Abstract
The aim of this study was to review the role of clinical trial networks in orthopaedic surgery. A total of two electronic databases (MEDLINE and EMBASE) were searched from inception to September 2013 with no language restrictions. Articles related to randomised controlled trials (RCTs), research networks and orthopaedic research, were identified and reviewed. The usefulness of trainee-led research collaborations is reported and our knowledge of current clinical trial infrastructure further supplements the review. Searching yielded 818 titles and abstracts, of which 12 were suitable for this review. Results are summarised and presented narratively under the following headings: 1) identifying clinically relevant research questions; 2) education and training; 3) conduct of multicentre RCTs and 4) dissemination and adoption of trial results. This review confirms growing international awareness of the important role research networks play in supporting trials in orthopaedic surgery. Multidisciplinary collaboration and adequate investment in trial infrastructure are crucial for successful delivery of RCTs.
Cite this article: Bone Joint Res 2014;3:169–74.
Introduction
Randomised controlled trials (RCTs) of healthcare interventions are generally considered to provide the highest level of primary evidence to inform clinical practice. RCTs are less common in surgical specialties, and the quality of surgical RCTs and the standard of reporting are often considered low.1 The number of RCTs in surgery is, however, increasing and this trend can also be seen within orthopaedic surgery. In a review of research published since 1975, Hanzlik et al2 demonstrate that the percentage of RCTs amongst studies published in the Journal of Bone & Joint Surgery (American Volume) has risen from 4% to 21%.2 Clinical trial networks in orthopaedic surgery play a crucial role in the success of RCTs. In this review, we discuss how orthopaedic clinical trial networks have been used internationally to meet the challenges of helping to promote RCTs in orthopaedic surgery. Using recent developments in the United Kingdom as an example, we indicate how clinical trial networks have evolved to identify important research questions, support education and training, promote and co-ordinate RCTs, and disseminate the results of RCTs. We also discuss the emergence of United Kingdom trainee research collaboratives and their role as clinical research networks in fostering collaborations and training clinicians in research methods.
Materials and Methods
For this scoping review, two electronic databases were searched from their inception to September 2013, with no language restrictions: MEDLINE (Ovid SP) (from 1948) and EMBASE (Ovid SP) (from 1974). Reference lists of included papers were also searched for potentially relevant studies and to check the quality of the search strategy. Articles were considered for inclusion if they satisfied the following three criteria: articles were related to the field of RCTs, articles discussed the use of research networks, and articles discussed the field of orthopaedic research. Search terms were developed within these three groups of terms, with both free text and MeSH terms combined, to produce a more comprehensive search strategy.
Searching yielded 818 titles and abstracts, of which 43 full text articles were retrieved for consideration in this review. Two authors (LC and LJ) reviewed full text articles for relevance, which resulted in the 12 articles that were included in this review. Disagreements were resolved through discussion, with no arbitration required. Data were extracted by two reviewers (LC and LJ) using a form based on the Cochrane Collaboration guidelines3 and refined so that they related to the area under review.
