Abstract
Aims
This study aimed to evaluate sagittal spinopelvic alignment (SSPA) in the early stage of rapidly destructive coxopathy (RDC) compared with hip osteoarthritis (HOA), and to identify risk factors of SSPA for destruction of the femoral head within 12 months after the disease onset.
Methods
This study enrolled 34 RDC patients with joint space narrowing > 2 mm within 12 months after the onset of hip pain and 25 HOA patients showing femoral head destruction. Sharp angle was measured for acetabular coverage evaluation. Femoral head collapse ratio was calculated for assessment of the extent of femoral head collapse by RDC. The following parameters of SSPA were evaluated using the whole spinopelvic radiograph: pelvic tilt (PT), sacral slope (SS), pelvic incidence (PI), sagittal vertical axis (SVA), thoracic kyphosis angle (TK), lumbar lordosis angle (LL), and PI-LL.
Results
The HOA group showed higher Sharp angles compared with the RDC group. PT and PI-LL were higher in the RDC group than the HOA group. SS and LL were lower in the RDC group than the HOA group. No difference was found in PI, SVA, or TK between the groups. Femoral head collapse ratio was associated with PT, SS, SVA, LL, and PI-LL. A PI-LL > 20° and a PT > 30° correlated with greater extent of femoral head destruction by RDC. From regression analysis, SS and SVA were significantly associated with the femoral head collapse ratio within 12 months after disease onset.
Conclusion
Compared with HOA, RDC in the early stage correlated with sagittal spinopelvic malalignment. SS and SVA may partially contribute to the extent of femoral head destruction by RDC within 12 months after the onset of hip pain. The present study indicates a potential role of SSPA assessment in identification of RDC patients at risk for subsequent bone destruction.
Cite this article: Bone Jt Open 2022;3(1):77–84.
Take home message
Compared with hip osteoarthritis, rapidly destructive coxopathy is potentially linked to increased pelvic tilt, decreased sacral slope, loss of lumbar lordosis, and spinopelvic mismatch as sagittal spinopelvic malalignment.
Sacral slope and sagittal vertical axis may be associated with the extent of femoral head destruction by rapidly destructive coxopathy within 12 months after the disease onset.
Introduction
Rapidly destructive coxopathy (RDC), also known as rapidly destructive osteoarthritis of the hip, is a rare disorder. The first manifestation of the disease is rapid chondrolysis with progressive loss of the joint space. RDC is diagnosed when the hip shows joint space narrowing at the pace of > 2 mm/year.1 RDC generally occurs in elderly females, and requires surgical treatment due to severe pain and disability.2 Lack of sequential radiographs by rapid progression of the disease makes it difficult to clarify the process of disease progression in the early stage.3 Although the pathogenesis of RDC is still under investigation, one of the proposed mechanisms of RDC development is an increase in posterior pelvic tilt (PT).4 Some hips with RDC develop massive destruction of the femoral head during the first six to 24 months after the onset.3,5 Recent studies have shown that femoral head destruction within 12 months following the disease onset may be linked to increased posterior PT in patients with RDC.6,7
Patients with spinal disorders present the interaction between the sagittal spinopelvic alignment (SSPA) and the compensatory mechanism.8 Of SSPA parameters,9 pelvic incidence (PI) is a fixed fundamental anatomical parameter of the pelvis since the sacroiliac joint has limited mobility. PT and sacral slope (SS) determine the sagittal pelvic orientation on the femoral head. The relationship is defined with the equation: PI = PT+ SS. PT quantifies the pelvic rotation around the femoral heads. Sagittal vertical axis (SVA) quantifies the progressive anterior translation of the head away from the pelvis to assess alignment of the whole spine. Thoracic kyphosis and lumbar lordosis are also evaluated as SSPA.
Since the proposal of hip-spine syndrome,10 there has been increasing evidence on potential relationship between SSPA and hip disease development. Increased posterior PT by lumbar degenerative kyphosis decreases anterior acetabular coverage of the femoral head, which may cause hip osteoarthritis (HOA) in the absence of dysplasia (or with mild dysplasia) in elderly adults.4,11Similarly, previous studies have suggested that stress concentration in the reduced acetabular coverage by posterior PT with loss of lumbar lordosis could lead to RDC development.4,11,12 Indeed, Morimoto et al12 have demonstrated that female patients with end-stage RDC exhibit a significant increase in posterior PT, lower lumbar lordosis, lumbar range of motion, and SS compared with those with HOA. However, the authors have evaluated sagittal lumbopelvic alignment without the whole spinopelvic radiographs, and have provided no data of patients in the early stage of RDC. In addition to pelvic parameters, the seventh cervical vertebra (C7) positioning is essential for assessment of the global spine balance of SSPA.13 At present, no information is available about SSPA in the early stage of RDC.
Using an anteroposterior pelvic radiograph in a supine position, previous studies have shown possible relationship of RDC progression with increased posterior PT.6,7 However, pelvic inclination may alter from a supine to a standing position.12 The whole spinopelvic radiograph in a standing position is required for accurate assessment of the effect of SSPA on disease progression in the early stage of RDC. This study aimed to evaluate SSPA using the whole spinopelvic lateral radiograph in a standing position in the early stage of RDC in comparison with HOA, and to identify risk factors of SSPA for destruction of the femoral head within 12 months after the disease onset.
