Abstract
Objectives
The purpose of this study was to evaluate in vivo biocompatibility of novel single-walled carbon nanotubes (SWCNT)/poly(lactic-co-glycolic acid) (PLAGA) composites for applications in bone and tissue regeneration.
Methods
A total of 60 Sprague-Dawley rats (125 g to 149 g) were implanted subcutaneously with SWCNT/PLAGA composites (10 mg SWCNT and 1gm PLAGA 12 mm diameter two-dimensional disks), and at two, four, eight and 12 weeks post-implantation were compared with control (Sham) and PLAGA (five rats per group/point in time). Rats were observed for signs of morbidity, overt toxicity, weight gain and food consumption, while haematology, urinalysis and histopathology were completed when the animals were killed.
Results
No mortality and clinical signs were observed. All groups showed consistent weight gain, and the rate of gain for each group was similar. All groups exhibited a similar pattern for food consumption. No difference in urinalysis, haematology, and absolute and relative organ weight was observed. A mild to moderate increase in the summary toxicity (sumtox) score was observed for PLAGA and SWCNT/PLAGA implanted animals, whereas the control animals did not show any response. Both PLAGA and SWCNT/PLAGA showed a significantly higher sumtox score compared with the control group at all time intervals. However, there was no significant difference between PLAGA and SWCNT/PLAGA groups.
Conclusions
Our results demonstrate that SWCNT/PLAGA composites exhibited in vivo biocompatibility similar to the Food and Drug Administration approved biocompatible polymer, PLAGA, over a period of 12 weeks. These results showed potential of SWCNT/PLAGA composites for bone regeneration as the low percentage of SWCNT did not elicit a localised or general overt toxicity. Following the 12-week exposure, the material was considered to have an acceptable biocompatibility to warrant further long-term and more invasive in vivo studies.
Cite this article: Bone Joint Res 2015;4:70–7
Article focus
To investigate the in vivo biocompatibility of single-walled carbon nanotubes (SWCNT)/poly(lactic-co-glycolic) (PLAGA) composites in a subcutaneous implant rat model.
We hypothesised that SWCNT/PLAGA composites are biocompatible, non-toxic, and ideal candidates for bone and tissue regeneration.
Key messages
SWCNT/PLAGA composites exhibited biocompatibility similar to PLAGA, a well-known FDA approved biocompatible polymer over the 12-week study period.
The results obtained demonstrate the potential of SWCNT/PLAGA composites for bone tissue engineering and regeneration applications as a low percentage of SWCNT did not elicit a localised or generally overt toxicity.
Strengths and limitations
Strength: this is a well established surgical model used to evaluate biocompatibility of materials for orthopaedic applications.
Limitation: the duration of the study was short.
Introduction
Bone defects and nonunions caused by trauma, resection of tumour, pathological deformation and peri-prosthetic fractures occur within both young and ageing populations accounting for more than three million surgeries annually.1,2 With this high level of demand, the repair of these bone defects poses a great challenge to the field of orthopaedic surgery.3 Current methods for bone repair and regeneration rely heavily on the use of autografts, allografts and synthetic bone graft substitutes. However, to circumvent the limitations posed by them, bone tissue engineering (BTE) has evolved as an alternative approach that relies on the use of biodegradable polymers with or without the use of cells and growth factors.4,5 Biodegradable polymers are of interest in medicine and are an ideal candidate for BTE because of their commercial availability, biocompatibility, degradation into non-toxic products and the ability to control the material's characteristics such as mechanical properties, porosity and surface charges.6
Poly(lactic-co-glycolic acid) (PLAGA) is widely used as a composite material for BTE as it exhibits the properties of an ideal bone graft but lacks adequate mechanical strength.7-9 Reinforcing PLAGA with a second-phase material can increase the mechanical properties of PLAGA. Carbon-based biomaterials such as diamond-like carbon, pyrolytic carbon and carbon fibres have been used as fillers and coatings in implants, due to intrinsic properties like a low coefficient of friction, chemical inertness, hardness, high wear and resistance to corrosion.9,10 These materials are relevant to medicine due to their biocompatibility.11 Carbon nanotubes (CNT) have also been researched for their use in biomedical systems due to their unique properties in terms of size, strength and surface area. CNT possess high tensile strength, are ultra lightweight, and act as an exceptional substrate for cell growth and differentiation.12-16 These properties make CNT an excellent candidate for use as nanofillers in polymeric materials in order to increase their mechanical properties.
A previous study in our laboratory17 demonstrated that reinforcing PLAGA with single-walled CNT (SWCNT), produced a novel SWCNT/PLAGA composite, excellent cell growth, gene expression and mineralisation. The results demonstrated that the degradation rate of PLAGA remained unaffected by the addition of SWCNT, and the addition of 10 mg SWCNT resulted in the highest cell proliferation rate compared with 5 mg, 20 mg, 40 mg and 100 mg of SWCNT. In another study,18 we demonstrated that the addition of 10 mg SWCNT to fabricate three-dimensional (3D) SWCNT/PLAGA composites led to a greater compressive modulus and ultimate compressive strength in addition to a higher cell proliferation rate and gene expression, when compared with PLAGA alone. These results demonstrated the potential use of our SWCNT/PLAGA composites for BTE and bone regeneration.
Although in vitro results indicated the biocompatibility of SWCNT/PLAGA composites, adequate testing of their biocompatibility in vivo is necessary for possible use in biological systems.19 Cellular response to biomaterials range from no response to localised inflammation, and the degree of cellular response is determined by the extent of fibrous tissue encapsulation of the implant.20 Inert biomaterials often cause fibrous tissue encapsulation, while toxic biomaterials lead to the death of cells.21 Composites must be certified as biocompatible and non-toxic to ensure that they are safe for use in clinical applications. The rat is a widely accepted non-clinical animal model for toxicity studies and has been used to study the biocompatibility of composites both when implanted subcutaneously as in our study, and when implanted in the area of clinical interest.22,23 Based on our previous studies, we demonstrated that use of a lesser amount of SWCNT provided increased cell proliferation, gene expression and strength to SWCNT/PLAGA composites. Use of less SWCNT could cause less toxicity, which could make these composites useful for BTE applications. The goal of this study was to evaluate the in vivo biocompatibility of SWCNT/PLAGA composites and compare them with a Food and Drug Administration (FDA) approved biocompatible polymer, PLAGA, in a subcutaneous implant rat model. We hypothesised that SWCNT/PLAGA composites are biocompatible, non-toxic, and ideal candidates for bone regeneration and orthopaedic applications.
Materials and Methods
Fabrication of PLAGA and SWCNT/PLAGA composites
SWCNT (Sigma-Aldrich, St Louis, Missouri) and PLAGA (Purasorb PLG8523, Purac Biomaterials, The Netherlands) were obtained and stored in the desiccator and at -800C, respectively. PLAGA and SWCNT/PLAGA (10 mg SWCNT and 1 g PLAGA) composites were fabricated using the method described in our laboratory.18 The thin films obtained were bored into circular disks (12 mm diameter) and placed in a desiccator for 24 hours to remove the residual solvent.
