Abstract
High-quality randomised controlled trials (RCTs) evaluating surgical therapies are fundamental to the delivery of evidence-based orthopaedics. Orthopaedic clinical trials have unique challenges; however, when these challenges are overcome, evidence from trials can be definitive in its impact on surgical practice. In this review, we highlight several issues that pose potential challenges to orthopaedic investigators aiming to perform surgical randomised controlled trials. We begin with a discussion on trial design issues, including the ethics of sham surgery, the importance of sample size, the need for patient-important outcomes, and overcoming expertise bias. We then explore features surrounding the execution of surgical randomised trials, including ethics review boards, the importance of organisational frameworks, and obtaining adequate funding.
Cite this article: Bone Joint Res 2014;3:161–8.
Introduction
Surgical innovations have conventionally been accepted through the endorsements of renowned experts or through the findings of weak scientific evidence.1,2 Transitioning from eminence-based medicine to evidence-based medicine (EBM), however, has become the widely accepted new paradigm. Not only does EBM advocate the use of evidence in clinical decision-making, it emphasises the particular need for high-quality evidence. Accordingly, randomised controlled trials (RCTs) remain at the cornerstone of EBM as the highest level for a therapeutic intervention in the hierarchy of evidence (Fig. 1), however, has become the widely accepted new paradigm. Not only does EBM advocate the use of evidence in clinical decision-making, it emphasises the particular need for high-quality evidence. Accordingly, randomised controlled trials (RCTs) remain at the cornerstone of EBM as the highest level for a therapeutic intervention in the hierarchy of evidence (Fig. 1) remain at the cornerstone of EBM as the highest level for a therapeutic intervention in the hierarchy of evidence (Fig. 1).
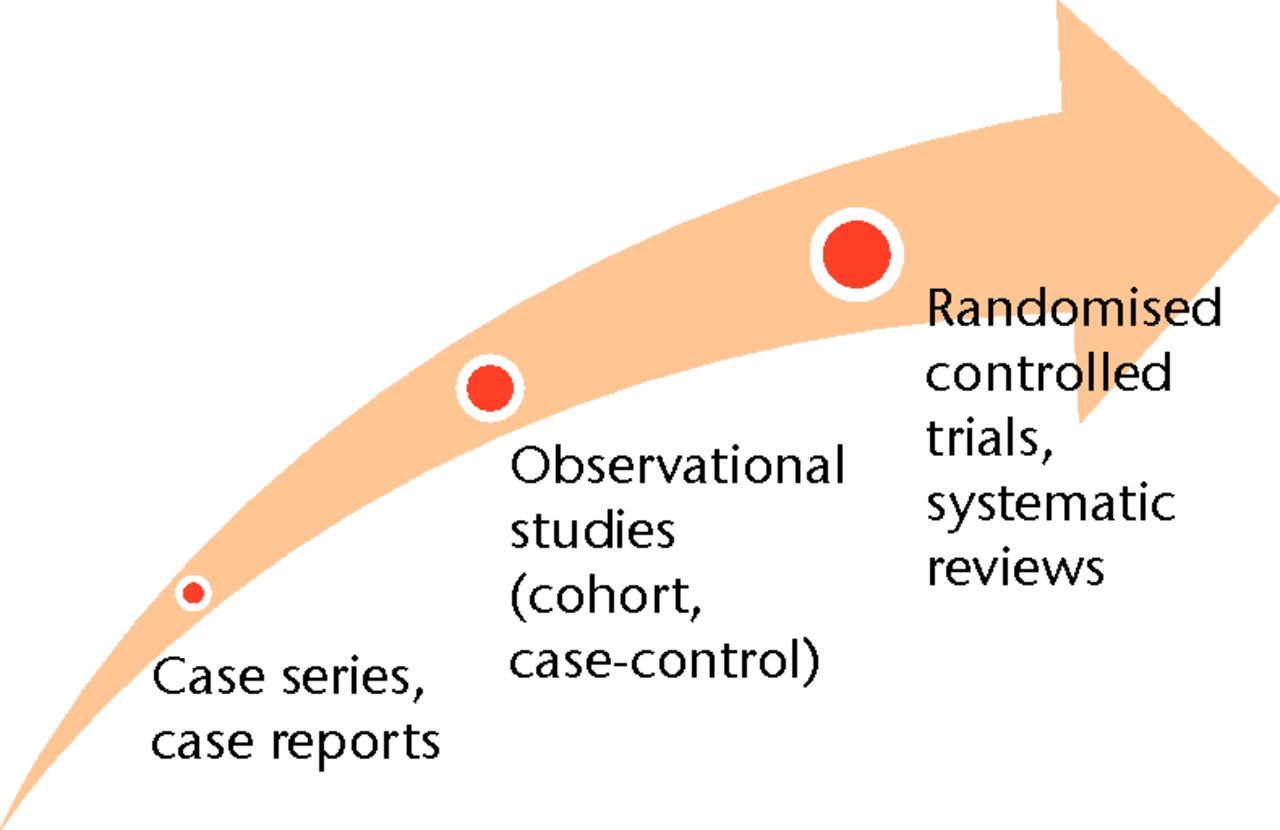
Fig. 1
Image demonstrating the hierarchy of evidence
Many of the interventions in orthopaedic surgery are aimed at improving patient function and quality of life.1 Randomising alternative approaches, such as surgical techniques or implants, provides a robust assessment of the superiority of one treatment over another. While randomised trials are well respected and often quoted in orthopaedic surgery, there is a relative paucity of RCTs in orthopaedic journals3 representing approximately 3% of the orthopaedic literature.4,5
Surgical trials proportionally are two-fold less common than trials in internal medicine.6 This scarcity may in large part be attributable to several perceived barriers associated with the design and execution of randomised trials of surgical interventions.
We highlight several challenges in the conduct of surgical trials. Our discussion extends to both methodological issues in relation to study design and to pragmatic issues relating to the execution of RCTs.
Design of modern-day RCTs
Randomised trials in orthopaedic surgery have evolved considerably over the last decade. However, several challenges remain. Among these, four common issues prevail: i)Â identifying the most robust controls and the ethics of sham (or ‘placebo’) surgery, ii)Â achieving large enough sample sizes to be meaningful, iii) using patient important outcomes in lieu of ‘convenient’ outcomes, and iv) managing the inherent differential expertise biases that exist in all surgical trials.