Results
The selection process we used for identification of studies can be found in the PRISMA4 flowchart in Figure 1. In total, 12 articles were included in this review,5-16 of which one was a non-English language study.8
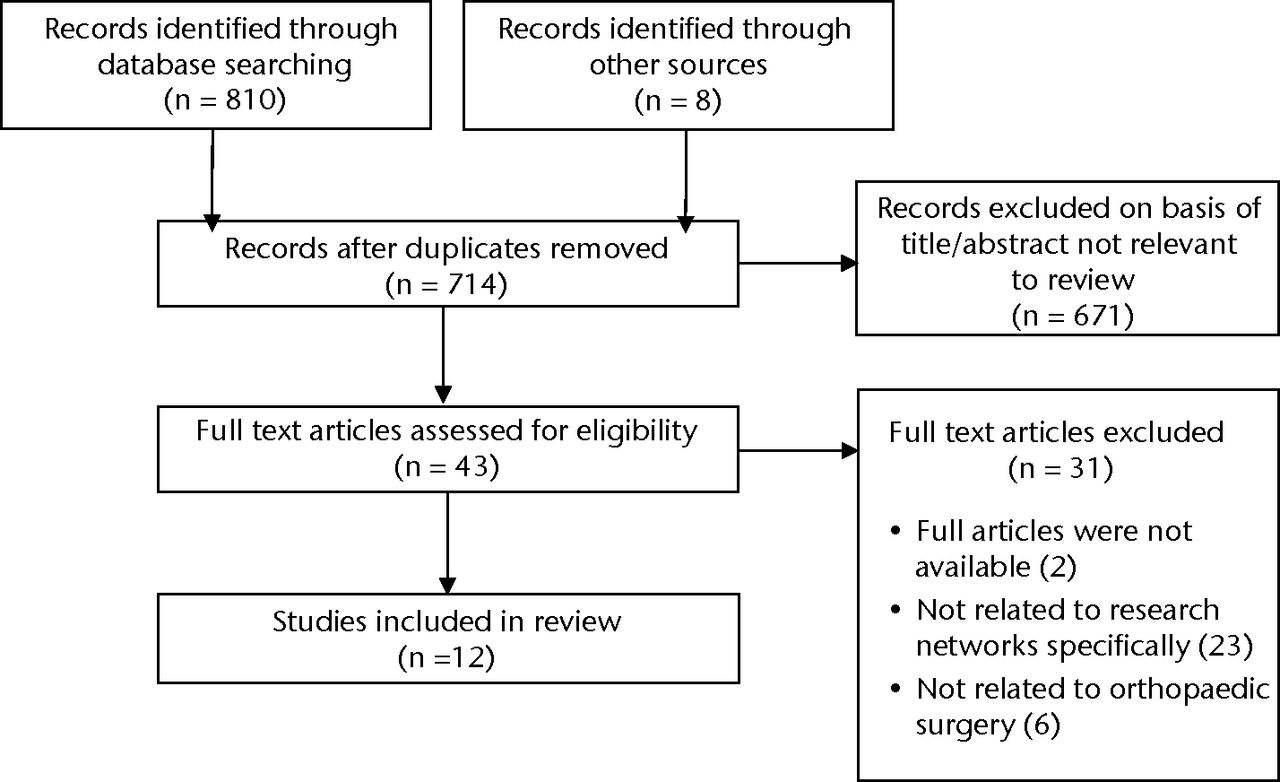
Fig. 1
Flowchart showing the study selection process
Articles suggest a growing international awareness of the important role that research networks play in encouraging and supporting RCTs in orthopaedics, with literature from Canada, Germany, the United Kingdom and the United States identified in this review. These findings will be summarised narratively under the following headings: identifying clinically relevant research questions; education and training; conduct of multicentre RCTs and dissemination and adoption of trial results.
Identifying clinically relevant research questions
Six studies identified in this review discussed the role of networks and societies in fostering collaborations between orthopaedic surgeons as a means to identify and prioritise areas for future research.5-7,9-11 Rangan et al9 suggest that consulting clinicians early in the research design process, for example through research priority-setting exercises, may instil a greater level of ‘ownership’ over research projects. This unified approach to identifying research questions may also help to prevent duplication of effort. In addition, the ability to access expertise from a wider pool of researchers and clinicians may benefit trial design and decision making; for example, Bhandari et al5 report the benefits of involving an expert panel in the estimation of recruitment rates for orthopaedic trials. Engagement of clinicians together with researchers, funders, policy makers, and health service users is advocated by the COMET (Core Outcome Measures in Effectiveness Trials) initiative, which was set up in 2010 to develop standardised core outcome sets through agreement and collaboration with a wide range of representatives.17 In terms of research prioritisation in the field of orthopaedic surgical trials, examples below highlight how clinicians have engaged in discussions either through conferences or using research methods such as a Delphi approach.
