Abstract
Objectives
The aim of this study was to examine whether asymmetric loading influences macrophage elastase (MMP12) expression in different parts of a rat tail intervertebral disc and growth plate and if MMP12 expression is correlated with the severity of the deformity.
Methods
A wedge deformity between the ninth and tenth tail vertebrae was produced with an Ilizarov-type mini external fixator in 45 female Wistar rats, matched for their age and weight. Three groups were created according to the degree of deformity (10°, 30° and 50°). A total of 30 discs and vertebrae were evaluated immunohistochemically for immunolocalisation of MMP12 expression, and 15 discs were analysed by western blot and zymography in order to detect pro- and active MMP12.
Results
No MMP12 expression was detected in the nucleus pulposus. Expression of MMP12 in the annulus progressively increased from group I to groups II and III, mainly at the concave side. Many growth plate chondrocytes expressed MMP12 in the control group, less in group I and rare in groups II and III. Changes in cell phenotype and reduction of cell number were observed, together with disorganisation of matrix microstructure similar to disc degeneration. ProMMP12 was detected at the area of 54 kDa and active MMP12 at 22 kDa.
Conclusions
Expression of MMP12 after application of asymmetric loading in a rat tail increased in the intervertebral disc but decreased in the growth plate and correlated with the degree of the deformity and the side of the wedged disc.
Cite this article: Bone Joint Res 2014;3:273–9.
Article focus
The goal of this study was to examine whether asymmetric loading influences macrophage elastase (MMP12) expression in different parts of a rat tail intervertebral disc and growth plate, and if MMP12 expression was correlated with the severity of the deformity.
Key messages
Expression ofMMP12 after application of asymmetric loading in rat tail increased in the intervertebral disc but decreased in the growth plate and correlated with the degree of the deformity and the side of the wedged disc.
Disc cells were reduced in number after asymmetric loading, identified with changes in their phenotype, and in a large proportion expressed MMP12.
Strengths and limitations
Strengths: This is an experimental study which detects MMP12 expression after application of asymmetric loading in an animal model’s intervertebral disc with immunohistochemical analysis, as well as with western blot analysis and zymography. Application of asymmetric loading was achieved with an accurate and reproducible method.
Limitation: detection and quantification of the MMP12 gene with a real-time polymerase chain reaction was not performed.
Introduction
Spinal motion is a complex process in which the spine undergoes continuous asymmetric loading modes, a process which is associated with intervertebral disc metabolism and altered growth rate. Application of loading magnitude above a critical threshold can initiate the degenerative process, inducing molecular changes and causing structural damage to the disc.1 Numerous factors result in an imbalance between degradation and synthesis of the extracellular matrix in the intervertebral disc and in vertebral growth plates. After mechanical compression and distraction forces, degradation prevails over synthesis and is evident at both the microscopic and macroscopic level. Possible aetiological factors can be divided into those that affect the biology and those that affect the biomechanical environment, both at the disc and the vertebral growth plates, leading to histological and molecular changes at the chondrocytes of the hypertrophic zone.2 A crucial question that has to be answered is whether metabolism disorders or changes in biomechanics initiate the cascade of degradation.