Using robust controls: the ethics of sham surgery
The double-blinded, placebo-controlled, randomised trial remains the reference standard in drug trial designs.1 The importance of blinding in randomised trials has been well documented in the medical literature.5,7,8 In a comprehensive review of meta-epidemiologic Studies (reviews of meta-analyses), Savovićet al7 studied the effect of blinding on treatment outcomes in 1057 randomised trials identified from 104 meta-analyses. They demonstrated that lack of, or unclear, double-blinding was associated with a statistically significant exaggeration in treatment effect (13% increase in odds ratio, CI 4% to 21%) when compared with studies that were double-blinded.
In a review of RCTs published in TheJournal of Bone & Joint Surgery[American Volume] between 1988 and 2000,4 it was demonstrated that drug trials more often reported being double-blinded in comparison with trials of surgical intervention. Practically, blinding surgeons in a surgical intervention trial is impossible. However, outcomes assessors, data analysts and patients, may be blinded. Among the 30 surgical RCTs in this study, only 17% made a clear statement about blinding of patients, and only 33% made a statement regarding blinding of outcome assessors.4 These findings are concerning given the above mentioned influence lack of blinding has on inflating treatment effect size.5,7,8
Furthermore, blinding works to quell the placebo effect of an intervention—the positive change in symptoms that can be attributed to a patient’s expectations of their treatment as opposed to the treatment itself.9,10 Blinding warrants even further attention in orthopaedic research, as there is often a heavy emphasis on subjective outcome measures that are vulnerable to placebo effects, such as pain, function, and quality of life.11
Blinding of surgeons is always impractical (if not impossible) and blinding of patients is often difficult.6 To achieve the latter, the issue of sham surgery has garnered much attention in the conduct of surgical RCTs. Although a sham surgery control group can offer a clear benefit to a trial’s methodological rigour, there are ethical considerations.11 The main concern is that sham surgeries offer no therapeutic benefit, while exposing patients to unnecessary risks—a threat to the ethical principles of beneficence and non-maleficence.1,9 Furthermore, sham surgeries compromise the integrity of the physician-patient relationship, as patients must be kept unaware of their sham surgery in follow-up visits to preserve blinding.3 However, universally labelling all sham surgeries as unethical may be an unfair argument. From a theoretical standpoint, it has been argued that the ethics of clinical research are not tantamount to the ethics of daily clinical practice, as the objective of the former is to answer a research question while minimising bias.1,9 Sham surgeries may be considered akin to blood draws, lumbar punctures, and biopsies routinely performed in medical trials, as investigators are ethically bound to minimise patient risk, but not completely eliminate it.1,9,11 From a pragmatic standpoint, sham surgeries may be justified when there is a true lack of consensus as to whether surgical management is superior to no treatment, referred to as clinical equipoise.1,9,10 Moreover, both the risks and potential benefits of sham surgery must be critically evaluated. Sham surgery may be suitable if it is minimally invasive, low risk, and may offer research findings that significantly benefit society.1,11 Obtaining informed consent is a matter of critical importance in a trial with sham surgery, as patients must acknowledge the risks and benefits of participating in a trial where they may potentially receive no therapeutic benefit.9,11 Finally, an additional surgeon independent of the trial may be recruited to carry out all follow-up visits, in order to to avoid the deception otherwise required by the operating surgeon.10
Moseley et al12 randomised 180 patients with painful osteoarthritis of the knee to treatment with arthroscopic debridement, arthroscopic lavage, or sham surgery. The study found no significant differences in pain or function between the treatment groups compared with the sham surgery group.12 This study serves as a suitable prototype for when sham surgery may be appropriate in a clinical trial. There was clinical equipoise around the arthroscopic management of knee osteoarthritis, the placebo surgery was low risk as it entailed intravenous sedation and three small incisions, and the consent process ensured patient understanding with regard to the trial.12
Given the ethical concerns and the highly specific circumstances that are amenable to sham surgery, however, the use of placebo-controlled, randomised trials remain rare in orthopaedic surgery. As future surgical advancements are likely to progress in modest steps from current non-placebo interventions, the more suitable control groups may be gold-standard interventions and the need for sham surgeries may become a less pressing concern.6
How big is big enough? – the challenge of sample size
Regardless of the methodological safeguards used to limit bias in orthopaedic trials, a small study with an inadequate sample size may be misleading and insufficient to guide clinical practice.13,14 There is perhaps no better example in the orthopaedic forum of the importance of adequate sample-size recruitment than the findings of the Study to Prospectively evaluate Reamed Intramedullary Nails in Patients with Tibial fractures (SPRINT).15 In this multicentre trial, over 1200 patients with both closed and open tibial shaft fractures were randomised to reamed or unreamed intramedullary nailing. After the recruitment of 50 patients, the study’s findings suggested that reamed nailing leads to a higher risk of re-operation (RR 1.85, 0.64 to 5.35). However, as a greater number of patients were recruited, the findings of this study shifted. In the final analysis of 1226 patients, reamed nailing showed a trend towards lower re-operation rates in comparison with unreamed nailing (RR 0.89, 0.70 to 1.14).16 The importance of full patient recruitment has been further demonstrated in a comprehensive review by Bassler et al,17 in which RCTs that had been stopped early for apparent ‘benefit’ were compared with non-truncated RCTs evaluating the same research questions. In their analysis of 91 truncated RCTs and 424 matching non-truncated RCTs, it was found that truncated RCTs were significantly more likely to overestimate treatment effects (ratio of relative risks = 0.71, p < 0.001).
Performing sample size calculations prior to a study and ensuring adequate patient recruitment, is a crucial component of preserving a trial’s methodological rigour. An insufficient sample size directly decreases the statistical power of a study—i.e. the ability to detect a statistically significant difference when a true difference indeed exists between treatment arms. Alternatively stated, there is an increased risk of a false-negative study finding (a type-II or beta error). However, simply recruiting more patients may indeed identify small statistical differences that are clinically unimportant.13,14 Investigators must establish the primary outcome of interest a priori and the minimally important clinical difference associated with that outcome measure. Once established, a sample-size calculation can be performed prior to commencing a trial to ensure that investigators can correlate statistical significance to clinical significance and appropriately power their study.
