Abstract
Aims
Endoprosthetic reconstruction with a distal femoral arthroplasty (DFA) can be used to treat distal femoral bone loss from oncological and non-oncological causes. This study reports the short-term implant survivorship, complications, and risk factors for patients who underwent DFA for non-neoplastic indications.
Methods
We performed a retrospective review of 75 patients from a single institution who underwent DFA for non-neoplastic indications, including aseptic loosening or mechanical failure of a previous prosthesis (n = 25), periprosthetic joint infection (PJI) (n = 23), and native or periprosthetic distal femur fracture or nonunion (n = 27). Patients with less than 24 months’ follow-up were excluded. We collected patient demographic data, complications, and reoperations. Reoperation for implant failure was used to calculate implant survivorship.
Results
Overall one- and five-year implant survivorship was 87% and 76%, respectively. By indication for DFA, mechanical failure had one- and five-year implant survivorship of 92% and 68%, PJI of 91% and 72%, and distal femur fracture/nonunion of 78% and 70% (p = 0.618). A total of 37 patients (49%) experienced complications and 27 patients (36%) required one or more reoperation. PJI (n = 16, 21%), aseptic loosening (n = 9, 12%), and wound complications (n = 8, 11%) were the most common complications. Component revision (n = 10, 13.3%) and single-stage exchange for PJI (n = 9, 12.0 %) were the most common reoperations. Only younger age was significantly associated with increased complications (mean 67 years (SD 9.1)) with complication vs 71 years (SD 9.9) without complication; p = 0.048).
Conclusion
DFA is a viable option for distal femoral bone loss from a range of non-oncological causes, demonstrating acceptable short-term survivorship but with high overall complication rates.
Cite this article: Bone Jt Open 2022;3(3):173–181.
Take home message
Distal femoral arthroplasty (DFA) is a viable option for significant non-oncological distal femoral bone loss.
Patients who underwent DFA for mechanical failure demonstrated the highest survivorship as well as highest complication rate.
Those who underwent DFA for trauma and its sequelae demonstrated the highest failure rate.
Introduction
Over the last two decades, the number of primary and revision total knee arthroplasties (TKAs) performed in the USA, and the resultant economic burden, have increased dramatically and are projected to continue to rise.1,2 This has led to an increase in patients with distal femoral bone loss relating to osteolysis, component failure, periprosthetic joint infection (PJI), periprosthetic distal femur fractures, and cumulative loss from multiple revisions.3-5 There is debate around how best to address these defects. Traditionally, distal femoral bone loss has been managed with augmentation (cones and/or sleeves),6,7 structural allografts,5,8 modular prostheses,9 and endoprosthetic replacement (EPR) in the form of distal femoral arthroplasty (DFA). 4,10-12 Compared to the salvage alternatives of arthrodesis and amputation, endoprostheses demonstrate improved cost-effectiveness, more rapid recovery, earlier weightbearing, and superior psychological, physical, and functional outcomes.10,13-15 Despite high complication rates, the benefits of megaprosthesis implantation in revision knee arthroplasty have been shown to outweigh the risks.10,13 For these reasons, EPR has become increasingly popular for non-neoplastic indications.9-11
There is additional interest in DFA in the setting of geriatric distal femoral fractures. To date, DFA is considered primarily in highly comminuted intra-articular fractures, patients with severe pre-existing knee arthritis, those with periprosthetic fracture with a loose component or inadequate distal bone stock for fixation, and those with fracture non- or malunion.16,17 These often low-energy fractures occur in a similar patient population as low-energy hip fractures, and have reported nonunion rates of up to 24% following open reduction internal fixation (ORIF),18 with up to 25% one-year mortality rates. Treatment with internal fixation often requires prolonged limited weightbearing in this already susceptible population. Arthroplasty for displaced geriatric femoral neck fractures has demonstrated improved functional scores and decreased revision rates when compared to ORIF.19,20 There is early suggestion DFA in geriatric native distal femur fractures may have similar benefits: it has shown decreased rates of wheelchair dependence at one year (0% vs 23%) compared to ORIF, albeit without significant differences in reoperation rate.16 In periprosthetic distal femur fractures deemed unfixable, DFA had decreased estimated blood loss (EBL), operating time, and length of hospital stay compared to reconstruction with allograft-prosthesis composite, or revision components with augments.21 The major downside of EPR is the potential complications of infection, aseptic loosening, implant mechanical failure, and periprosthetic fracture.4,12,22-24
Published literature has shown favourable outcomes following DFA in patients with oncological indications;25,26 however, there is a scarcity of studies describing megaprosthesis use and outcomes for non-oncological reconstructions. The primary aim of this study was to determine the survivorship, complications, and outcomes for patients who presented to our institution with non-neoplastic disease for DFA. The secondary aim was to identify predictors and risk factors that influenced postoperative outcomes.
Methods
Demographic data
We performed a retrospective review to identify all cases of DFA performed at our single institution between January 2002 and April 2019. Included in this study were patients with minimum two-year clinical follow-up and non-neoplastic indications for DFA, including acute native and periprosthetic distal femoral fracture, distal femoral nonunion, PJI, and aseptic loosening or mechanical failure of a previous prosthesis. This study was approved by our institutional review board.
Overall, 75 patients met the inclusion criteria. The demographic details of the study’s population are shown in Table I. For analysis, indications for DFA were categorized as mechanical failure (n = 25), PJI (n = 23), and trauma (n = 27). Within the trauma group, 20 (74%) of distal femur fractures were periprosthetic and 7 (26%) were in native femora.
Table I.
