Abstract
Objectives
The objective of this study was to compare the elution characteristics, antimicrobial activity and mechanical properties of antibiotic-loaded bone cement (ALBC) loaded with powdered antibiotic, powdered antibiotic with inert filler (xylitol), or liquid antibiotic, particularly focusing on vancomycin and amphotericin B.
Methods
Cement specimens loaded with 2 g of vancomycin or amphotericin B powder (powder group), 2 g of antibiotic powder and 2 g of xylitol (xylitol group) or 12 ml of antibiotic solution containing 2 g of antibiotic (liquid group) were tested.
Results
Vancomycin elution was enhanced by 234% in the liquid group and by 12% in the xylitol group compared with the powder group. Amphotericin B elution was enhanced by 265% in the liquid group and by 65% in the xylitol group compared with the powder group. Based on the disk-diffusion assay, the eluate samples of vancomycin-loaded ALBC of the liquid group exhibited a significantly larger inhibitory zone than samples of the powder or the xylitol group. Regarding the ALBCs loaded with amphotericin B, only the eluate samples of the liquid group exhibited a clear inhibitory zone, which was not observed in either the xylitol or the powder groups. The ultimate compressive strength was significantly reduced in specimens containing liquid antibiotics.
Conclusions
Adding vancomycin or amphotericin B antibiotic powder in distilled water before mixing with bone cement can significantly improve the efficiency of antibiotic release than can loading ALBC with the same dose of antibiotic powder. This simple and effective method for preparation of ALBCs can significantly improve the efficiency of antibiotic release in ALBCs.
Cite this article: Bone Joint Res 2014;3:246–51.
Article focus
This study aimed to find a simple, effective and ready-to-use method to improve the efficiency of antibiotic release in antibiotic-loaded bone cement (ALBC) for the treatment of clinical orthopaedic infection.
We compared the elution characteristics, antimicrobial activity, and mechanical properties of ALBC loaded with powder antibiotic, powder antibiotic with inert filler (xylitol), or liquid antibiotic, particularly focusing on vancomycin and amphotericin B.
Key messages
Adding vancomycin or amphotericin B antibiotic powder in distilled water before mixing with bone cement can provide a significantly higher efficiency of antibiotic release than that of bone cement loaded with the same dose of antibiotic powder.
This simple, inexpensive and effective method for the preparation of ALBCs can significantly improve the efficiency of antibiotic release in bone cement.
Strengths and limitations
Strengths: This is the first study to compare the efficiency of antibiotic elution of ALBCs with different preparations.
This study can provide a simple and ready-to-use method for orthopaedic surgeons to prepare ALBC for treatment of infection in orthopaedics.
Limitation: this is an in vitro study, which does not necessarily reflect actual clinical circumstances.
Introduction
Antibiotic-loaded polymethylmethacrylate (PMMA) bone cements have been widely used in the treatment of osteomyelitis. The ability to deliver high local concentrations of antimicrobial agents has made antibiotic-loaded PMMA one of the standards of treatment for patients with chronic infection of the skeletal system, including periprosthetic joint infection (PJI).1
The efficiency of the release of antibiotics from bone cement is a critical factor which determines the antibacterial activity of antibiotic-loaded bone cement (ALBC). High porosity would be expected to increase the release of antibiotics from ALBC. To increase the porosity and thereby enhance the elution of antibiotics, various inert fillers such as glycine and xylitol are added to bone cement.2 These inert filler materials, though effective for augmenting antibiotic release, are not readily available in sterile form. Many powder antibiotics are effectively released from bone cement.3-5 In contrast, liquid antibiotics have not been widely used in bone cement because the mechanical integrity of the cement decreases by up to 50%.6 Liquid gentamicin in bone cement has been reported to elute effectively and maintain its bactericidal activity,7,8 and liquid gentamicin combined with vancomycin in the same cement sample demonstrated a positive reciprocal effect on the elution of both antibiotics.8 The positive effect on the elution of antibiotics with the addition of liquid gentamicin to bone cement is caused by the increased porosity of the cement.8 The addition of a liquid antibiotic can therefore increase the porosity of ALBCs, and it is reasonable to speculate that dissolution of antibiotic powder in distilled water before mixing it with bone cement, may provide more efficient antibiotic release than loading it with the same dose of antibiotic powder.
To our knowledge, no reports have compared the elution characteristics, antimicrobial activity and mechanical properties of ALBCs loaded with powder antibiotics, powder antibiotics with inert filler (xylitol), or liquid antibiotics in an in vitro model. Vancomycin, an antibiotic commonly loaded in ALBC, and amphotericin B, an antibiotic expected to have a poor release profile in ALBC,9 were used in this experiment. We attempted to determine the difference in the profiles of antibiotic release among ALBC with liquid antibiotic, powder antibiotic, or powder antibiotic augmented with xylitol, particularly focusing on vancomycin and amphotericin B, and the effect of liquid antibiotics on the compressive strength of ALBC.
Materials and Methods
Antibiotic-loaded cement specimen
Surgical Simplex bone cement (Stryker Orthopaedics, Limerick, Ireland) with powder (powder group), powder/xylitol (xylitol group) or liquid (liquid group) vancomycin (Gentle Pharmaceutical Co., Yulin, Taiwan) or amphotericin B (China Chemical & Pharmaceutical Co. Ltd, Taichung, Taiwan) was tested.