Given the challenges and resources required to perform a surgical RCT, it would be unfavourable to forego a sample size calculation at the risk of under-powering a trial and reaching an equivocal conclusion.18 Freedman et al18 have demonstrated that the statistical power of orthopaedic RCTs is severely compromised.18 In a review of 33 orthopaedic RCTs published in premier orthopaedic journals (The Journal of Bone & Joint Surgery American Volume (JBJS [Am]), The Journal of Bone & Joint Surgery British Volume (JBJS [Br]), Clinical Orthopaedics and Related Research (CORR)), they found that only 9% of studies performed statistical sample size calculations and that nearly half of all studies with insignificant findings were too underpowered to detect even a large treatment effect.18 Similar findings were reported by Bhandari et al4 in the review of 72 orthopaedic randomised trials.4 Specifically, they demonstrated that 94% of trials failed to calculate the effective sample size needed to provide acceptable study power.4
Despite the need for sufficient patient recruitment, most RCTs often overestimate their ability to recruit participants and are also hindered by patient dropout.3 In a review of the prospective and retrospective screening studies used for the SPRINT trial, it was demonstrated that both methods grossly overestimated actual patient recruitment in the definitive trial. Despite the similar estimates provided by both approaches, they both lacked validity as they overestimated recruitment by nearly 70%.19
Proposed strategies to improve patient recruitment have included providing patients with detailed information about the benefits and risks of trial participation, altering study design to cater to patient preferences (i.e. no placebo arm, two surgical arms), improving the consent process, and offering incentives.3 Ultimately, however, establishing multicentre trials will afford investigators an effective and efficient means of conducting large trials that are adequately powered.
There are several benchmark trials to suggest that multicentre trials are not only feasible, but should be the current norm. For example, The Heart Outcomes Prevention Evaluation (HOPE) study was a multinational, randomised trial that recruited 9297 patients across 276Â centres from 19 different countries. Patients with cardiovascular risk factors were randomised to receive either ramipril (angiotensin-converting enzyme inhibitor) or placebo medication. At a mean follow-up of five years, patients treated with ramipril had significantly lower rates of myocardial infarction, stroke, and cardiovascular death.20 Such landmark trials in cardiology have had a substantial clinical impact, as the mortality associated with coronary disease has fallen by nearly 40% in the past several decades and approximately half of this reduction is directly due to evidence-based medical therapies.21 Large, multicentre RCTs are by no means exclusive to nonsurgical trials. The SPRINT trial recruited 1339Â patients from 29 different sites in Canada, USA, and The Netherlands.15 Currently underway, the Fluid Lavage of Open Wounds (FLOW) trial is a multicentre randomised trial evaluating both irrigation solution (soap versus saline) and pressure (gravity, low, high) on the re-operation rates for open fracture management. With a recruitment goal of 2280 patients, the study currently has already crossed the 2000 patient mark, with participating sites from Canada, Australia, USA, Norway, and India.22,23 The establishment of large orthopaedic research networks, by means of orthopaedic associations and specialist societies, can foster a culture in which these large, multicentre trials are feasible. Such networks can allow surgeons to collectively identify important research topics, endorse RCTs, educate surgeons about trial methodology, assist in the co-ordination of large trials, and ultimately, help disseminate research findings.24
Patient-important outcomes
Sample size is clearly tied to an orthopaedic trial’s primary outcome measure. The decision to use a scale like the SF-36 or a dichotomous (yes/no) measure like re-operation will have significant implications on the number of patients required for a trial to show incremental benefits.14
The World Health Organization defines health as a state of complete physical, mental, and social well-being.25,26 This definition of health is of direct relevance to orthopaedic surgeons and investigators, as patients afflicted with orthopaedic conditions often suffer limitations in function and quality of life, which inevitably compromise their state of health. Accordingly, outcome measures used in a trial should be patient-important and as objective as possible.27 In medical trials, it is frequently possible to couple objective evaluation with patient importance, through measures such as death, thromboembolic events, and bleeding rates. This remains a greater challenge in orthopaedic trials, as patient-important outcomes must include more complex subjective evaluations to appreciate how interventions influence day-to-day activities, participation in social activities, and fulfilment of societal expectations. Further underpinning the need for such subjective measures is the fact that physiological outcomes do not consistently correlate with self-reported health.25 The FLOW trial serves as a notable example, with its inclusive focus on important outcomes. As mentioned, the primary outcome measure of this trial is re-operation for infection, managing wound healing and promoting bone healing. To supplement re-operation rates, the study is also assessing function and quality of life with the Short-Form 12 and Euro-Qol-5 Dimensions.22