Demographic details of patients undergoing distal femoral arthroplasty, subdivided by surgical indication.
| Variable | Mech failure (n = 25) | PJI (n = 23) | Fracture (n = 27) | All (n = 75) | p-value |
|---|---|---|---|---|---|
| Mean age, yrs (SD) | 68.9 (9.5) | 64.8 (9.5) | 72.9 (8.9) | 69.1 (9.7) | 0.012† |
| Female, n (%) | 13 (52) | 12 (52) | 22 (78) | 47 (63) | 0.041‡ |
| Mean BMI, kg/m2 (SD) | 33.7 (6.0) | 32.4 (7.6) | 32.6 (10.9) | 32.9 (8.4) | 0.847† |
| Mean CCI* (SD) | 0.83 (0.98) | 1.05 (1.82) | 0.58 (1.06) | 0.81 (1.31) | 0.456† |
| Mean ASA grade (SD) | 2.8 (0.4) | 2.9 (0.4) | 2.75 (0.4) | 2.8 (0.4) | 0.414† |
| Diabetes mellitus, n (%) | 5 (20) | 2 (9) | 5 (18) | 12 (16) | 0.512‡ |
| Rheumatoid arthritis, n (%) | 0 | 1 (4) | 2 (7) | 3 (4) | 0.394‡ |
| Active smoker, n (%) | 11 (44) | 9 (39) | 10 (37) | 30 (40) | 0.872‡ |
| Mean no. prior knee surgeries (SD) | 3.5 (2.6) | 4.6 (1.7) | 1.5 (0.9) | 3.1 (2.3) | < 0.001† |
-
*
Age was not adjusted.
-
†
Analysis of variance.
-
‡
Chi-squared test.
-
ASA, American Society of Anesthesiologists; CCI, Charlson Comorbidity Index; SD, standard deviation.
Surgical data
In the cohort, three types of implants were used: 61 Global Modular Arthroplasty System (GMRS; Stryker, USA), 11 Limb Preservation System (LPS; Depuy Synthes, USA), and three Orthopaedic Salvage System (OSS; Zimmer Biomet, USA). Implant type as well as the use of adjunctive fixation options, such as hydroxyapatite (HA) collar, cone, and sleeve choice, were based on surgeon preference. In 70 patients the DFA component was cemented, and in five it was press-fit. All patients received a rotating hinge implant. A total of 19 patients’ implants included segmental modular extension components for distal femoral length. The mean operating time for the entire cohort was 172 minutes (standard deviation (SD) 49.1) and the mean estimated blood loss (EBL) was 461 ml (SD = 790).
Radiological data
The Anderson Orthopaedic Research Institute (AORI) classification was used preoperatively based on radiograph images to determine degree of femoral bone loss in each patient. Overall, 16 were type 2 A, 17 type 2B, and 42 type C.27
Follow-up data
The clinical follow-up duration was recorded for all patients. The mean follow-up duration was 60.3 months (SD 35.8; 24.2 to 203). The Kaplan-Meier survivorship analysis was only performed using patients who fulfilled the minimum two-year clinical follow-up.
Statistical analysis
The rates of complications and reoperations were determined from hospital electronic medical record (EMR) notes and physician follow-up records. Survivorship of the DFA was established using Kaplan-Meier curves with revision as the endpoint.28 Survival was calculated for the entire cohort as well as for each category of surgical indication. All p-values for parametric data were calculated using independent-samples t-test or analysis of variance (ANOVA), and all nonparametric data were calculated using the Mann-Whitney U test. All p-values for categorical data were calculated using Fisher’s exact test or chi-squared test. All p-values for Kaplan meier curves were calculated using log rank test. The mean and SD were calculated for age, BMI, previous surgeries, and CCI. All analyses were performed using R studio software version 3.5.1 (R Foundation for Statistical Computing, Austria).
Results
Survivorship
With any implant revision, including polyethylene exchange, as the final endpoint, Kaplan-Meier analysis of the 75-patient cohort (95% CI 7.73 to 9.97) demonstrated a survival rate of 87% at one year and 76% at five years (Figure 1). Patients with an original indication of mechanical failure (n = 25 (95% CI 5.28 to 9.96)) demonstrated a survival rate of 92% at one year and 68% at five years. Those with an indication for PJI (n = 23 (95% CI 7.50 to 11.8)) had a one-year survival rate of 91% and a five-year survival rate of 72%. Patients with an initial indication of trauma (n = 27 (95% CI 6.11 to 9.64)) had a one-year survival rate of 78% and a five-year survival rate of 70% (Figure 2). There was no statistically significant difference between groups (p = 0.618).
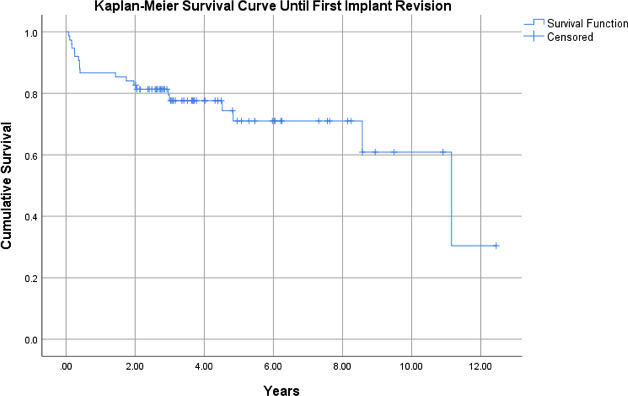
Fig. 1
Kaplan-Meier survival curve for all patients’ status post-distal femoral arthroplasty for non-oncological indications, with endpoint of time until first reoperation for implant revision (95% confidence interval 7.73 to 9.97).