Willett et al10 describe a Delphi approach that was used to develop consensus amongst members of the AOUK (the United Kingdom branch of the Swiss AO Foundation; a group which considers research questions in surgical fracture fixation). This collaborative approach led to the successful identification of ten high priority research questions and methodological discussions about their implementation.10
Findings from a symposium of the American Academy of Orthopaedic Surgeons (AAOS) and the Orthopaedic Research Society (ORS) in 2009 are presented in three papers included in this review, authored by Katz, Losina and Wright.6,7,11 To promote the research culture in orthopaedics, Wright et al11 advocate the involvement of leaders in the field to encourage the participation of colleagues in multicentre trials. Katz et al6 describe a framework which aims to enable researchers to set priorities for future research by considering aspects such as cost, feasibility and anticipated benefit of conducting a RCT.6 This article also presents specific questions for future prioritisation in the field of orthopaedic trials that were considered at this symposium.6
Similarly, the International Hip Fracture Research Collaborative (IHFRC), based in Canada and comprised of 310 international members, with expertise in orthopaedics and trial methodology, aims to identify research priority areas and inform the design and co-ordination of large multinational RCTs in the field of hip fractures.4 In their overview of the IHFRC collaborative, Bhandari et al5 suggest that larger groups such as this may bridge the gap between multiple smaller groups internationally, and act as a single point of reference for researchers and clinicians. A total of two multicentre and multinational trials have since been initiated through the IHFRC group: The FAITH trial (Fixation using Alternative Implants for the Treatment of Hip fractures: a multicentre randomised trial) and the HEALTH trial (Hip fracture Evaluation with ALternatives of Total Hip arthoplasty versus hemi-arthroplasty: a multicentre trial).5
Education and training
The need for greater education and training amongst clinicians has been widely acknowledged, with studies in this review demonstrating the role that research networks can have in implementing these improvements in the field of orthopaedics. In the United Kingdom, the Department of Health’s commitment of ‘Best Research for Best Health’ in 2006,18 led to the development of the United Kingdom Clinical Research Network (UKCRN) and 25 Comprehensive Local Research Networks (CLRNs) with the aim of supporting researchers running or co-ordinating studies throughout the network. As part of the UKCRN, national Specialty Groups exist in a range of areas, of which two groups are of relevance to orthopaedic research: the ‘Musculoskeletal’ and ‘Injuries and Emergencies’ groups.19 These networks offer a number of functions that promote good research practice in the United Kingdom by facilitating the successful completion of ongoing trials and fostering collaborations with clinicians and researchers with similar research interests. For ongoing trials, recruitment is monitored monthly for the networks’ ‘portfolio’ of studies, with independent peer support and guidance offered to trials to ensure they deliver research in time and on target.19 Meanwhile, for those wishing to develop research ideas or collaborate as principal investigators in current national trials, these specialty groups provide local and national contact details of clinicians with research interests in similar fields, lists of ongoing trials that are currently open to recruitment and support on Patient and Public Involvement in research. In addition, Rangan et al9 note that these research networks can now be used to facilitate training in areas such as the International Conference on Harmonisation’s Good Clinical Practice (ICH-GCP), which is a pre-requisite for undertaking RCTs in the United Kingdom as it outlines key aspects of research governance.
Meanwhile, in the United States, Trippel et al15 have highlighted the importance of orthopaedic specialist societies, such as the Clinical Trials Network, established in the United States by the Paediatric Orthopaedic Society of North America, which builds research infrastructure through the development of guidelines and training for investigators. Wright et al11 also stress the importance of greater training provision for clinicians in their summary report from the AAOS and ORS symposium.