Animals, housing and implantation of scaffolds
All animal experiments were performed after receiving approval from the Laboratory Animal Care and Use Committee of Southern Illinois University School of Medicine. In total, 60 (five animals per group/time point), 36 to 40 day-old (125 g to 149 g) male Sprague-Dawley rats were purchased (Harlan, Indianapolis, Indiana) and acclimatised for one week before surgical procedures were performed. The animals were housed individually in animal rooms with an environmentally controlled temperature, relative humidity and a 12-hour light/dark cycle. Animals were randomly assigned numbers one to 60 and randomly allocated into groups. Animals were anaesthetised using isoflurane which was delivered using an anaesthetic vaporiser. The dorsum of the animals was shaved and made sterile with betadine and alcohol. Two incisions approximately 15 mm in length (15 mm apart) were made laterally on the dorsum using a surgical scalpel blade no.10. A subcutaneous pouch on opposite sides of each incision was made using the blunt-dissection technique. The sterilised PLAGA and SWCNT/PLAGA disks were unsealed in a sterilised environment and each animal was implanted with two polymer disks of the same type. Sham-implanted rats were used as negative controls. Following implantation, the skin was closed using sterile auto-clips (Fig. 1), 36 to 40 day-old (125 g to 149 g) male Sprague-Dawley rats were purchased (Harlan, Indianapolis, Indiana) and acclimatised for one week before surgical procedures were performed. The animals were housed individually in animal rooms with an environmentally controlled temperature, relative humidity and a 12-hour light/dark cycle. Animals were randomly assigned numbers one to 60 and randomly allocated into groups. Animals were anaesthetised using isoflurane which was delivered using an anaesthetic vaporiser. The dorsum of the animals was shaved and made sterile with betadine and alcohol. Two incisions approximately 15 mm in length (15 mm apart) were made laterally on the dorsum using a surgical scalpel blade no.10. A subcutaneous pouch on opposite sides of each incision was made using the blunt-dissection technique. The sterilised PLAGA and SWCNT/PLAGA disks were unsealed in a sterilised environment and each animal was implanted with two polymer disks of the same type. Sham-implanted rats were used as negative controls. Following implantation, the skin was closed using sterile auto-clips (Fig. 1) male Sprague-Dawley rats were purchased (Harlan, Indianapolis, Indiana) and acclimatised for one week before surgical procedures were performed. The animals were housed individually in animal rooms with an environmentally controlled temperature, relative humidity and a 12-hour light/dark cycle. Animals were randomly assigned numbers one to 60 and randomly allocated into groups. Animals were anaesthetised using isoflurane which was delivered using an anaesthetic vaporiser. The dorsum of the animals was shaved and made sterile with betadine and alcohol. Two incisions approximately 15 mm in length (15 mm apart) were made laterally on the dorsum using a surgical scalpel blade no.10. A subcutaneous pouch on opposite sides of each incision was made using the blunt-dissection technique. The sterilised PLAGA and SWCNT/PLAGA disks were unsealed in a sterilised environment and each animal was implanted with two polymer disks of the same type. Sham-implanted rats were used as negative controls. Following implantation, the skin was closed using sterile auto-clips (Fig. 1) and acclimatised for one week before surgical procedures were performed. The animals were housed individually in animal rooms with an environmentally controlled temperature, relative humidity and a 12-hour light/dark cycle. Animals were randomly assigned numbers one to 60 and randomly allocated into groups. Animals were anaesthetised using isoflurane which was delivered using an anaesthetic vaporiser. The dorsum of the animals was shaved and made sterile with betadine and alcohol. Two incisions approximately 15 mm in length (15 mm apart) were made laterally on the dorsum using a surgical scalpel blade no.10. A subcutaneous pouch on opposite sides of each incision was made using the blunt-dissection technique. The sterilised PLAGA and SWCNT/PLAGA disks were unsealed in a sterilised environment and each animal was implanted with two polymer disks of the same type. Sham-implanted rats were used as negative controls. Following implantation, the skin was closed using sterile auto-clips (Fig. 1) were made laterally on the dorsum using a surgical scalpel blade no.10. A subcutaneous pouch on opposite sides of each incision was made using the blunt-dissection technique. The sterilised PLAGA and SWCNT/PLAGA disks were unsealed in a sterilised environment and each animal was implanted with two polymer disks of the same type. Sham-implanted rats were used as negative controls. Following implantation, the skin was closed using sterile auto-clips (Fig. 1). The animals were given buprenorphine (0.05 mg/kg subcutaneously) for pain management and were allowed to recover. At specific time points post-implantation (two, four, eight and 12 weeks), the animals were killed as per protocol by carbon dioxide inhalation. The implants, surrounding tissues and major organs (adrenal glands, lungs, spleen, heart, liver and kidneys) were harvested for further evaluation.
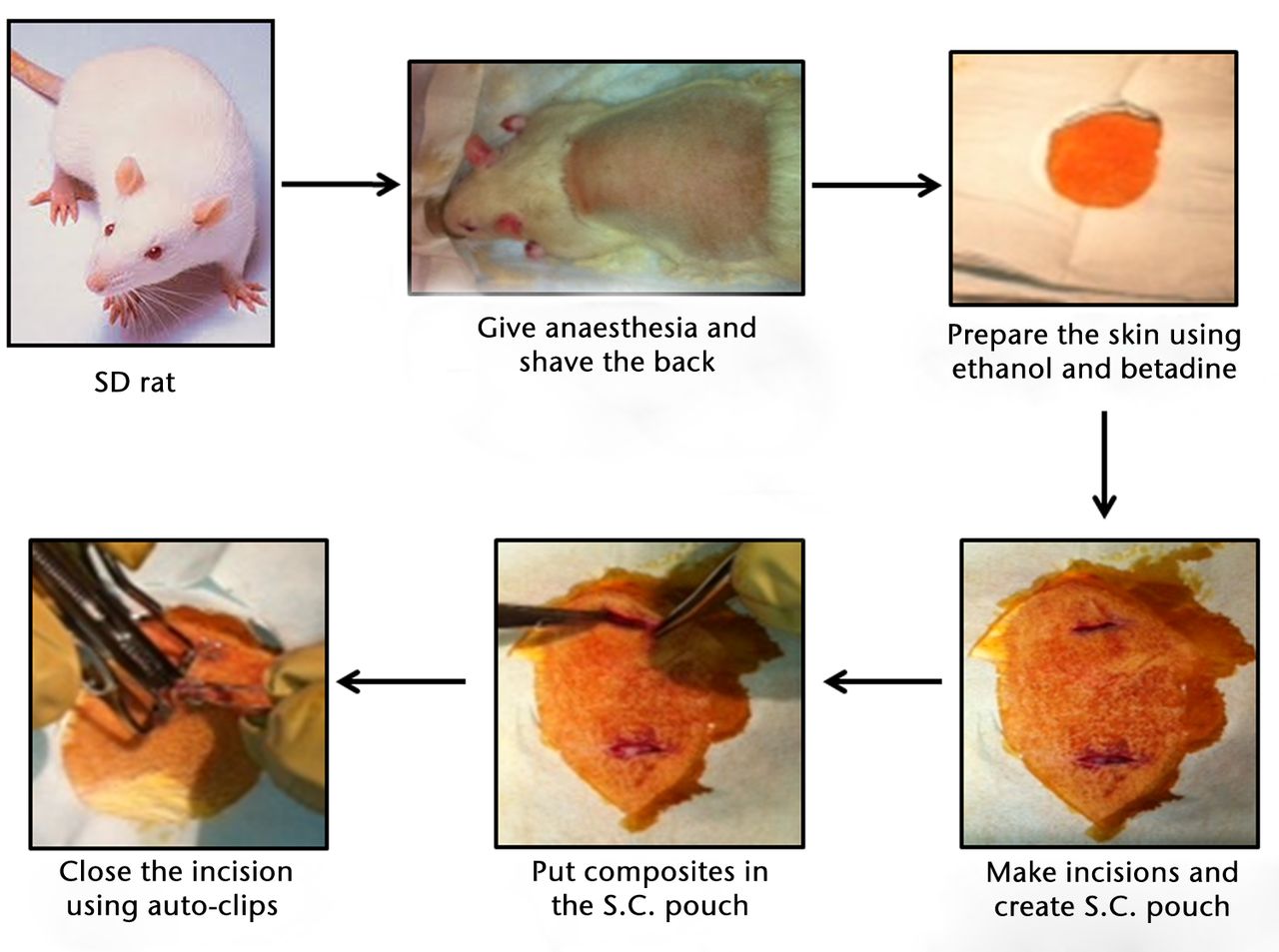
Fig. 1
Schematic representation of the surgical procedure involved in subcutaneous implantation of sham, poly(lactic-co-glycolic acid) (PLAGA) and single-walled carbon nanotubes (SWCNT)/PLAGA composites in the rat.