Health Related Quality of Life (HRQOL) measures are an important tool in the armamentarium of outcome measures available to orthopaedic investigators and are becoming more commonplace in orthopaedic trials.14 There are two types of HRQOL measures—generic and disease-specific. Generic HRQOL instruments measure a patient’s general health status by assessing the physical, functional and emotional aspects of health.25 These measures are typically useful for comparing the health of patients with different diseases, severity of disease or undergoing different interventions. Due to their broad scope, however, they may be less sensitive in detecting small but meaningful changes in the health of patients. Disease-specific HRQOL instruments assess specific aspects of physical, mental and social health that are relevant to the condition of interest. Given the narrower focus, these instruments are able to detect smaller differences in health status, but are often not comparable across different disease states.14,25 Ideally, both a generic and disease-specific instrument should be used in a trial.14 The challenge for investigators is ensuring that optimal instruments are selected. In doing so, a clear understanding of the trial’s objectives is needed such that outcome instruments that run parallel to these goals, can be selected. Furthermore, HRQOL measures must be valid (measure what they are intended to measure), reliable (consistent when applied repeatedly in a stable population) and responsive (detect important changes in health state).25
Limiting differential expertise bias: novel designs
If significant discrepancy exists in a surgeon’s skill or expertise between the various treatment arms, there is a threat of introducing differential expertise bias into the trial.28 The second challenge facing surgical RCTs is that of clinical equipoise. Although community equipoise may exist for a given condition among surgeons as a whole, individual surgeons often favour a particular intervention.2 This lack of clinical equipoise, which unfortunately is often based on weak scientific findings, serves as a barrier and ethical challenge to surgeon participation in RCTs.3,29
In light of these challenges, there has been increasing interest in the expertise-based RCT. In this design, a surgeon with expertise for one intervention is paired with a surgeon who holds expertise in the other intervention. Patients are then randomised to each surgeon directly, who is solely responsible for performing their procedure of expertise (Fig. 2).29
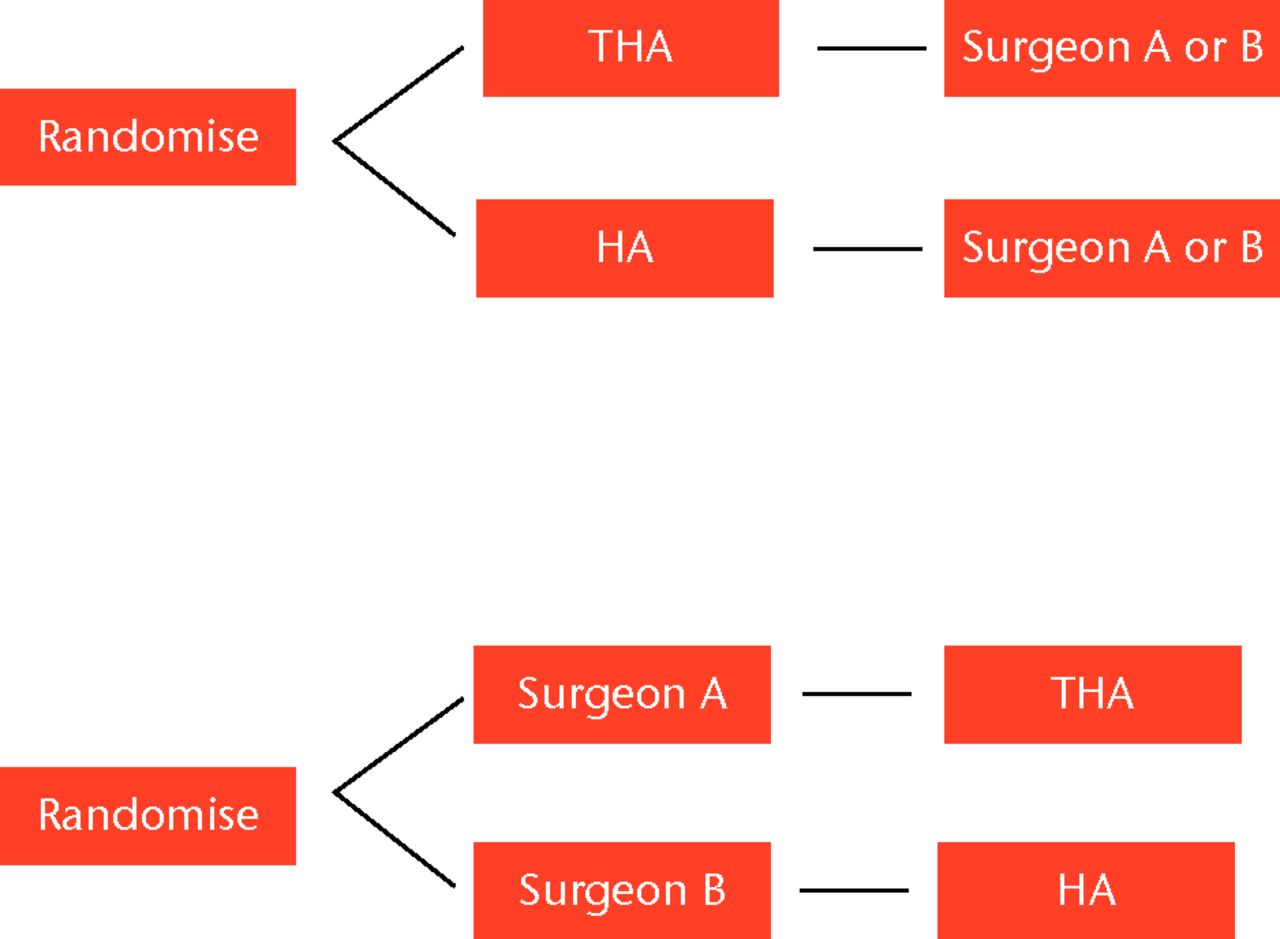
Fig. 2
Flow diagrams showing conventional randomisation a) versus b) expertise-based randomisation.
The Hip Fracture Evaluation with Alternatives of Total Hip Arthroplasty versus Hemi-arthroplasty (HEALTH) trial is an international, multicentre randomised trial currently underway that has employed an expertise-based design (ClinicalTrials.gov identifier: NCT00556842). This design is fitting given that a recent international survey demonstrated that surgeons vary widely in their preferred choice of arthroplasty when managing patients between 60 and 80 years old with completely displaced fractures of the femoral neck.30
Ideally, this study design will prevent differential expertise bias, avoid ethical challenges faced by surgeons, foster surgeon participation, and reduce procedural cross-over rates.28 The concern surrounding expertise-based RCTs extends to the potential loss of generalisability, as community surgeons may not be as skilled as trial experts.2 Nevertheless, the expertise-based RCT offers a pragmatic solution to these unavoidable challenges of surgeon experience and equipoise.
Clinical trial execution
Despite the fact that a clinical trial is well-designed, the integrity of collected data and the ensuing conclusions may be seriously undermined if the trial is not executed with assiduous attention to ethical and logistical issues. In our experience, these issues become especially pronounced in large multicentre clinical trials, owing to the volume of sites, personnel, and data. In this section, we will highlight three prominent themes that are central to the successful execution of clinical trials: i) ethical and logistical issues associated with institutional ethics review boards (IRBs); ii) importance of an appropriate organisational framework; and iii) securing sufficient funding.
Ethical research conduct and ethics review boards
Founded on the principles of the Declaration of Helsinki, the Good Clinical Practice (GCP) Guidelines produced by the International Conference on Harmonisation (ICH) were designed to guide the ethical conduct of clinical trials.31 IRBs are bestowed the responsibility to uphold this mandate through the review of clinical trial protocols and ensuring that they are ethically and methodologically sound. The latter is of paramount importance, as methodologically poor research is unethical research, offering not only misleading conclusions, but also compromising patients’ time and draining societal resources. We advise researchers to view each IRB application as an opportunity to refine study design and conduct.