Matrix metalloproteinases (MMPs) are a family of proteolytic enzymes that share structural and functional characteristics with different substrate specificities.3 Weiler et al4 have investigated the role of MMPs in intervertebral disc degeneration. MMPs appear to be involved in cell attachment, proliferation, differentiation, and apoptosis of growth plate chondrocytes.5 Furthermore, MMP expression is dependent on the duration and intensity of mechanical loading and thus participates in growth modulation.6
Macrophage elastase (MMP12) is secreted as a 54 kDa pro-form and in its active form (22 kDa) degrades mainly elastin.7 The protein was originally identified in a conditioned medium from murine peritoneal inflammatory macrophages, and is expressed in several cell types, such as human airway smooth muscle cells, corneal epithelial cells and hypertrophic chondrocytes.8 Besides elastin, MMP12 degrades various extracellular matrix proteins, including type IV collagen, fibronectin, laminin, gelatin, vitronectin and chondroitin sulfate proteoglycans.9 It also upregulates other MMPs.10
The aim of our present study was to examine whether asymmetric loading produced by an Ilizarov-type mini external fixator influences MMP12 expression in different parts of a rat tail intervertebral disc and growth plate and if MMP12 expression was correlated with the severity of the deformity.
Materials and Methods
Animal model
A total of 45 female eight-week old albino Wistar rats (National Center for Scientific Research Demokritos, Athens, Greece), weighing 180 g ± 10 g, were included in the study. All animals were selected after control breeding and were matched for age and weight. Animals were included in the study before reaching adulthood in order to take into account the impact of growth in intervertebral disc changes, and were kept in a safe laboratory environment with stable humidity, temperature of 21°C and artificial daylight for 12 hours. Animal procedures were reviewed and approved by the Veterinary Ethics Committee and performed under the standards of the Greek State and European Community on the Protection, Care and Use of Animals for experimental purposes (licence number K/8404/08). All efforts were taken to minimise pain or discomfort to the animals.
The rats were anaesthetised and an Ilizarov-type mini external fixator was applied under fluoroscopic control. The apparatus was applied for five weeks between the ninth and tenth vertebrae at the base of the rat tail. The two rings of the fixator were placed at a predetermined angle so that an accurate deformity of the intermediate disc was achieved. The intact intervertebral discs outside of the fixator were used as controls. According to the degree of the deformity, the subjects were categorised in three groups, namely group I (10o), group II (30o) and group III (50o) (Fig. 1) (Fig. 1). The reliability of this animal model is well documented in the literature.1,11,12Rats were killed on day 35, when they had reached approximately 90% of skeletal maturity.13 From the total of 45 discs, 30 were randomly selected for immunohistochemical analysis and 15 were selected for western blot analysis and zymography. All discs were divided in the above three groups according to the degree of the deformity.
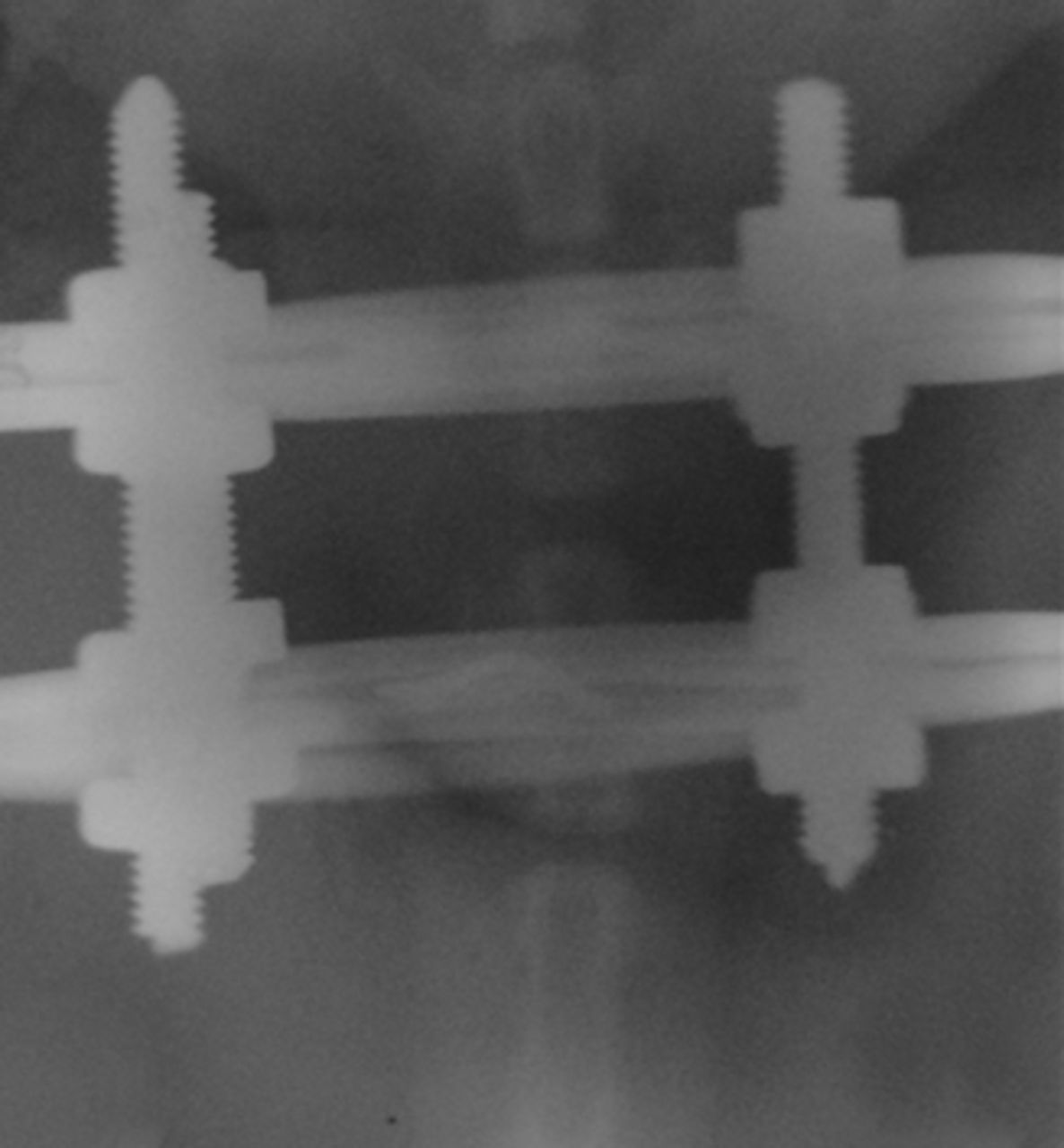
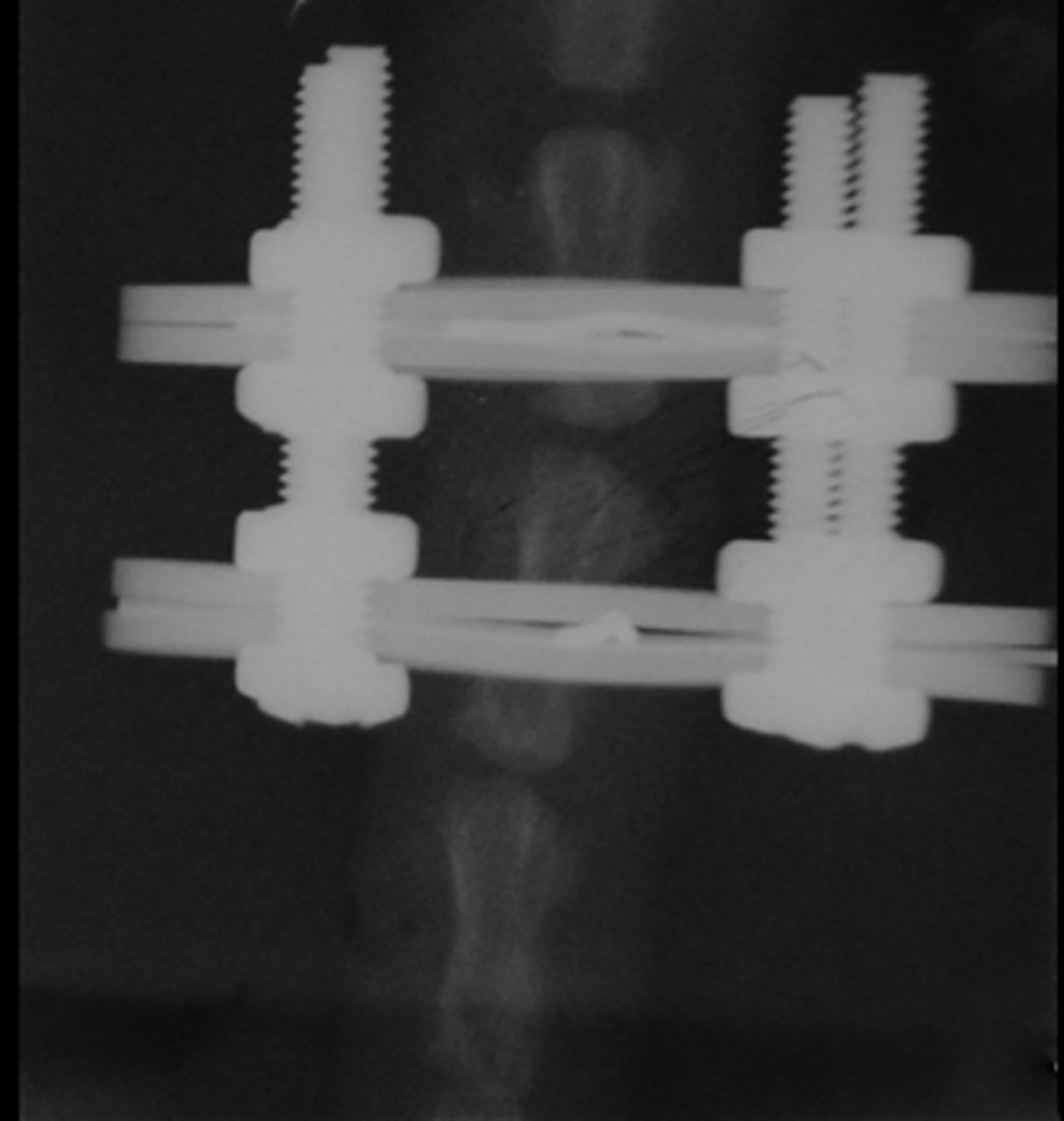
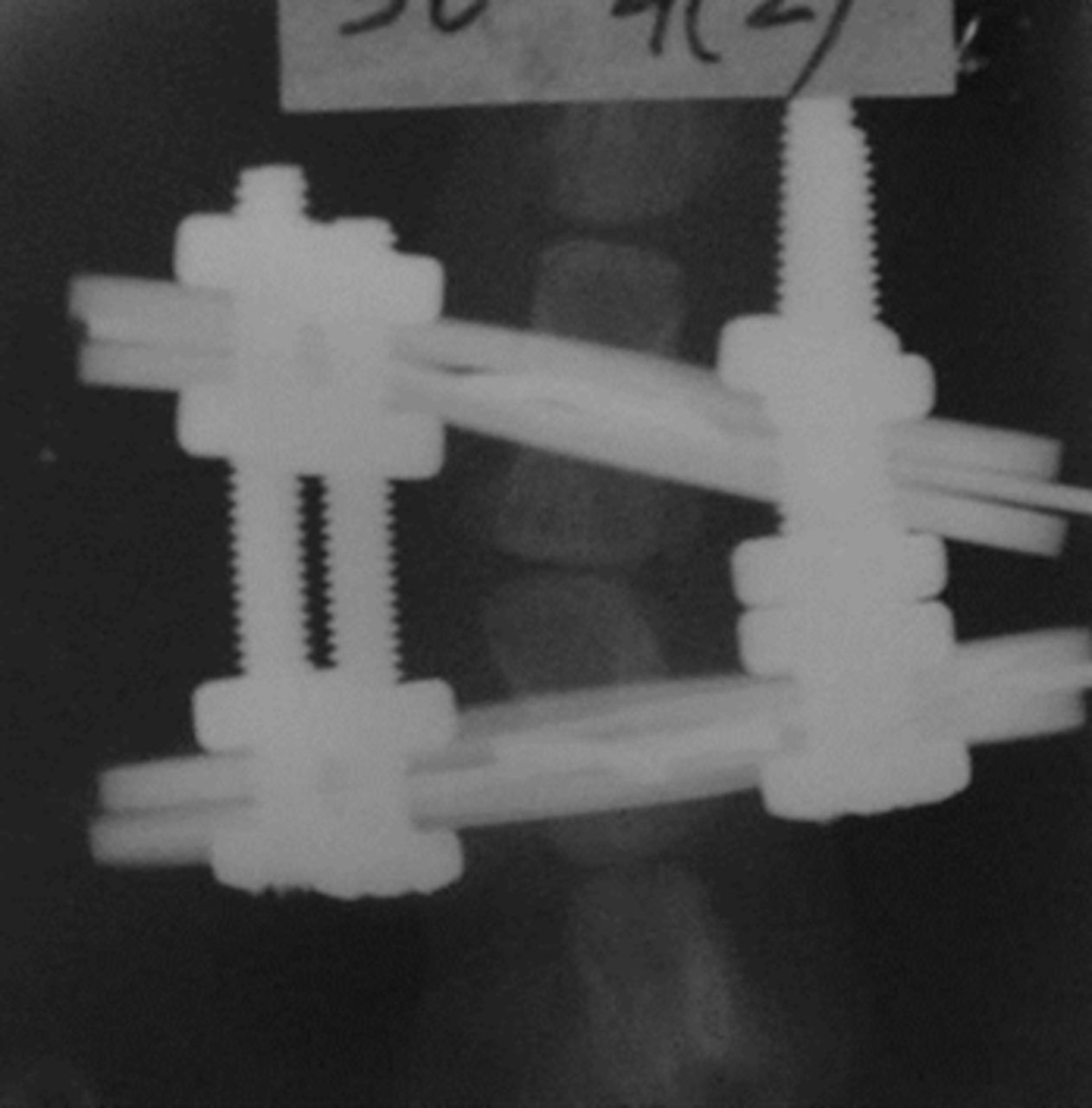
Figs. 1a - 1c
Radiographs of asymmetrically loaded rat tail intervertebral discs with a) 10°, b) 30° and c) 50° of deformity.