Morbidity and clinical signs
Rats were observed once a day for signs of morbidity and adverse clinical signs throughout the study including visual examination, physical examination and/or palpation. Rats were also observed for any implant-associated lesions or abnormalities in behaviour or function (AG, TAL).
Body weights
Individual body weights were recorded before surgery and at days one, three and seven post-surgery for the first week, and weekly thereafter.
Food consumption
This was measured for individual rats as an assessment of general wellbeing at days one, three and seven post-operatively for the first week, and weekly thereafter. The amount of food was weighed before it was supplied to each cage and remnants of food were weighed at the next time point to calculate the difference. Amounts were then used to calculate the daily food consumption (g/animal/day).
Urinalysis
Urine was collected from five animals per group once at 12 weeks post-implantation. Rats were placed in metabolic cages on the day of collection for four hours with access to water, and the urine was collected. The parameters measured included pH, specific gravity, leucocytes, nitrite, protein, ketone, ascorbic acid, urobilinogen, bilirubin, glucose and occult blood using an Urispec 11-way test strips (Henry Schein, Melville, New York).
Haematology
Blood samples were collected at two, four, eight and 12 weeks post-implantation in EDTA-containing tubes using cardiac puncture after euthanasia. The parameters including white blood cell (WBC) count, red blood cell count, haemoglobin concentration, mean corpuscular volume and mean corpuscular haemoglobin (MCH) were determined using an automated haematology machine (VetScanHM2, Abaxis, California). WBC differential counts including neutrophil, lymphocyte, eosinophil, basophil and monocyte were determined from a Wright-Giemsa (Sigma-Aldrich) stained blood smear.
Necropsy
The animals were killed at the specified intervals and observed for macroscopic abnormalities. Major organs including heart, lungs, liver, spleen, kidneys and adrenal glands were collected, weighed (absolute and relative to body weights) and observed for abnormalities.
Histopathology
The collected organs along with the implant and the surrounding tissues were fixed in 10% neutral-buffered formalin solution for at least seven days. The samples were embedded in paraffin, sectioned using a microtome to 4 µm to 5µm thickness, and stained with haematoxylin and eosin (H and E). All organs and the implant sites were analysed by a veterinary pathologist blinded to the treatment group. Two implant sites were evaluated for each animal. For the implant sites, a scoring system using 11 parameters was used to calculate a final summary toxicity score for each animal. Scores for the 11 individual parameters ranged from 0 (no finding) to 4 (severe) with the parameters including necrosis, inflammation, polymorphonuclear neutrophils (PMNs), macrophages, lymphocytes, plasma cells, giant cells, fibroplasia, fibrosis, presence of lipid material and relative size. Summary toxicity (sumtox) scores could range from 0 (no findings) to a maximum score of 44.
Statistical analysis
The mean and standard error of the mean (sem) values along with statistical analysis using one-way ANOVA with Tukey post hoc test, were performed for body weight, food consumption, haematology, absolute and relative organ weights and histopathology. For urinalysis, qualitative interpretations were presented descriptively and mean sem values, along with statistical analysis using one-way ANOVA with Tukey post hoc test, were performed for the volume of urine. The results were considered significant when p < 0.05.
Results
Morbidity and clinical signs
No mortality, behavioural changes, treatment-related adverse clinical signs or signs of physical self-mutilation indicating localised or neurological toxicity were observed during the post-operative examinations, or at the time of euthanasia in any of the groups.
Body weight
All the groups showed consistent weight gain and followed the same pattern of rate of gain throughout the study period (Fig. 2).
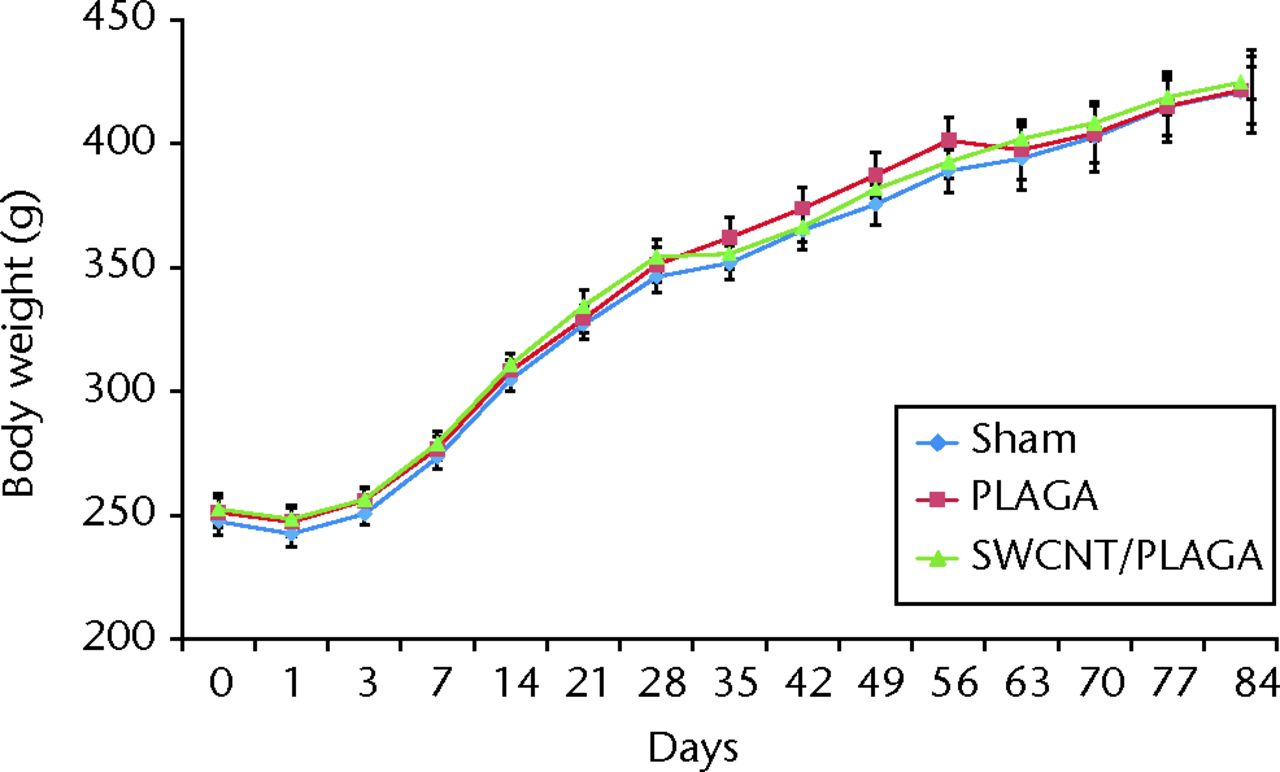
Fig. 2
Graph showing the changes in body weight in rats implanted with Sham, poly (lactic-co-glycolic acid) (PLAGA) and single-walled carbon nanotubes (SWCNT)/PLAGA composites. Data represent mean with standard error of the mean, and p < 0.05 was considered significant.
Food consumption
The food consumption for rats implanted with Sham, PLAGA and SWCNT/PLAGA exhibited a similar pattern by 14 days (two weeks), 28 days (four weeks) and 84 days (12 weeks) (Fig. 3), 28 days (four weeks) and 84 days (12 weeks) (Fig. 3) and 84 days (12 weeks) (Fig. 3) (Fig. 3). However, the food consumption of rats implanted with PLAGA was significantly higher than that of Sham at day 35 of the 56-day (eight-week) period. All groups showed an increase in food consumption initially following the post-surgical period, and then food consumption levelled out.