In the powder group, the antibiotic–cement mixture consisted of 2 g of antibiotic powder mixed with 40 g of bone cement polymer prior to the addition of the monomer component. In the xylitol group, the antibiotic–cement mixture consisted of 2 g of antibiotic powder and 2 g of xylitol (Hiliga International Development Co. Ltd, Taipei, Taiwan) mixed with 40 g of bone cement polymer prior to the addition of the monomer component. In the liquid group, 2 g of antibiotic powder was added in 12 ml of the distilled water and vortex for 30 seconds before mixing with bone cement. Subsequently, the antibiotic liquid was added to 20 mL of monomer and mixed by hand with a metal spatula before the powdered component was added.8 The cement–antibiotic mixture was also mixed by hand in a ceramic container for two minutes until it achieved a doughy consistency, and was then manually pressed into a plastic mold to form uniform cylindrical specimens. The cement cylinders, 20 mm in height and 15 mm in diameter, were cured at room temperature for one hour. A total of six antibiotic preparations were added to bone cement: powder vancomycin, powder amphotericin B, xylitol/powder vancomycin, xylitol/powder amphotericin B, liquid vancomycin, or liquid amphotericin B. Bone cement without antibiotics served as a control.
Antibiotic broth elution assay
Each cement cylinder was immersed in a polypropylene tube with 5 mL of phosphate-buffered saline (PBS, pH 7.3) and shaken in a rotator at 37°C for 48 hours. The cement cylinders were then transferred into a new test tube with PBS once they had been washed three times a day in saline. The cumulative and daily antibiotic release was measured, and 2 mL elution samples of PBS were collected at seven time points over a period of ten days (Day 0, 1, 2, 3, 5, 7, and 10) and subsequently stored at -80°C until further analysis.
The concentration of vancomycin was determined by using high-performance liquid chromatography (HPLC). The method of HPLC analyses was modified from previously published methods.3,10 In brief, the chromatography assay was performed using a model ALC 717 chromatograph (Waters Associates, Milford, Massachusetts) with a stainless steel column (for vancomycin: RP18 column, 100 mm × 4.6 mm, 5 µm particle size). The mobile phase was water/acetonitrile/100 mm ammonium formate (composite ratio: 78/12/10). The HPLC system had a sensitivity of 0.5 µg/mL for vancomycin. The concentration of vancomycin in the cement cylinders was obtained from the HPLC analysis through comparisons made with the prepared daily standard curves, where the peak areas were related with antibiotic concentrations.
The concentration of amphotericin B was measured using visual spectrum spectroscopy absorbance at 415 nm on a BMG Fluo Star Omega (TECAN Infinite M200 PRO, Tocan Group Ltd, Switzerland). Standard curves were generated from prepared samples with known concentrations of amphotericin B. Amphotericin B was solubilised by mixing the eluate samples 50:50 with dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St Louis, Missouri) to disperse the micelles. The visual spectrum spectroscopy system had a sensitivity of 0.1 μg/mL for amphotericin B, the concentration of which was estimated by interpolation from standard curves.
Bioassay of antibiotic activity
The bioactivity of joint fluid was estimated using an agar disk diffusion bioassay with methicillin-resistant Staphylococcus aureus (MRSA) (ATCC 43300) and Candida albicans (ATCC 90028) strains. The technique was based on the inhibitory activity of disks (PDM Diagnostic Discs, AB Biodisk, Sweden) containing 35 µl of test samples and standardised concentrations of vancomycin or amphotericin B. The disks were placed on MRSA- or C. albicans-seeded agar and incubated overnight at 37°C. The diameter of the inhibition zones was measured using a caliper. All samples were tested three times. The growth was visually compared with that of the control (without the antibiotic) and standard samples with different concentrations of antibiotics.
Ultimate compression test
The cement specimens (both before and after antibiotic broth elution) in each group were tested for failure in axial compression by using a material testing system (Bionix 858; MTS Corp, Eden Prairie, Minnesota). A specially designed push rod with a self-alignment function was used as a plunger to ensure full surface contact between the specimen and the push rod. Each specimen was compressed at a displacement rate of 0.1 mm/s. Force, displacement and time were recorded simultaneously for each specimen in increments of 0.05 mm by MTS Test Star software (MTS Corp.). The ultimate compressive force was compared among groups. A cement specimen without antibiotics was used as a control.
Statistical analysis
The antimicrobial concentration of elution samples and the ultimate compressive force of cements with different preparations were tested three times. The results were reported as the mean and standard error of mean (sem). We used an ANOVA to determine the statistical difference in the ultimate compression force and efficiency of antibiotic release between cements with different preparation methods. A p-value of < 0.05 was considered statistically significant.
Results
All tested samples showed burst release during the first day of the broth elution assay, with the release rates rapidly decreasing by the next time point (Fig. 1). The cement samples of the liquid or xylitol group showed a significantly higher efficiency of antibiotic release than that of the powder group in both the bone cement loaded with vancomycin and that with amphotericin B (Fig. 1). During the ten-day study period, vancomycin elution was enhanced by 234% in the liquid group (total amount of vancomycin released: 1212 µg/ml; sem 59) and by 12% in the xylitol group (total amount of vancomycin released: 406 µg/ml; sem 28) compared with the powder group (total amount of vancomycin released: 362 µg/ml; sem 32; p < 0.05). Similar findings were observed for ABLCs loaded with amphotericin B: Amphotericin B elution was enhanced by 265% in the liquid group (total amount of amphotericin B released: 190 µg/ml; sem 19) and by 65% in xylitol group (total amount of amphotericin B released: 86 µg/ml; sem 18) compared with the powder group (total amount of amphotericin B released: 52 µg/ml; sem 10; p < 0.05). Regarding the duration of release of the antibiotic in ALBCs, vancomycin-loaded ALBCs released the antibiotic for up to ten days. Amphotericin B-loaded ALBCs released the antibiotic for five days. The durations of antibiotic release were not significantly different among groups, i.e., those consisting of ALBC loaded with vancomycin or amphotericin B (p > 0.05).