Cartilage homogenisation and western blot analysis
We evaluated expression of MMP12 in specimens obtained from 15 rat tails, five from each group, which were immediately washed with normal saline under sterile conditions and stored at -80°C until used. The discs and the growth plate, which correspond to the lower third of each vertebra, were homogenised with liquid nitrogen using a special bone and cartilage homogeniser (BioPulverizer, Biospec Products, Bartlesville, Oklahoma). Homogenisation was performed at 4°C in a 2 ml extraction buffer, containing 500 mM Tris-HCl, pH 7.6, 200 mM NaCl, 1% Triton X-100, 10 mM CaCl2, PMSF 1 mM and Na3VO4 20 nM. An equal amount of total proteins from each sample (100 μg) was analysed by SDS-PAGE and transferred to Immobilon P membranes. Blocking was performed by incubating the membranes with Tris-buffered saline pH 7.4 supplemented with 0.1% Tween (TBS-T), containing 5% non-fat dry milk for beta-actin or 3% bovine serum albumin for MMP12. Membranes were incubated with primary antibodies for 16 hours at 4°C under continuous agitation, washed three times with TBS-T, and incubated with secondary antibodies for one hour at room temperature. Primary antibodies used were rabbit anti-MMP12 (1:1000) and mouse anti-beta-actin (1:1000) (both from Santa Cruz Biotechnology Inc. Heidelberg, Germany). Detection of immunoreactive bands was performed using the enhanced chemiluminescence detection kit (Pierce Biotechnology, Rockford, Illinois). The protein levels that corresponded to the immunoreactive bands were quantified using the ImagePC image analysis software (Scion Corp., Frederick, Maryland) and the ratio MMP-2/beta-actin was calculated for each sample.
Casein zymography
The discs and the growth plate, which correspond to the lower third of each vertebra, were homogenised as described above and 50 μg of total proteins were analysed by 10% SDS-PAGE containing 1% casein zymography. After electrophoresis, gels were incubated in 2.5% Triton X-100 (5×20 minutes) at room temperature and then for 48 hours at 37°C in developing buffer (50 mM Tris-HCI pH 7.5, 200 mM NaCl and 10 mM CaCl2). After incubation, the gels were stained with Coomassie Brilliant Blue R for 45 minutes at room temperature, de-stained in methanol-acetic acid-water and photographed using a digital camera.
Immunohistochemical analysis
The 30 specimens for immunohistochemical analysis, ten from each group, were surgically prepared and after skin removal were fixed in 10% buffer formalin for 24 to 36 hours, decalcified in neutral ethylenediaminetetraacetic acid (EDTA) for six to eight weeks in room temperature, and embedded in paraffin blocks. Histological sections 3 µm thick were deparaffinised in xylene and degraded alcohols and immersed in distilled water. Blockage of endogenous peroxidase was achieved with 3% H2O2 for 30 minutes in a dark chamber at room temperature. The sections were then washed once in distilled water and three times with TBS, incubated for one hour at room temperature with anti-MMP12 (Santa Cruz sc-30072), diluted 1:50 in antibody diluent (DAKO REAL S2022), incubated for 45 minutes at room temperature with peroxidase-labeled anti-mouse/rabbit IgG (En-vision Kit, DAKO Detection System, Peroxidase/DAB+, Rabbit/Mouse K5007, Agilent Technologies Dako, Glostrup, Denmark), washed three times with TBS, and stained for ten minutes in a dark chamber at room temperature with 3-amino-9-ethylcarbazole/H2O2, washed in distilled water and counterstained with haematoxylin.
Each specimen was scored semi-quantitatively for immunopositivity by determining the proportion of disc cells that were positive for MMP12. In both the convex and concave side, the clearly positive disc cells were counted in ten random high power fields. Each microscope field was scored as 0, 1, 2 and 3, when the number of positive disc cells was 0, 1 to 10, 11 to 20 and > 20, respectively.
Statistical analysis
This was performed with the use of an unpaired t-test. Values (mean value ± standard deviation (sd) or standard error (sem)) were compared between each group and the control group.
Results
Western blot analysis and casein zymography
Pro-MMP12 was detected at 54 kDa and its total expression normalised for beta-actin was not significantly affected as the deformity progressed compared with the control group. The increase was found to be significant in group III (p < 0.01) with severe angulation of 50o (Fig. 2).
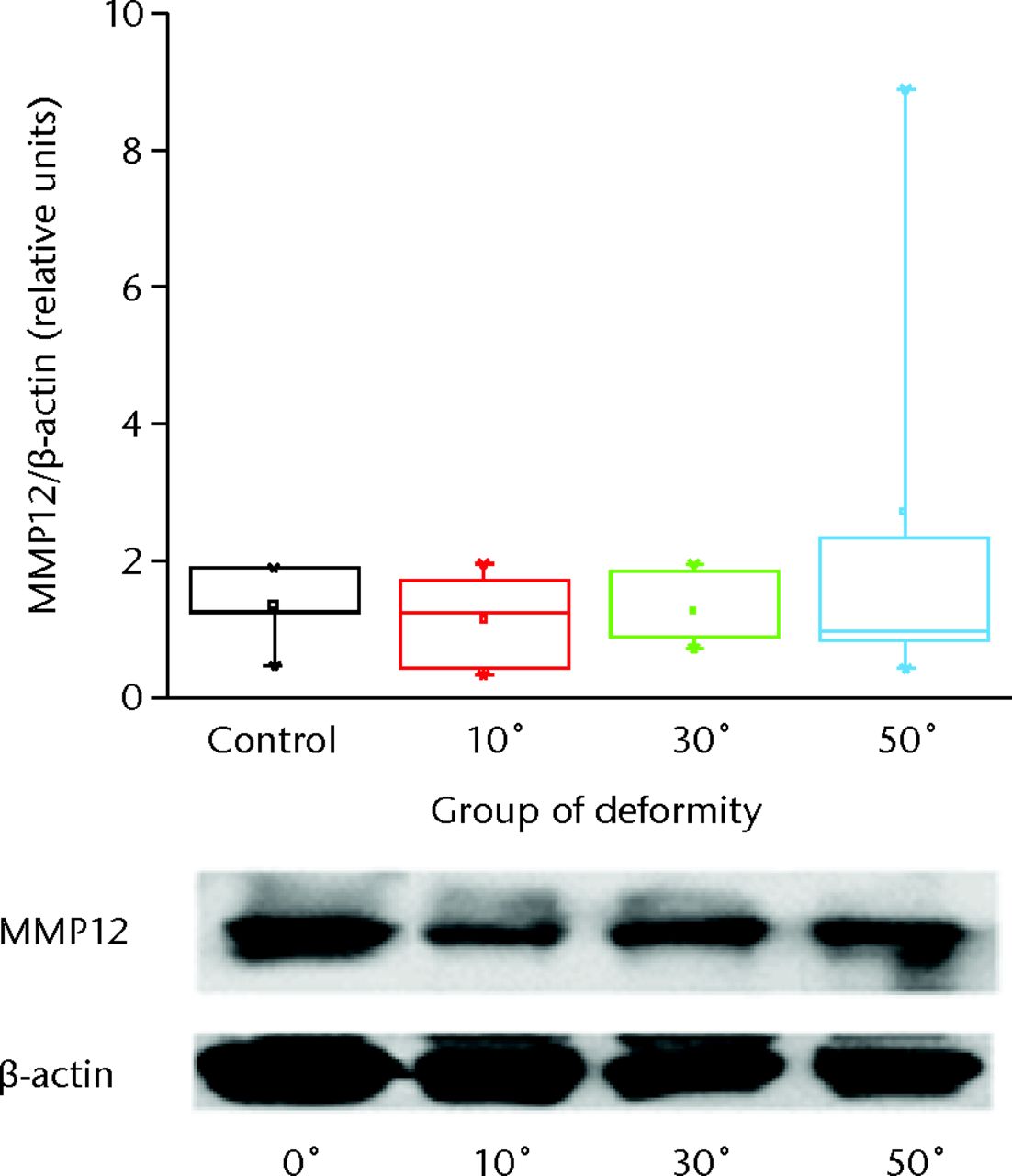
Fig. 2
Representative picture of Western blot analysis for macrophage elastase (MMP12) and beta-actin from the growth plate and intervertebral disc of the animal model tails. The immunoreactive bands were quantified by densitometric analysis and the ratio MMP12/beta-actin was calculated for each lane. Results are shown as the mean ±semcompared with the controls.
Similarly to pro-MMP12 expression, levels of active MMP12 detected by zymography at 22 kDa and normalised for beta-actin, were increased in group III (p < 0.01) (Fig. 3) (Fig. 3).