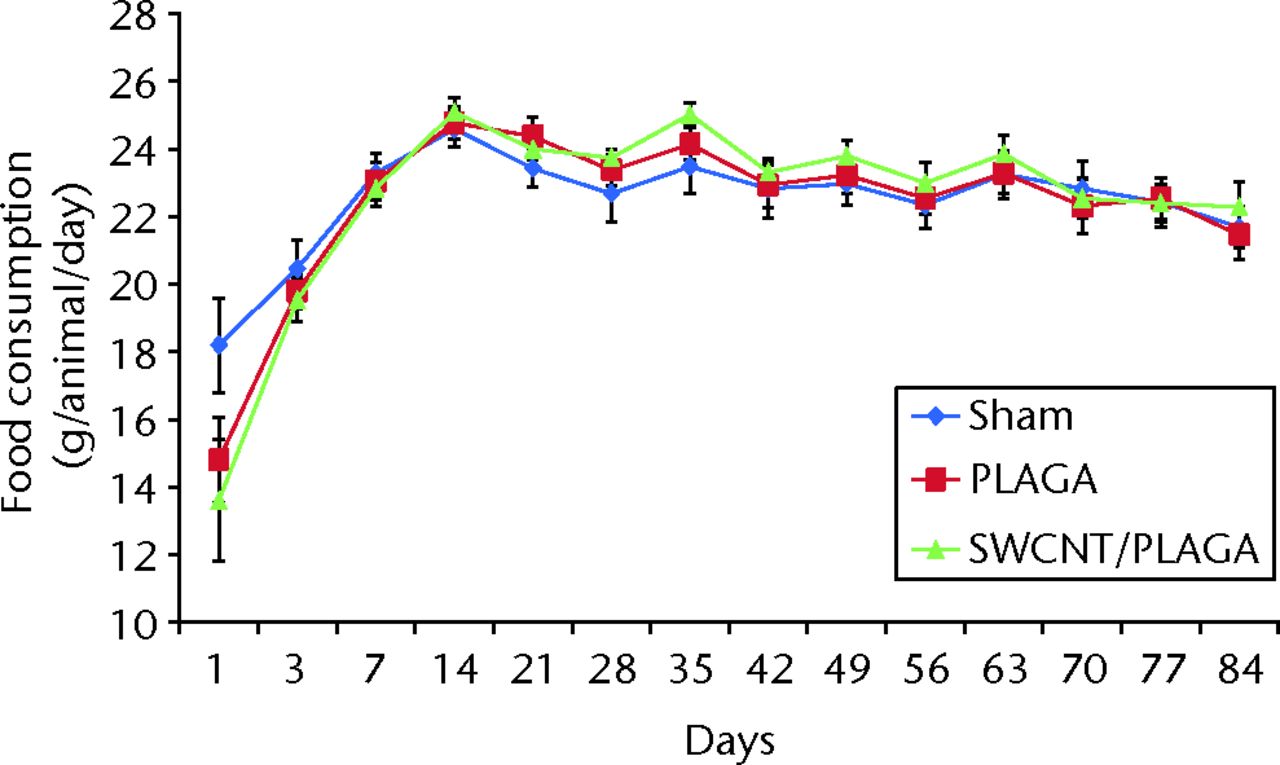
Fig. 3
Graph showing the food consumption in rats implanted with Sham, poly (lactic-co-glycolic acid) (PLAGA) and single-walled carbon nanotubes (SWCNT)/PLAGA composites at 12 weeks post-implantation. Data represent mean with standard error of the mean and p < 0.05 was considered significant.
Urinalysis
All groups showed no significant difference for any of the 11 urinalysis parameters measured at termination. However, the urine volume collected for the SWCNT/PLAGA group was significantly higher compared with the PLAGA. There was no significant difference in the volume of urine collected between Sham and PLAGA or between Sham and SWCNT/PLAGA groups.
Haematology
Significantly higher values for MCH were observed for Sham and SWCNT/PLAGA compared with PLAGA at four weeks, as well as a higher granulocyte percentage (GR%) value for Sham compared with SWCNT/PLAGA at 12 weeks. No significant difference was observed for any of the other haematological parameters tested at two and eight weeks.
For the WBC differential count (Fig. 4), the segmented neutrophils count was significantly higher for PLAGA compared with Sham at four weeks. No other statistically significant difference was observed for any other parameters tested at two, eight and 12 weeks. All of these statistically significant differences observed for haematological parameters and WBC differential count were within the normal range of values reported for rats.
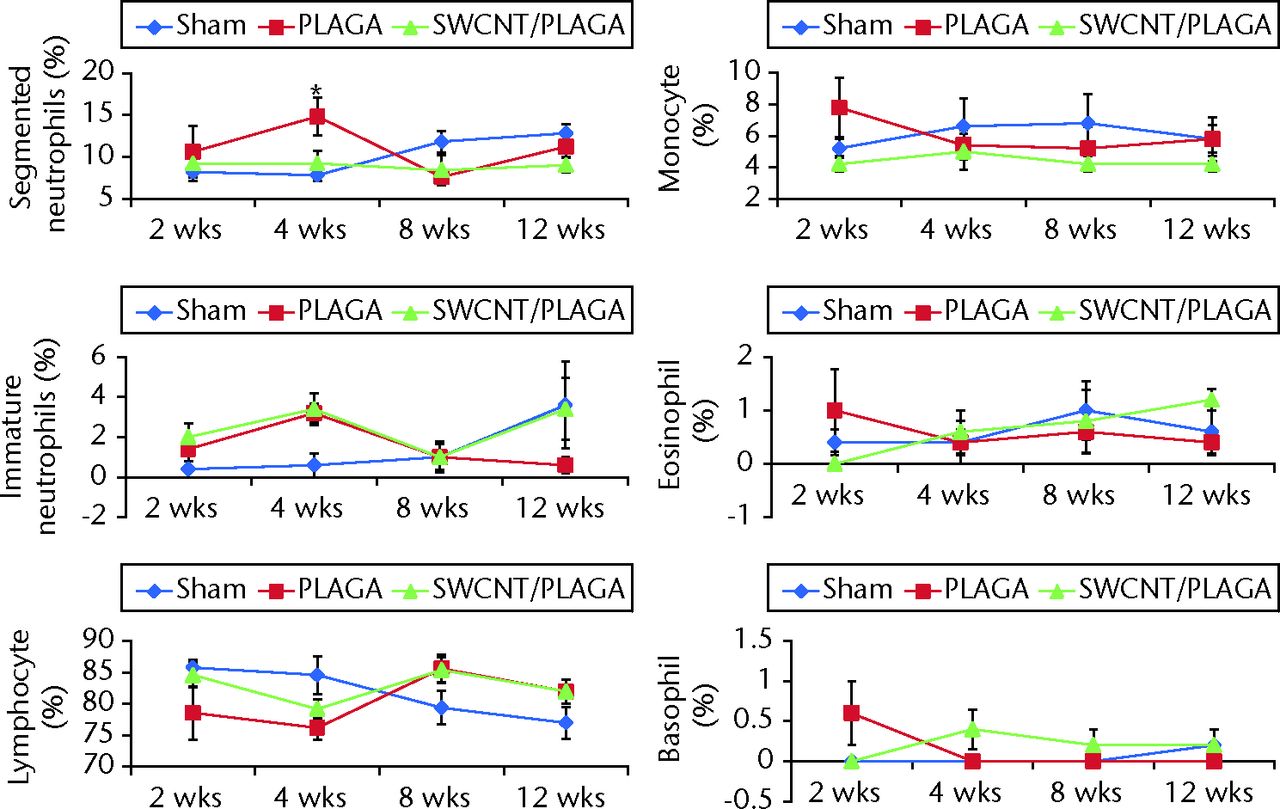
Fig. 4
Graphs showing white blood cell differential count of rats implanted with Sham, poly(lactic-co-glycolic acid) (PLAGA) and single-walled carbon nanotubes (SWCNT)/PLAGA composites. The parameters include segmented neutrophils, immature neutrophils, lymphocyte, monocyte, eosinophil and basophil. Data represent mean with standard error of the mean and p < 0.05 was considered significant (PLAGA was significantly different from Sham).
