated with cannulated screws or compression screw and side plate, however, the surgeon should be aware that in moderate to severe cases this frequently can lead to a shepherd crook deformity, malunion or fracture below the plate.13,40,41 Due to the abnormal nature of the bone, it may be difficult to get sufficient fixation in the bone with screws and plates alone, especially in cases when a deformity is present.12,42 Curettage and use of cortical graft over cancellous graft along with open reduction and internal fixation with an intramedullary rod with fixation is highly recommended to avoid deformity13,41 (Fig. 3). Guille et al41 reported that 18 of 27 patients (66.6%) with fibrous dysplasia and Shepherd’s crook deformity treated surgically by several different methods required repeat operation or casting as a result of recurrence or microfractures, while Yang et al42 reported no progression in all 14 patients treated surgically by the four-step procedure for the lesion, valgus osteotomy for correction of the deformity and intramedullary nail with neck cross pinning.
Malignant bone tumours
Patients presenting with a pathological fracture of a primary bone malignancy are rare. These patients encompass approximately between 5% and 10% primary malignancies of bone.43,44 Osteosarcoma and Ewing sarcoma are the most common primary bone malignancies in childhood. Failure to recognise these fractures as being pathological can lead to inappropriate treatment and potentially worse outcomes.45
Fractures may occur from minimal trauma or spontaneously due to the loss of bone matrix from the high cellularity of the tumour, or secondary to biopsy.43,46 The incidence of osteosarcoma presenting with a pathological fracture ranges from 5% to 13%.43,47 Of 397 patients with Ewing sarcoma, Fuchs et al46 reported a pathological fracture in 35 patients (8.8%). Of these, 14 fractures (40%) were sustained either before or after initial treatment, while the remainder sustained a fracture subsequent to radiation.46 Larger, proximal, diaphyseal, lytic and telangiectatic or fibroblastic osteosarcoma subtypes have been found to be more prone to fracture.44,47,48
Standard initial management of malignant bone tumours includes neoadjuvant chemotherapy, after appropriate biopsy and staging studies have been performed. Plain radiographs and MRI of the entire bone are paramount to assess the primary tumour, evaluate for a soft-tissue mass, proximity to neurovascular structures and to rule out skip metastases. A whole body nuclear medicine bone scan is necessary to rule out bony metastases and a CT of the lung to rule out pulmonary metastases.