Methods
Patients
This monocentric, retrospective study, using anonymized data with a general opt-out procedure, was approved by the institutional review board of the authors’ institution (Kobe City Medical Center General Hospital, Japan). This study enrolled patients with sufficient clinical records including the onset of hip pain, age, a series of hip radiographs taken at intervals of every two to three months during the period of > 12 months from the onset of hip pain, and anteroposterior and lateral radiographs of the whole spine-pelvis in the free-standing position with the clavicle position14 taken within 12 months after the onset of hip pain. Based on a survey over a consecutive series of patients with hip pain from 2016 to 2020, the hip joints of 34 patients met the diagnostic criteria of RDC; chondrolysis > 2 mm in one year.1 In each case, the disease was unilateral with no evidence of antecedent HOA, osteonecrosis, infection, neuropathy, or inflammatory disease like rheumatoid arthritis. Magnetic resonance images were used to exclude osteonecrosis in some cases. Of the 34 hips with RDC, 25 hips developed femoral head destruction confirmed by radiographs and/or CT within 12 months after the disease onset (Figure 1). Of patients who underwent total hip arthroplasty for unilateral HOA showing femoral head destruction confirmed by radiographs and/or CT (Figure 1), we found 25 patients with preoperative anteroposterior and lateral radiographs of the whole spine-pelvis, whose ages and BMI at the time of the radiological studies were similar to those of the 34 patients with RDC. A series of radiographs of the HOA patients showed osteophyte formation in the acetabulum and/or femoral head with joint space narrowing at the pace of < 2 mm/year (Figure 1).
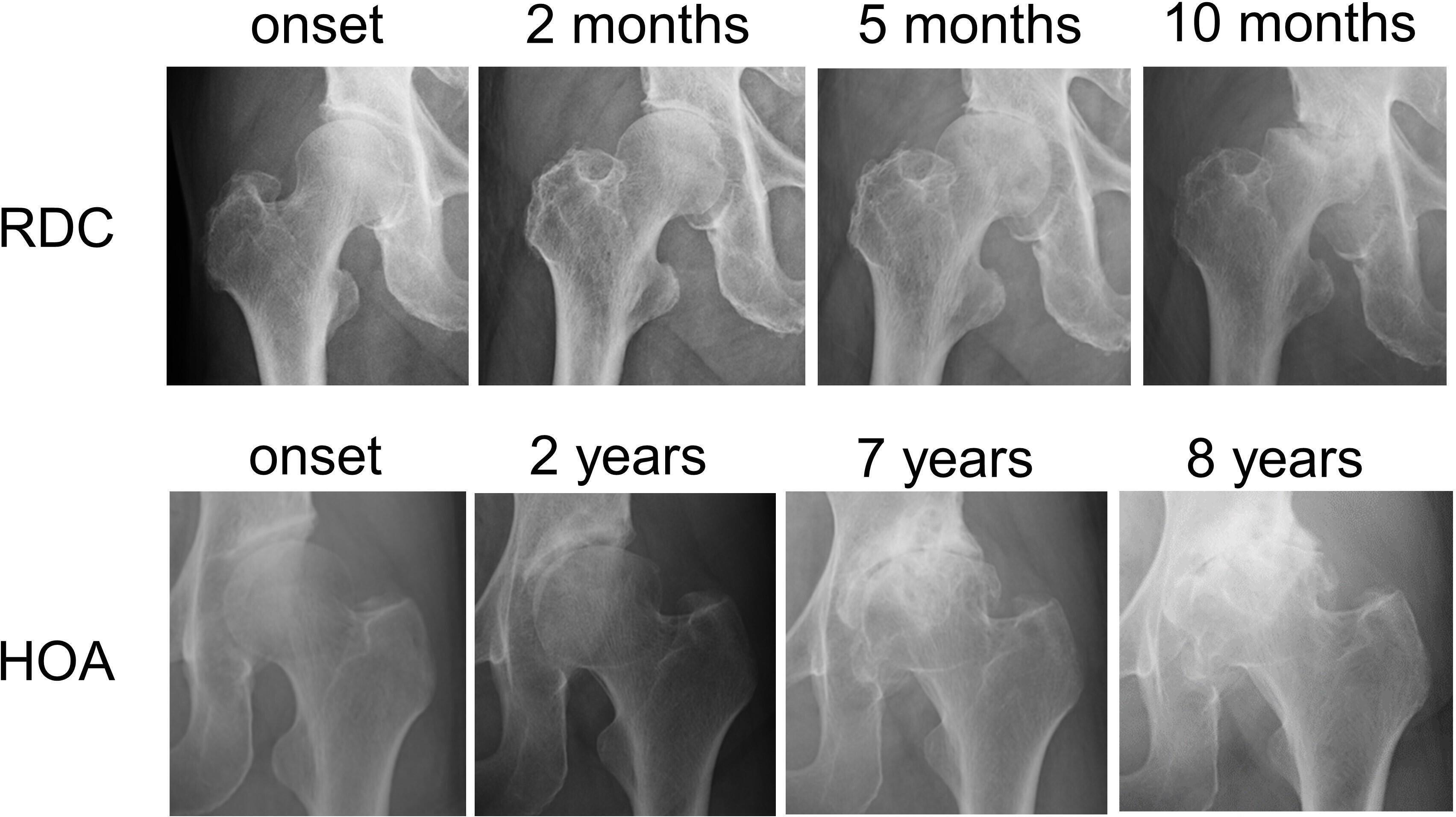
Fig. 1
Disease progression of rapidly destructive coxopathy (RDC) and hip osteoarthritis (HOA). Right hip joint with RDC demonstrating chondrolysis at two months, partial destruction of the femoral head at 5 months, and its massive destruction at ten months after the onset of hip pain. Sharp angle of the right hip was 42° at the onset of hip pain. Left hip joint with HOA showing slight joint space narrowing at two years, partial destruction of the femoral head with osteophyte formation in the acetabulum and femoral head at seven years, and exacerbating femoral head destruction at eight years after the onset of hip pain. Sharp angle of the left hip was 50° at the onset of hip pain.