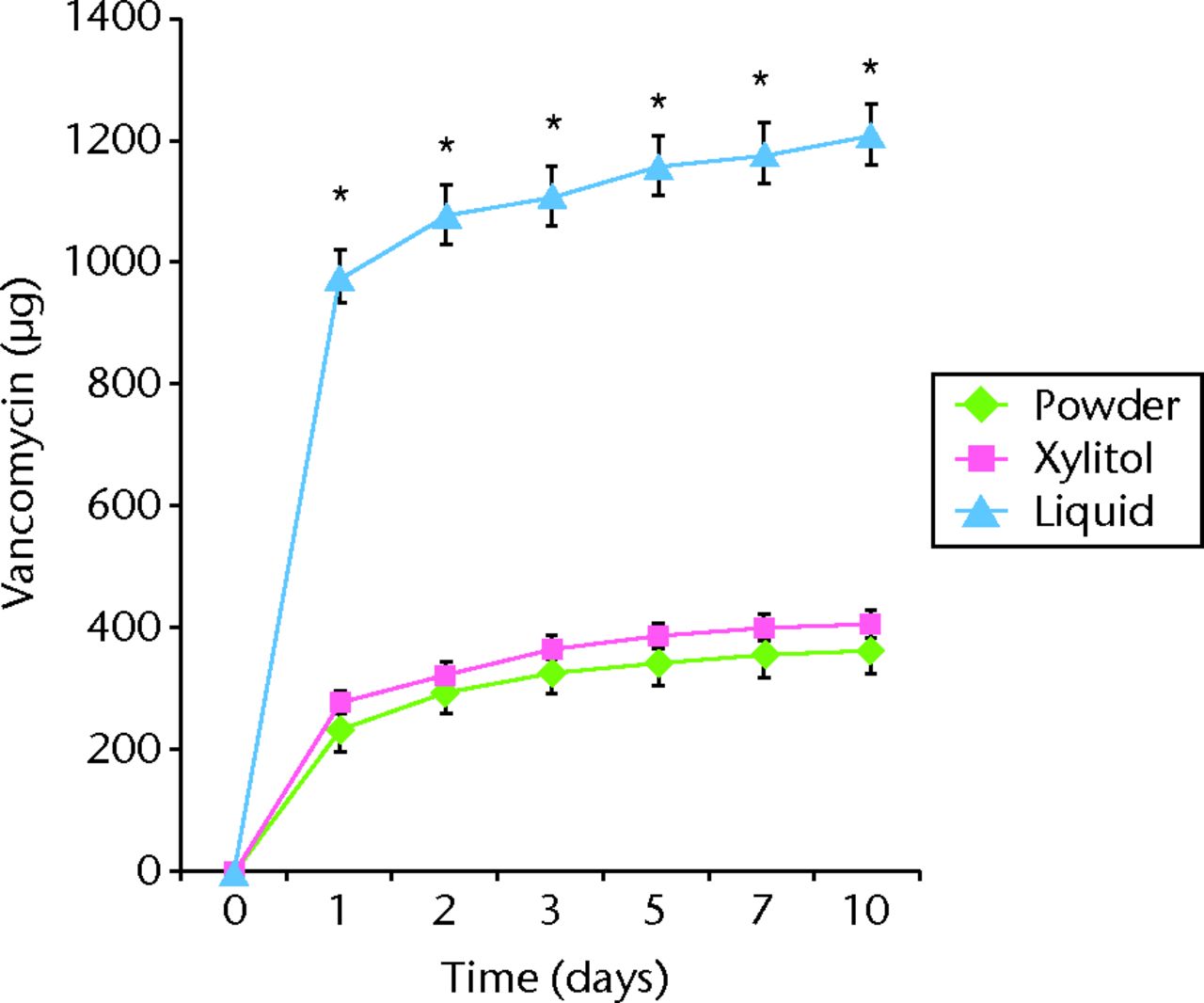
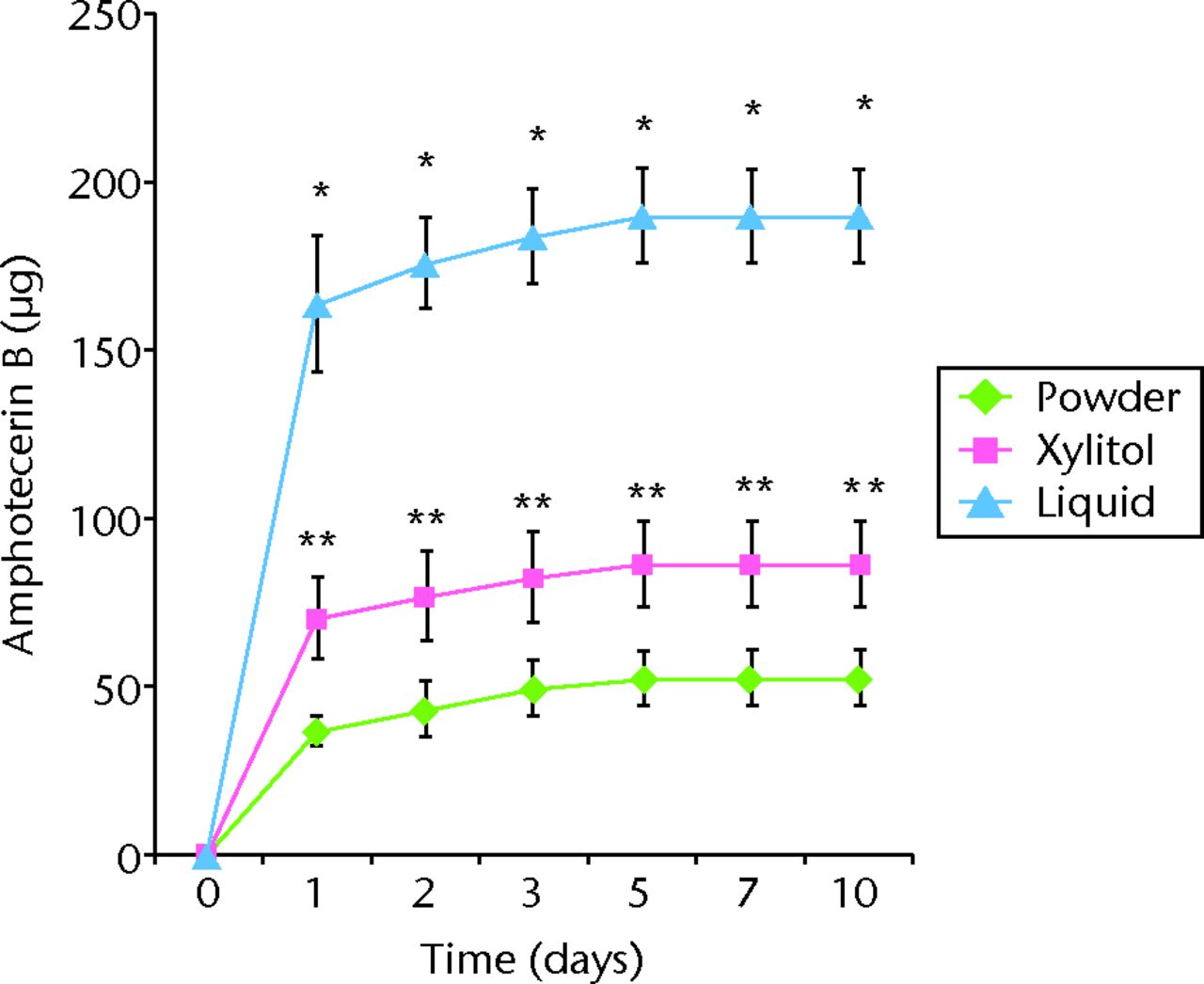
Figs. 1a - 1b
Graphs showing cumulative antibiotic released from cement specimens loaded with different preparations of a) vancomycin or b) amphotericin B in broth elution assay for ten days. The data are presented as the cumulative release of a) vancomycin or b) amphotericin B from cement specimens with different preparations. Values are shown as the mean and standard error of the mean for triplicate specimens in each group. *Significant difference (p < 0.05) between powder and liquid groups. **Significant difference (p < 0.05).
Regarding the mechanical strength of the cement samples, the mean compression modulus was significantly lower for specimens containing liquid antibiotic than it was for control specimens and those containing other preparations, both before and after the elution of the broth (p < 0.05; Fig. 2). The ultimate compressive strength was reduced by 35% and 55% in specimens containing vancomycin liquid and amphotericin B liquid, respectively, in comparison with the control. In