Gross findings at necropsy, absolute and relative organ weights
Implants did not migrate from their original location, even though no immobilisation (sutures, adhesives, etc.) was used, and they maintained their structural integrity for the 12-week study period. No macroscopic abnormalities were noted in any of the animals at two, four, eight and 12 weeks post-implantation. Subcutaneous tissue surrounding the implants appeared grossly normal, with no overt evidence of inflammation, and all incision sites were healed (Fig. 5) was used, and they maintained their structural integrity for the 12-week study period. No macroscopic abnormalities were noted in any of the animals at two, four, eight and 12 weeks post-implantation. Subcutaneous tissue surrounding the implants appeared grossly normal, with no overt evidence of inflammation, and all incision sites were healed (Fig. 5) (data shown for eight and 12 weeks). No significant differences in absolute and relative organ weights (Fig. 6). No significant differences in absolute and relative organ weights (Fig. 6) were observed among all groups at all the intervals.
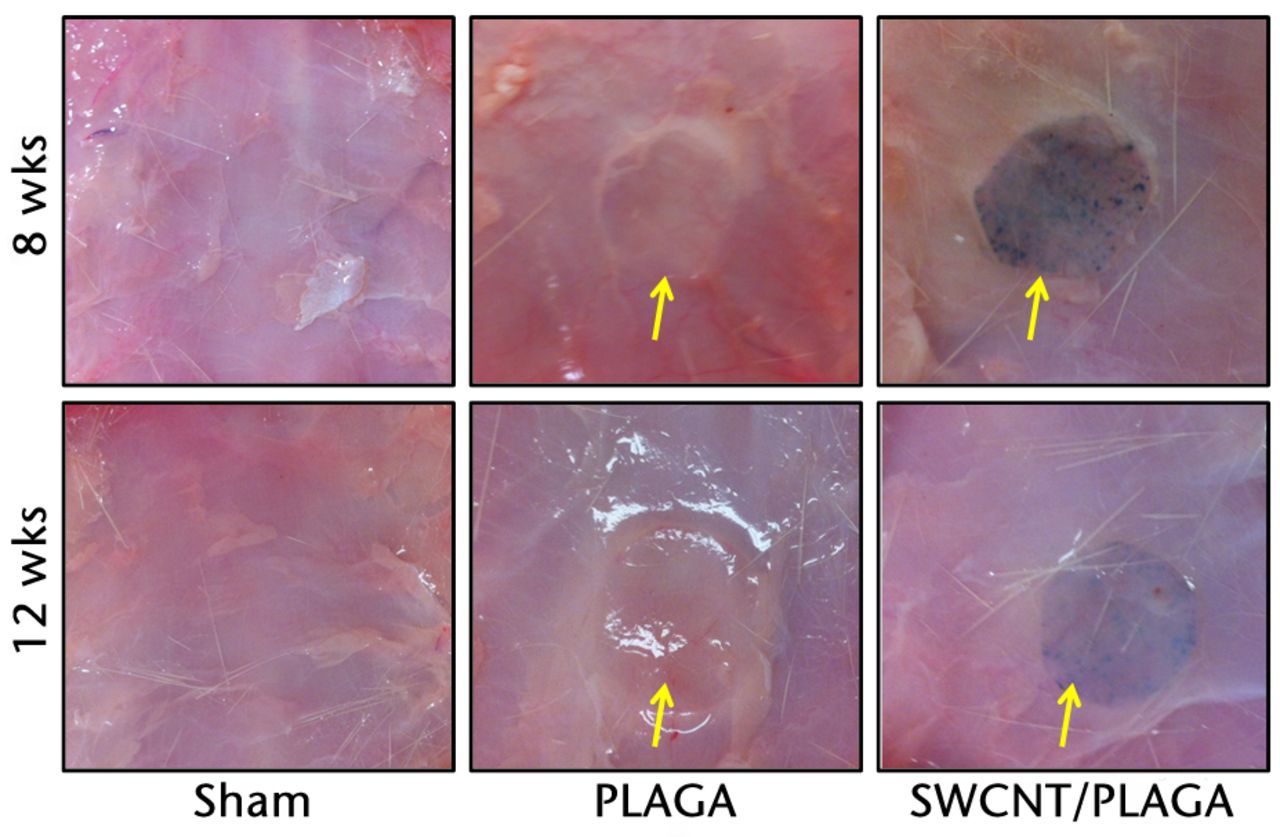
Fig. 5
Gross pathological images of subcutaneous tissue surrounding the implants (Sham, poly (lactic-co-glycolic acid) (PLAGA) and single-walled carbon nanotubes; SWCNT/PLAGA) at eight and 12 weeks post-implantation. All incision sites were healed and the tissue surrounding the implants appeared grossly normal, with no overt evidence of inflammation.
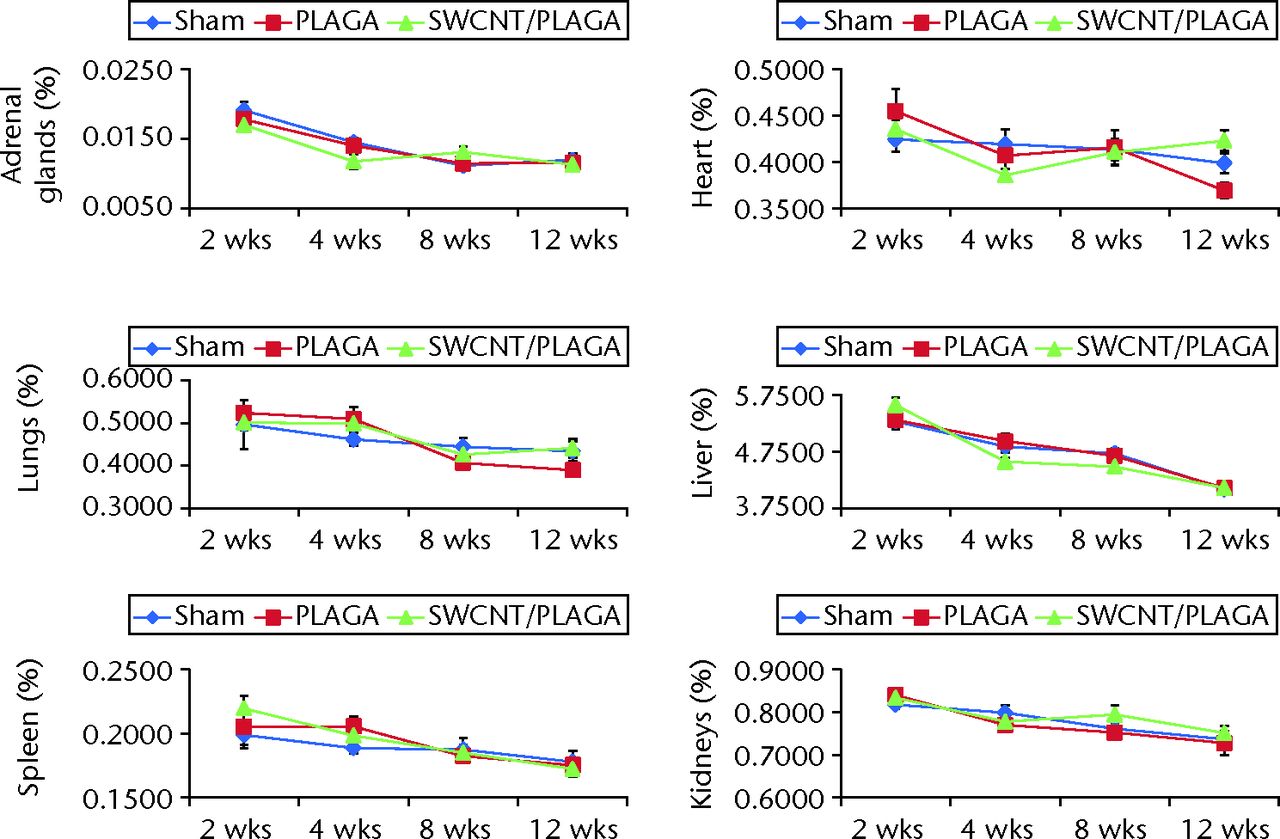
Fig. 6
Graphs showing the relative organ weight in rats implanted with Sham, poly(lactic-co-glycolic acid) (PLAGA) and single-walled carbon nanotubes (SWCNT)/PLAGA composites. The parameters include adrenal glands, lungs, spleen, heart, liver and kidneys. Data represent mean with standard error of the mean and p < 0.05 was considered significant.