Through greater involvement in trials and collaboration with more experienced lead clinicians, it is hoped that the infrastructure and research culture may improve in orthopaedics. For example, international fellowships such as the American British Canadian (ABC) travelling fellowship, have been successfully used to promote international collaboration.9 Yeung and Bhandari12 urge that orthopaedic surgeons in countries proficient in undertaking trials should foster collaborations with colleagues internationally in order to promote the research culture and provide necessary training, but also to improve the external validity of trials to specific settings. Through exploring the rates of RCTs published on hip fractures internationally, this article demonstrates high levels of variance across countries in terms of their contributions in this area. Trials were most common in the United Kingdom and Scandinavian countries, with Canada and the US contributing only one tenth of the number of trials conducted in Europe.12
Conduct of multicentre RCTs
Studies included in this review suggest a number of ways through which trial networks and societies can aid the conduct of multicentre RCTs in orthopaedics, such as the identification of and co-ordination between skilled research staff. Their role may be even more important where multinational trials are concerned, with localised systems of gaining research approvals and language and communication barriers creating difficulties. The IHFRC collaboration based in Canada have a central ‘Methods Centre’ where experienced trial methodologists co-ordinate the FAITH and HEALTH trials, with specific ‘Country Offices’ responsible for the day-to-day liaison with centres.5
Research networks can be used to develop links between research communities, which may be vitally important, as effective liaison between clinical, academic and regulatory roles in trials is crucial to their success. In an abstract presented at the British Society of Rheumatology, Scott14 made recommendations for a ‘National Arthritis Research Network’ to facilitate the recruitment of patients and participation of rheumatology and orthopaedic clinicians in trials in this field. This paper suggests a specific network such as this could act as a point of liaison with researchers, clinicians, Research and Development departments and local Clinical Research Networks, to overcome regulatory issues associated with implementing research studies, as well as providing advice and training for clinicians wishing to participate in research.14
In Germany, Otto et al8 have advocated the use of research networks on a central and regional basis to generate support and links between researchers in orthopaedic trials.8 This article draws upon the authors’ experiences of setting up a Clinical Trials Unit for trauma and orthopaedic surgery, and highlights the role that networks can play in obtaining skilled project staff.8 This point is also raised by Carr and Cooper13 in their presentation of recruitment data from a multicentre RCT of treatments for rotator cuff injuries, the UKUFF trial. This paper suggests a beneficial effect on recruitment was obtained when sites were supported through the local research network (i.e. through research nurse provision), with supported sites recruiting almost twice as many patients over the course of the trial.13 Rangan et al9 have also described how the ProFHER trial, a multicentre RCT of surgical versus non-surgical intervention for fractures of the proximal humerus,20 has benefited from the support of the CLRNs in the UK, which were able to allocate research nurse or physiotherapist support to the study through either service support costs or research capability funding.
Aside from the practical assistance that networks may provide in the day-to-day running of trials, Vitale et al16 describe the involvement of a clinical research network, launched in 1999 by the Paediatric Orthopaedic Society of North America, in developing a trauma registry to record and manage clinical outcome data in paediatric musculoskeletal trauma across five centres. The authors, however, acknowledge that the usefulness of such registers to promote multicentre research activity, may be limited by regulatory challenges associated with storing patient-identifiable data.16
Dissemination and adoption of trial results
The active promotion of trials amongst the medical community is important not only during the early phases of design and eventual conduct of the trial, but also crucially when disseminating trial results, in order for them to be adopted into medical practice. Larger multicentre RCTs with wider participation improves generalisability and facilitates adoption of trial findings into clinical practice. In the United Kingdom, Clinical Commissioning Groups and bodies such as National Institute for Health and Care Excellence (NICE)21 use trial findings to inform healthcare commissioning, thus facilitating adoption into clinical practice. Rangan et al9 have described the importance of this at a national and international level, and suggest specialist societies, such as the British Elbow and Shoulder Society (BESS) can be used to provide this interface between researchers and the medical community. Furthermore, these societies may produce guidelines for best practice for their members based upon the reports of RCTs in the field.9
Trainee-led research collaboratives
Current United Kingdom health research policy, based on the Cooksey report,22 encourages collaborative research, with the National Health Service (NHS) becoming a partner as opposed to a mere receptacle in research. This in turn has led to development of trainee research collaboratives, which empower trainees in these roles.23,24 They offer an environment in which trainees can support each other, develop research ideas and attain the necessary skills to remain research active beyond completion of their higher surgical training.
In 2007, general surgical trainees in the West Midlands set up the first trainee-led research collaborative in the United Kingdom.25 The West Midlands Research Collaborative (WMRC) aimed to use the natural network formed by surgical trainees to run multicentre RCTs. Their initial success encouraged other regions to develop like-minded research groups. With over 15 active research groups covering England, Scotland and Wales, the general surgical trainees now have an effective research network, which has enabled them to complete a range of prospective clinical trials and co-ordinated audit projects.26-29 Clinical audits, in addition to a variety of surveys,29-31 systematic reviews/meta-analyses,32,33 and cohort studies28 have helped to identify gaps in current knowledge and highlight variations in clinical practice. Such information has been successfully used to generate hypotheses and research questions, and provides the background and justification for subsequent prospective randomised trials.27
The general surgical model has subsequently been adopted by a number of other surgical specialties including neurosurgery, urology, paediatric surgery, ENT, cardiothoracic surgery, plastic/reconstructive surgery and orthopaedics.34 The importance of effective communication, interaction and information-sharing, both within and between groups, is recognised and encouraged and is supported by national events such as the National Research Collaborative Meeting.35
To date, trainee-led research groups in surgical specialties have attracted almost £2 million in research funding, mainly from the National Institute for Health Research (NIHR) for trials like ROSSINI (ISRCTN40402832), DREAMS (ISRCTN21973627) and Hughes Abdominal Repair Trial (HART) (ISRCTN25616490).