contrast, the ultimate compressive strength was increased by 5% in specimens containing amphotericin B powder in comparison with the control (Fig. 2). The ultimate compressive strength was reduced by 35% and 55% in specimens containing vancomycin liquid and amphotericin B liquid, respectively, in comparison with the control. In contrast, the ultimate compressive strength was increased by 5% in specimens containing amphotericin B powder in comparison with the control (Fig. 2). The compressive strength of ALBC did not significantly decrease over ten days in elution, regardless of the use of ALBC with different preparations (Fig. 2).
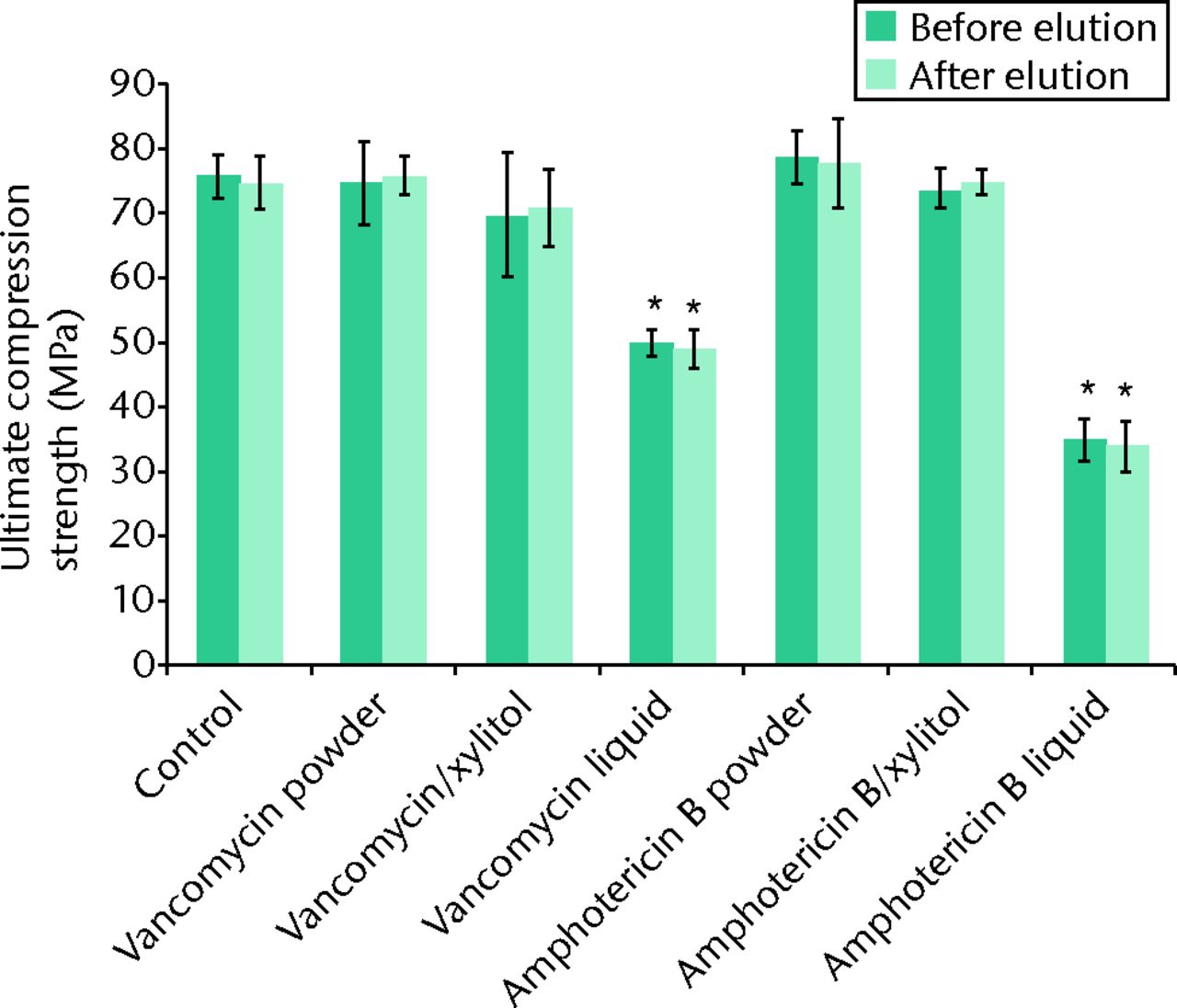
Fig. 2
Bar graph showing the mean ultimate compressive strength of the cement samples before and after the ten-day broth elution assay relative to cement without antibiotics (control). Values are shown as the mean and standard error of the mean for triplicate specimens for each group. *Significant difference (p < 0.05) when compared with the control.