Patients presenting with a fracture require immobilisation during this initial phase of treatment. Cast immobilisation, traction or external fixation can be used to stabilise the fracture,49 based on the location of the lesion and type of fracture (Fig. 4). The use of external fixator pins or traction pins requires careful planning to ensure the pins are placed away from the tumour to reduce the risk of potential pin site and/or soft-tissue seeding. Protected weightbearing or cast immobilisation after bone biopsy may also be necessary. Frequently, malignant tumours have a large soft-tissue component and this can be sampled.
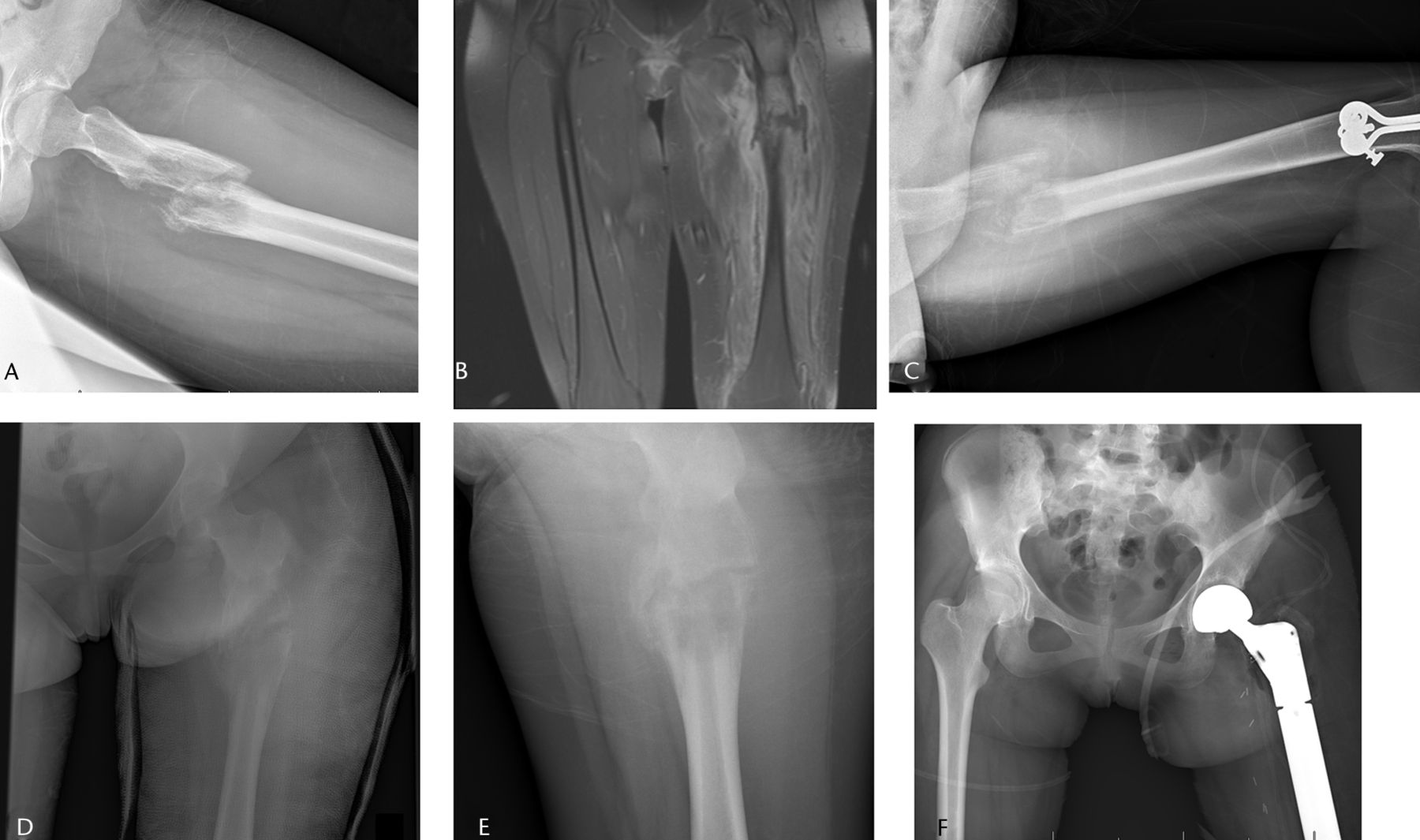
Fig. 4
Imaging in a 15-year-old female patient, a) radiograph at presentation, showing a pathological fracture through a malignant-appearing lesion of the proximal femur, b) coronal STIR T1-weighted post-contrast MRI showing the fracture, associated haematoma and soft-tissue mass, after which a biopsy confirmed a diagnosis of Ewing sarcoma. The patient underwent six weeks in traction (c) and six further weeks in a spica cast (d). After neoadjuvant chemotherapy, the fracture healed (e) and patient underwent a wide resection of a proximal femoral mass with endoprosthetic replacement (f).
Pathological fractures have shown potential to heal while on neoadjuvant chemotherapy. Jaffe et al50 described 13 patients with pathological fractures, 11 of which healed while on chemotherapy. Scully et al51 showed that fractures that healed had increased necrosis after final histopathologic analysis. Fractures may also occur while on chemotherapy perhaps due to increased tumour necrosis.
Limb salvage surgery is the preferred treatment for malignant bone tumours in paediatric patients at most institutions; however, amputation still plays a role, especially when a satisfactory surgical margin cannot be obtained, or the residual limb will be non-functional.52 Several studies have demonstrated no adverse effect on local recurrence and overall survival in patients undergoing limb salvage surgery compared with those undergoing amputation.53-55
The question of whether a pathological fracture affects disease-free and overall survival has been reviewed in many studies. A fracture through a primary bone tumour will result in the formation of a haematoma that may contaminate adjacent tissue.43 Some authors had theorised that this may also lead to distant haematogenous spread of the tumour, due to damage to the microcirculation.56 Earlier reports had advocated for amputation in patients with pathological fractures due to this theorised risk.57 In Abudu et al’s43 retrospective series of 40 patients with pathological fractures of osteosarcoma, there was not a significant difference in overall survival between patients treated with limb salvage versus those treated with amputation. Moradi et al58 also found no difference in the disease-free and overall survival outcomes when amputation was compared with limb salvage in 447 patients treated for a pathological fracture of a malignant bone tumour.