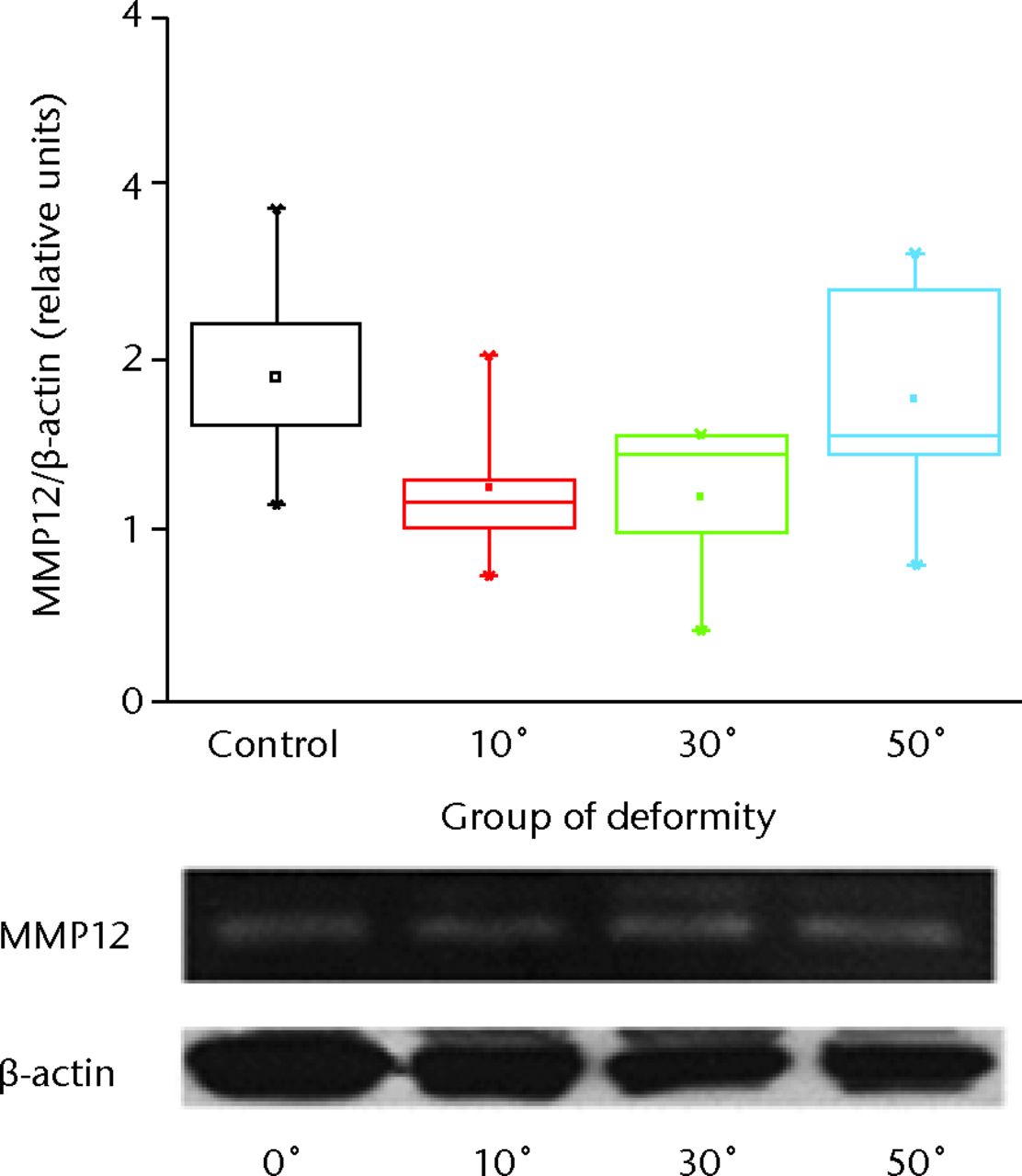
Fig. 3
Representative picture of zymography analysis for MMP12. The active MMP12 bands were quantified by densitometric analysis. Results are the mean ±sem of the ratio-active MMP12/beta-actin compared with the controls.
Immunohistochemistry
MMP12 was detected in disc cells of annulus fibrosus of all the examined discs with various degrees of deformity and was not detected in the nucleus pulposus.
In the control group, MMP12 was expressed by the chondrocytes in the hypertrophic zone of the growth plate (Fig. 4) but was not detected in the intervertebral disc.
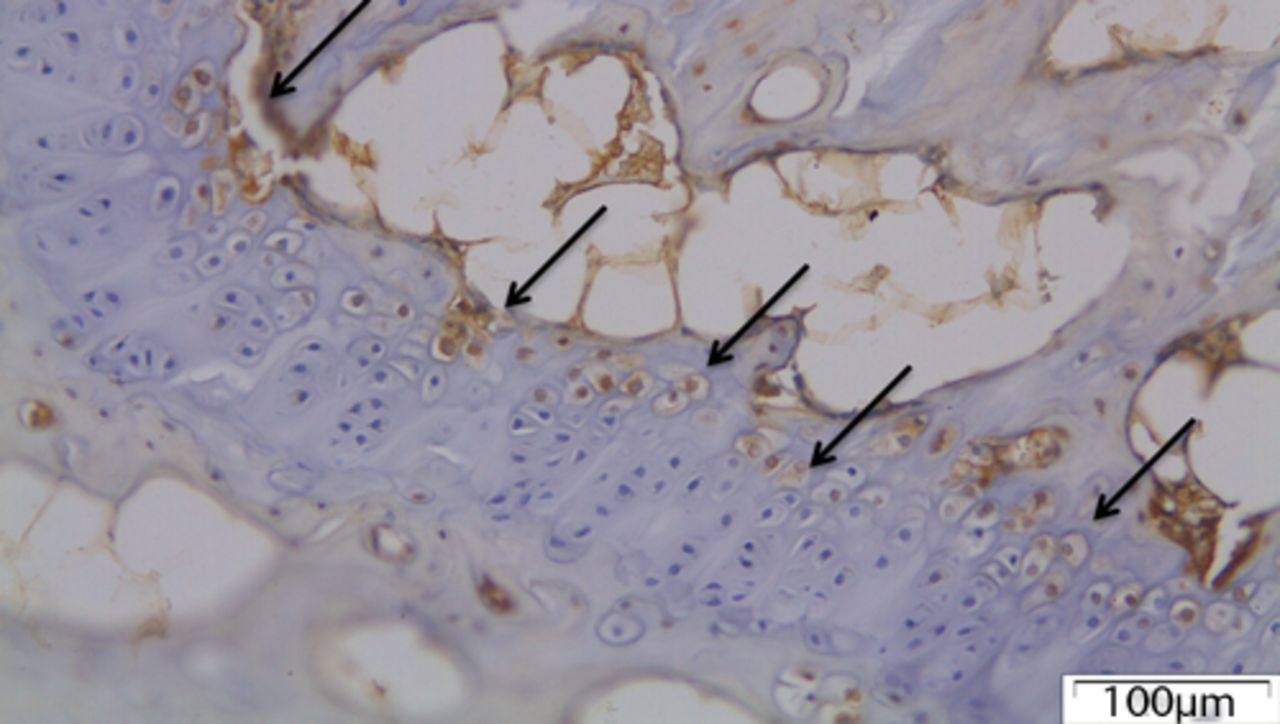
Fig. 4
The growth plate of a control disc with chondrocytes expressing MMP12 (arrows).
In group I (mild deformity of 10o) MMP12 staining was slightly increased at the concave side of the disc. A chondral metaplasia at the periphery of annulus fibrosus was noted mainly at the concave side. Chondrocytes did not stain for MMP12. Approximately 20% of fibroblasts were positively stained for MMP12 (Fig. 5) MMP12 staining was slightly increased at the concave side of the disc. A chondral metaplasia at the periphery of annulus fibrosus was noted mainly at the concave side. Chondrocytes did not stain for MMP12. Approximately 20% of fibroblasts were positively stained for MMP12 (Fig. 5). On the contrary, MMP12 expression was decreased in the chondrocytes of the hypertrophic zone compared with the control group.