Trainees in orthopaedic surgery have established similar clinical research networks. The Collaborative Orthopaedic Research NETwork (CORNET), set up by trainees in the Northern Deanery36 and the South Yorkshire Trainees’ Orthopaedic Research Collaborative (STORC) in the Yorkshire Deanery are examples of regional collaboratives. To supplement the work of these regional groups, the British Orthopaedic Trainees Association (BOTA) recently announced the launch of the British Orthopaedic Network Environment (BONE). This trainee-led initiative aims to provide a platform for nationally co-ordinated research and audit while also supporting orthopaedic leadership and education.
Discussion
Established collaborations exist between clinicians, allied health professionals and supporting networks for delivery of clinical care. Using such networks for running clinical trials has proved effective, with examples highlighted in this review. Although there are variations in the structure of such collaborations in different countries, the underlying principles remain the same. An appropriate skill mix including clinicians, patients, trial methodologists, systematic reviewers, health economists, epidemiologists and statisticians, is essential for effective design and efficient conduct of trials. Collaborative work between clinical trial units, academic institutions and clinical networks, with sufficient investment to build research capacity within the clinical networks, generates an efficient system for running clinical trials.
The increasing number of RCTs in orthopaedic surgery demonstrates that surgeons are willing and able to randomise patients into clinical trials. There is also an appetite to answer fundamental questions facing the specialty by conducting RCTs to generate high-quality evidence. Involvement of clinicians from prioritisation of research questions through to conduct and dissemination of trial findings is crucial, and there is evidence of a high level of engagement both from fully trained practising surgeons and from trainees. Clinician involvement at these different stages in their careers should help provide continuity to a programme of research, which should include RCTs that produce high-quality evidence to inform clinical practice.
Large UK-wide multicentre RCTs like the Proximal Fracture of the Humerus: Evaluation by Randomisation (ProFHER), UKUFF and Distal Acute Fracture Fixation trial (DRAFFT), which have successfully completed recruitment, have benefited considerably from research associate (RA) support from the CLRN.37 We have found that trained RAs assume a position of equipoise effectively (whereby there is no preferred treatment and a balanced account of both treatment options is given to patients) and spend sufficient time with patients explaining the study, which has resulted in good consent rates from eligible patients. RAs also help ensure that clinical trials are run in compliance with good clinical practice (ICH-GCP) and research governance regulations. In conjunction with principal investigators drawn from specialist societies of the British Orthopaedic Association, the RAs have played an essential part in the successful conclusion of these trials in the UK. The infrastructure of the current UK trials is sufficiently robust to ensure that national portfolio clinical trials are run to a high methodological and ethical standard. A consequence of establishing such a successful trials network is the opportunity to develop more challenging trial designs to address more complex issues. In order to further improve generalisability and acceptability of trial findings, international collaboration in trials becomes important, and developing wider multinational clinical networks should be part of our clinical trials strategy for the future.