Scully et al51 investigated osteosarcoma patients with (n = 52) and without pathological fracture (n = 55) in a multicentre study. A pathological fracture was an independent multivariate risk factor for local recurrence and a univariate risk factor for decreased survival.51 The degree of displacement of the fracture and whether it occurred prior to presentation or during treatment had no effect on outcomes. However, not all patients received neoadjuvant chemotherapy, as this study reviewed patients from over a 30-year follow-up period.
Bacci et al44 showed no difference in disease-free survival and overall survival in a comparison of a cohort of 46 patients with a pathological fracture of an osteosarcoma to a larger group without a fracture. All patients in this study received neoadjuvant chemotherapy. There was also no difference between those that underwent an amputation compared with those who had limb salvage surgery.
Bramer et al48 in 2007 reviewed outcomes after pathological fracture at a single institution. In 484 patients with osteosarcoma, rates of local recurrence were similar between groups with or without a fracture; however, overall survival was worse among the fracture group. More aggressive tumours had an increased risk for fracture, and perhaps already carry a worse prognosis, and hence were likely to have a worse outcome. In 156 patients with Ewing sarcoma, no difference in local recurrence or overall survival was found. Another study by Ferguson et al47 also found lowered overall five-year survival among patients with a pathological fracture in osteosarcoma, compared with those without a fracture (41% versus 60%). Limb sparing surgery can be performed in patients with a pathological fracture, where appropriate, although more aggressive tumours, which may be more prone to fracture, carry a worse overall outcome in patients with osteosarcoma.
Fractures can also occur after limb sparing surgery, most commonly after allograft reconstruction, but also in patients with Ewing sarcoma after radiation treatment. Reports of failure ranges from 16% to 60%.59-62
Late fractures in patients with Ewing sarcoma treated with radiation for local control can occur due to the effect radiation has on bone healing potential. Of a total of 35 patients with pathological fractures, Fuchs et al46 described the development of a late pathological fracture in 21 (60%) at a mean of four years after treatment with radiation. Wagner et al63 found a trend towards a relationship between external beam radiation > 40 Gy and incidence of pathological fracture, although small numbers meant that statistical significance was not reached. One-third of the patients with a late fracture had active disease or a secondary neoplasm, reinforcing the need for continued surveillance of these patients with malignancies.
Summary
Pathological fractures can occur due to benign and malignant conditions. A thorough history, physical examination and review of plain radiographs are crucial to determine the cause and guide treatment. In most benign cases the fracture will heal and the lesion can be addressed at the time of the fracture or after the fracture is healed. A step-wise and multidisciplinary approach is necessary in caring for paediatric patients with malignancies. Pathological fractures do not have to be treated with amputation; these fractures can heal and limb salvage can be performed when indicated.
1 Dormans JP , PillSG. Fractures through bone cysts: unicameral bone cysts, aneurysmal bone cysts, fibrous cortical defects, and nonossifying fibromas. Instr Course Lect2002;51:457–467.PubMed Google Scholar
2 Snyder BD , Hauser-KaraDA, HippJA, et al.Predicting fracture through benign skeletal lesions with quantitative computed tomography. J Bone Joint Surg [Am]2006;88-A:55–70.CrossrefPubMed Google Scholar