Histopathology
There were no lesions observed in the major organs related to implantation of PLAGA, SWCNT/PLAGA and control. The H and E stain (Fig. 7) showed the formation of a fibrous capsule surrounding both the PLAGA and SWCNT/PLAGA composites at all the intervals. A mild to moderate inflammatory response was observed for both composites characterised by the presence of inflammatory cells such as PMNs, and was reported as a sumtox score. The control animals were without any observable response to the Sham operation, with a sumtox score of zero for all four time periods post-operatively. Both composites induced a mild to moderate inflammatory response, showed a significant decrease in sumtox score from two to four weeks and no change was observed for four to eight weeks and eight to 12 weeks and between two and eight weeks, and two and 12 weeks. In addition, at all the time intervals, both composites showed a significantly higher sumtox score compared with control. However, there was no significant difference between PLAGA and SWCNT/PLAGA at all the time intervals (Fig. 8).
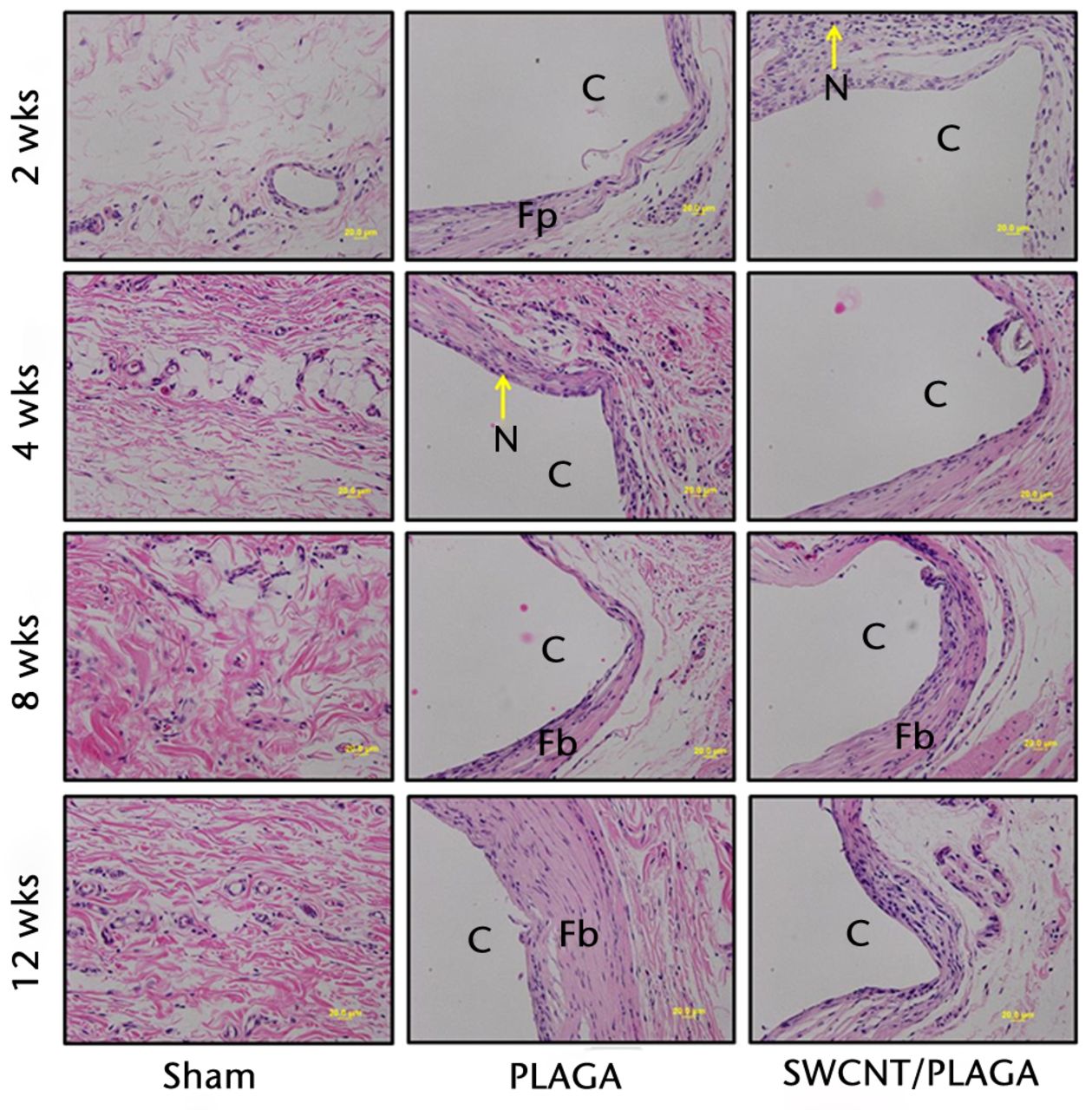
Fig. 7
Micrograph of subcutaneous skin tissues of rats implanted with Sham, poly (lactic-co-glycolic acid) (PLAGA) and single-walled carbon nanotubes (SWCNT)/PLAGA at two, four, eight and 12 weeks post-implantation stained with haematoxylin and eosin (×20 magnification). C, composite (PLAGA or SWCNT/PLAGA) implant site; M, muscular tissue; N, polymorphonuclear neutrophils; Fp, fibroplasia; Fb, fibrosis.