The final IRB-approved protocol must be adhered-to meticulously throughout the execution of a clinical trial. Deviations from the protocol should be justifiable and approved by the IRB. This will ensure that any protocol deviation mid-trial does not unintentionally (or intentionally) bias the final outcome data. The importance of this principle is such that many granting agencies and journals now require trial pre-registration.32,33 Various clinical registries are available for this purpose, including ISRCTN.org and ClinicalTrials.gov. Publication of protocols in the scientific literature, where they are available for widespread peer review, has also been advocated.32,33
From a logistical perspective, IRB applications can result in a significant delay to the start-up of a trial, especially in multinational, multicentre designs.34 Identifying these delays early can help investigators circumvent or, at the very least, anticipate them. Firstly, there is the inherent delay in waiting for IRB approval, which is unavoidable and certainly necessary. Often, there is at least one, but often more, revisions. There is also variability among legislations, protocols, and efficiencies between different geographical regions. For instance, in the Fixation using Alternative Implants for the Treatment of Hip Fractures (FAITH) trial, there were significant differences seen between the Netherlands and both USA and Canada in terms of median time to receive IRB approval (104 days versus 53 days and 104 versus 55 days, respectively).34
After receiving full IRB approval, there are always further delays in getting a trial moving forward, as certain organisational aspects of a trial can only be organised once IRB approval is obtained. Data from the FAITH trial again reinforces this point; median time between receiving ethics approval and start-up ranged from 41 days (the Netherlands) to 232 days (Canada).34 In that instance, it was determined in retrospect that a central regional co-ordinator could potentially reduce the logistical component of the delay by circumventing the need for contract negotiations with individual study sites.34 Ultimately, anticipating logistical delays to start-up and a dedicated team that can help study sites traverse these challenges, is fundamental to successful and efficient trial execution.
Multicentre trial organisation
There are several organisational committees that must exist to ensure the ethical and efficient execution of a clinical trial (Fig. 3).
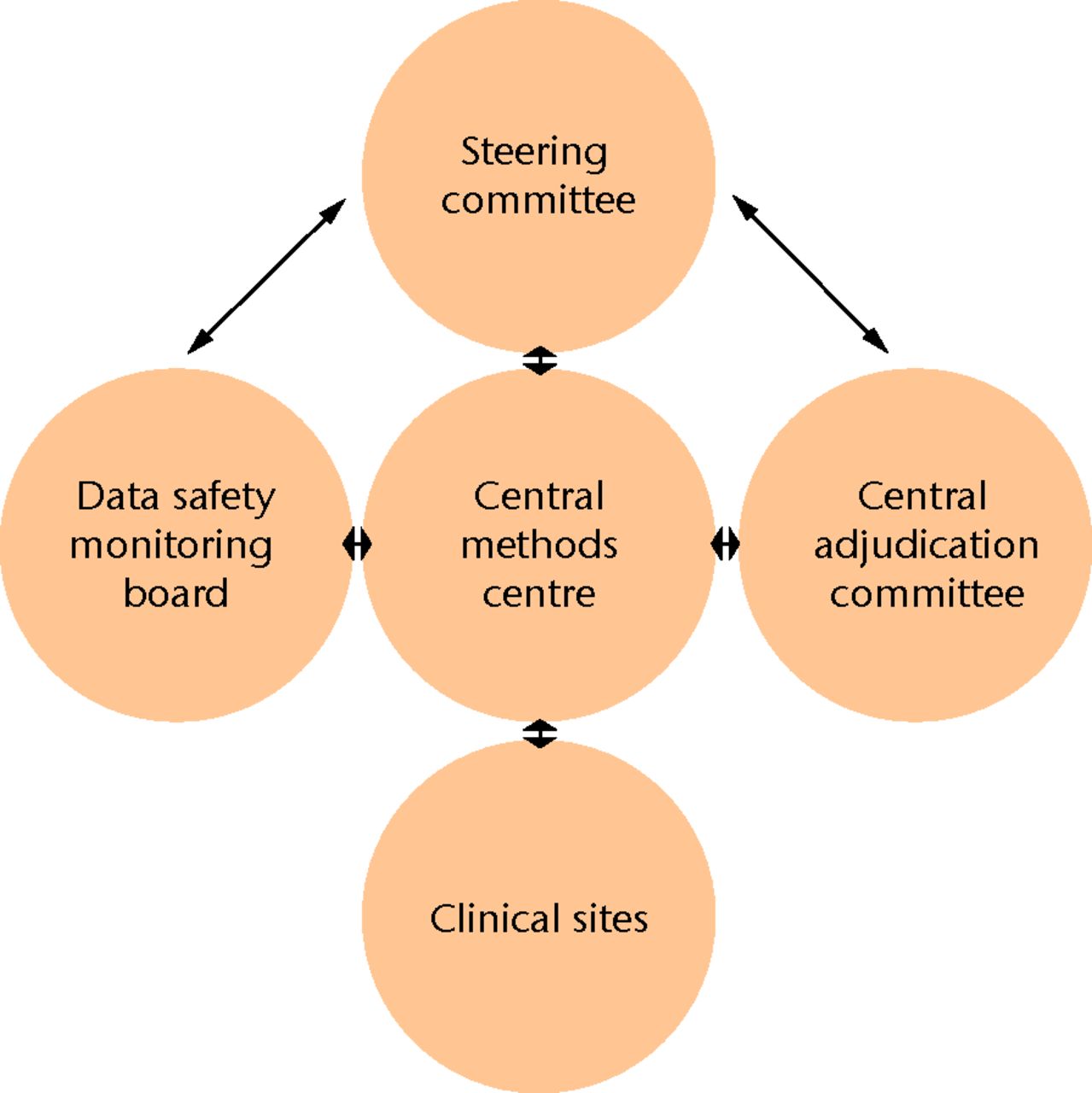
Fig. 3
Diagram showing the multicentre trial committee organisational framework
Steering committee
The steering committee is the key to the organisation of a clinical trial. It develops the trial design and oversees its conduct, and is typically composed of the principal investigator alongside relevant clinical experts, biostatisticians, and research methodologists. The committee should be diverse enough to provide critical insight, but small enough to prevent dysfunction and inefficiencies.35 The committee is tasked with designing the protocol, continually interacting with the methods centre to resolve issues as they arise, and ultimately leading the analysis and dissemination of results.