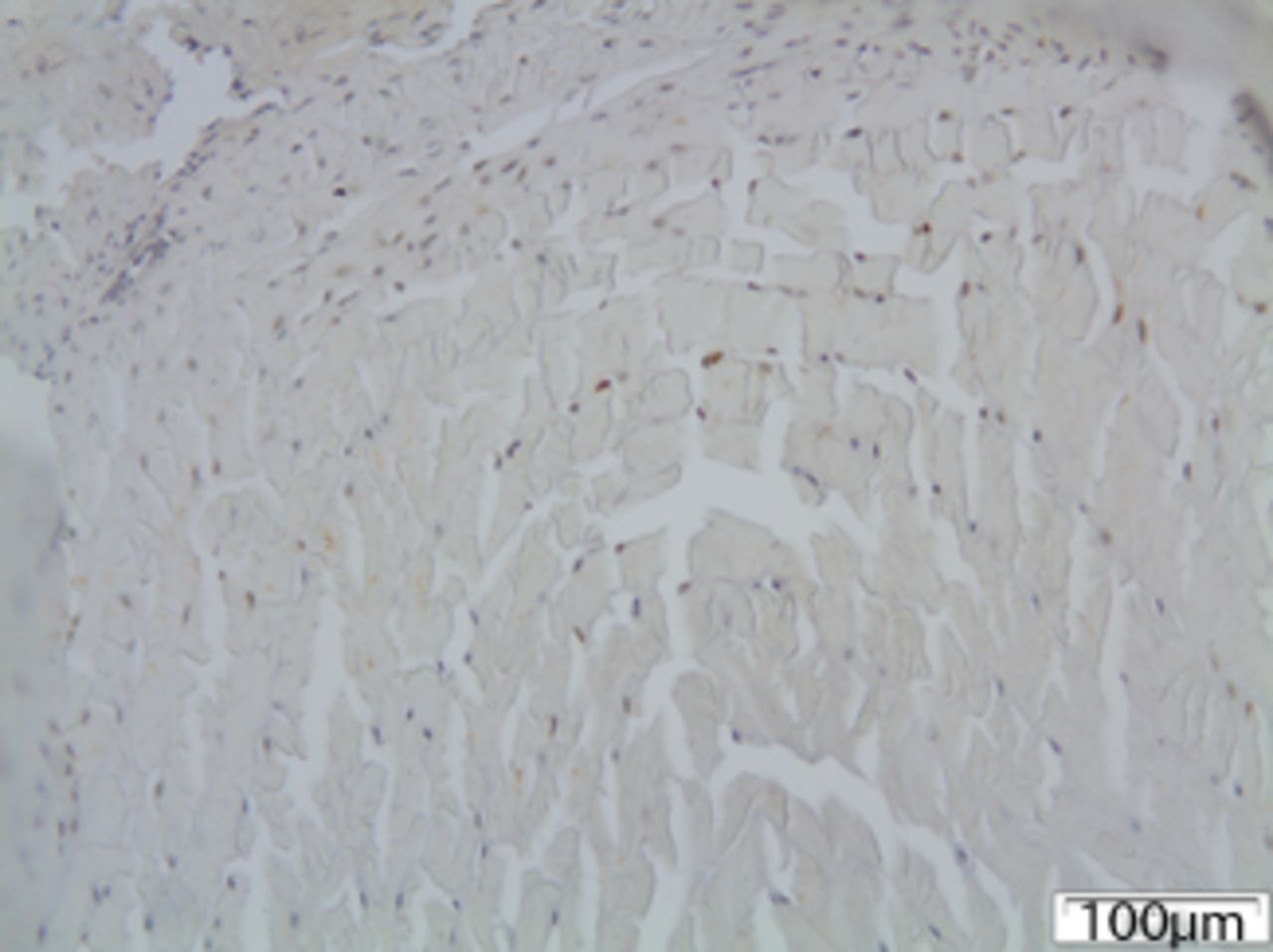
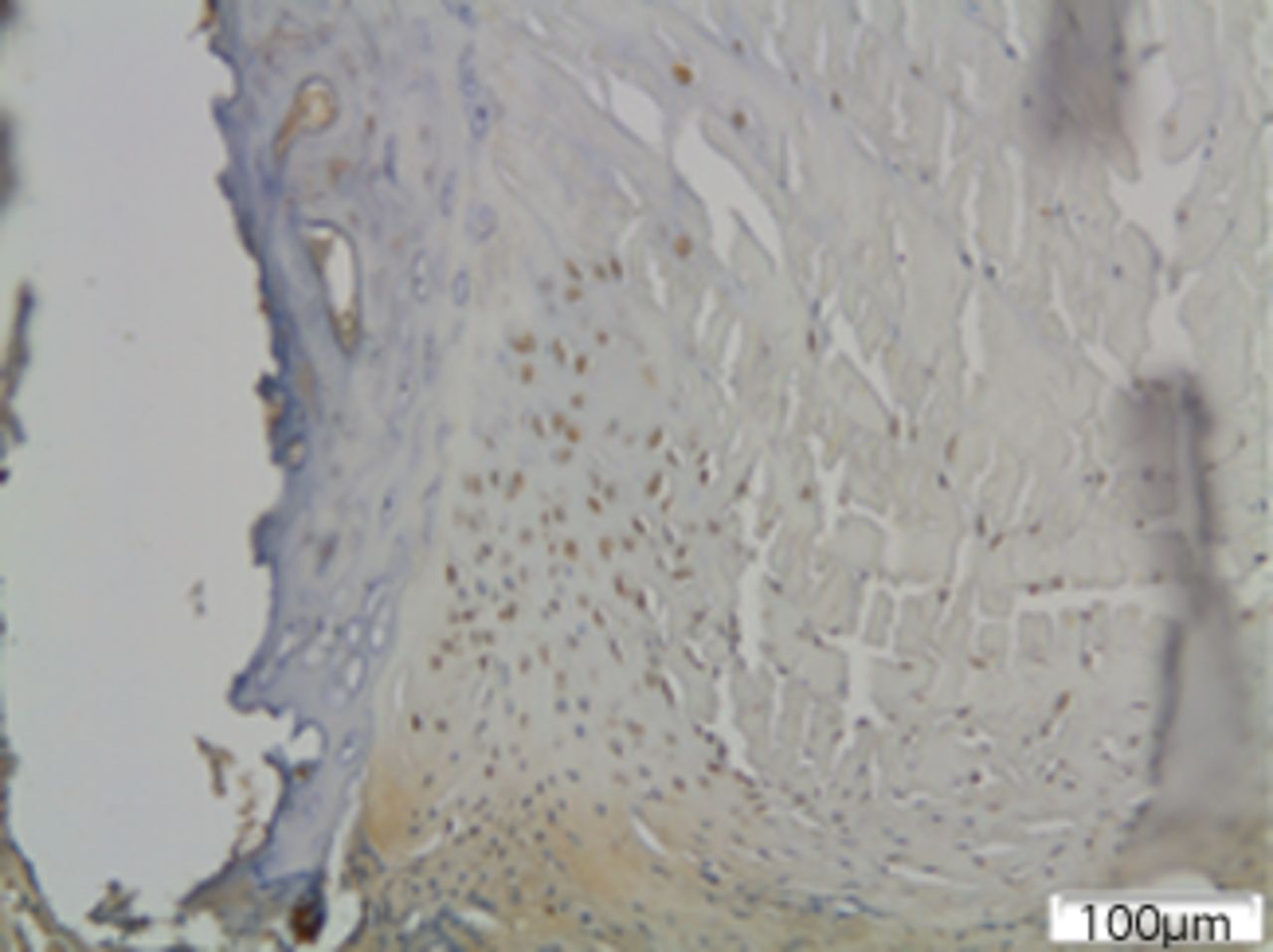
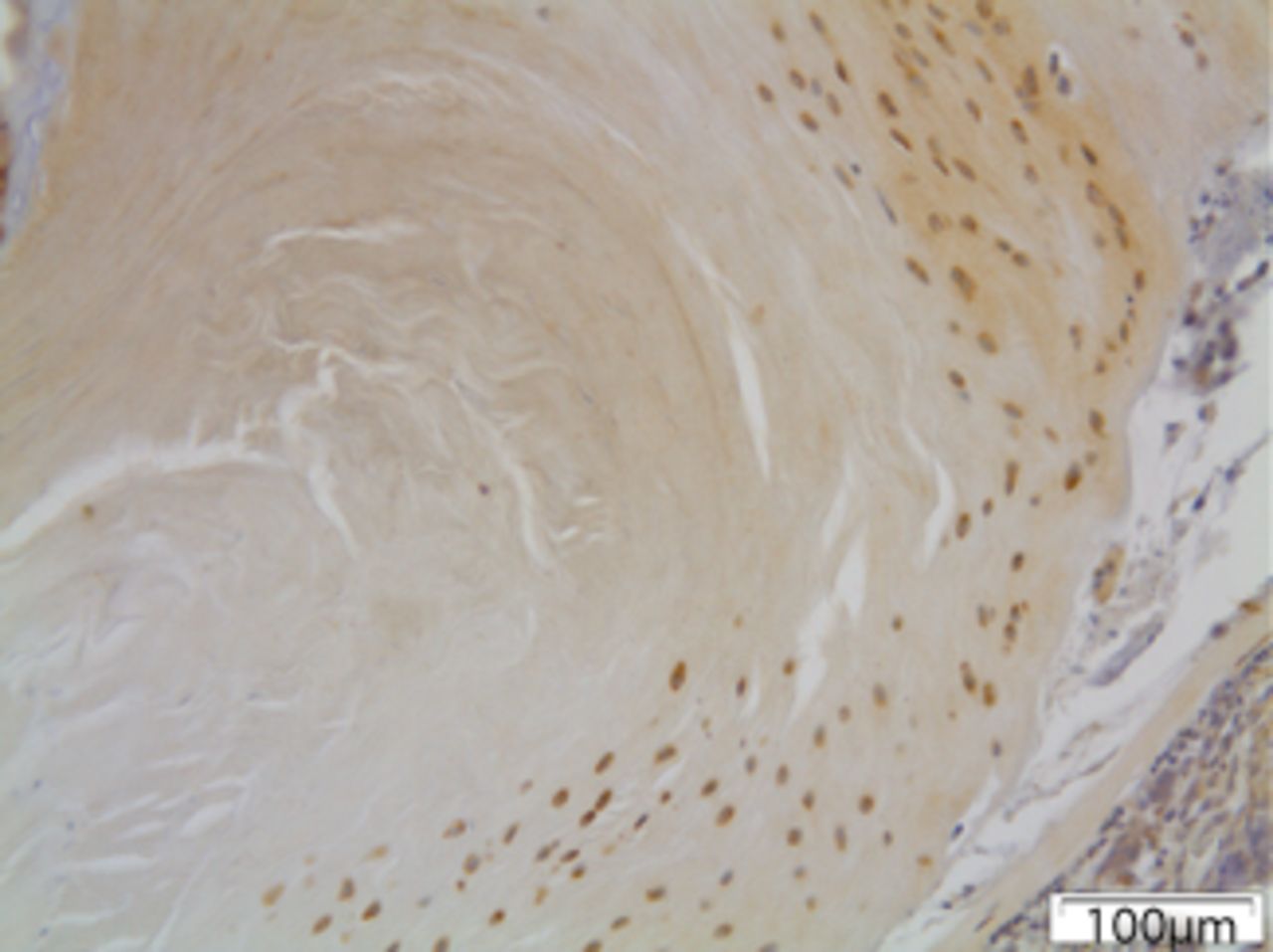
Figs. 5a - 5c
Immunohistochemical images of a) the periphery of annulus fibrosus in group I with a small number of fibroblasts expressing MMP12; b) the periphery of annulus fibrosus in group II, where the number of disc cells expressing MMP12 is increased and includes both fibroblasts and chondrocytes and c) the periphery of annulus fibrosus in group III, where disc cells are absent from the periphery of the annulus fibrosus and most of the remaining cells from central area are expressing MMP12.