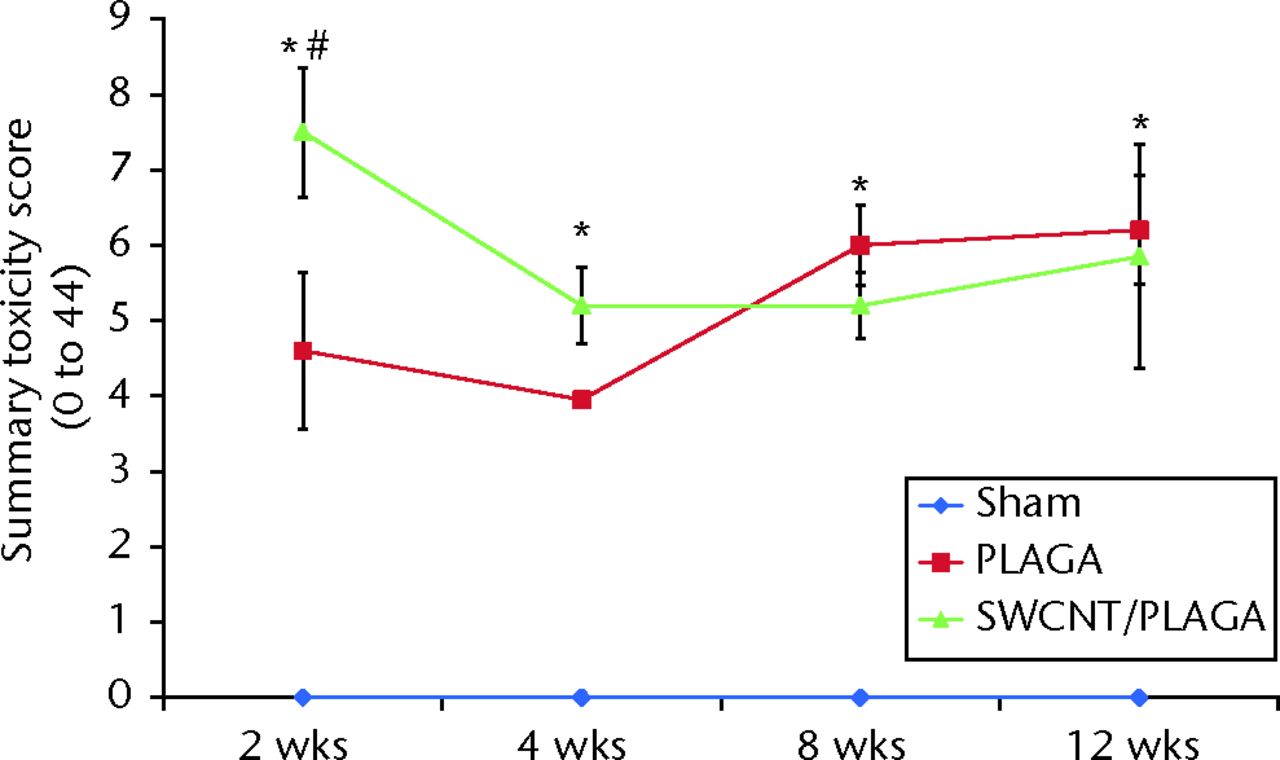
Fig. 8
Graph showing the histopathological changes to Sham, poly(lactic-co-glycolic acid) (PLAGA) and single-walled carbon nanotubes (SWCNT)/PLAGA in rat subcutaneous tissue as a function of the summary toxicity score on a scale of 0 to 44 over a period of 12 weeks post-implantation. Data represent mean with standard error of the mean and p < 0.05 was considered significant. PLAGA and SWCNT/PLAGA were significantly different from Sham; both PLAGA and SWCNT/PLAGA showed significant decrease from week two to week four.
Discussion
Biodegradable composites are of interest in orthopaedics, and polymers such as PLAGA are ideal for BTE applications and are currently used in the biomedical industry.24-26 Our previously published in vitro studies have demonstrated that SWCNT/PLAGA composites are biocompatible with significantly enhanced cell proliferation and mechanical strength compared with a PLAGA alone.17,18 However, before a composite can be applied in a clinical application, it has to be certified as biocompatible and non-toxic.27 The goal of this study was to evaluate the in vivo biocompatibility of a SWCNT/PLAGA composite in Sprague-Dawley rats for a period of 12 weeks to demonstrate that SWCNT/PLAGA composites are biocompatible, non-cytotoxic, and safe for orthopaedic applications. Rats which were implanted with Sham (control), PLAGA and SWCNT/PLAGA composites subcutaneously had no mortality, visible inflammation, behavioural changes or visible signs of physical self-mutilation during the post-operative examinations and at the time they were killed. All the groups showed consistent weight gain throughout the study appropriate for the age of the animals and the rate of gain for each group was similar. The food consumption, as a measure of general health, in all the groups followed the same pattern. All groups showed a significant increase in food consumption initially following the post-surgical period; from then on food consumption plateaued. This pattern could be attributed to the necessity of more food consumption during the wound healing period and to the normal growth pattern for this age of rat. The urinalysis showed no significant difference between all the groups for any of the 11 urinalysis parameters tested, indicating a lack of acute toxic effects on renal function after 12 weeks. For the haematological analysis and WBC differential count, the statistically significant differences observed for MCH, GR% and segmented neutrophils values were within the normal range reported for Sprague-Dawley rats. Therefore, it was considered that there were no acute haematological effects due to implantation of SWCNT/PLAGA compared with the control and PLAGA groups.
No lesions were observed in the major organs of the rats related to implantation of composites. The absolute and relative organ weight in all the groups at all the intervals was similar and didn’t show any significant difference. The Sham (control) group didn’t show any response to the operation, with a sumtox score of zero for the duration of the study. The formation of a fibrous capsule surrounding both the PLAGA and SWCNT/PLAGA composites at all the intervals was observed. A mild to moderate inflammatory response was observed for both composites, and is reflected in the sumtox score. At all the time intervals, both composites showed a significantly higher sumtox score compared with the Sham group. However, there was no significant difference between PLAGA and SWCNT/PLAGA-implanted groups. Our results are in agreement with the recently published study by Bhattacharya et al28 who demonstrated that six weeks after SWCNT composites were implanted in a rat calvarial defect, there were no histological signs of inflammation or graft rejection. It is documented that inflammation around implants is a process of normal host defence mechanisms brought about by the result of surgical implantation, along with the presence of the implanted material.24,29 In a polymeric implant, the inflammatory reaction is dependent on several factors such as the extent of injury or defect, size, shape, polymer degradation rate, as well as the physical, chemical and mechanical properties of the implant material.30-32 Biodegradable polymers such as polyphosphazenes, polyanhydrides, polyester (PLAGA) and many non-degradable polymers have been shown to produce inflammatory responses.33-35 In this study, no macroscopic abnormalities were observed in any of the animals at any time interval. Subcutaneous tissue surrounding the implants appeared grossly normal, with no overt evidence of inflammation, and all incision sites were healed. The integrity of both the composites assessed visually appeared to be maintained over the 12-week study period as a result of the slow rate of degradation (around one year) of the PLAGA (85:15) used, which led to decreased release rates of SWCNT from the SWCNT/PLAGA composite keeping the composites intact, as shown in Figure 5. This slow degradation likely accounts for the lack of adverse clinical and pathology effects after 12 weeks. This may be beneficial in longer-term studies as the slow release of SWCNT combined with the small percentage of SWCNT in the composite could greatly decrease the toxicological potential of our implant material. Future studies will be designed to evaluate the efficacy of SWCNT/PLAGA composites in bone regeneration in a nonunion ulnar bone defect rabbit model. At the conclusion of this proposed study, the PLAGA will be degraded completely to allow for an enhanced release of SWCNT from SWCNT/PLAGA composites, thus prolonging the exposure of SWCNT with host tissue. This will help us to evaluate the long-term biocompatibility and bone regeneration capability of the SWCNT/PLAGA composites.
In conclusion, our SWCNT/PLAGA composites, which possess high mechanical strength and mimic the microstructure of human trabecular bone,18 displayed biocompatibility similar to PLAGA, a well-known FDA-approved biocompatible polymer over the 12-week study period. Thus, the results obtained demonstrate the potential of SWCNT/PLAGA composites for BTE and bone regeneration applications, and will have a significant impact on the ability of clinicians to restore greater functional activity in injured patients.