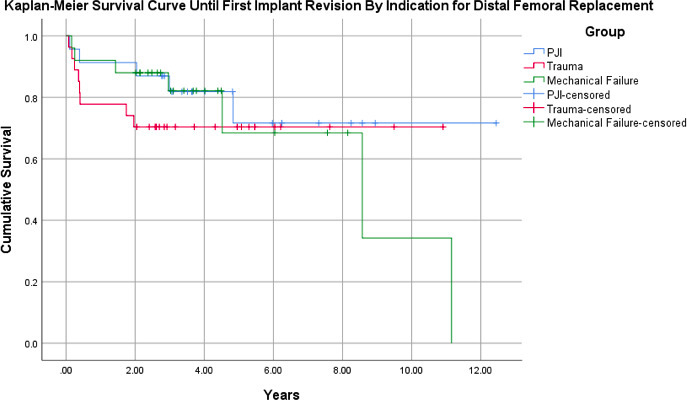
Fig. 2
Kaplan-Meier survival curve for patients status post-distal femoral arthroplasty for non-oncological indications, subdivided by initial indication for megaprosthesis, with endpoint of time until first reoperation for implant revision. Patients with an original indication of mechanical failure (n = 25): 95% confidence interval (CI) 5.28 to 9.96; periprosthetic joint infection (PJI) (n = 23): 95% CI 7.50 to 11.8; and trauma (n = 27): 95% CI 6.11 to 9.64.
With any reoperation as the endpoint, Kaplan-Meier analysis of the 75-patient cohort (95% CI 5.81 to 8.66) demonstrated a survival rate of 77% at one year and 62% at five years (Figure 3). Patients with an original indication of mechanical failure (n = 25 (95% CI 4.60 to 8.81)) demonstrated a survival rate of 80% at one year and 70% at five years. Those with an indication for PJI (n = 23 (95% CI 6.08 to 10.9)) had a one-year survival rate of 83% and a five-year survival rate of 63%. Patients with an original indication of trauma (n = 27 (95% CI 4.67 to 8.57)) had a one-year survival rate of 70% and a five-year survival rate of 56% (Figure 4). There was no statistically significant difference between groups (p = 0.630).
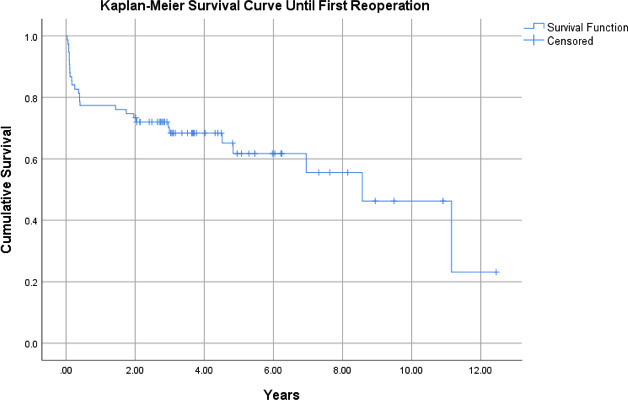
Fig. 3
Kaplan-Meier survival curve for all patients status post-distal femoral arthroplasty for non-oncological indications, with endpoint of time until first reoperation for any cause (95% confidence interval 5.81 to 8.66).
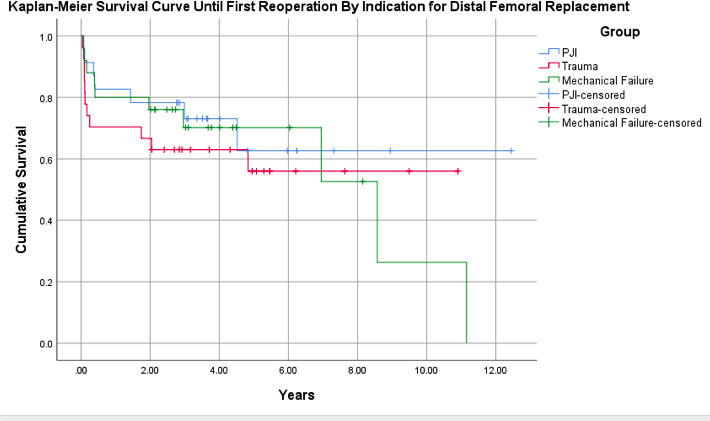
Fig. 4
Kaplan-Meier survival curve for patients status post-distal femoral arthroplasty for non-oncological indications, subdivided by initial indication for megaprosthesis, with endpoint of time until first reoperation for any cause. Patients with an original indication of mechanical failure (n = 25): 95% confidence interval (CI) 4.60 to 8.81; periprosthetic joint infection (PJI) (n = 23): 95% CI 6.08 to 10.9; and trauma (n = 27): 95% CI 4.67 to 8.57.
Complications and reoperations
A total of 38 patients (51%) did not experience any complications. For the remaining patients, ten (13%) had complications managed exclusively nonoperatively, while 27 (36%) had at least one reoperation (Table II). There were 13 patients (17%) who underwent multiple reoperations (Table III). Broken down by initial indication for DFA, the mechanical failure cohort had 14 (56%) patients with any complications, with nine (36%) undergoing a total of 23 additional surgeries. PJI was again the most common complication, occurring in six (24%) patients. Two patients underwent knee arthrodesis and two patients underwent above-knee amputations. The PJI cohort had 12 (52%) patients with one or more complication, with five (22%) managed nonoperatively and seven (30%) underdoing a total of 21 additional surgeries. Recurrent PJI was the most common complication, occurring in five (22%) patients. One patient underwent knee arthrodesis, and one underwent above-knee amputation. The trauma cohort had 11 (41%) patients with any complications, with all 11 undergoing at least one reoperation for a total of 24 additional surgeries. PJI was again the most common complication, occurring in five (19%) patients. Three patients underwent above-knee amputations. Overall, among the entire population the most common complications were PJI (n = 16, 21%), aseptic loosening (n = 9, 12%), and wound complications (n = 8, 11%). Of the factors analyzed, only younger age was significantly associated with increased risk of complication (Table IV).
Table II.