In group II (moderate deformity of 30o) MMP12 quantity was increased and the number of positive stained MMP12 cells was more prominent at the concave side of the disc. In this group, there were roughly 30% of chondrocytes and 50% of fibroblasts positive for MMP12. Immunopositivity was higher at the periphery of the annulus fibrosus. A large number of positively stained fibroblasts were noted at the margin of the intervertebral disc adjacent to the vertebral end plates. At the periphery of the disc, clusters of disc cells that were differentiated into chondrocytes and expressing MMP12 were observed (Fig. 5) MMP12 quantity was increased and the number of positive stained MMP12 cells was more prominent at the concave side of the disc. In this group, there were roughly 30% of chondrocytes and 50% of fibroblasts positive for MMP12. Immunopositivity was higher at the periphery of the annulus fibrosus. A large number of positively stained fibroblasts were noted at the margin of the intervertebral disc adjacent to the vertebral end plates. At the periphery of the disc, clusters of disc cells that were differentiated into chondrocytes and expressing MMP12 were observed (Fig. 5). MMP12 was not detected in the growth plate.
In group III (severe deformity of 50o) the proportion of positively stained fibroblasts was nearly 90%, and 50% of chondrocytes were positive for MMP12, although there was a significant reduction in cell population and a severe disorganisation of matrix microstructure, predominantly at the concave side of the disc. No disc cells could be viewed at the periphery of the disc (Fig. 5) the proportion of positively stained fibroblasts was nearly 90%, and 50% of chondrocytes were positive for MMP12, although there was a significant reduction in cell population and a severe disorganisation of matrix microstructure, predominantly at the concave side of the disc. No disc cells could be viewed at the periphery of the disc (Fig. 5).
MMP12 expression in the intervertebral disc rose significantly with the severity of deformity (p < 0.001). When analysing the difference between the convex and concave side, it was not statistically significant in group I (p = 0.36), but was found to be statistically significant in group II (p < 0.01) and in group III (p < 0.01), with cells in the concave side predominately expressing MMP12 (Fig. 6). When analysing the difference between the convex and concave side, it was not statistically significant in group I (p = 0.36), but was found to be statistically significant in group II (p < 0.01) and in group III (p < 0.01), with cells in the concave side predominately expressing MMP12 (Fig. 6), but was found to be statistically significant in group II (p < 0.01) and in group III (p < 0.01), with cells in the concave side predominately expressing MMP12 (Fig. 6) and in group III (p < 0.01), with cells in the concave side predominately expressing MMP12 (Fig. 6), with cells in the concave side predominately expressing MMP12 (Fig. 6). In contrast, the expression of MMP12 in the hypertrophic zone of the growth plate was decreased in group I, and eliminated in group II and III, compared with the control group.
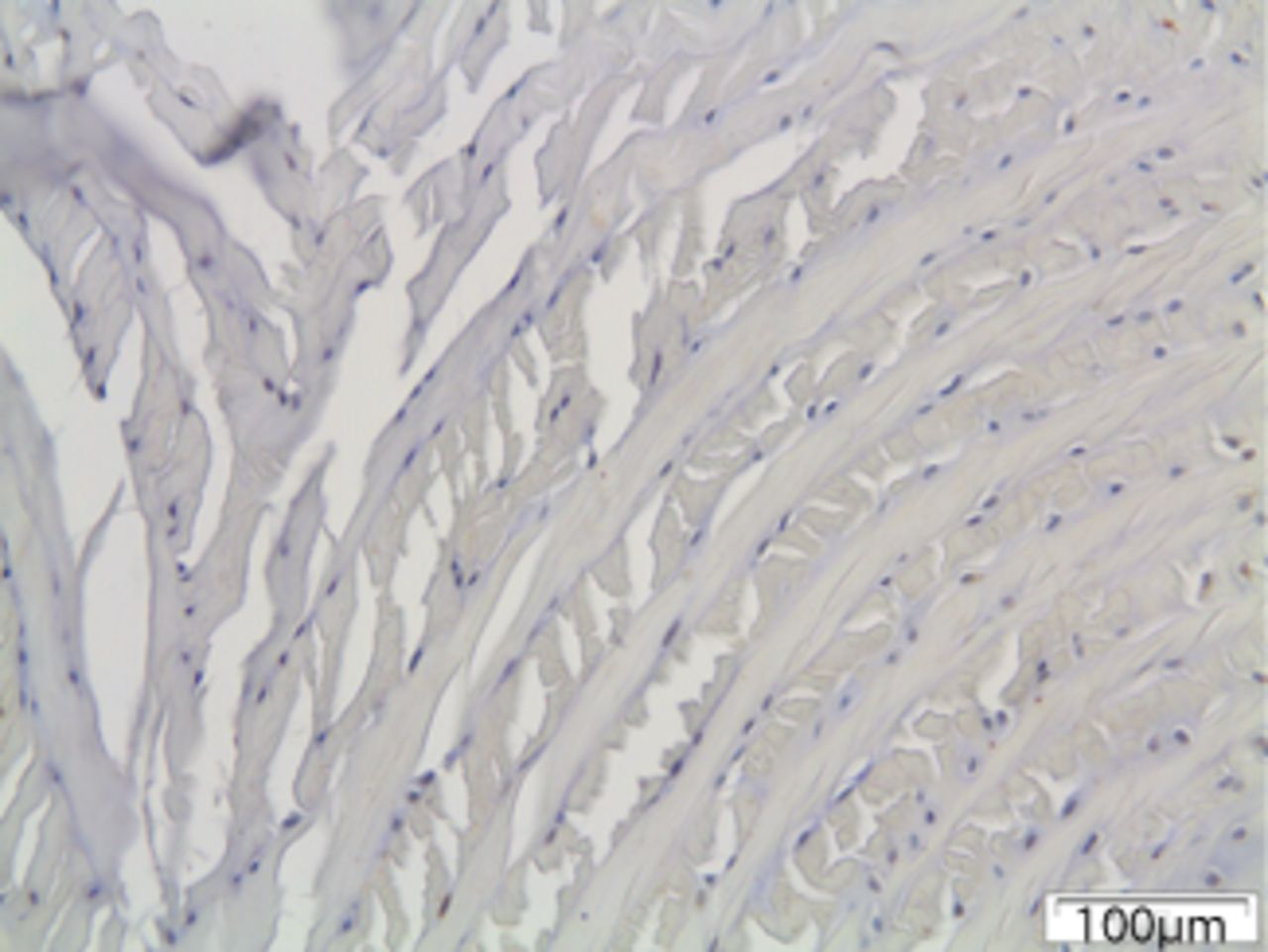
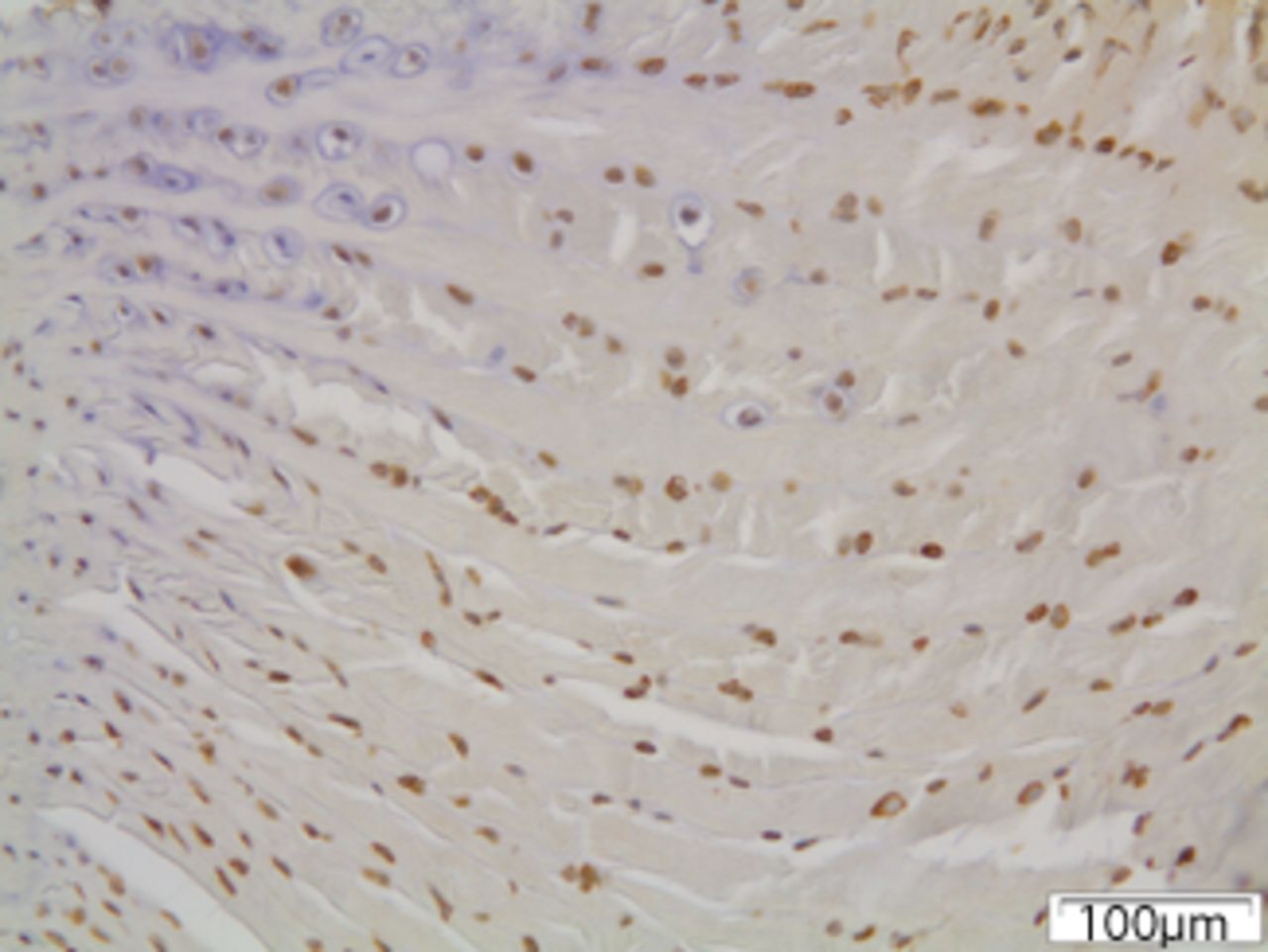
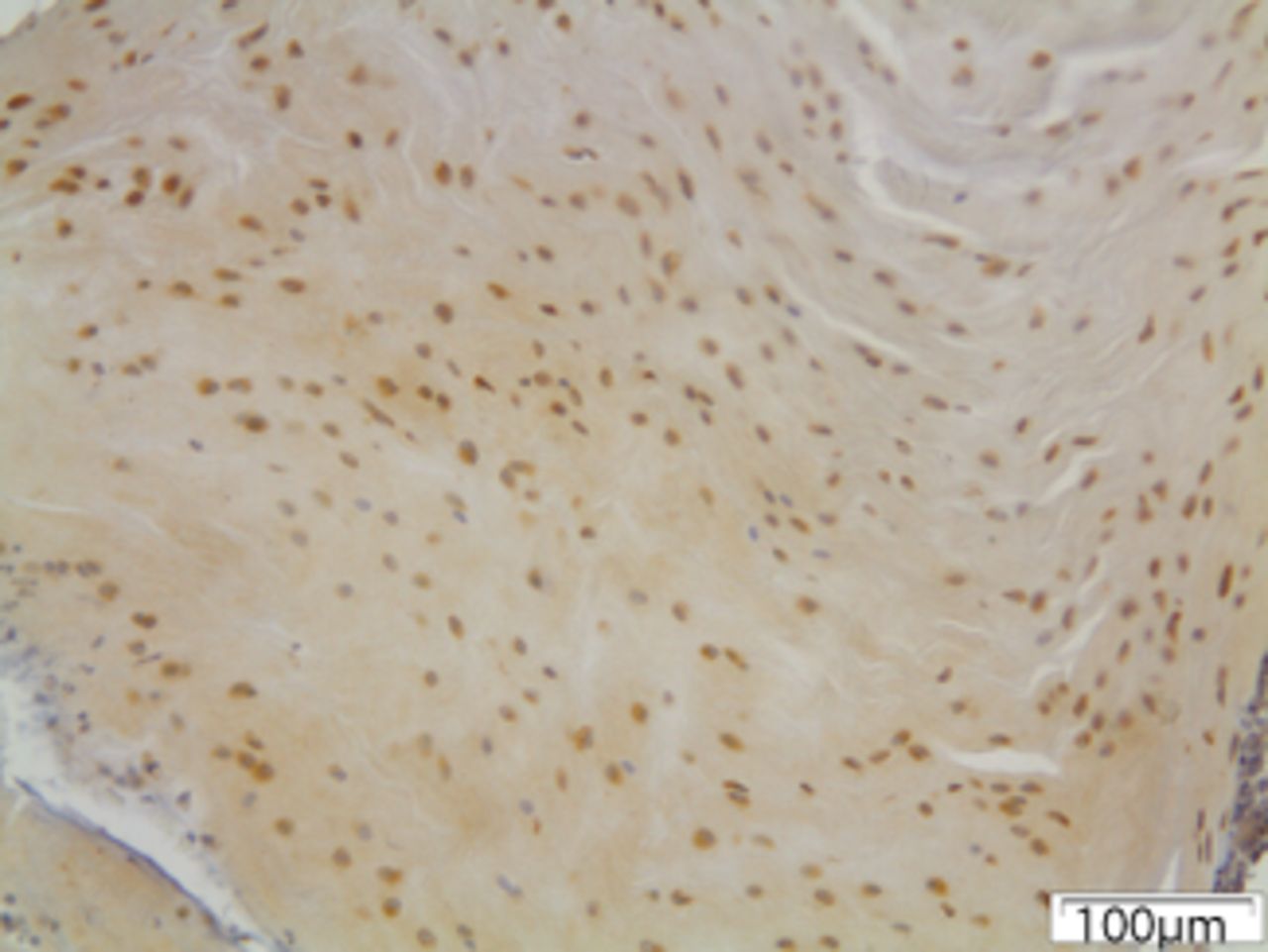
Figs. 6a - 6c
Immunohistochemical images of the annulus fibrosus of the control group with a) absence of expression of MMP12, b) the convex side of a disc specimen from group III, where a large number of disc cells, both fibroblasts and chondrocytes are expressing MMP12: note the disorganisation of microstructure of the lamellae of annulus fibrosus, and c) the concave side of the same disc specimen as in Figure 6b, where all disc cells are expressing MMP12. A reduction of cell population and a complete disorganisation of microstructure are evident at the concave side.
Discussion