Methods Centre
The Methods Centre may be thought of as the operational arm of the Steering Committee. It is a group of individuals that ensure the committee’s protocol is executed seamlessly. In a multicentre clinical trial, the Methods Centre typically operates out of a central geographical location, which may be a contract research organisation or academic medical centre. It is usually composed of a project manager, research co-ordinator and assistants, and a data analyst/statistician.
The Methods Centre, or Central Methods Centre, is tasked with overseeing day-to-day activities during the clinical trial and communicating with each clinical site to identify issues and provide relevant support. Each clinical site or geographical region may have its own local co-ordinator who serves as an intermediary to the Central Methods Centre.34 Specific examples of the Methods Centre’s responsibilities include distributing study resources and case report forms, operating a central telephone or computer-based randomisation system, identifying and communicating issues to the Steering Committee, and collecting data through courier, facsimile, or electronic data capture systems.
Data safety and monitoring committee
In accordance with the ethical duties of all researchers, data safety and monitoring is a key consideration in any clinical research trial, although a specific committee may not be necessary in every instance to accomplish this goal.36 However, in most large multicentre orthopaedic trials, which evaluate morbidity or mortality of an intervention, a dedicated Data Safety and Monitoring Committee ensures that the continuation of the trial is both meaningful and safe for patients. This committee is formed by a group of experts who are completely independent of the trial investigators. The committee must comprise the expertise to make an informed decision regarding the continuation or cessation of an orthopaedic trial. As such, committee membership generally spans the fields of orthopaedic surgery, clinical trial methodology, biostatistics, and medical ethics.37
A data monitoring plan is typically developed a priori, outlining operational details for the committee; including data to be collected, time frames for assessments and recommendations, and trial-stopping rules. Subsequently, the Data Safety and Monitoring Committee makes recommendations to the Steering Committee (and occasionally other government regulatory organisations) regarding the continuation, modification, or cessation of the clinical trial.
Adjudication Committee
A Central Adjudication Committee (CAC) provides a systematic and unbiased assessment of study outcome data, including eligibility criteria, protocol deviations, and endpoint data. CACs have been used successfully in numerous clinical trials,38-42 although they are still a relative rarity in the orthopaedic literature.43 The greatest use of an Adjudication Committee is predominantly in the assessment of outcomes that have a degree of subjectivity associated with them. For instance, in orthopaedic surgery fracture trials, Adjudication Committees may serve a useful function in the assessment of fracture healing.43,44
An Adjudication Committee typically requires at least three individuals. Larger committees may provide additional insight and perspective, but have not been shown to influence trial results significantly.45 Adjudication Committee members are selected based on expertise in the assessment of the study outcomes. For instance, orthopaedic surgeons or diagnostic radiologists (or some combination thereof) would be the appropriate members to board a CAC tasked to determine radiological fracture healing.
Adjudication Committee members must have access to all relevant study data (such as radiographs, clinic and operative notes) and, if possible, be blinded to treatment allocation. Prior to assessment, criteria for outcome evaluation in an objective and systematic manner should be established. Standardised measurement instruments, if available, may be employed at this stage. For example, the Radiographic Union Scale for Tibial Fractures (RUST) is a standardised measurement tool that was developed to measure fracture healing of patients with tibial shaft fractures.46,47 All outcome data are assessed independently by each member of the Adjudication Committee. Discrepancies in assessment are then identified and subsequently resolved by consensus among the members. Discrepancy identification has been typically performed by the project management team and resolved through in-person or teleconference consensus meetings. This approach has tended to delay the final analysis and dissemination of study results, which rely upon timely adjudication of outcomes. An automated system is another option investigators may employ to streamline the efficiency of this process. The Global AdjudicatorTM (www.globaladjudicator.ca), for instance, is one such automated web-based system developed by a Contract Research Organisation specialising in orthopaedic trials.48
Clinical trial funding
Multinational, multicentre clinical trials inevitably require a large amount of funding. The design, organisation, and execution of these trials require resources and personnel that may cost millions. There are several major sources of funding available for clinical researchers. The closest and most accessible source is often at one’s own local institution. Universities and academic medical centres often have funds allocated for research being conducted at their institutions, or by investigators from within the institution. Unfortunately, the amount of this funding is limited and often insufficient given the scale of large clinical trials. Research foundations and associations are another source of funding, albeit also limited by their size. These organisations are often specialty-specific, such as the Orthopedic Research and Education Foundation (OREF) and the Orthopaedic Trauma Association (OTA).
A larger amount of funding is available from government organisations interested in promoting clinical research. The Canadian Institutes for Health Research (CIHR) and the National Institutes of Health (NIH) are two such organisations that provide funds, occasionally into the millions, for well-designed clinical research trials. These organisations are accountable to the public and therefore attempt to fund research that is likely to be successful and beneficial to society. As such, they consider factors such as investigator qualifications, success in pilot studies, and a research track record, through a process that endeavours to be impartial through peer review. Unfortunately, the increasing costs of clinical trials and the recent economic climate have made obtaining such funding increasingly difficult, as governments worldwide strive to cut deficits. Orthopaedic surgeons may encounter particular difficulties obtaining this funding as these organisations are less comfortable and familiar with medical device and implant trials than pharmaceutical trials, resulting in substantially less funding for surgical research.49,50 Perhaps the largest source of funding in present day clinical trials is from industry. Over the past decade, the number of clinical trials funded by industry has seen a substantial increase.51,52 There may be a number of reasons for this, including increasing costs of trials, decreases in government funding, and increasing interest by industry to provide funds due to regulatory requirements, or other reasons.52-54 The mutually beneficial relationship between researchers and industry has long been recognised.52 However, there are several concerns which were recently brought to the forefront by a number of research articles and media reports. Disproportionately pro-industry conclusions, biased study design, and suppression of negative results are overt ways in which industry-funded research has developed a poor reputation.51 There are several strategies to ensure that the benefits of the academia-industry relationship are harnessed without propagating factors that have led to its disrepute. This issue is beyond the subject of this review and we therefore refer readers to other sources.51,52
Conclusion
Although challenging, orthopaedic investigators must continue to pursue high-quality research in the form of randomised trials. Foregoing design considerations—such as sample size calculations, patient-important outcomes, and surgeon expertise—can ultimately threaten the validity of a surgical trial. In the setting of multicentre trials, neglecting the execution strategies of ethical conduct, trial organisation and funding procurement poses both ethical and pragmatic barriers to the successful conduct of a RCT. If given generous consideration, orthopaedic surgeon researchers will ensure that high quality evidence forms the basis for further evolution in orthopaedic surgery.