Radiological parameters
Sharp angle15 was measured on the initial radiograph of RDC and HOA at the onset of hip pain using a PACS (Picture Archiving and Communication System) workstation (GE Healthcare Japan). Intraclass correlation coefficient (ICC) of intrarater reliability for Sharp angle was 0.99.
On the last anteroposterior radiograph of patients with RDC taken within 12 months after the onset of hip pain, the vertical distance was measured using a PACS workstation between the two separate lines parallel to the radiological teardrop line drown through the most proximal and distal portions of the femoral head. According to the previous study,7 femoral head collapse ratio for assessment of the extent of femoral head collapse was calculated with the method as demonstrated in Figure 2. ICC for femoral head collapse ratio was 0.99.
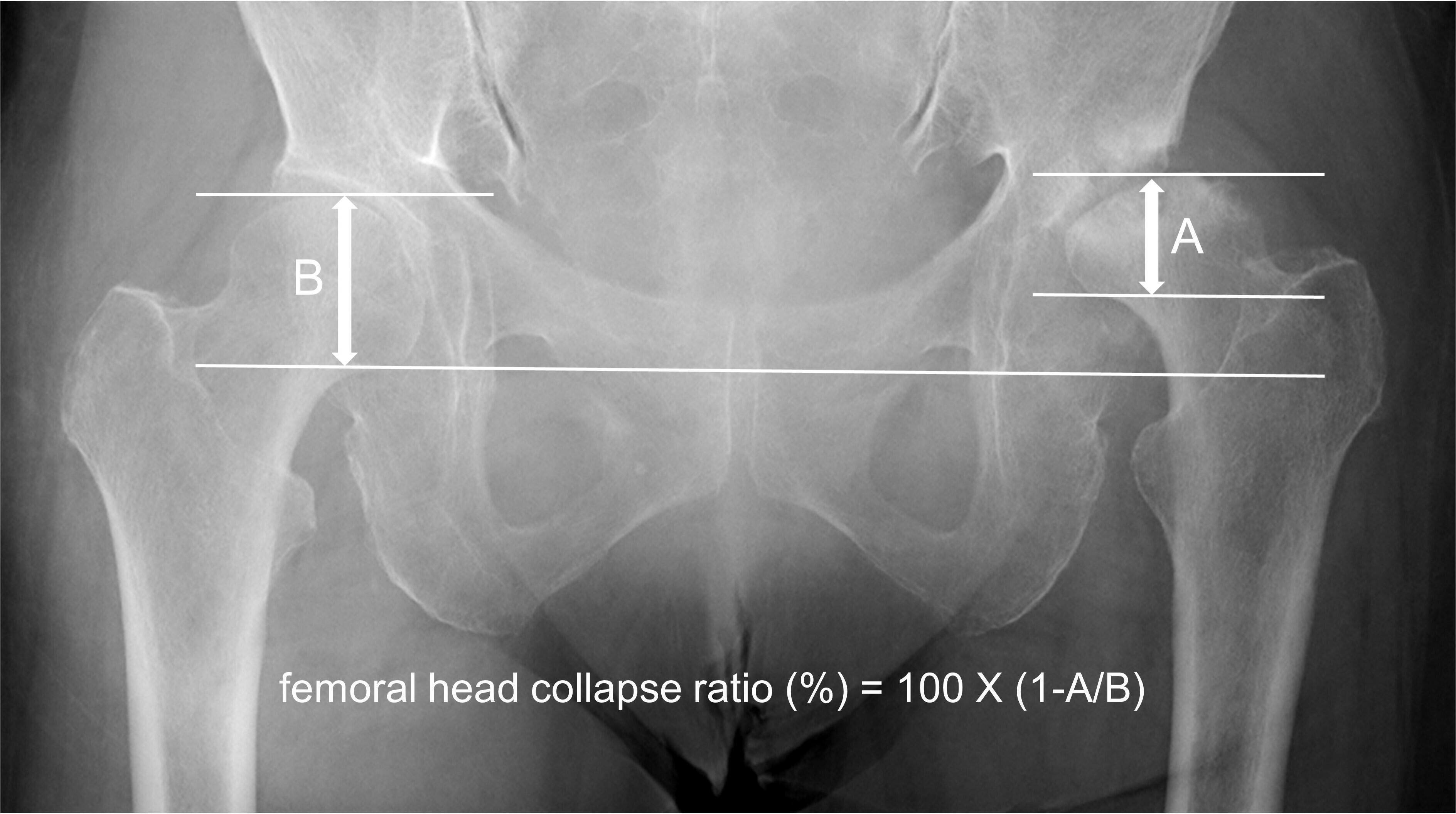
Fig. 2
Measurement of the femoral head collapse ratio. On the last anteroposterior radiograph taken within 12months after the onset of hip pain, the vertical distance is measured between the two separate lines parallel to the radiological teardrop line drown through the most proximal and distal portions of the affected (A) and non-affected (B) femoral heads. The femoral head collapse ratio (%) is calculated with the equation as femoral head collapse ratio (%) = 100×(1 A/B).