In this study we demonstrate that MMP12 is expressed in the intervertebral disc and in the growth plate chondrocytes and its expression depends on the magnitude of the mechanical loading. MMP12, which was originally found in alveolar macrophages of cigarette smokers,14 is associated with many diseases, such as intestinal ulcerations, cutaneous diseases, breast cancer, skin cancer, emphysema, atherosclerosis, aneurysms15 and rheumatoid arthritis. To the best of our knowledge, this is the first study that examines the role of MMP12 in a vertebrae growth plate and in the intervertebral disc degeneration.
Several in vitro and in vivo studies have demonstrated the distribution of MMP2, MMP9, MMP13 and MMP14 in the human or animal growth plate, implying an important regulatory role during normal growth.6 Moreover, it has been shown that MMP13 may have a critical role in growth plate angiogenesis and MMP9 in chondrocyte apoptosis and mineralisation of the matrix.16
Mechanical loading induces changes, not only in the orientation and the morphology of the hypertrophic chondrocytes, but at the molecular level as well, which are dependent on the duration and intensity of loading.6 MMP-expression is increased when a physiological load is applied. However, long-term excessive mechanical loading leads to reductive expression of MMPs in the chondrocytes of the hypertrophic zone.17 This is in line with our study which shows significant expression of MMP12 in the hypertrophic zone of the growth plate in the control group, but a reduction of MMP12 expression in 10° of asymmetrical loading and a complete elimination of its expression in 30° and 50°, respectively. These findings indicate that excessive asymmetrical loading may have a harmful effect on the growth plate.
On the other hand, over-expression of MMPs was evidenced in human disc degeneration.18 Several MMPs are upregulated in disc degeneration. MMP1 is over-expressed in degenerated, especially herniated, discs.19 A number of MMPs, such as MMP1, 2, 3, 7, 9 and 13 were found to be increased in rat tail discs that sustained static compression, while asymmetric loading caused a significant difference of MMP1 gene expression between the convex and concave side of the wedged discs.20 In a recent study, MMP12 was detected in human intervertebral disc and additionally in chondrocytes of the vertebral endplate of an older sand rat’s degenerating disc.21 Our study shows that there is a positive correlation between the degree of the deformity and the amount of disc cells that express MMP12. Mechanical deformation after application of asymmetric forces over the intervertebral disc stimulates MMP12 production, mainly in the outer annulus fibrosus. A similar pattern was found in a previous study for MMP1.22 Interestingly, no MMP12 expression was found in the nucleus pulposus, a finding which is in agreement with Cui et al,23 who identified MMP12 as a possible marker to distinguish bovine nucleus pulposus cells from articular chondrocytes. The concave side of the disc was found to sustain increased MMP12 expression in more severe deformations (groups II and III). Asymmetric loading progressively produced changes in cell phenotype and reduction of cell number, together with disorganisation of matrix microstructure similar to disc degeneration. Disc-cell population was found to be affected by the degree of deformation, with the population of the cells decreasing as the deformity progresses. Additionally, at the margins of intervertebral disc adjacent to the vertebral end plates, a reduction of fibroblast-like cells and an increased number of chondrocytes positive in MMP12 expression was observed. Whether this observation could be explained by chondrocyte migration at the area or by differentiation of fibroblasts to chondrocytes, or even by fibroblast cell death, it cannot be answered by the present study. Court et al24 reported similar changes in cell phenotype in the inner annulus fibrosus of the concave side in bent mouse tails.