All complications following distal femoral arthroplasty, by initial surgical indication.
| Complication | Indication, n (%) | ||
|---|---|---|---|
| Mech fail (n = 25) |
PJI (n = 23) |
Fracture (n = 27) |
|
| Total complications | 14 (56) | 12 (52) | 11 (41) |
| PJI | 6 (24) | 5 (22) | 5 (18) |
| Aseptic loosening | 4 (16) | 4 (17) | 2 (7) |
| Wound complication | 3 (12) | 2 (9) | 3 (11) |
| Surgical site infection | 2 (8) | 1 (4) | 1 (4) |
| Periprosthetic fracture | 1 (4) | 3 (13) | 2 (7) |
| Extensor mechanism disruption | 1 (4) | 3 (13) | 0 (0) |
| Haematoma | 0 (0) | 3 (13) | 0 (0) |
| Nerve palsy | 1 (4) | 1 (4) | 0 (0) |
| Dislocation | 0 (0) | 0 (0) | 1 (4) |
| Arthrofibrosis | 0 (0) | 0 (0) | 1 (4) |
-
PJI, periprosthetic joint infection.
Table III.
Reoperations following distal femoral arthroplasty, by initial surgical indication.
| Variable |
Indication | ||
|---|---|---|---|
| Mech fail (n = 25) | PJI (n = 23) | Trauma (n = 27) | |
| Patients undergoing reoperations, n (%) | 9 (36) | 7 (30) | 11 (41) |
| Total reoperations | 23 | 21 | 24 |
| One reoperation | 3 | 3 | 8 |
| Two reoperations | 3 | 1 | 1 |
| Three reoperations | 0 | 1 | 0 |
| Four reoperations | 2 | 0 | 0 |
| Five reoperations | 0 | 0 | 0 |
| Six reoperations | 1 | 1 | 1 |
| Seven reoperations | 0 | 1 | 1 |
| Single-stage exchange (PJI), n (%) | 4 (17) | 4 (19) | 2 (8) |
| Revision (aseptic loosening), n (%) | 4 (17) | 4 (19) | 3 (12) |
| Irrigation and debridement (infection), n (%) | 4 (17) | 0 (0) | 9 (38) |
| Amputation, n (%) | 2 (9) | 1 (5) | 3 (12) |
| Explant and antibiotic spacer, n (%) | 2 (9) | 3 (14) | 3 (12) |
| Irrigation and debridement (wound), n (%) | 3 (13) | 2 (10) | 1 (4) |
| Knee arthrodesis, n (%) | 3 (13) | 1 (5) | 0 (0) |
| Spacer removal and reimplantation, n (%) | 1 (4) | 2 (10) | 0 (0) |
| Flap, n (%) | 0 (0) | 0 (0) | 1 (4) |
| ORIF, n (%) | 0 (0) | 1 (5) | 0 (0) |
| Other, n (%) | 0 (0) | 3 (14) | 1 (4) |
-
ORIF, open reduction internal fixation; PJI, periprosthetic joint infection
Table IV.
Complications following distal femoral arthroplasty by patient and surgical factors.
| Factor | No complication (n = 38) |
Complication (n = 37) |
p-value |
|---|---|---|---|
| Mean age, yrs (SD) | 71.2 (9.9) | 66.8 (9.1) | 0.049† |
| Female, n (%) | 27 (71) | 20 (54) | 0.128‡ |
| Mean BMI, kg/m2 (SD) | 32.3 (8.9) | 33.6 (7.8) | 0.504† |
| Mean CCI* (SD) | 0.94 (1.2) | 0.65 (1.5) | 0.358† |
| Mean ASA grade (SD) | 2.8 (0.5) | 2.8 (0.4) | 1.000† |
| Diabetes Mellitus, n (%) | 6 (16) | 6 (16) | 1.000‡ |
| Rheumatoid arthritis, n (%) | 2 (5) | 1 (2.7) | 1.000‡ |
| Active smoker, n (%) | 17 (45) | 13 (35) | 0.396‡ |
| Mean no. prior knee surgeries (SD) | 3.1 (2.4) | 3.1 (2.1) | 1.000† |
| Estimated blood loss, ml (SD) | 512 (1,068) | 407 (298) | 0.566† |
| Operating time, mins (SD) | 162 (40) | 183 (56) | 0.065† |
-
*
Not age-adjusted.
-
†
Independent-samples t-test.
-
‡
Chi-squared test.
-
ASA, American Society of Anesthesiologists score; CCI, Charlson Comorbidity Index; SD, standard deviation.
The mean time until the first reoperation was 21.1 months (SD 34.0). The types of reoperations varied based upon indication for surgery (Table III). The most common reoperations throughout the entire population were component revision (13.3 %) and single-stage exchange for PJI (12.0 %). The mean time to explant in those whose implant failed was 27.3 months (SD 36.7).
Discussion
As the volume of patients with distal femoral bone loss increases, it is imperative that the arthroplasty surgeon be knowledgeable on outcomes of available treatment methods. The aim of this study was to identify whether DFA for non-neoplastic indications allows for acceptable outcomes and prosthesis survivorship in patients presenting with extreme distal femoral bone loss, and to report on predictive factors for prosthesis outcomes. It highlights the efficacy of DFA in these challenging cases, and identifies differing outcomes based on the indication for DFA, with trauma showing a non-significant decrease in implant survivorship at one and five years. The severity of complications also varied, with DFA indicated for trauma or mechanical loosening, resulting in higher rates of arthrodesis or amputation compared to DFA for PJI.