The following SSPA parameters9 were measured using a sagittal radiograph of the whole spine-pelvis in each patient (Figure 3). PT is the angle between the vertical and the line from the centre of the femoral heads to the midpoint of the sacral endplate. SS is the angle between the horizontal and the first sacral endplate. PI is calculated using following equation: PI = PT + SS. The SVA is defined as the sagittal offset of the C7 plumb line from the posterosuperior corner of the first sacral endplate. Thoracic kyphosis angle (TK) is the angle between the upper endplate of the fourth thoracic vertebra and the lower endplate of the twelfth thoracic vertebra. Lumbar lordosis angle (LL) is the angle between the upper endplate of the first lumbar vertebra and the first sacral endplate. ICC for the SPAA parameters ranged from 0.87 to 0.99. The SRS-Schwab classification modifiers were used to investigate the degree of sagittal malalignment.16 Accordingly, the degree of PI-LL was classified as < 10°, 10 to 20°, and > 20°. The degree of PT was categorized as < 20°, 20 to 30°, and > 30°. The degree of SVA was stratified as < 40 mm, 40 to 95 mm, and > 95 mm.
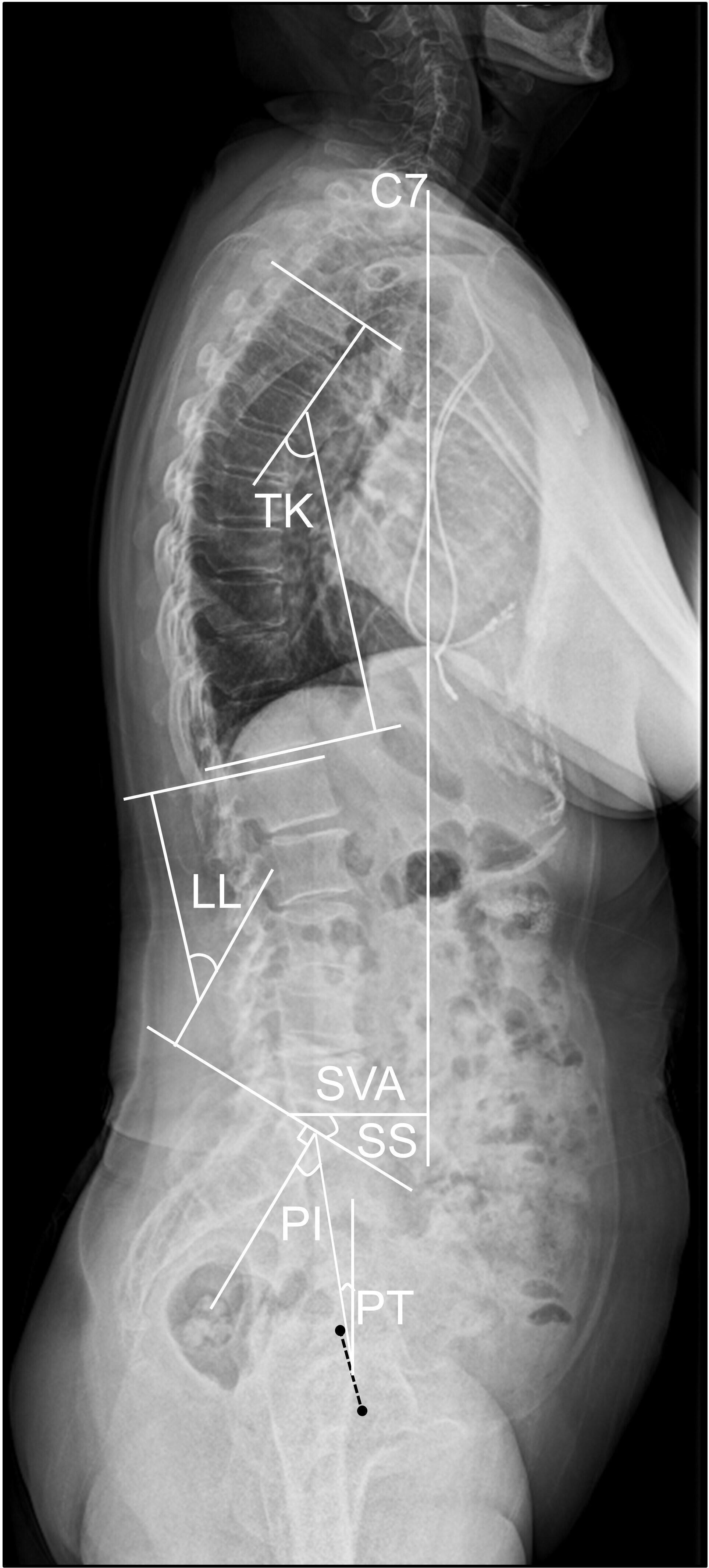
Fig. 3
Illustration of the radiological parameters of the sagittal alignment of the spine and pelvis. PI, pelvic incidence; PT, pelvic tilt; SS, sacral slope; LL, lumbar lordosis angle; TK, thoracic kyphosis angle; SVA, sagittal vertical axis.