1 Ziebland S , FeatherstoneK, SnowdonC, et al.Does it matter if clinicians recruiting for a trial don't understand what the trial is really about? Qualitative study of surgeons' experiences of participation in a pragmatic multi-centre RCT. Trials2007;8:4.CrossrefPubMed Google Scholar
2 Hanzlik S , MahabirRC, BaynosaRC, KhiabaniKT. Levels of evidence in research published in The Journal of Bone and Joint Surgery (American Volume) over the last thirty years. J Bone Joint Surg [Am]2009;91-A:425–428.CrossrefPubMed Google Scholar
3 Reeves BC , DeeksJJ, HigginsJPT, WellsGAIncluding non-randomized studies. In: Higgins JPT, Green, S, ed. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.2: updated September 2009. Available from www.cochrane-handbook.org: The Cochrane Collaboration, 2009 (date last accessed 16 April 2014). Google Scholar
4 Moher D , LiberatiA, TetzlaffJ, AltmanDG. ; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol2009;62:1006–1012. Google Scholar
5 Bhandari M , SpragueS, SchemitschEH. ; International Hip Fracture Research Collaborative. Resolving controversies in hip fracture care: the need for large collaborative trials in hip fractures. J Orthop Trauma2009;23:479–484. Google Scholar
6 Katz JN , WrightJG, LosinaE. Clinical trials in orthopaedics research. Part II. Prioritization for randomized controlled clinical trials. J Bone Joint Surg [Am]2011;93-A:30.CrossrefPubMed Google Scholar
7 Losina E , WrightJ, KatzJN. Clinical trials in orthopaedics research. Part III. Overcoming operational challenges in the design and conduct of randomized clinical trials in orthopaedic surgery. J Bone Joint Surg [Am]2012;94-A:35.CrossrefPubMed Google Scholar
8 Otto C , SieweJ, ZarghooniK, et al.[Clinical research in orthopaedics--creation of a clinical trial unit in orthopaedics/trauma surgery]. Z Orthop Unfall2010;148:145-148. [Article in German].CrossrefPubMed Google Scholar
9 Rangan A , BrealeyS, CarrA. Orthopaedic trial networks. J Bone Joint Surg [Am]2012;94-A(Suppl1):97–100.CrossrefPubMed Google Scholar
10 Willett KM , GrayB, MoranCG, GiannoudisPV, PallisterI. Orthopaedic trauma research priority-setting exercise and development of a research network. Injury2010;41:763–767.CrossrefPubMed Google Scholar
11 Wright JG , KatzJN, LosinaE. Clinical trials in orthopaedics research. Part I. Cultural and practical barriers to randomized trials in orthopaedics. J Bone Joint Surg [Am]2011;93-A:15.CrossrefPubMed Google Scholar
12 Yeung M , BhandariM. Uneven global distribution of randomized trials in hip fracture surgery. Acta Orthop2012;83:328–333.CrossrefPubMed Google Scholar
13 Carr A , CooperC. The UKUFF trial and the NIHR comprehensive local research networks. Should Elbow2009;1:63–64. Google Scholar
14 Scott D . Building a UK clinical research network in rheumatology. Rheumatology2011;50:26. Google Scholar
15 Trippel SB , BosseMJ, HeckDA, WrightJG. Symposium. How to participate in orthopaedic randomized clinical trials. J Bone Joint Surg [Am]2007;89-A:1856–1864.CrossrefPubMed Google Scholar
16 Vitale MG , VitaleMA, LehmannCL, et al.Towards a National Pediatric Musculoskeletal Trauma Outcomes Registry: the Pediatric Orthopaedic Trauma Outcomes Research Group (POTORG) experience. J Pediatr Orthop2006;26:151–156.CrossrefPubMed Google Scholar
17 Williamson, P, Clarke M. The COMET (Core Outcome Measures in Effectiveness Trials) Initiative: Its Role in Improving Cochrane Reviews. Cochrane Database Syst Rev 2012;5:ED000041. Google Scholar
18 No authors listed. Department of Health: Best Research for Best Health. A new national health research strategy https://www.gov.uk (date last accessed 16 April 2014). Google Scholar
19 No authors listed. National Institute for Health Research Clinical Research Network http://www.crn.nihr.ac.uk/ (date last accessed 8 May 2014). Google Scholar
20 Handoll H , BrealeyS, RanganA, et al.Protocol for the ProFHER (PROximal Fracture of the Humerus: Evaluation by Randomisation) trial: a pragmatic multi-centre randomised controlled trial of surgical versus non-surgical treatment for proximal fracture of the humerus in adults. BMC Musculoskelet Disord2009;10:140.CrossrefPubMed Google Scholar