Distal femoral bone loss and fracture occur disproportionately in geriatric populations.1,2 These patients are prone to prolonged bed rest and partial weightbearing periods, increasing their risk of associated complications such as deconditioning, falls, pneumonia, and venous thromboembolism following surgery.29,30 Though used, megaprosthesis for these indications is an exceptional indication, as demonstrated by the small number of cases and limited published work on the procedure. In fact, the decision to place a megaprosthesis is sometimes only made during surgery.4,31 This decision is based on intraoperative factors such as bone quality, nonunion, and damage observed following cement spacer or existing prosthesis removal, and may differ from the original plan based on preoperative imaging and exam. A key benefit of DFA is the ability for early or immediate postoperative weightbearing and range of motion. In most cases, the pathologies necessitating megaprosthetic reconstruction are in fact limb- and even life-threatening. Previous research has shown that lower limb salvage is feasible for a majority of patients, thus sparing them the negative psychological, social, physical, and functional effects associated with amputation.4,32-34
The survivorship of our entre cohort, regardless of indications, was 87% at one year and 76% at five years. In an earlier published series of 37 patients who received 39 DFAs for non-neoplastic indications, Berend and Lombardi4 reported a 12-month implant survivorship of 97% at and a 46-month implant survivorship of 87%. This study’s rate was 87% at one year and 78% at 46 weeks, lower than survivorship reported in other studies. Perhaps this lower value reflects the larger number of patients in our cohort with a history of PJI and the higher mean number of previous surgeries on the knee. Although the survivorship numbers appear to be low, they are higher than the one- and five-year survivorship of other rotating hinge prostheses that are used in salvage revision TKA. 35,36Additionally, the limb salvage was ultimately successful in 92% (n = 69) of patients, similar to the previously reported 95% by Berend and Lombardi.
When looking at survivorship based on indication, our study showed one- and five-year implant survivorship for mechanical failure of 92% and 68%, PJI of 91% and 72%, and distal femur fracture/nonunion of 78% and 70% (p = 0.618, log-rank test). We were underpowered and unable to detect a significant difference in survivorship between cohorts; however, there are no current studies comparing these three indications. Although low, our survivorship and cohort size compare to recently published literature on a cohort-to-cohort basis (Table V). For PJI (n = 41), Theil et al37 reported 66% survivorship at two years and 50% at five years. Matar et al38 reported a five-year, 80% survivorship for a cohort composed of patients who underwent DFA for PJI (n = 16) and aseptic loosening (n = 17).
Table V.
Summary of cited literature data for use of distal femoral arthroplasty in non-oncological patients.
| Study | Indication for DFA | Patients, n | Median follow-up, mths | Complication rate, % | Reoperation rate, % | Survivorship 1 yr, % | Survivorship 5 yrs, % |
|---|---|---|---|---|---|---|---|
| Present study | 75 | 60 | 49 | 36 | 87 | 76 | |
| Fracture/nonunion | 27 | - | 41 | 41 | 78 | 70 | |
| PJI | 23 | - | 52 | 30 | 91 | 72 | |
| Aseptic loosening/mechanical failure | 25 | - | 56 | 36 | 92 | 68 | |
| Theil et al37 | PJI | 41 | 59 | - | 47 | 66 at 2 yrs | 50 |
| Matar et al39 | Periprosthetic fracture | 27 | 48 | 7.4 | 3.7 | - | - |
| Matar et al38 | 33 | 60 | 12 | - | - | 80 | |
| PJI | 16 | - | - | - | - | - | |
| Aseptic loosening | 17 | - | - | - | - | - | |
| Berend and Lombardi4 | 37 | 46 | 18 | 14 | 97 | 83 | |
| Revision TKA | 11 | - | - | - | - | - | |
| Fracture/nonunion | 15 | - | - | - | - | - | |
| Aseptic loosening/mechanical failure | 11 | - | - | - | - | - | |
| Höll et al13 | 21 | 34 | 55 | 24 | - | - | |
| PJI | 5 | - | - | - | - | - | |
| Fracture/nonunion | 14 | - | 57 | - | - | - | |
| Aseptic loosening | 2 | - | - | - | - | - | |
| Mortazavi et al40 | Periprosthetic fracture | 22 | 59 | 46 | 23 | - | - |
-
Dashes signify that a particular data point was not reported in the cited work.
-
DFA, distal femoral arthroplasty; PJI, periprosthetic joint infection.