Statistical analysis
Continuous data were expressed as means ± standard deviation (SD). Student’s t-test was performed for continuous normally distributed data, and the Mann-Whitney U test was performed for non-normally distributed data. Cohen’s d was calculated for the comparison between two means. Categorical data were compared using chi-squared test or Fisher’s exact test. Pearson’s correlation coefficients were calculated to determine associations between SSPA parameters and the femoral head collapse ratio. Stepwise multiple regression analysis was performed to identify significant predictors of SSPA parameters for the femoral head collapse ratio. Multicollinearity between predictors was judged by variance inflation factors (VIF). Statistical analyses were conducted in SPSS for Windows version 25 (SPSS, USA). The level of significance was set at p < 0.05.
Results
There was no difference in the ages between the RDC (74.6 years (SD 8.1)) and HOA (71.9 years (SD 6.4)) groups (p = 0.102, Mann-Whitney U test). Sex distribution showed no difference between the RDC (one male and 33 females) and the HOA (four males and 21 females) groups (p = 0.097, Fisher’s exact test). There was no difference in BMI between the RDC (23.2 kg/m2 (SD 3.1)) and HOA (24.0 kg/m2 (SD 4.7)) groups (p = 0.842, Mann-Whitney U test).
Comparison of radiological parameters between RDC and HOA
The HOA patients showed higher Sharp angles compared with the RDC patients. We measured PT, SS, PI, SVA, TK, LL, and PI-LL as SSPA parameters on the whole spine-pelvis lateral radiograph in the standing position (Figure 3). When we compared the radiological parameters between the patients with RDC and HOA (Table I), PT and PI-LL were significantly higher in the RDC group than the HOA group. SS and LL were significantly lower in the RDC group than the HOA group. There was no significant difference in PI, SVA, or TK between the groups.
Table I.
Comparison of radiological parameters and the SRS-Schwab classification modifiers16 between rapidly destructive coxopathy (RDC) and hip osteoarthritis (HOA).
| Variable | RDC (n = 34) | HOA (n = 25) | p-value | Cohen’s d |
|---|---|---|---|---|
| Radiological parameters, mean (SD; 95% CI) | ||||
| Sharp angle, ° | 40.4 (3.5; 39.2 to 41.6) | 48.8 (3.4; 47.6 to 50.1) | < 0.001* | 2.43 |
| PT, ° | 25.3 (15.2; 20.0 to 30.6) | 13.1 (8.7; 9.5 to 16.6) | < 0.001† | 0.96 |
| SS, ° | 24.3 (11.8; 20.2 to 28.4) | 32.7 (7.9; 29.5 to 36.0) | 0.003† | 0.82 |
| PI, ° | 49.6 (11.6; 45.6 to 53.6) | 45.8 (10.0; 41.6 to 49.9) | 0.189† | 0.35 |
| SVA, mm | 70.5 (50.4; 52.9 to 88.1) | 52.5 (46.7; 36.1 to 68.9) | 0.145† | 0.39 |
| TK, ° | 29.2 (13.3; 24.5 to 33.8) | 31.1 (10.1; 26.9 to 35.3) | 0.537† | 0.16 |
| LL, ° | 29.1 (18.6; 22.6 to 35.6) | 43.5 (12.0; 38.5 to 48.4) | 0.004* | 0.89 |
| PI-LL, ° | 20.5 (21.8; 12.9 to 28.1) | 2.3 (11.3; -2.3 to 7.0) | < 0.001† | 1.00 |
| SRS-Schwab classification modifiers | ||||
| PI-LL, n | ||||
| < 10° | 12 | 19 | 0.005‡ | |
| 10 to 20° | 9 | 4 | ||
| > 20° | 13 | 2 | ||
| PT, °, n | ||||
| < 20° | 12 | 18 | 0.001* | |
| 20 to 30° | 9 | 7 | ||
| > 30° | 13 | 0 | ||
| SVA, mm, n | ||||
| < 40 | 11 | 11 | 0.645‡ | |
| 40 to 95 | 14 | 9 | ||
| > 95 | 9 | 5 | ||
-
*
Mann-Whitney U test.
-
†
Student’s t-test.
-
‡
Chi-squared test.
-
LL, lumbar lordosis angle; PI, pelvic incidence; PT, pelvic tilt; SD, standard deviation; SS, sacral slope; SVA, sagittal vertical axis; TK, thoracic kyphosis angle.
Incidence of spinopelvic malalignment in patients with RDC and HOA
Patient distribution of spinopelvic malalignment according to the sagittal modifiers in the SRS-Schwab classifications16 are shown in Table I. The distributions of PI-LL and PT were significantly different between patients with RDC and HOA. Of 34 patients with RDC, 13 showed both a PI-LL > 20° and a PT > 30°.