With regard to the bioactivity of ALBC with different preparations against test organisms, vancomycin and amphotericin B both retained their antibacterial effects after incorporation into PMMA in powder, xylitol/powder or liquid form. To test the bioactivity of vancomycin or amphotericin B-loaded PMMA, MRSA and C. albicans, respectively, were used. Based on the disk-diffusion assay of vancomycin, the eluate samples of the liquid group exhibited a significantly larger inhibitory zone than ALBC samples loaded with powder antibiotic or powder antibiotic/xylitol (p < 0.05; Fig.3a). On the C. albicans-seeded agar dish, only the eluate samples of the liquid amphotericin B group exhibited a clear inhibitory zone (Fig. 3b). This inhibitory zone was not observed in the xylitol or powder groups of amphotericin B-loaded PMMA.
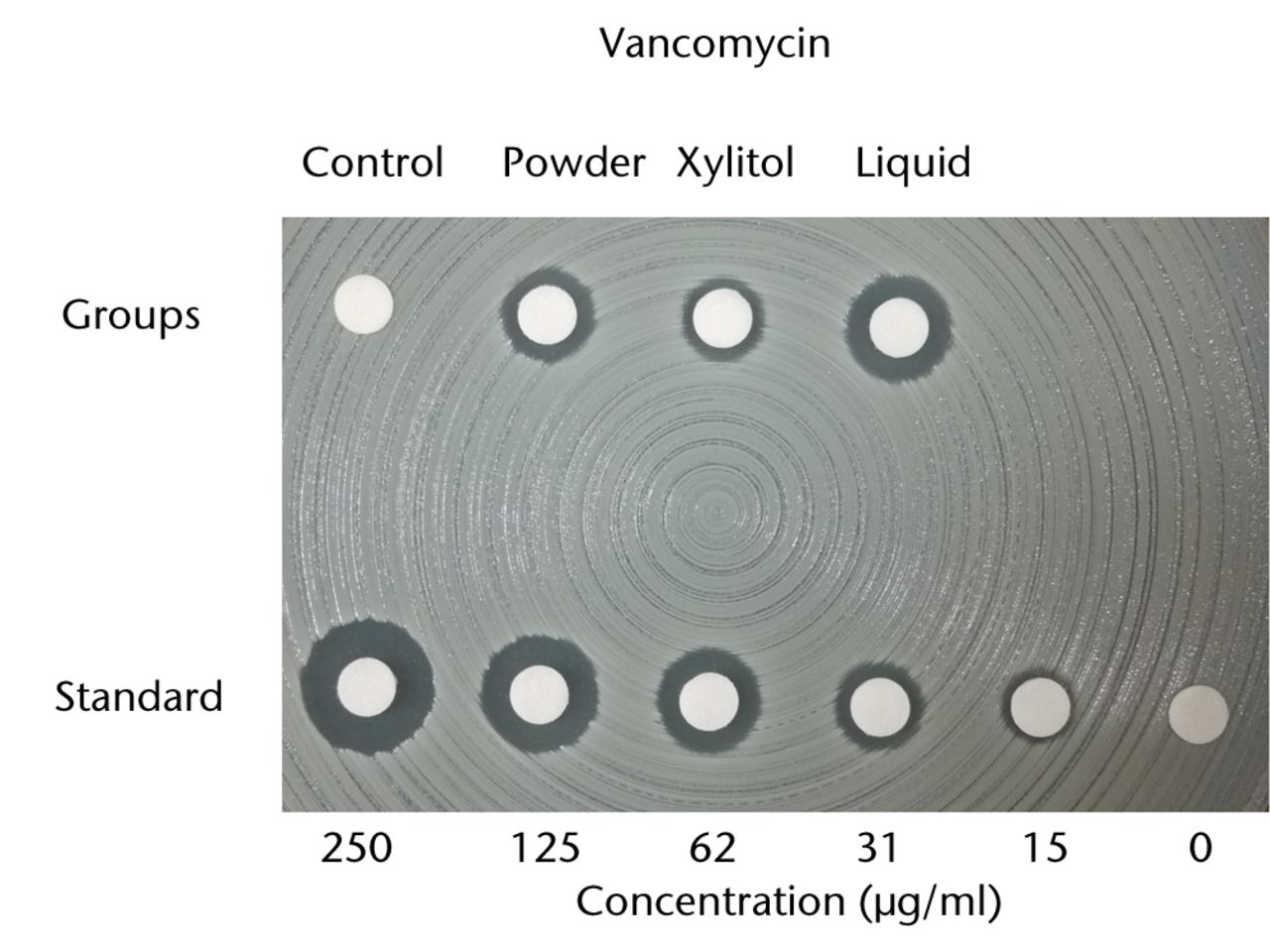
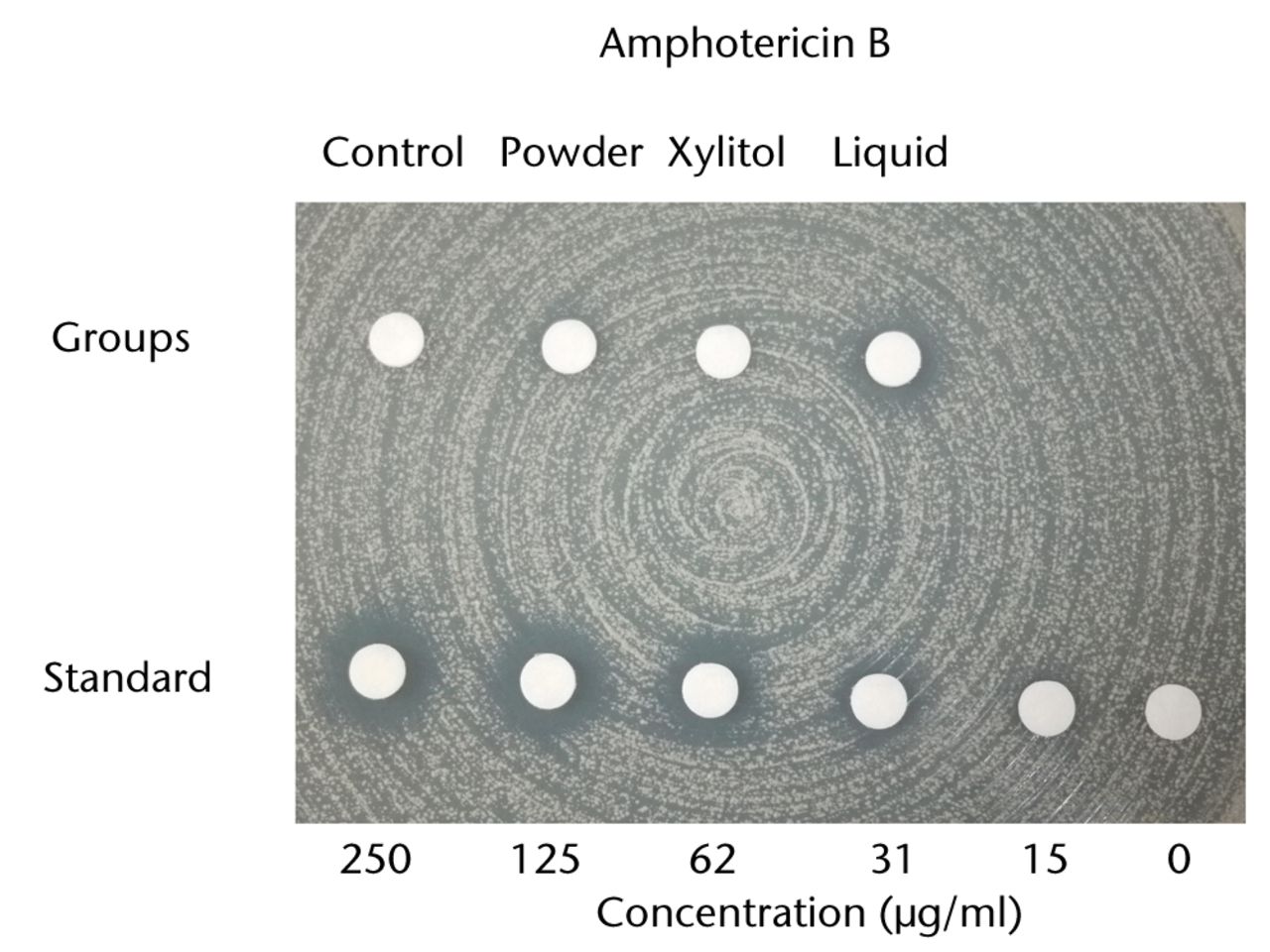
Figs. 3a - 3b
Charts showing antibacterial activity of eluate samples from bone cement with different preparations, as determined by agar-disk diffusion bioassay. The data are presented in terms of inhibition of the test organisms: figure 1a - methicillin-resistant Staphylococcus aureus and b) Candida albicans. The growth was visually compared with standard samples containing different concentrations of a) vancomycin and b) amphotericin B.
Discussion
Implant-related skeletal infection is one of the most devastating complications of orthopaedic surgery. The most common pathogens involved in skeletal infection are the Staphylococcus species, which constitute 50% to 60% of all isolates.11,12 Vancomycin, a glycopeptide antibiotic, remains the standard therapeutic agent used in most MRSA infections. Vancomycin has been shown to be stable in PMMA and is released in a microbiologically-active form.3 Vancomycin-loaded bone cement has become one of the most common ALBC in the treatment of skeletal infection. In this study, we found that ALBC loaded with liquid vancomycin presented a 3.3-fold higher vancomycin release than ALBC loaded with powder vancomycin. This simple, inexpensive, and effective method for the preparation of ALBC can provide better release kinetics than traditional ALBC made with antibiotic powder.