1 Borrelli J Jr , PrickettWD, RicciWM. Treatment of nonunions and osseous defects with bone graft and calcium sulfate. Clin Orthop Relat Res2003;411:245–254.CrossrefPubMed Google Scholar
2 Giannoudis PV , DinopoulosH, TsiridisE. Bone substitutes: an update. Injury2005;36(Suppl 3):S20–S27.CrossrefPubMed Google Scholar
3 Korompilias AV , LykissasMG, SoucacosPN, KostasI, BerisAE. Vascularized free fibular bone graft in the management of congenital tibial pseudarthrosis. Microsurgery2009;29:346–352.CrossrefPubMed Google Scholar
4 Soucacos PN , JohnsonEO, BabisG. An update on recent advances in bone regeneration. Injury2008;39(suppl 2):S1–S4.CrossrefPubMed Google Scholar
5 Petite H , ViateauV, BensaïdW, et al.Tissue-engineered bone regeneration. Nat Biotechnol2000;18:959–963.CrossrefPubMed Google Scholar
6 Laurencin CT , AmbrosioAM, BordenMD, CooperJA Jr. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng1999;1:19–46.CrossrefPubMed Google Scholar
7 Lu L , MikosAG. The importance of new processing techniques in tissue engineering. MRS Bull1996;21:28–32.CrossrefPubMed Google Scholar
8 Athanasiou KA , NiederauerGG, AgrawalCM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials1996;17:93–102.CrossrefPubMed Google Scholar
9 Lu L , GarciaCA, MikosAG. In vitro degradation of thin poly(DL-lactic-co-glycolic acid) films. J Biomed Mater Res1999;46:236–244.CrossrefPubMed Google Scholar
10 Laurencin CT, Nair LS. Nanotechnology and Tissue Engineering: The Scaffold. Boca Raton, FL: CRC Press, 2008. Google Scholar
11 Cui FZ , LiDJ. A review of investigations on biocompatibility of diamond-like carbon and carbon nitride films. Surf Coat Tech2000;131:481–487. Google Scholar
12 Smart SK , CassadyAI, LuGQ, MartinDJ. The biocompatibility of carbon nanotubes. Carbon2006;44:1034–1047. Google Scholar
13 Sinha N , YeowJT. Carbon nanotubes for biomedical applications. IEEE Trans Nanobioscience2005;4:180–195. Google Scholar
14 Armentano I , DottoriM, PugliaD, KennyJM. Effects of carbon nanotubes (CNTs) on the processing and in-vitro degradation of poly(DL-lactide-co-glycolide)/CNT films. J Mater Sci Mater Med2008;19:2377–2387.CrossrefPubMed Google Scholar
15 Saito N , UsuiY, AokiK, et al.Carbon nanotubes: biomaterial applications. Chem Soc Rev2009;38:1897–1903.CrossrefPubMed Google Scholar
16 Hu H , NiY, MontanaV, HaddonRC, ParpuraV. Chemically Functionalized Carbon Nanotubes as Substrates for Neuronal Growth. Nano Lett2004;4:507–511.CrossrefPubMed Google Scholar
17 Gupta A , WoodsMD, IllingworthKD, et al.Single walled carbon nanotube composites for bone tissue engineering. J Orthop Res2013;31:1374–1381.CrossrefPubMed Google Scholar
18 Gupta A , MainBJ, TaylorBL, et al.In vitro evaluation of three-dimensional single-walled carbon nanotube composites for bone tissue engineering. J Biomed Mater Res A2014;102:4118–4126.CrossrefPubMed Google Scholar
19 Sethuraman S , NairLS, El-AminS, et al.In vivo biodegradability and biocompatibility evaluation of novel alanine ester based polyphosphazenes in a rat model. J Biomed Mater Res A2006;77:679–687.CrossrefPubMed Google Scholar
20 Kasemo B , LausmaaJ. Material-tissue interfaces: the role of surface properties and processes. Environ Health Perspect1994;102(suppl 5):41–45.CrossrefPubMed Google Scholar
21 Hench LL , WilsonJ. Surface-active biomaterials. Science1984;226:630–636.CrossrefPubMed Google Scholar
22 Watanabe T , BanS, ItoT, et al.Biocompatibility of composite membrane consisting of oriented needle-like apatite and biodegradable copolymer with soft and hard tissues in rats. Dent Mater J2004;23:609–612.CrossrefPubMed Google Scholar
23 van der Zande M , WalboomersXF, OlaldeB, et al.Effect of nanotubes and apatite on growth factor release from PLLA scaffolds. J Tissue Eng Regen Med2011;5:476–482.CrossrefPubMed Google Scholar
24 Ibim SM , UhrichKE, BronsonR, et al.Poly(anhydride-co-imides): in vivo biocompatibility in a rat model. Biomaterials1998;19:941–951.CrossrefPubMed Google Scholar
25 Laurencin CT , AttawiaMA, ElgendyHE, HerbertKM. Tissue engineered bone-regeneration using degradable polymers: the formation of mineralized matrices. Bone1996;19(suppl):93S–99S.CrossrefPubMed Google Scholar
26 Lim TY , PohCK, WangW. Poly (lactic-co-glycolic acid) as a controlled release delivery device. J Mater Sci Mater Med2009;20:1669–1675.CrossrefPubMed Google Scholar
27 Khouw IM , van WachemPB, MolemaG, et al.The foreign body reaction to a biodegradable biomaterial differs between rats and mice. J Biomed Mater Res2000;52:439–446.CrossrefPubMed Google Scholar
28 Bhattacharya M , Wutticharoenmongkol-ThitiwongsawetP, HamamotoDT, et al.Bone formation on carbon nanotube composite. J Biomed Mater Res A2011;96:75–82.CrossrefPubMed Google Scholar
29 Menei P , DanielV, Montero-MeneiC, et al.Biodegradation and brain tissue reaction to poly(D,L-lactide-co-glycolide) microspheres. Biomaterials1993;14:470–478.CrossrefPubMed Google Scholar
30 Homsy CA . Bio-compatibility in selection of materials for implantation. J Biomed Mater Res1970;4:341–356.CrossrefPubMed Google Scholar
31 Little K , ParkhouseJ. Tissue reactions to polymers. Lancet1962;2:857–861.CrossrefPubMed Google Scholar
32 Wood NK , KaminskiEJ, OglesbyRJ. The significance of implant shape in experimental testing of biological materials: disc vs. rod. J Biomed Mater Res1970;4:1–12.CrossrefPubMed Google Scholar
33 Laurencin C , DombA, MorrisC, et al.Poly(anhydride) administration in high doses in vivo: studies of biocompatibility and toxicology. J Biomed Mater Res1990;24:1463–1481.CrossrefPubMed Google Scholar
34 Brady JM , CutrightDE, MillerRA, BarristoneGC. Resorption rate, route, route of elimination, and ultrastructure of the implant site of polylactic acid in the abdominal wall of the rat. J Biomed Mater Res1973;7:155–166.CrossrefPubMed Google Scholar
35 Turner JE , LawrenceWH, AutianJ. Subacute toxicity testing of biomaterials using histopathologic evaluation of rabbit muscle tissue. J Biomed Mater Res1973;7:39–58.CrossrefPubMed Google Scholar
Funding statement:
A grant has been received from the Orthopaedic Research and Education Foundation and the Robert J Gladden Orthopaedic Society (#5-61480-13) to Southern Illinois University which is related to this article.
Author contributions:
A. Gupta: MMICB graduate student thesis project, Study design, Data collection, Manuscript preparation.
T. A. Liberati: Tissue and data collection, Study design, Manuscript preparation
S. J. Verhulst: Data analysis, Edited manuscript
B. J. Main: Data collection, Data analysis
M. H. Roberts: Data collection, Data analysis
A. G. R. Potty: Assisted with surgery, Manuscript preparation
T. K. Pylawka: Data analysis, Manuscript preparation
S. F. El-Amin III: Principal Investigator, Thesis Advisor, Study design, Performed Surgeries, Manuscript Preparation
ICMJE Conflict of Interest:
None declared
©2015 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.
Supplementary material. Graphs showing further data regarding consumption of food and absolute organ weight, and tables giving urinalysis and haematological values are available alongside the online version of this article at www.bjj.boneandjoint.org.uk