Association of SSPA parameters with the extent of femoral head collapse in the early stage of RDC
We further investigated associations between radiological SSPA parameters and the femoral head collapse ratio in the patients with RDC. The femoral head collapse ratio was a mean of 16.3% (0 to 66.7). The femoral head collapse ratio significantly correlated with PT, SS, SVA, LL, and PI-LL (Figure 4). No correlation of the femoral head collapse ratio was found with PI (Figure 4) or TK (r = −0.09). Whereas 13 patients with RDC showed both a PI-LL > 20° and a PT > 30°, 21 patients exhibited a PI-LL < 20° and a PT < 30° (Figure 4). We compared the two subgroups to evaluate association of the SRS-Schwab classification modifiers with the extent of femoral head destruction. Whereas the femoral head collapse ratio was 29.2% (SD 25.1%) in the patients with both a PI-LL > 20° and a PT > 30°, the ratio was 8.3% (SD 12.4%) in those with a PI-LL < 20° and a PT < 30°. The difference was statistically significant (p = 0.006, Mann-Whitney U test).
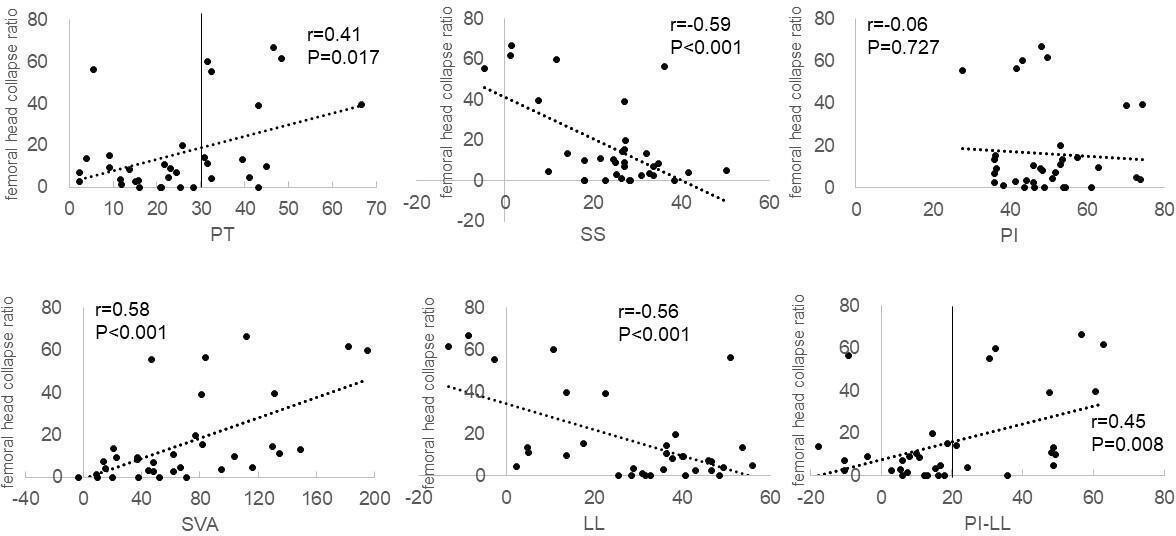
Fig. 4
Scatter plots of correlation between the femoral head collapse ratio and radiological parameters in patients with rapidly destructive coxopathy. Pearson’s correlation coefficients (r) are shown with p-values. LL, lumbar lordosis angle; PI, pelvic incidence; PT, pelvic tilt; SS, sacral slope; SVA, sagittal vertical axis; TK, thoracic kyphosis angle.
Stepwise regression analysis was performed using PT, SS, SVA, LL, and PI-LL to identify predictive variables for the femoral head collapse ratio in the early stage of RDC. Of the five SSPA parameters, SS and SVA were found to be the significant predictors. SS and a combination of SS and SVA explained 32.3% and 42.9%, respectively, of the femoral head collapse ratio within 12 months after the onset of hip pain (Table II).
Table II.
Stepwise regression analysis with predictor variables of sagittal spinopelvic alignment.
| Model | Predictor | Partial regression coefficient (SE) | Standardized partial regression coefficient | t-ratio | p-value* | 95% CI | Adjusted R2 | VIF |
|---|---|---|---|---|---|---|---|---|
| 1 | Sacral slope | -1.026 (0.251) | -0.586 | -4.091 | < 0.001 | -1.537 to –0.515 | 0.323 | 1.000 |
| 2 | Sacral slope | -0.697 (0.262) | -0.398 | -2.659 | 0.012 | -1.231 to –0.162 | 0.429 | 1.294 |
| Sagittal vertical axis | 0.162 (0.061) | 0.395 | 2.639 | 0.013 | 0.037 to 0.287 | 1.294 |
-
*
Multiple regression analysis.
-
CI, confidence interval; R2, coefficient of determination; SE, standard error; VIF, variance inflation factor.
Discussion
The inter-relationship between hip pathology and adult spine deformity with sagittal spinopelvic malalignment has received increasing attention. Using lateral lumbopelvic radiographs, Morimoto et al12 have demonstrated that patients with end-stage RDC exhibit increased PT, decreased SS, and loss of LL compared with those with HOA. The present study using the whole spine-pelvis radiographs has provided additional evidence that patients in the early stage of RDC show increased PT, decreased SS, and loss of LL in comparison with those with HOA.