3 Easley ME , KneislJS. Pathologic fractures through nonossifying fibromas: is prophylactic treatment warranted?J Pediatr Orthop1997;17:808–813.PubMed Google Scholar
4 Kaelin AJ , MacEwenGD. Unicameral bone cysts: natural history and the risk of fracture. Int Orthop1989;13:275–282. Google Scholar
5 Leong NL , AndersonME, GebhardtMC, SnyderBD. Computed tomography-based structural analysis for predicting fracture risk in children with benign skeletal neoplasms: comparison of specificity with that of plain radiographs. J Bone Joint Surg [Am]2010;92-A:1827–1833.CrossrefPubMed Google Scholar
6 Pireau N , De GheldereA, Mainard-SimardL, LascombesP, DocquierPL. Fracture risk in unicameral bone cyst: is magnetic resonance imaging a better predictor than plain radiography?Acta Orthop Belg2011;77:230–238.PubMed Google Scholar
7 Arkader A, Dormans JP. Pathologic fractures associated with tumors and unique conditions of the musculoskeletal system. In: Beaty JH, Skaggs DL, Flynn JM, Waters K, eds. Rockwood and Wilkins’ fractures in children. Seventh ed. Philadelphia: Lippincott Williams & Wilkins, 2010:120–191. Google Scholar
8 Peabody TD , SimonMA. Making the diagnosis: keys to a successful biopsy in children with bone and soft-tissue tumors. Orthop Clin North Am1996;27:453–459.PubMed Google Scholar
9 Wilkins RM . Unicameral bone cysts. J Am Acad Orthop Surg2000;8:217–224.CrossrefPubMed Google Scholar
10 Reynolds J . The “fallen fragment sign” in the diagnosis of unicameral bone cysts. Radiology1969;92:949–953. Google Scholar
11 Neer CS , FrancisKC, JohnstonAD, KiernanHA. Current concepts on the treatment of solitary unicameral bone cyst. Clin Orthop Relat Res1973;97:40–51.CrossrefPubMed Google Scholar
12 Jackson WF , TheologisTN, GibbonsCL, MathewsS, KambouroglouG. Early management of pathological fractures in children. Injury2007;38:194–200.CrossrefPubMed Google Scholar
13 Ortiz EJ , IslerMH, NaviaJE, CanosaR. Pathologic fractures in children. Clin Orthop Relat Res2005;432:116–126.CrossrefPubMed Google Scholar
14 Tey IK , MahadevA, LimKB, LeeEH, NathanSS. Active unicameral bone cysts in the upper limb are at greater risk of fracture. J Orthop Surg (Hong Kong)2009;17:157–160.CrossrefPubMed Google Scholar
15 Donaldson S , WrightJG. Recent developments in treatment for simple bone cysts. Curr Opin Pediatr2011;23:73–77.CrossrefPubMed Google Scholar
16 Hou HY , WuK, WangCT, et al.Treatment of unicameral bone cyst: surgical technique. J Bone Joint Surg [Am]2011;93-A(Suppl 1):92–99.CrossrefPubMed Google Scholar
17 Dormans JP , SankarWN, MorozL, ErolB. Percutaneous intramedullary decompression, curettage, and grafting with medical-grade calcium sulfate pellets for unicameral bone cysts in children: a new minimally invasive technique. J Pediatr Orthop2005;25:804–811.CrossrefPubMed Google Scholar
18 Canavese F , WrightJG, ColeWG, HopyanS. Unicameral bone cysts: comparison of percutaneous curettage, steroid, and autologous bone marrow injections. J Pediatr Orthop2011;31:50–55.CrossrefPubMed Google Scholar
19 Wright JG , YandowS, DonaldsonS, MarleyL. A randomized clinical trial comparing intralesional bone marrow and steroid injections for simple bone cysts. J Bone Joint Surg [Am]2008;90-A:722–730.CrossrefPubMed Google Scholar
20 Lokiec F , EzraE, KhermoshO, WeintroubS. Simple bone cysts treated by percutaneous autologous marrow grafting: a preliminary report. J Bone Joint Surg [Br]1996;78-B:934–937. Google Scholar
21 Yandow SM , LundeenGA, ScottSM, CoffinC. Autogenic bone marrow injections as a treatment for simple bone cyst. J Pediatr Orthop1998;18:616–620.CrossrefPubMed Google Scholar
22 Scaglietti O , MarchettiPG, BartolozziP. The effects of methylprednisolone acetate in the treatment of bone cysts: results of three years follow-up. J Bone Joint Surg [Br]1979;61-B:200–204. Google Scholar
23 Donaldson S, Stephens D, Wright JG. Simple bone cysts: better with age? Procs Pediatric Orthopaedic Society of North America, Hawaii, 2010. Google Scholar