Other options for knee reconstruction are available for patients in need of lower limb preservation. One commonly used method is a structural allograft. However, complications following this procedure occur in 23% to 55% of cases and may include resorption, nonunion, and infection.9,13,41-43 Perhaps the worst impediment of the allograft is the varying time it takes for the patient to become weightbearing.44 In this series of 75 patients, 49% (n = 37) suffered at least one complication, with nine patients (13%) needing revisions. This is consistent with other published literature, showing overall complication rates following DFA of up to 46% for periprosthetic fracture as the presenting indication,40 and 55% across all non-oncological indications.13 Revisions have been shown to range from 4% in patients with DFA for periprosthetic fracture to 18% for all patients who underwent DFA without tumour indications.4,13,39
PJI was the most common complication. A total of 16 (21.3%) of our patients experienced PJI following implantation of their megaprosthesis (Figure 5). Though high, this rate is mirrored in published literature describing rates of PJIs with megaprostheses, with reported rates of 20% following DFA.13,45 In our study, PJI was a major driver of implant survivorship failure (n = 13). Another common complication in this study was aseptic loosening. A study by Myers et al10 reported an aseptic loosening rate of 35% following prostheses over a period of ten years. The loosening rate in this study was predictably lower at only 12%, due to shorter follow-up time. However, in our study, loosening was a driver for survivorship failure. Overall, an increase in aseptic loosening can be expected with longer follow-up durations, and may decrease in populations with limited walking capacity.6,13
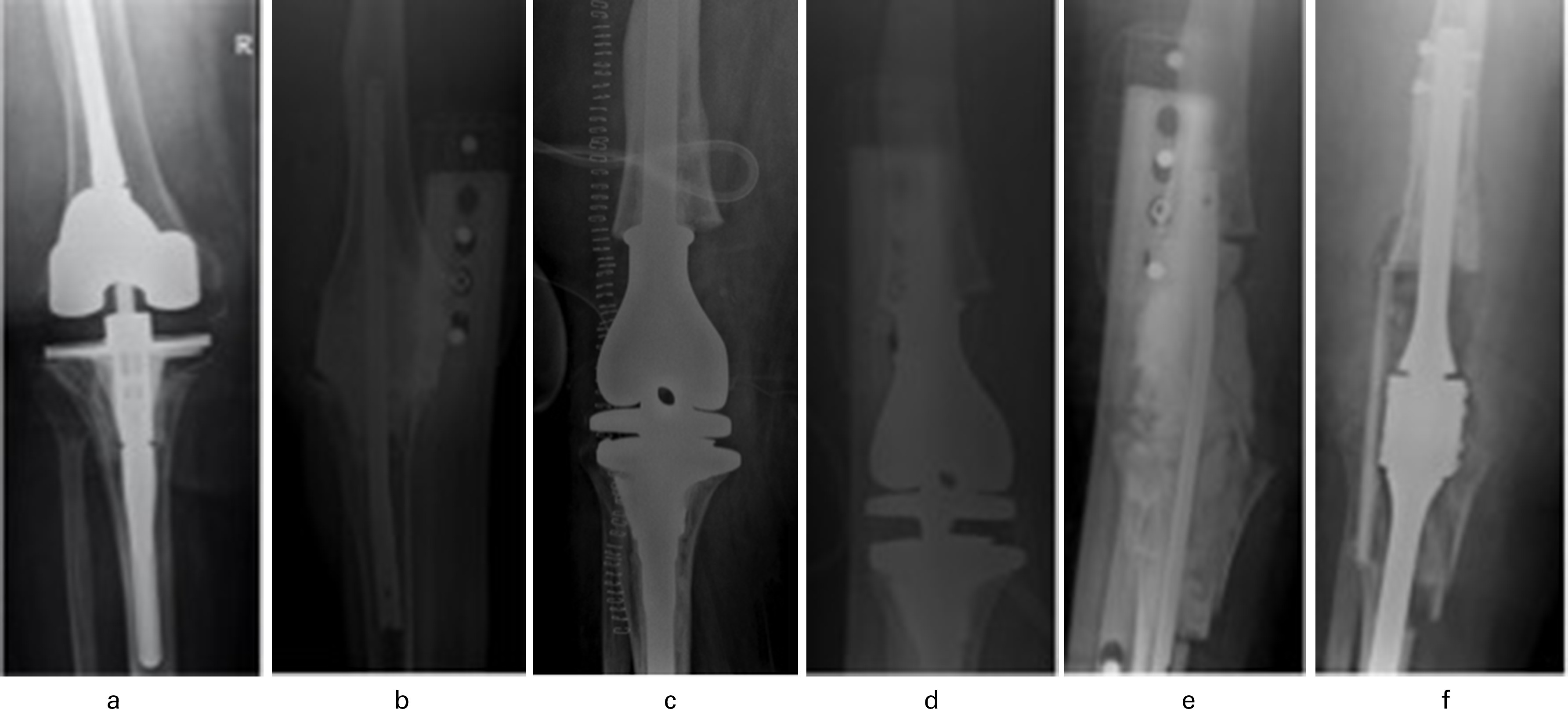
Fig. 5
Pre- and postoperative anteroposterior radiographs of a 60-year-old female with prior total knee arthroplasty (2009) and periprosthetic joint infection (PJI) and revision (2010) presenting with a) imaging of her existing total knee arthroplasty. b) Six years after the revision knee arthroplasty and multiple failed antibiotic courses for recurrent methicillin-sensitive Staphylococcus aureus PJI, the implant was explanted and an antibiotic cement spacer was placed. c) Four months later she underwent distal femoral arthroplasty (DFA). d) Due to suspected ongoing PJI she then underwent a polyethylene exchange and irrigation and debridement three weeks later. e) Without resolution of the PJI, she underwent DFA explantation and placement of antibiotic spacer after another two months and, lastly, f) after an additional three months she underwent arthrodesis of the right knee. There have been no signs of PJI since fusion.
This study has several limitations. The number of patients for each surgical indication is small, and powered analysis could not be conducted due to the infrequent performance of this procedure, though the size of each group is similar to reported populations in other studies. Additionally, it was a retrospective analysis and lacked a control group. Furthermore, despite our attempts, we were not able to obtain enough patient-reported pre- and postoperative functional outcome scores to include in this study. We suspect this is due to the length of our inclusion timeframe (starting in 2002), which may mean patients further removed from their procedure have since become mentally incapacitated or deceased.
This topic has much potential for future study. The field of arthroplasty would benefit greatly from an appropriately powered prospective comparison of the treatment options for distal femoral bone loss for each of the previously discussed indications, with inclusion of functional outcomes. Another area of future study is research into techniques for limiting aseptic loosening following DFA, possibly with methods such as press fitting implants, the use of cones/sleeves, or using hybrid cemented fixation.
To summarize, in this retrospective cohort study, we demonstrated that DFA is a viable surgical option for those patients with significant distal femoral bone loss or fracture. Patients should be counselled preoperatively about relatively high complications rates, likelihood of implant survivorship, and the reality of the often limb-threatening nature of their diagnoses.