This study has revealed for the first time that SS is associated with the extent of femoral head destruction in the early stage of RDC. Because the slope of the first sacrum provides the stage for the lumbar curve, small SS correlates with small LL and large PT. Pelvic retroversion leads to increased PT.9 In this study, patients in the early stage of RDC exhibited increased PT, decreased SS, and loss of LL. Consequently, a compensatory mechanism adopted to maintain upright posture in individuals with such sagittal spinopelvic malalignment could cause retroversion of the pelvis to restore sagittal balance. The present results of SSPA are in accordance with the previous finding that patients in the early stage of RDC show an increase in posterior PT.6,7
Most patients with HOA in the present study showed a larger Sharp angle than the normal value of Sharp angle (< 44°).17 In those HOA patients, decreased femoral head coverage as a result of acetabular dysplasia may contribute to femoral head destruction. In contrast, the patients with RDC demonstrated a normal Sharp angle. The extent of femoral head destruction correlated with increased PT, decreased SS, and loss of LL. Posterior pelvic tilt by alterations in SSPA can change acetabular position. Loss of LL and increased PT in close association with decreased SS may result in reduction of anterior acetabular coverage of the femoral head in the absence of dysplasia.4,11 Thus, both loss of LL and increased PT in close association with decreased SS likely act as a mechanical factor and enhance stress concentration in the anterior portion of the femoral head during weightbearing, leading to bone destruction in the early stage of RDC during 12 months after the onset of hip pain. However, absence of sequential data of SSPA from the disease onset through development of femoral head destruction needs further studies to determine the causative role of SSPA in RDC progression.
The present study has also demonstrated for the first time that SVA is additionally associated with the extent of femoral head destruction in the early stage of RDC. SVA is the direct consequence of loss of LL in spite of pelvic compensation by increased PT18 because the loss of lumbar lordosis drives sagittal plane deterioration. Due to the broad range of normative values and the impact of pelvic morphology on spinal curvatures, LL should be evaluated in relation to PI by PI-LL methodology. PI-LL quantifies the mismatch between pelvic morphology and the lumbar curve. Since compensatory mechanisms can control global spinal malalignment, PT may mask an abnormal SVA and be a sensitive marker of spinopelvic mismatch.9 Collectively, measurement of spinopelvic parameters (PI-LL and PT) in addition to SVA provides a more comprehensive evaluation of SSPA. Compared with those with HOA, more patients with RDC exhibited severe spinopelvic mismatch (both a PI-LL > 20° and a PT > 30°) in association with greater extent of femoral head destruction. There is potential concern that sagittal visualization of vertebral landmarks depends on positioning patient’s arms.14 Without a whole spinopelvic radiograph, SS, PT, PI, and LL could readily be assessed on a lumbopelvic radiograph.12 Thus, a sagittal lumbopelvic radiograph should be taken for SSPA evaluation at the time before initiation of femoral head destruction to predict subsequent destruction in the hip with RDC showing only joint space narrowing.
Bone stock deficiency as a result of severe bone destruction makes arthroplasty difficult to be performed for some patients with RDC.5 This indicates importance of early identification of RDC patients at risk before initiation of bone destruction. From the regression analysis, SSPA cannot fully explain the extent of femoral head destruction by RDC within 12 months after the onset of hip pain. In addition to spinopelvic malalignment, biological factors may contribute to femoral head destruction in the early stage of RDC. RDC with femoral head destruction within one year after the onset is associated with increased serum levels of matrix metalloproteinase-3, tartrate-resistant acid phosphatase 5b, and bone alkaline phosphatase.6,19 Actually, mature and activated osteoclasts are found in the synovium of RDC with femoral head destruction in contrast to their absence in HOA.20 Combined assessment of SSPA and such serum markers could offer a better prediction model of femoral head destruction in the early stage of RDC, which should be investigated in our future study.
This study has several limitations. First, this study was retrospective with some selection bias. Second, this study investigated a small number of subjects with the absence of a healthy control. However, recruitment of a larger number of patients with RDC with a complete set of data for 12 months following disease onset could be difficult due to a rarity of this disease.3,6,7,19
In conclusion, patients in the early stage of RDC showed increased PT, decreased SS, loss of LL, and increased PI-LL as sagittal spinopelvic malalignment compared with those with HOA. SS and SVA could at least partially correlate with the extent of femoral head destruction in the early stage of RDC. Further studies are required to determine the role of SSPA assessment in identification of RDC patients at risk for subsequent bone destruction.