1 Wolf BR , BuckwalterJA. Randomized surgical trials and "sham" surgery: relevance to modern orthopaedics and minimally invasive surgery. Iowa Orthop J2006;26:107–111.PubMed Google Scholar
2 Cook JA . The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials2009;10:9.CrossrefPubMed Google Scholar
3 Campbell AJ , BagleyA, Van HeestA, JamesMA. Challenges of randomized controlled surgical trials. Orthop Clin North Am2010;41:145–155.CrossrefPubMed Google Scholar
4 Bhandari M , RichardsRR, SpragueS, SchemitschEH. The quality of reporting of randomized trials in the Journal of Bone and Joint Surgery from 1988 through 2000. J Bone Joint Surg [Am]2002;84-A:388–396.CrossrefPubMed Google Scholar
5 Poolman RW , StruijsPA, KripsR, et al.Reporting of outcomes in orthopaedic randomized trials: does blinding of outcome assessors matter?J Bone Joint Surg [Am]2007;89-A:550–558.CrossrefPubMed Google Scholar
6 Simunovic N , DevereauxPJ, BhandariM. Design considerations for randomised trials in orthopaedic fracture surgery. Injury2008;39:696–704.CrossrefPubMed Google Scholar
7 Savović J , JonesHE, AltmanDG, et al.Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med2012;157:429–438.CrossrefPubMed Google Scholar
8 Kjaergard LL , VillumsenJ, GluudC. Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med2001;135:982–989.CrossrefPubMed Google Scholar
9 Kishen TJ , HarrisIA, DiwanAD. Primum non nocere and randomised placebo-controlled surgical trials: a dilemma?ANZ J Surg2009;79:508–509.CrossrefPubMed Google Scholar
10 Dowrick AS , BhandariM. Ethical issues in the design of randomized trials: to sham or not to sham. J Bone Joint Surg [Am]2012;94-A(Suppl1):7–10. Google Scholar
11 Mehta S , MyersTG, LonnerJH, HuffmanGR, SennettBJ. The ethics of sham surgery in clinical orthopaedic research. J Bone Joint Surg [Am]2007;89-A:1650–1653.PubMed Google Scholar
12 Moseley JB , O'MalleyK, PetersenNJ, et al.A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med2002;347:81–88.PubMed Google Scholar
13 Karlsson J , EngebretsenL, DaintyK. ; ISAKOS Scientific Committee. Considerations on sample size and power calculations in randomized clinical trials. Arthroscopy2003;19:997–999. Google Scholar
14 Zlowodzki M , BhandariM. Outcome measures and implications for sample-size calculations. J Bone Joint Surg [Am]2009;91-A(Suppl3):35–40.CrossrefPubMed Google Scholar
15 Bhandari M , GuyattG, et al.Randomized trial of reamed and unreamed intramedullary nailing of tibial shaft fractures. J Bone Joint Surg [Am]2008;90-A:2567–2578.PubMed Google Scholar
16 Bhandari M , TornettaP 3rd, et al.(Sample) size matters! An examination of sample size from the SPRINT trial study to prospectively evaluate reamed intramedullary nails in patients with tibial fractures. J Orthop Trauma2013;27:183–188.CrossrefPubMed Google Scholar
17 Bassler D , BrielM, MontoriVM, et al.Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA2010;303:1180–1187.CrossrefPubMed Google Scholar
18 Freedman KB , BackS, BernsteinJ. Sample size and statistical power of randomised, controlled trials in orthopaedics. J Bone Joint Surg [Br]2001;83-B:397–402.PubMed Google Scholar
19 Kooistra BW , DijkmanBG, GuyattGH, et al.Prospectively screening for eligible patients was inaccurate in predicting patient recruitment of orthopedic randomized trials. J Clin Epidemiol2011;64:537–542.CrossrefPubMed Google Scholar
20 Yusuf S , SleightP, PogueJ, et al.Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med2000;342:145–153. Google Scholar
21 Ford ES , AjaniUA, CroftJB, et al.Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med2007;356:2388–2398.CrossrefPubMed Google Scholar
22 Flow Investigators. Fluid lavage of open wounds (FLOW): design and rationale for a large, multicenter collaborative 2 x 3 factorial trial of irrigating pressures and solutions in patients with open fractures BMC Musculoskelet Disord2010;11:85. Google Scholar
23 No authors listed. ClinicalTrials.gov: Fluid Lavage of Open Wounds (FLOW), 2013. http://clinicaltrials.gov/ct2/show/NCT00788398 [date last accessed 28 February 2014]. Google Scholar
24 Rangan A , BrealeyS, CarrA. Orthopaedic trial networks. J Bone Joint Surg [Am]2012;94-A(Suppl1):97–100.CrossrefPubMed Google Scholar
25 Bryant DM , SandersDW, ColesCP, et al.Selection of outcome measures for patients with hip fracture. J Orthop Trauma2009;23:434–441.CrossrefPubMed Google Scholar
26 No authors listed. World Health Organization: Definition of health. http://www.who.int/about/definition/en/print.html [date last accessed 28 February 2014]. Google Scholar
27 Bhandari M , PetrisorB, SchemitschE. Outcome measurements in orthopedic. Indian J Orthop2007;41:32–36.CrossrefPubMed Google Scholar
28 Walter SD , IsmailaAS, DevereauxPJ. ; SPRINT Study Investigators. Statistical issues in the design and analysis of expertise-based randomized clinical trials. Stat Med2008;27:6583–6596. Google Scholar
29 Bednarska E , BryantD, DevereauxPJ. ; Expertise-Based Working Group. Orthopaedic surgeons prefer to participate in expertise-based randomized trials. Clin Orthop Relat Res2008;466:1734–1744. Google Scholar
30 Bhandari M , DevereauxPJ, TornettaP 3rd, et al.Operative management of displaced femoral neck fractures in elderly patients. An international survey. J Bone Joint Surg [Am]2005;87-A:2122–2130.CrossrefPubMed Google Scholar