References
1. Kurtz S , Ong K , Lau E , Mowat F , Halpern M . Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030 . J Bone Joint Surg Am . 2007 ; 89-A ( 4 ): 780 – 785 . Crossref PubMed Google Scholar
2. Kurtz SM , Ong KL , Schmier J , et al. Future clinical and economic impact of revision total hip and knee arthroplasty . J Bone Joint Surg Am . 2007 ; 89 Suppl 3 ( suppl_3 ): 144 – 151 . Crossref PubMed Google Scholar
3. Hamilton DF , Howie CR , Burnett R , Simpson A , Patton JT . Dealing with the predicted increase in demand for revision total knee arthroplasty: challenges, risks and opportunities . Bone Joint J . 2015 ; 97-B ( 6 ): 723 – 728 . Crossref PubMed Google Scholar
4. Berend KR , Lombardi AV . Distal femoral replacement in nontumor cases with severe bone loss and instability . Clin Orthop Relat Res . 2009 ; 467 ( 2 ): 485 – 492 . Crossref PubMed Google Scholar
5. Backstein D , Safir O , Gross A . Management of bone loss: structural grafts in revision total knee arthroplasty . Clin Orthop Relat Res . 2006 ; 446 : 104 – 112 . Crossref PubMed Google Scholar
6. Lombardi AV , Berend KR , Adams JB . Management of bone loss in revision TKA: it’s a changing world . Orthopedics . 2010 ; 33 ( 9 ): 662 . Google Scholar
7. Kim HJ , Lee OS , Lee SH , Lee YS . Comparative analysis between cone and sleeve in managing severe bone defect during revision total knee arthroplasty: a systematic review and meta-analysis . J Knee Surg . 2018 ; 31 ( 7 ): 677 – 685 . Crossref PubMed Google Scholar
8. Ajoy SM , Mahesh M , RangaSwamy BT . Distal femur fractures with massive bone loss: treatment option primarily using bone allograft. Orthopaedic Proceedings . 2016 ; 98-B ( SUPP_14 ): 6 . Google Scholar
9. Bauman RD , Lewallen DG , Hanssen AD . Limitations of structural allograft in revision total knee arthroplasty . Clin Orthop Relat Res . 2009 ; 467 ( 3 ): 818 – 824 . Crossref PubMed Google Scholar
10. Myers GJC , Abudu AT , Carter SR , Tillman RM , Grimer RJ . Endoprosthetic replacement of the distal femur for bone tumours: long-term results . J Bone Joint Surg Br . 2007 ; 89-B ( 4 ): 521 – 526 . Crossref PubMed Google Scholar
11. Simon MA , Aschliman MA , Thomas N , Mankin HJ . Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. 1986 . J Bone Joint Surg Am . 2005 ; 87-A ( 12 ): 2822 . Crossref PubMed Google Scholar
12. Kawai A , Muschler GF , Lane JM , Otis JC , Healey JH . Prosthetic knee replacement after resection of a malignant tumor of the distal part of the femur. Medium to long-term resultsProsthetic Knee Replacement after Resection of a Malignant Tumor of the Distal Part of the Femur. Medium to Long-Term Results* . J Bone Joint Surg Am . 1998 ; 80-A ( 5 ): 636 – 647 . Google Scholar
13. Höll S , Schlomberg A , Gosheger G , et al. Distal femur and proximal tibia replacement with megaprosthesis in revision knee arthroplasty: a limb-saving procedure . Knee Surg Sports Traumatol Arthrosc . 2012 ; 20 ( 12 ): 2513 – 2518 . Crossref PubMed Google Scholar
14. Grimer RJ , Carter SR , Pynsent PB . The cost-effectiveness of limb salvage for bone tumours . J Bone Joint Surg Br . 1997 ; 79-B ( 4 ): 558 – 561 . Crossref PubMed Google Scholar
15. Bradish CF , Kemp HB , Scales JT , Wilson JN . Distal femoral replacement by custom-made prostheses. Clinical follow-up and survivorship analysis . J Bone Joint Surg Br . 1987 ; 69-B ( 2 ): 276 – 284 . Crossref PubMed Google Scholar
16. Hart GP , Kneisl JS , Springer BD , Patt JC , Karunakar MA . Open reduction vs distal femoral replacement arthroplasty for comminuted distal femur fractures in the patients 70 years and older . J Arthroplasty . 2017 ; 32 ( 1 ): 202 – 206 . Crossref PubMed Google Scholar
17. Atrey A , Hussain N , Gosling O , et al. A 3 year minimum follow up of Endoprosthetic replacement for distal femoral fractures - An alternative treatment option . J Orthop . 2017 ; 14 ( 1 ): 216 – 222 . Crossref PubMed Google Scholar
18. Moloney GB , Pan T , Van Eck CF , Patel D , Tarkin I . Geriatric distal femur fracture: are we underestimating the rate of local and systemic complications? Injury . 2016 ; 47 ( 8 ): 1732 – 1736 . Crossref PubMed Google Scholar
19. Ravikumar KJ , Marsh G . Internal fixation versus hemiarthroplasty versus total hip arthroplasty for displaced subcapital fractures of femur--13 year results of a prospective randomised study . Injury . 2000 ; 31 ( 10 ): 793 – 797 . Crossref PubMed Google Scholar
20. Rogmark C , Carlsson A , Johnell O , Sernbo I . A prospective randomised trial of internal fixation versus arthroplasty for displaced fractures of the neck of the femur. Functional outcome for 450 patients at two years . J Bone Joint Surg Br . 2002 ; 84-B ( 2 ): 183 – 188 . Crossref PubMed Google Scholar
21. Saidi K , Ben-Lulu O , Tsuji M , Safir O , Gross AE , Backstein D . Supracondylar periprosthetic fractures of the knee in the elderly patients: a comparison of treatment using allograft-implant composites, standard revision components, distal femoral replacement prosthesis . J Arthroplasty . 2014 ; 29 ( 1 ): 110 – 114 . Crossref PubMed Google Scholar
22. Jeys L , Grimer R . The long-term risks of infection and amputation with limb salvage surgery using endoprostheses . In : Treatment of Bone and Soft Tissue Sarcomas . Berlin, Germany : Springer , 2009 : 75 – 84 . Google Scholar
23. Jeys LM , Grimer RJ , Carter SR , Tillman RM . Periprosthetic infection in patients treated for an orthopaedic oncological condition . J Bone Joint Surg Am . 2005 ; 87-A ( 4 ): 842 – 849 . Crossref PubMed Google Scholar