References
1. Lequesne M . Rapid destructive coxarthritis . Rhumatologie . 1970 ; 2 : 51 – 63 . [ In French ]. Google Scholar
2. Bock GW , Garcia A , Weisman MH , et al. Rapidly destructive hip disease: clinical and imaging abnormalities . Radiology . 1993 ; 186 ( 2 ): 461 – 466 . Crossref PubMed Google Scholar
3. Sugano N , Ohzono K , Nishii T , et al. Early MRI findings of rapidly destructive coxopathy . Magn Reson Imaging . 2001 ; 19 ( 1 ): 47 – 50 . Crossref PubMed Google Scholar
4. Watanabe W , Sato K , Itoi E , Yang K , Watanabe H . Posterior pelvic tilt in patients with decreased lumbar lordosis decreases acetabular femoral head covering . Orthopedics . 2002 ; 25 ( 3 ): 321 – 324 . Crossref PubMed Google Scholar
5. Zazgyva A , Gurzu S , Gergely I , Jung I , Roman CO , Pop TS . Clinico-radiological diagnosis and grading of rapidly progressive osteoarthritis of the hip . Medicine (Baltimore) . 2017 ; 96 ( 12 ): e6395 . Crossref PubMed Google Scholar
6. Yasuda T , Matsunaga K , Hashimura T , et al. Characterization of rapidly progressive osteoarthritis of the hip in its early stage . Eur J Rheumatol . 2020 ; 7 ( 3 ): 130 – 134 . Crossref PubMed Google Scholar
7. Yasuda T , Matsunaga K , Hashimura T , et al. Characterization of femoral head destruction in the early stage of rapidly progressive osteoarthritis of the hip . Austin J Orthopade & Rheumatol . 2019 ; 6 ( 2 ): 1081 . Google Scholar
8. Roussouly P , Nnadi C . Sagittal plane deformity: an overview of interpretation and management . Eur Spine J . 2010 ; 19 ( 11 ): 1824 – 1836 . Crossref PubMed Google Scholar
9. Diebo BG , Varghese JJ , Lafage R , Schwab FJ , Lafage V . Sagittal alignment of the spine: What do you need to know? Clin Neurol Neurosurg . 2015 ; 139 : 295 – 301 : S0303-8467(15)30055-X . Crossref PubMed Google Scholar
10. Offierski CM , MacNab I . Hip-spine syndrome . Spine (Phila Pa 1976) . 1983 ; 8 ( 3 ): 316 – 321 . Crossref PubMed Google Scholar
11. Yoshimoto H , Sato S , Masuda T , et al. Spinopelvic alignment in patients with osteoarthrosis of the hip: A radiographic comparison to patients with low back pain . Spine (Phila Pa 1976) . 2005 ; 30 ( 14 ): 1650 – 1657 . Crossref PubMed Google Scholar
12. Morimoto T , Kitajima M , Tsukamoto M , Yoshihara T , Sonohata M , Mawatari M . Sagittal spino-pelvic alignment in rapidly destructive coxarthrosis . Eur Spine J . 2018 ; 27 ( 2 ): 475 – 481 . Crossref PubMed Google Scholar
13. Roussouly P , Pinheiro-Franco JL . Biomechanical analysis of the spino-pelvic organization and adaptation in pathology . Eur Spine J . 2011 ; 20 ( Suppl 5 ): 609 – 618 . Crossref PubMed Google Scholar
14. Horton WC , Brown CW , Bridwell KH , Glassman SD , Suk S-. I , Cha CW . Is there an optimal patient stance for obtaining a lateral 36" radiograph? A critical comparison of three techniques . Spine (Phila Pa 1976) . 2005 ; 30 ( 4 ): 427 – 433 . Crossref PubMed Google Scholar
15. Sharp IK . Acetabular dysplasia. the acetabular angle . J Bone Joint Surg Br . 1961 ; 43-B ( 2 ): 268 – 272 . Google Scholar
16. Schwab F , Ungar B , Blondel B , et al. Scoliosis Research Society-Schwab adult spinal deformity classification: a validation study . Spine (Phila Pa 1976) . 2012 ; 37 ( 12 ): 1077 – 1082 . Crossref PubMed Google Scholar
17. Nakamura S , Ninomiya S , Nakamura T . Primary osteoarthritis of the hip joint in Japan . Clin Orthop Relat Res . 1989 ; 241 : 190 . PubMed Google Scholar
18. Glassman SD , Berven S , Bridwell K , Horton W , Dimar JR . Correlation of radiographic parameters and clinical symptoms in adult scoliosis . Spine (Phila Pa 1976) . 2005 ; 30 ( 6 ): 682 – 688 . Crossref PubMed Google Scholar
19. Yasuda T , Matsunaga K , Hashimura T , et al. Bone turnover markers in the early stage of rapidly progressive osteoarthritis of the hip . Eur J Rheumatol . 2020 ; 8 ( 2 ): 57 – 61 . Crossref PubMed Google Scholar
20. Ogawa K , Mawatari M , Komine M , et al. Mature and activated osteoclasts exist in the synovium of rapidly destructive coxarthrosis . J Bone Miner Metab . 2007 ; 25 ( 6 ): 354 – 360 . Crossref PubMed Google Scholar
Author contributions
E. Onishi: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
S. Ota: Data curation, Investigation, Resources, Validation, Writing – review & editing.
S. Fujita: Data curation, Investigation, Methodology, Resources, Validation, Visualization, Writing – review & editing.
Y. Tsukamoto: Data curation, Investigation, Resources, Validation, Writing – review & editing.
S. Yamashita: Data curation, Investigation, Resources, Validation, Writing – review & editing.
T. Hashimura: Data curation, Investigation, Resources, Validation, Writing – review & editing.
K. Matsunaga: Data curation, Investigation, Resources, Validation, Writing – review & editing.
T. Yasuda: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding statement
The author(s) received no financial or material support for the research, authorship, and/or publication of this article.
ICMJE COI statement
The authors confirm that they have no conflicts of interest.
Ethical review statement
Ethics committee approval was received for this study from the Ethics Committee of Kobe City Medical Center General Hospital, Japan (approval date: 1 June 2019; approval no: k190516). Informed consent was not obtained due to the nature of this study.
Open access funding
The authors report that the open access funding for this manuscript was self-funded.
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