Fungal skeletal infection is rare as it is estimated to occur in approximately 1% of all cases of skeletal infection,13,14 and remains a therapeutic challenge.14 The efficacy of antifungal-loaded bone cement spacers in the treatment of fungal skeletal infection is controversial. Amphotericin B is a commonly used antifungal agent. Placement of amphotericin B-loaded cement spacers has been reported to successfully eradicate fungal skeletal infection.15,16 However, amphotericin B has a highly hydrophobic region that has been found to form covalent crosslinks in the PMMA matrix.9,17 Complete absence of release of amphotericin B in an elution study using amphotericin B-loaded bone cement has been reported.9 Poor efficiency of antibiotic release of amphotericin B-loaded ABLCs was also noted in this study. The accumulated release of amphotericin B powder-loaded ALBC (52 µg/ml; sem 10) was much worse than that of vancomycin power-loaded ALBC (362 µg/ml; sem 32) in a ten-day broth elution assay. The eluate samples of amphotericin B powder-loaded ALBCs did not exhibit a clear inhibitory zone in the disk diffusion assay. These findings suggest that amphotericin B powder-loaded ALBCs may not provide sufficient fungal eradication. In our previous report, 50% of patients treated with two-stage exchange arthroplasty for fungal PJI had recurrence or lack of control of the infection.14 Poor antibiotic elution from the bone cement may explain why patients with amphotericin B-impregnated cement spacers did not have a better outcome than those without such spacers in our previous report.14 In this study, adding 2 g of amphotericin B in 12 ml of distilled water before mixture with PMMA powder increased amphotericin B release by a factor of 3.6, relative to loading with only 2 g of amphotericin B powder. The eluate samples of ALBC with liquid amphotericin B exhibited a clear inhibitory zone on the C. albicans-seeded agar dish, which was not observed in ALBC with powder amphotericin B augmented with or without xylitol. Therefore, liquid amphotericin B ALBC may be a more effective measure in the treatment of fungal infection.
The kinetics of antibiotic release in ALBC depends on the penetration of dissolution fluids into the polymer matrix and subsequent diffusion of the dissolved drug from the ALBC. To increase antibiotic release, soluble fillers such as glycine, xylitol, sucrose, or erythritol can be added into the bone cement to increase its porosity and consequently increase the penetration of dissolution fluids.2,18,19 Xylitol has been reported to be more effective than other soluble fillers in increasing the amount of antibiotic release.2 In this study, addition of xylitol can improve the efficiency of ALBC loaded with vancomycin or amphotericin B. However, even with the addition of xylitol, the eluate samples from the amphotericin B-loaded bone cement still did not exhibit a clear inhibitory zone in the disk-diffusion assay. This finding indicates that even with the addition of xylitol, amphotericin B-loaded ALBC is still unable to provide a sufficient anti-fungal capacity. An additional drawback of using xylitol as a filler is the lack of availability of aseptic xylitol, thus resulting in a lack of readiness for its use in clinical applications. In contrast, dissolution of the antibiotic powder in distilled water before mixing with bone cement can significantly improve the efficiency of antibiotic release (p < 0.05), even for amphotericin B-loaded ALBC, which has been recognised as an ALBC with poor drug release.
Liquid antibiotics in acrylic bone cement have been shown to significantly compromise the mechanical properties of the cement (p < 0.05).7,20 International industrial standards state that bone cement used for definitive fixation must have an ultimate compressive strength of at least 70 MPa. This standard was established to reduce the incidence of premature cement breakdown.21,22 In this study, we found that the ultimate compressive strength of cement specimens was significantly compromised by loading with liquid antibiotics (p < 0.05). The ultimate compressive strength of liquid antibiotic-loaded ALBC was below 70 MPa both before and after broth elution. Therefore, liquid-antibiotic-loaded ABLCs do not provide sufficient mechanical strength for use in prosthesis fixation in total joint replacement. However, it should be noted that the compressive strength of PMMA is many times higher than that required in a clinical setting, and that even a 35% to 55% decrease should not compromise its function as a spacer. Additionally, ALBC beads are used as a therapeutic measure and are temporary because they are typically exchanged or removed from three to 14 days.
Our study has some limitations. First, this is an in vitro study of specimens prepared in a laboratory environment, which does not necessarily reflect actual clinical circumstances. The in vivo quantity and flow of body fluids, limb mobility, host response, and antibiotic stability were not taken into consideration. Second, only one type of bone cement and one dose of antibiotic were used. Third, ATCC bacteria were selected as test organisms in the experiment. The bioactivity of the cement specimens against other bacteria, especially clinical isolates, remains unclear.
To summarise, dissolution of 2 g of vancomycin or amphotericin B antibiotic powder in 12 ml of distilled water before mixing with bone cement can provide significantly higher efficiency of release than that of bone cement loaded with the same dose of antibiotic powder. However, inferior mechanical properties limit the use of the liquid–antibiotic-loaded cement to temporary beads or spacers used in the treatment of active infection and preclude it from being used for the implantation of a prosthesis. This simple, inexpensive, and effective method for preparation of ALBCs can significantly improve the efficiency of bone cement in releasing antibiotics. Further clinical studies are needed to determine the anti-infection efficiency and safety of ALBCs prepared using this protocol.