31 No authors listed. US Food and Drug Administration: ICH E6: Good Clinical Practice: Consolidated Guidance. http://www.fda.gov/scienceresearch/specialtopics/runningclinicaltrials/guidancesinformationsheetsandnotices/ucm219488 (date last accessed 1 May 2014). Google Scholar
32 De Angelis C , DrazenJM, FrizelleFA, et al.Clinical trial registration: a statement from the Internal Committee of Medical Journal Editors. Ann Intern Med . 2004;141:477–478. Google Scholar
33 Laine C , De AngelisC, DelamotheT, et al.Clinical trial registration: looking back and moving ahead. Ann Intern Med2007;147:275–277.PubMed Google Scholar
34 Zielinski SM , ViveirosH, HeetveldMJ, et al.Central coordination as an alternative for local coordination in a multicenter randomized controlled trial: the FAITH trial experience. Trials2012;13:5.CrossrefPubMed Google Scholar
35 Sprague S , MattaJM, BhandariM, et al.Multicenter collaboration in observational research: improving generalizability and efficiency. J Bone Joint Surg [Am]2009;91-A(Suppl3):80–86.CrossrefPubMed Google Scholar
36 Poolman RW , HansonB, MartiRK, BhandariM. Conducting a clinical study: A guide for good research practice. Indian J Orthop2007;41:27–31.CrossrefPubMed Google Scholar
37 DAMOCLES Study Group, NHS Health Technology Assessment Programme. A proposed charter for clinical trial data monitoring committees: helping them to do their job well Lancet2005;365:711–722. Google Scholar
38 Walter SD , CookDJ, GuyattGH, KingD, TroyanS. Outcome assessment for clinical trials: how many adjudicators do we need? Canadian Lung Oncology Group. Control Clin Trials1997;18:27–42.CrossrefPubMed Google Scholar
39 Cook D , WalterS, FreitagA, et al.Adjudicating ventilator-associated pneumonia in a randomized trial of critically ill patients. J Crit Care1998;13:159–163.CrossrefPubMed Google Scholar
40 Mahaffey KW , HarringtonRA, AkkerhuisM, et al.Systematic adjudication of myocardial infarction end-points in an international clinical trial. Curr Control Trials Cardiovasc Med2001;2:180–186.CrossrefPubMed Google Scholar
41 McGarvey LP , JohnM, AndersonJA, et al.Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax2007;62:411–415.CrossrefPubMed Google Scholar
42 Pogue J , WalterSD, YusufS. Evaluating the benefit of event adjudication of cardiovascular outcomes in large simple RCTs. Clin Trials2009;6:239–251.CrossrefPubMed Google Scholar
43 Vannabouathong C , SpragueS, BhandariM. Guidelines for fracture healing assessments in clinical trials. Part I: definitions and endpoint committees. Injury2011;42:314–316.CrossrefPubMed Google Scholar
44 Lefaivre KA , SlobogeanG, StarrAJ, et al.Methodology and interpretation of radiographic outcomes in surgically treated pelvic fractures: a systematic review. J Orthop Trauma2012;26:474–481.CrossrefPubMed Google Scholar
45 Simunovic N , WalterS, et al.Outcomes assessment in the SPRINT multicenter tibial fracture trial: Adjudication committee size has trivial effect on trial results. J Clin Epidemiol2011;64:1023–1033.CrossrefPubMed Google Scholar
46 Kooistra BW , DijkmanBG, BusseJW, et al.The radiographic union scale in tibial fractures: reliability and validity. J Orthop Trauma2010;24Suppl1:S81–S86.CrossrefPubMed Google Scholar
47 Whelan DB , BhandariM, StephenD, et al.Development of the radiographic union score for tibial fractures for the assessment of tibial fracture healing after intramedullary fixation. J Trauma2010;68:629–632.CrossrefPubMed Google Scholar
48 Kuurstra N , VannabouathongC, SpragueS, BhandariM. Guidelines for fracture healing assessments in clinical trials. Part II: electronic data capture and image management systems--Global Adjudicator™ system. Injury2011;42:317–320.CrossrefPubMed Google Scholar
49 Mann M , TendulkarA, BirgerN, HowardC, RatcliffeMB. National institutes of health funding for surgical research. Ann Surg2008;247:217–221.CrossrefPubMed Google Scholar
50 Rangel SJ , EfronB, MossRL. Recent trends in National Institutes of Health funding of surgical research. Ann Surg2002;236:277-86;discussion 286-287.:.CrossrefPubMed Google Scholar
51 Okike K , KocherMS, MehlmanCT, BhandariM. Industry-sponsored research. Injury2008;39:666–680.CrossrefPubMed Google Scholar
52 Chopra SS . MSJAMA: Industry funding of clinical trials: benefit or bias?JAMA2003;290:113–114. Google Scholar
53 Zuckerman JD , PrasarnM, KubiakEN, KovalKJ. Conflict of interest in orthopaedic research. J Bone Joint Surg [Am]2004;86-A:423–428.CrossrefPubMed Google Scholar
54 Sade RM . Dangerous liaisons? Industry relations with health professionals. J Law Med Ethics2009;37:398–400.CrossrefPubMed Google Scholar
Funding statement:
M. Bhandari reports personal fees from Smith & Nephew, Stryker, Amgen, Zimmer, Moximed, Bioventus, and grants from Smith & Nephew, DePuy, Eli Lilly, and Bioventus which are not related to this article.
Author contributions:
R. Mundi: Conception and design, Data collection/analysis, Writing paper, Reviewed paper critically for intellectual content, Final approval
H. Chaudhry: Conception and design, Data collection/analysis, Writing paper, Reviewed paper critically for intellectual content, Final approval
S. Mundi: Data collection/analysis, Writing paper, Reviewed paper critically for intellectual content, Final approval
K. Godin: Data collection, Writing paper, Final approval
M. Bhandari: Conception and design, Reviewed paper critically for intellectual content, Final approval
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