The structure and composition of the extracellular matrix is responsible for the mechanical properties of the intervertebral disc.25 The main components are the collagen fibres and proteoglycans. Additionally, elastin is the main structural component of elastic fibres which form a network within the annulus fibrosus of the intervertebral disc. They are strongly associated with collagen fibres, a finding which implies a close functional relationship.25 They have an indirect mechanical role in the alignment of collagen fibres in stress.26 A potential absence of elastin from matrix may result in a progressive disorganisation after asymmetric loading and eventually cause disc degeneration, thus resulting in changes in its mechanical properties.26 A similar disorganisation was observed in asymmetric-loaded discs from patients suffering from scoliosis.25 Considering the significant contribution of elastin in disc biomechanics, elastin degradation should be a key factor in understanding the multifactorial aetiology of disc degeneration.26
MMP12 gene detection and quantification was not performed with a real-time polymerase chain reaction (RT-PCR), which is a limitation of the present study. A future study could emphasise RT-PCR, providing us with additional and more detailed information about the expression of MMP12 following the application of asymmetric forces on the intervertebral disc.
Conclusion
MMP12 is expressed in the hypertrophic zone of the physiologic vertebra growth plate. Asymmetrical mechanical loading inhibits the expression of MMP12 in the growth plate chondrocytes. On the contrary, the expression of MMP12 in rat tail intervertebral discs increases after application of asymmetric loading and is correlated with the degree of the deformity and the side of the wedged disc. Future research could investigate any potential benefit after inhibition of MMP12 in patients with disc degeneration.
1 Iatridis JC , MentePL, StokesIAF, AronsonDD, AliniM. Compression-Induced changes in intervertebral disc properties in a rat tail model. Spine1999;24:996–1002.CrossrefPubMed Google Scholar
2 Stokes IA , IatridisJC. Mechanical conditions that accelerate intervertebral disc degeneration: Overload versus Immobilization. Spine (Phila PA 1976)2004;29:2724–2732.CrossrefPubMed Google Scholar
3 Brinckerhoff CE , MatrisianLM. Matrix metalloproteinases: A tail of a frog that became a prince. Nat Rev Mol Cell Biol2002;3:207–214.CrossrefPubMed Google Scholar
4 Weiler C , NerlichAG, ZippererJ, BachmeierBE, BoosN. SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J2002;11:308–320. Google Scholar
5 Ortega N , BehonickD, StickensD, WerbZ. How proteinases regulate bone morphogenesis. Ann N Y Acad Sci2003;995:109–116. Google Scholar
6 Pichler K , HerbertV, SchmidtB, et al.Expression of matrix metalloproteinases in human growth plate chondrocytes is enhanced at high levels of mechanical loading: A possible explanation for overuse injuries in children. Bone Joint J2013;95-B:568–573.CrossrefPubMed Google Scholar
7 Raza SL , NehringLC, ShapiroSD, CorneliusLA. Proteinase-activated receptor-1 regulation of macrophage elastase (MMP-12) secretion by serine proteinases. J Biol Chem2000;275:41243–41250.CrossrefPubMed Google Scholar
8 Lyu J , JooCK. Wnt-7a up-regulates matrix metalloproteinase-12 expression and promotes cell proliferation in corneal epithelial cells during wound healing. J Biol Chem2005;280:21653–21660.CrossrefPubMed Google Scholar
9 Gronski TJ Jr , MartinRL, KobayashiDK, et al.Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J Biol Chem1997;272:12189–12194.CrossrefPubMed Google Scholar
10 Oh H , YangS, ParkM, ChunJS. Matrix metalloproteinase (MMP)-12 regulates MMP-9 expression in interleukin-1beta-treated articular chondrocytes. J Cell Biochem2008;105:1443–1450.CrossrefPubMed Google Scholar
11 Grivas TB , VasiliadisES, KaspirisA, KhaldiL, KletsasD. Expression of matrix metalloproteinase-1 (MMP-1) in Wistar rat's intervertebral disc after experimentally induced scoliotic deformity. Scoliosis2011;6:9.CrossrefPubMed Google Scholar
12 Mente PL , StokesIA, SpenceH, AronssonDD. Progression of vertebral wedging in an asymmetrically loaded rat tail model. Spine1997;22:1292–1296.CrossrefPubMed Google Scholar
13 Hughes PC , TannerJM. The assessment of skeletal maturity in the growing rat. J Anat1970;106:371–402.PubMed Google Scholar
14 Shapiro S , KobayashiD, LeyTJ. Cloning and characterization of a unique elastolytic metalloproteinase produced by human alveolar macrophages. J Biol Chem1993;268:23824–23829.PubMed Google Scholar
15 Morris DR , BirosE, CroninO, KuivaniemiH, GolledgeJ. The association of genetic variants of matrix metalloproteinases with abdominal aortic aneurysm: a systematic review and meta-analysis. Heart2014;100:295–302.CrossrefPubMed Google Scholar
16 Vu TH , ShipleyJM, BergersG, et al.MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell1998;93:411–422.CrossrefPubMed Google Scholar
17 Monfort J , Garcia-GiraltN, López-ArmadaMJ, et al.Decreased metalloproteinase production as a response to mechanical pressure in human cartilage: a mechanism for homeostatic regulation. Arthritis Res Ther2006;8:R149.CrossrefPubMed Google Scholar
18 Vo NV , HartmanRA, YurubeT, et al.Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J2013;13:331–341.CrossrefPubMed Google Scholar
19 Salo J , MackiewiczZ, IndahlA, et al.Plasmin-matrix metalloproteinase cascades in spinal response to an experimental disc lesion in pig. Spine2008;33:839–844.CrossrefPubMed Google Scholar
20 Yurube T , TakadaT, SuzukiT, et al.Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration. Arthritis Res Ther2012;14:R51.CrossrefPubMed Google Scholar
21 Gruber HE , IngramJA, CoxMD, HanleyEN Jr. Matrix metalloproteinase-12 immunolocalization in the degenerating human intervertebral disc and sand rat spine: Biologic implications. Exp Mol Pathol.2014;97:1–5.CrossrefPubMed Google Scholar
22 Walter BA , KoreckiCL, PurmessurD, et al.Complex Loading Affects Intervertebral Disc Mechanics and Biology. Osteoarthritis Cartilage2011;19:1011–1018.CrossrefPubMed Google Scholar
23 Cui Y , YuJ, UrbanJP, YoungDA. Differential gene expression profiling of metalloproteinases and their inhibitors. A comparison between bovine intervertebral disc nucleus pulposus cells and articular chondrocytes. Spine2010;35:1101–1108. Google Scholar
24 Court C , ChinJR, LiebenbergE, ColliouOK, LotzJC. Biological and mechanical consequences of transient intervertebral disc bending. Eur Spine J2007;16:1899–1906.CrossrefPubMed Google Scholar
25 Yu J , TirlapurU, FairbankJ, et al.Microfibrils, elastin fibres and collagen fibres in the human intervertebral disc and bovine tail disc. J Anat2007;210:460–471.CrossrefPubMed Google Scholar
26 Smith LJ , FazzalariNL. The elastic fibre network of the human lumbar anulus fibrosus: architecture, mechanical function and potential role in the progression of intervertebral disc degeneration. Eur Spine J2009;18:439–448.CrossrefPubMed Google Scholar
Funding statement:
None declared
Author contributions:
E. S. Vasiliadis: Study design, Data collection, Data analysis, Performed surgeries, Writing the paper
A. Kaspiris: Study design, Performed the experiment, Data collection and analysis, Synthesising the data, Drafting the manuscript, Reviewing the manuscript
T. B. Grivas: Study design, Synthesising the data, Reviewing the manuscript
L. Khaldi: Immunohistochemical analysis, Reviewing the manuscript
M. Lamprou: Performed the western blot analysis and the casein zymography assay, Reviewing the manuscript
S. G. Pneumaticos: Study design, Synthesising the data, Reviewing the manuscript
K. Nikolopoulos: Study design, Synthesising the data, Reviewing the manuscript
D. S. Korres: Study design, Synthesising the data, Reviewing the manuscript
E. Papadimitriou: Supervised western blot analysis and the casein zymography assay, Synthesising the data, Drafting the manuscript, Reviewing the manuscript
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.p