21 No authors listed. National Institute for Health and Care Excellence, 2013. www.nice.org.uk (date last accessed 3 March 2014). Google Scholar
22 Cooksey D. A review of UK health research funding, 2006. http://www.official-documents.gov.uk/document/other/0118404881/0118404881.pdf (date last accessed 3 March 2014) Google Scholar
23 No authors listed. A trainee led research collaborative can facilitate trainee exposure to clinical research and provide excellent education and training Br J Surg2011;98(S3):74. Google Scholar
24 Kolias AG , CowieCJ, TarnarisA, et al.Ensuring a bright future for clinical research in surgery with trainee led research networks. BMJ2013;347:5225.CrossrefPubMed Google Scholar
25 No authors listed. West Midlands Research Collaborative. http://www.wmresearch.org.uk (date last accessed 12 May 2014). Google Scholar
26 National Surgical Research Collaborative. Multicentre observational study of performance variation in provision and outcome of emergency appendicectomy Br J Surg2013;100:1240–1252. Google Scholar
27 Pinkney TD , CalvertM, BartlettDC, et al.Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI Trial). BMJ2013;347:4305.CrossrefPubMed Google Scholar
28 Naqvi M , WardST, DowswellG, DonnellyJ. , SWIFT group collaborators & the West Midlands Research Collaborative (WMRC). The influence of key clinical practices on the knowledge of first year doctors about the patients under their care. Int J Clin Pract2013;67:181–188. Google Scholar
29 Jaunoo SS , HaleAL, MastersJP, JaunooSR. An international survey of opinion regarding investigation of possible appendicitis and laparoscopic management of a macroscopically normal appendix. Ann R Coll Surg Engl2012;94:476–480.CrossrefPubMed Google Scholar
30 Bhangu A , NepogodievD, GuptaA, et al.Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg2012;99:1501–1513.CrossrefPubMed Google Scholar
31 Aiken AM , HaddowJB, SymonsNR, et al.Use of antibiotic prophylaxis in elective inguinal hernia repair in adults in London and south-east England: a cross-sectional survey. Hernia2013;17:657–664.CrossrefPubMed Google Scholar
32 Gheorghe A , CalvertM, PinkneyTD, et al.Systematic review of the clinical effectiveness of wound-edge protection devices in reducing surgical site infection in patients undergoing open abdominal surgery. Ann Surg2012;255:1017–1029.CrossrefPubMed Google Scholar
33 Dias J, Marsh D. British Orthopaedic Association: Research Strategy, 2012. http://www.boa.ac.uk/LIB/LIBPUB/Documents/BOA%20Research%20Strategy.pdf (date last accessed 3 March 2014). Google Scholar
34 Bhangu A , KoliasAG, PinkneyT, HallNJ, FitzgeraldJE. Surgical research collaboratives in the UK. Lancet2013;382:1091–1092.CrossrefPubMed Google Scholar
35 No authors listed. National Research Collaborative. http://www.nationalresearch.org.uk/ (date last accessed 3 March 2014). Google Scholar
36 No authors listed. Collabative Orthopaedic Research Network, 2013. www.cornetresearch.co.uk (date last accessed 3 March 2014). Google Scholar
37 No authors listed. United Kingdom Clinical Research Network: Portfolio database. http://public.ukcrn.org.uk/Search/Portfolio.aspx (date last accessed 3 March 2014). Google Scholar
Funding statement:
Prof. A. Rangan reports grants from DePuy UK Ltd, and personal fees from JRI Ltd, which are not related to this article; in addition, Prof. A. Rangan has a UK and European patent pending that is not related to this article.
Author contributions:
A. Rangan: Co-ordinated and led the project, Contributed, edited and combined written sections to the paper, Produced and submitted final manuscript
L. Jefferson: Contributed written sections to the paper, Reviewed final manuscript, Undertook searching and initial screening of article titles and abstracts, Screened article full texts for inclusion and completed data extraction
P. Baker: Contributed written sections to the paper, Reviewed final manuscript
L. Cook: Contributed written sections to the paper, Reviewed final manuscript, Screened article full texts for inclusion, Completed data extraction.
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.
Supplementary material. A table showing the full search strategies used in this article is available alongside the online version of this article www.bjr.boneandjoint.org.uk