24. Unwin PS , Cannon SR , Grimer RJ , Kemp HBS , Sneath RS , Walker PS . Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb . J Bone Joint Surg Br . 1996 ; 78-B ( 1 ): 5 – 13 . PubMed Google Scholar
25. Roberts P , Chan D , Grimer RJ , Sneath RS , Scales JT . Prosthetic replacement of the distal femur for primary bone tumours . J Bone Joint Surg Br . 1991 ; 73-B ( 5 ): 762 – 769 . Crossref PubMed Google Scholar
26. Natarajan MV , Sivaseelam A , Ayyappan S , Bose JC , Sampath Kumar M . Distal femoral tumours treated by resection and custom mega-prosthetic replacement . Int Orthop . 2005 ; 29 ( 5 ): 309 – 313 . Crossref PubMed Google Scholar
27. Engh G . A classification of bone defects . In : Engh G , Rorabeck C . Revision Knee Arthroplasty . Baltimore, Maryland, USA : Williams & Wilkins , 1997 : 63 – 120 . Google Scholar
28. Murray DW , Carr AJ , Bulstrode C . Survival analysis of joint replacements . J Bone Joint Surg Br . 1993 ; 75-B ( 5 ): 697 – 704 . PubMed Google Scholar
29. Zuckerman JD , Skovron ML , Koval KJ , Aharonoff G , Frankel VH . Postoperative complications and mortality associated with operative delay in older patients who have a fracture of the hip . J Bone Joint Surg Am . 1995 ; 77-A ( 10 ): 1551 – 1556 . PubMed Google Scholar
30. Matsumoto H , Okuno M , Nakamura T , Yamamoto K , Hagino H . Fall incidence and risk factors in patients after total knee arthroplasty . Arch Orthop Trauma Surg . 2012 ; 132 ( 4 ): 555 – 563 . Crossref PubMed Google Scholar
31. Mortazavi SMJ , Kurd MF , Bender B , Post Z , Parvizi J , Purtill JJ . Distal femoral arthroplasty for the treatment of periprosthetic fractures after total knee arthroplasty . J Arthroplasty . 2010 ; 25 ( 5 ): 775 – 780 . Crossref PubMed Google Scholar
32. Harris IE , Leff AR , Gitelis S , Simon MA . Function after amputation, arthrodesis, or arthroplasty for tumors about the knee . J Bone Joint Surg Am . 1990 ; 72-A ( 10 ): 1477 – 1485 . PubMed Google Scholar
33. Rougraff BT , Simon MA , Kneisl JS , Greenberg DB , Mankin HJ . Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study . J Bone Joint Surg Am . 1994 ; 76-A ( 5 ): 649 – 656 . Crossref PubMed Google Scholar
34. Horgan O , MacLachlan M . Psychosocial adjustment to lower-limb amputation: a review . Disabil Rehabil . 2004 ; 26 ( 14–15 ): 837 – 850 . Crossref PubMed Google Scholar
35. Barrack RL . Evolution of the rotating hinge for complex total knee arthroplasty . Clin Orthop Relat Res . 2001 ; 392 : 292 – 299 . Crossref PubMed Google Scholar
36. Pour AE , Parvizi J , Slenker N , Purtill JJ , Sharkey PF . Rotating hinged total knee replacement: use with caution . J Bone Joint Surg Am . 2007 ; 89-A ( 8 ): 1735 – 1741 . Crossref PubMed Google Scholar
37. Theil C , Schneider KN , Gosheger G , et al. Revision TKA with a distal femoral replacement is at high risk of reinfection after two-stage exchange for periprosthetic knee joint infection . Knee Surg Sports Traumatol Arthrosc . 2021 ; 1 – 8 . Crossref PubMed Google Scholar
38. Matar HE , Bloch BV , James PJ . Outcomes of salvage endoprostheses in revision total knee arthroplasty for infection and aseptic loosening: Experience of a specialist centre . Knee . 2021 ; 29 : 547 – 556 . Crossref PubMed Google Scholar
39. Matar HE , Bloch BV , James PJ . Distal femoral replacements for acute comminuted periprosthetic knee fractures: Satisfactory clinical outcomes at medium-term follow-up . Arthroplast Today . 2021 ; 7 : 37 – 42 . Crossref PubMed Google Scholar
40. Mortazavi SMJ , Kurd MF , Bender B , Post Z , Parvizi J , Purtill JJ . Distal femoral arthroplasty for the treatment of periprosthetic fractures after total knee arthroplasty . J Arthroplasty . 2010 ; 25 ( 5 ): 775 – 780 . Crossref PubMed Google Scholar
41. Clatworthy MG , Ballance J , Brick GW , Chandler HP , Gross AE . The use of structural allograft for uncontained defects in revision total knee arthroplasty. A minimum five-year review . J Bone Joint Surg Am . 2001 ; 83-A ( 3 ): 404 – 411 . Crossref PubMed Google Scholar
42. Mnaymneh W , Emerson RH , Borja F , Head WC , Malinin TI . Massive allografts in salvage revisions of failed total knee arthroplasties . Clin Orthop Relat Res . 1990 ; 260 : 144 – 153 . PubMed Google Scholar
43. Mow CS , Wiedel JD . Structural allografting in revision total knee arthroplasty . J Arthroplasty . 1996 ; 11 ( 3 ): 235 – 241 . Crossref PubMed Google Scholar
44. Stulberg SD . Bone loss in revision total knee arthroplasty: graft options and adjuncts . J Arthroplasty . 2003 ; 18 ( 3 Suppl 1 ): 48 – 50 . Crossref PubMed Google Scholar
45. Hardes J , Gebert C , Schwappach A , et al. Characteristics and outcome of infections associated with tumor endoprostheses . Arch Orthop Trauma Surg . 2006 ; 126 ( 5 ): 289 – 296 . Crossref PubMed Google Scholar
Author contributions
K. R. Sobol: Investigation, Writing – original draft.
B. Fram: Formal analysis, Writing – original draft.
J. Strony: Investigation.
S. A. Brown: Writing – review & editing.
Funding statement
The authors received no financial or material support for the research, authorship, and/or publication of this article.
Acknowledgements
We would like to thank Mathew Sherman for his help with the statistics.
Ethical review statement
Approval for this study was obtained from our institutional review board (IRB ID 19D.112)
Open access funding
The authors confirm that the open access funding was provided by Thomas Jefferson University and Rothman Orthopaedics.
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