1 Hanssen AD . Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin Orthop Relat Res2005;437:91–96.CrossrefPubMed Google Scholar
2 McLaren AC , McLarenSG, SmeltzerM. Xylitol and glycine fillers increase permeability of PMMA to enhance elution of daptomycin. Clin Orthop Relat Res2006;451:25–28.CrossrefPubMed Google Scholar
3 Chang Y , ChenWC, HsiehPH, et al.In vitro activities of daptomycin-, vancomycin- and teicoplanin-loaded polymethylmethacrylate against methicillin-susceptible, methicillin-resistant, and vancomycin-intermediate strains of Staphylococcus aureus. Antimicrob Agents Chemother2011;55:5480–5484. Google Scholar
4 Hsieh PH , ShihCH, ChangYH, et al.Two-stage revision hip arthroplasty for infection: comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J Bone Joint Surg [Am]2004;86-A:1989–1997.PubMed Google Scholar
5 Ueng SW , HsiehPH, ShihHN, et al.Antibacterial activity of joint fluid in cemented total-knee arthroplasty: an in vivo comparative study of polymethylmethacrylate with and without antibiotic loading. Antimicrob Agents Chemother2012;56:5541–5546.CrossrefPubMed Google Scholar
6 Marks KE , NelsonCL, LautenschlagerEP. Antibiotic-impregnated acrylic bone cement. J Bone Joint Surg [Am]1976;58-A:358–364.PubMed Google Scholar
7 Seldes RM , WiniarskyR, JordanLC, et al.Liquid gentamicin in bone cement: a laboratory study of a potentially more cost-effective cement spacer. J Bone Joint Surg [Am]2005;87-A:268–272.CrossrefPubMed Google Scholar
8 Hsieh PH , TaiCL, LeePC, ChangYH. Liquid gentamicin and vancomycin in bone cement: a potentially more cost-effective regimen. J Arthroplasty2009;24:125–130.CrossrefPubMed Google Scholar
9 Goss B , LuttonC, WeinrauchP, et al.Elution and mechanical properties of antifungal bone cement. J Arthroplasty2007;22:902–908.CrossrefPubMed Google Scholar
10 Verdier MC , TributO, TattevinP, et al.Simultaneous determination of 12 beta-lactam antibiotics in human plasma by high-performance liquid chromatography with UV detection: application to therapeutic drug monitoring. Antimicrob Agents Chemother2011;55:4873–4879.CrossrefPubMed Google Scholar
11 Pulido L , GhanemE, JoshiA, PurtillJJ, ParviziJ. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res2008;466:1710–1715.CrossrefPubMed Google Scholar
12 Tsukayama DT , EstradaR, GustiloRB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg [Am]1996;78-A:512–523.CrossrefPubMed Google Scholar
13 Gamaletsou MN , KontoyiannisDP, SipsasNV, et al.Candida osteomyelitis: analysis of 207 pediatric and adult cases (1970-2011). Clin Infect Dis2012;55:1338–1351.CrossrefPubMed Google Scholar
14 Ueng SW , LeeCY, HuCC, HsiehPH, ChangY. What is the success of treatment of hip and knee candidal periprosthetic joint infection?Clin Orthop Relat Res2013;471:3002–3009.CrossrefPubMed Google Scholar
15 Wyman J , McGoughR, LimbirdR. Fungal infection of a total knee prosthesis: successful treatment using articulating cement spacers and staged reimplantation. Orthopedics2002;25:1391-1394;discussion 4.CrossrefPubMed Google Scholar
16 Wu MH , HsuKY. Candidal arthritis in revision knee arthroplasty successfully treated with sequential parenteral-oral fluconazole and amphotericin B-loaded cement spacer. Knee Surg Sports Traumatol Arthrosc2011;19:273–276.CrossrefPubMed Google Scholar
17 Vandermeulen G , RouxhetL, ArienA, BrewsterME, PreatV. Encapsulation of amphotericin B in poly(ethylene glycol)-block-poly(epsilon-caprolactone-co-trimethylenecarbonate) polymeric micelles. Int J Pharm2006;309:234–240.CrossrefPubMed Google Scholar
18 McLaren AC , NelsonCL, McLarenSG, DeCGR. The effect of glycine filler on the elution rate of gentamicin from acrylic bone cement: a pilot study. Clin Orthop Relat Res2004;427:25–27.CrossrefPubMed Google Scholar
19 McLaren AC , McLarenSG, HickmonMK. Sucrose, xylitol, and erythritol increase PMMA permeability for depot antibiotics. Clin Orthop Relat Res2007;461:60–63.CrossrefPubMed Google Scholar
20 Lautenschlager EP , MarshallGW, MarksKE, SchwartzJ, NelsonCL. Mechanical strength of acrylic bone cements impregnated with antibiotics. J Biomed Mater Res1976;10:837–845.CrossrefPubMed Google Scholar
21 Bridgens J , DaviesS, TilleyL, NormanP, StockleyI. Orthopaedic bone cement: do we know what we are using?J Bone Joint Surg [Br]2008;90-B:643–647.CrossrefPubMed Google Scholar
22 Pelletier MH , MalisanoL, SmithamPJ, OkamotoK, WalshWR. The compressive properties of bone cements containing large doses of antibiotics. J Arthroplasty2009;24:454–460.CrossrefPubMed Google Scholar
Funding statement:
This work was supported by internal funding (grant no. CMRPG3C0851).
Author contributions:
Y. H. Chang: Data collection, Data analysis, Performed surgeries, Writing the paper
C. L. Tai: Performed the biomechanical test
H. Y. Hsu: Performed the bioassay
P. H. Hsieh: Data analysis
M. S. Lee: Data analysis
S. W. N. Ueng: Initiated the idea for this study
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions license, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