24 Mik G , ArkaderA, ManteghiA, DormansJP. Results of a minimally invasive technique for treatment of unicameral bone cysts. Clin Orthop Relat Res2009;467:2949–2954.CrossrefPubMed Google Scholar
25 Betsy M , KupersmithLM, SpringfieldDS. Metaphyseal fibrous defects. J Am Acad Orthop Surg2004;12:89–95.CrossrefPubMed Google Scholar
26 Laus M , VicenziG. Histiocytic fibroma of bone (a study of 170 cases). Ital J Orthop Traumatol1979;5:343–348.PubMed Google Scholar
27 Arata MA , PetersonHA, DahlinDC. Pathological fractures through non-ossifying fibromas: review of the Mayo Clinic experience. J Bone Joint Surg [Am]1981;63-A:980–988. Google Scholar
28 Yanagawa T , WatanabeH, ShinozakiT, et al.The natural history of disappearing bone tumours and tumour-like conditions. Clin Radiol2001;56:877–886.CrossrefPubMed Google Scholar
29 Moretti VM , SlotcavageRL, CrawfordEA, LackmanRD, OgilvieCM. Curettage and graft alleviates athletic-limiting pain in benign lytic bone lesions. Clin Orthop Relat Res2011;469:283–288.CrossrefPubMed Google Scholar
30 Shimal A , DaviesAM, JamesSL, GrimerRJ. Fatigue-type stress fractures of the lower limb associated with fibrous cortical defects/non-ossifying fibromas in the skeletally immature. Clin Radiol2010;65:382–386.CrossrefPubMed Google Scholar
31 Drennan DB , MaylahnDJ, FaheyJJ. Fractures through large non-ossifying fibromas. Clin Orthop Relat Res1974;103:82–88.CrossrefPubMed Google Scholar
32 Cottalorda J , KohlerR, Sales de GauzyJ, et al.Epidemiology of aneurysmal bone cyst in children: a multicenter study and literature review. J Pediatr Orthop B2004;13:389–394.CrossrefPubMed Google Scholar
33 Dabska M , BuraczewskiJ. Aneurysmal bone cyst: pathology, clinical course and radiologic appearances. Cancer1969;23:371–389. Google Scholar
34 Dormans JP , HannaBG, JohnstonDR, KhuranaJS. Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin Orthop Relat Res2004;421:205–211.CrossrefPubMed Google Scholar
35 Godfrey LW , GreshamGA. The natural history of aneurysmal bone cyst. Proc R Soc Med1959;52:900–905.PubMed Google Scholar
36 Mankin HJ , HornicekFJ, Ortiz-CruzE, VillafuerteJ, GebhardtMC. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol2005;23:6756–6762.CrossrefPubMed Google Scholar
37 DiCaprio MR , EnnekingWF. Fibrous dysplasia: pathophysiology, evaluation, and treatment. J Bone Joint Surg [Am]2005;87-A:1848–1864. Google Scholar
38 Harris WH , DudleyHR, BarryRJ. The natural history of fibrous dysplasia: an orthopaedic, pathological, and roentgenographic study. J Bone Joint Surg [Am]1962;44-A:207–233. Google Scholar
39 Hoshi M , MatsumotoS, ManabeJ, et al.Malignant change secondary to fibrous dysplasia. Int J Clin Oncol2006;11:229–235.CrossrefPubMed Google Scholar
40 Ippolito E , BrayEW, CorsiA, et al.Natural history and treatment of fibrous dysplasia of bone: a multicenter clinicopathologic study promoted by the European Pediatric Orthopaedic Society. J Pediatr Orthop B2003;12:155–177.CrossrefPubMed Google Scholar
41 Guille JT , KumarSJ, MacEwenGD. Fibrous dysplasia of the proximal part of the femur: long-term results of curettage and bone-grafting and mechanical realignment. J Bone Joint Surg [Am]1998;80-A:648–658. Google Scholar
42 Yang L , JingY, HongD, Chong-QiT. Valgus osteotomy combined with intramedullary nail for Shepherd's crook deformity in fibrous dysplasia: 14 femurs with a minimum of 4 years follow-up. Arch Orthop Trauma Surg2010;130:497–502.CrossrefPubMed Google Scholar
43 Abudu A , SferopoulosNK, TillmanRM, CarterSR, GrimerRJ. The surgical treatment and outcome of pathological fractures in localised osteosarcoma. J Bone Joint Surg [Br]1996;78-B:694–698.PubMed Google Scholar
44 Bacci G , FerrariS, LonghiA, et al.Nonmetastatic osteosarcoma of the extremity with pathologic fracture at presentation: local and systemic control by amputation or limb salvage after preoperative chemotherapy. Acta Orthop Scand2003;74:449–454.CrossrefPubMed Google Scholar
45 Mankin HJ , MankinCJ, SimonMA. The hazards of the biopsy, revisited: Members of the Musculoskeletal Tumor Society. J Bone Joint Surg [Am]1996;78-A:656–663. Google Scholar
46 Fuchs B , ValenzuelaRG, SimFH. Pathologic fracture as a complication in the treatment of Ewing’s sarcoma. Clin Orthop Relat Res2003;415:25–30. Google Scholar
47 Ferguson PC , McLaughlinCE, GriffinAM, et al.Clinical and functional outcomes of patients with a pathologic fracture in high-grade osteosarcoma. J Surg Oncol2010;102:120–124.CrossrefPubMed Google Scholar
48 Bramer JA , AbuduAA, GrimerRJ, CarterSR, TillmanRM. Do pathological fractures influence survival and local recurrence rate in bony sarcomas?Eur J Cancer2007;43:1944–1951.CrossrefPubMed Google Scholar
49 Chandrasekar CR , GrimerRJ, CarterSR, et al.Pathological fracture of the proximal femur in osteosarcoma: need for early radical surgery?ISRN Oncol2012;2012:512389.CrossrefPubMed Google Scholar
50 Jaffe N , SpearsR, EftekhariF, et al.Pathologic fracture in osteosarcoma. Impact of chemotherapy on primary tumor and survival. Cancer1987;59:701–709.CrossrefPubMed Google Scholar
51 Scully SP , GhertMA, ZurakowskiD, ThompsonRC, GebhardtMC. Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. J Bone Joint Surg [Am]2002;84-A:49–57. Google Scholar
52 Hosalkar HS , DormansJP. Limb sparing surgery for pediatric musculoskeletal tumors. Pediatr Blood Cancer2004;42:295–310.CrossrefPubMed Google Scholar
53 Allison DC , CarneySC, AhlmannER, et al.A meta-analysis of osteosarcoma outcomes in the modern medical era. Sarcoma2012;2012:704872.CrossrefPubMed Google Scholar
54 Bacci G , FerrariS, LariS, et al.Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg [Br]2002;84-B:88–92. Google Scholar
55 Simon MA , AschlimanMA, ThomasN, MankinHJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg [Am]1986;68-A:1331–1337.CrossrefPubMed Google Scholar
56 O’Hara JM , HutterRV, FooteFW, MillerT, WoodardHQ. An analysis of thirty patients surviving longer than ten years after treatment for osteogenic sarcoma. J Bone Joint Surg [Am]1968;50-A:335–354.CrossrefPubMed Google Scholar
57 Finn HA , SimonMA. Limb-salvage surgery in the treatment of osteosarcoma in skeletally immature individuals. Clin Orthop Relat Res1991;262:108–118.PubMed Google Scholar
58 Moradi B , Zahlten-HinguranageA, LehnerB, ZeifangF. The impact of pathological fractures on therapy outcome in patients with primary malignant bone tumours. Int Orthop2010;34:1017–1023.CrossrefPubMed Google Scholar
59 Alman BA , De BariA, KrajbichJI. Massive allografts in the treatment of osteosarcoma and Ewing sarcoma in children and adolescents. J Bone Joint Surg [Am]1995;77-A:54–64.CrossrefPubMed Google Scholar
60 Brigman BE , HornicekFJ, GebhardtMC, MankinHJ. Allografts about the knee in young patients with high-grade sarcoma. Clin Orthop Relat Res2004;421:232–239.CrossrefPubMed Google Scholar
61 Campanacci L , ManfriniM, ColangeliM, AliN, MercuriM. Long-term results in children with massive bone osteoarticular allografts of the knee for high-grade osteosarcoma. J Pediatr Orthop2010;30:919–927.CrossrefPubMed Google Scholar
62 Muscolo DL , AyerzaMA, Aponte-TinaoL, FarfalliG. Allograft reconstruction after sarcoma resection in children younger than 10 years old. Clin Orthop Relat Res2008;466:1856–1862.CrossrefPubMed Google Scholar
63 Wagner LM , NeelMD, PappoAS, et al.Fractures in pediatric Ewing sarcoma. J Pediatr Hematol Oncol2001;239:568–571.CrossrefPubMed Google Scholar
Funding statement:
None declared
Author contributions:
C. B. R. De Mattos: Writing the paper, Critical revision
O. Binitie: Writing the paper, Critical revision
J. P. Dormans: Critical revision
ICMJE Conflict of Interest:
Dr. Dormans receives royalties from Elsevier, Mosby, Brooke’s Publishing and his department receives funding from AO Spine and OMEGA.
©2012 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










