Abstract
Objectives
Although many clinical and experimental investigations have shed light on muscle atrophy and intramuscular accumulation of fat after rotator cuff disruption, none have reported on their onset in the absence of muscle retraction.
Methods
In 30 rabbits, we detached one supraspinatus (SSP) tendon and repaired it immediately, thus preventing muscle retraction. The animals were killed in groups of 10 at one, two and six weeks. Both shoulders of 15 non-operated rabbits served as controls. We measured the weight and volume of SSP muscles and quantified the cross-sectional area of intramuscular fat (i-fat) histologically.
Results
There was significant loss of muscle weight and volume after one week (p = 0.004 and 0.003, respectively), and two weeks (both p < 0.001) in the experimental group; which recovered to control values after six weeks. I-fat accumulated one week after immediate repair, greater than in the control group and statistically significant at the mid-part of the muscle (mean 2.7% vs 1.5%, p = 0.008). I-fat continued to accumulate up to six weeks at all sites of the SSP muscle (all 3, p < 0.001). More fat accumulated closer to the musculotendinous junction than at the mid-part after two and six weeks (p = 0.012 and 0.019, respectively).
Conclusion
Muscle atrophy and i-fat accumulation occur early after SSP tendon tear and immediate repair. While early repair benefitted muscle recovery, it did not prevent fat accumulation. SSP muscle retraction was not essential to the muscle alterations. The divergent evolution of muscle and fat points to different pathophysiologies.
Cite this article: Bone Joint Res 2014;3:117–22.
Article focus
To elucidate the early appearance of muscle atrophy and intramuscular fat (i-fat) accumulation after supraspinatus tendon tear.
To measure the occurrence of these muscle changes in the absence of muscle retraction
To investigate the possibility of reversal of these changes.
Key messages
Muscle atrophy and i-fat accumulation develop early after supraspinatus tendon tear.
Muscle retraction is not essential for the development of these changes.
Very early reattachment leads to recovery of muscle atrophy but not fat accumulation.
Strengths and limitations
Direct reproducible measurements of early muscle changes.
Very early reattachment reverses muscle atrophy.
As in any animal model, the juxtaposition to the clinical situation is a limitation.
Introduction
The pathology of rotator cuff tears continues to arouse the interest of clinicians1,2 and researchers3-10 alike. In particular, the muscle atrophy and accumulation of intramuscular fat (i-fat) in the torn supraspinatus (SSP) muscle, as well as their clinical relevance, has been investigated and discussed.7,10-14 However, the timing and evolution of muscle and i-fat changes, in particular when related to the earliest moments of onset of muscle atrophy and of i-fat accumulation, remain unanswered.15 We reported i-fat accumulation four, eight and 12 weeks after transection of the SSP tendon.14 Then, Gayton et al16 reported on i-fat accumulation after transection of the SSP tendon in rabbits that was present three months post-operatively, the earliest time point of their investigation. In a recent study Rowshan et al17 sectioned the tendon of the subscapular muscle in rabbits and did not find any evidence of muscle atrophy, nor of i-fat accumulation at two weeks. After six weeks, however, both changes were present.
Animal models have included surgical detachment of rotator cuff tendons.3,14,17-19 Whether the SSP muscle alterations are related to the tendon tear, to retraction of the SSP muscle or to both, is unclear. Retraction of the SSP muscle may contribute to muscle atrophy by sarcomere loss through adaptive shortening and to the fat accumulation.20 Mechanical traction on the suprascapular nerve (SSN) at the scapular notch brought about by SSP muscle retraction may also contribute to the alterations.21 The contribution of muscle retraction to SSP muscle alterations is clinically important as it can influence the timing of surgical repair of a SSP tendon tear; an earlier repair preventing retraction may optimise clinical outcome.22
To find out the onset, differential recovery and role of retraction in muscle atrophy and i-fat accumulation, we devised an experiment in rabbits in which the SSP tendons would be detached unilaterally, and immediately reattached, to bring about the earliest possible repair, thus precluding any muscle retraction. We determined the volume and weight of the SSP muscles at one, two and six weeks post-operatively and measured the presence of i-fat histologically at three levels of the SSP muscles (proximal quarter, middle half, distal quarter) at the same time points both in experimental and control shoulders. We hypothesised that both muscle atrophy and i-fat accumulation occur early after transection of the SSP tendon and develop in the absence of muscle retraction.
Materials and Methods
Surgical repair of the rotator cuff
A total of 30 adult female white New Zealand rabbits (3.4 kg to 4.3 kg) received a ketamine, midazolam, and glycopyrrolate injection followed by isofluorane anesthesia pre-operatively. One shoulder of each rabbit was operated, alternating right and left sides. The protocol for the surgical detachment surgery has been described in a previous publication.14 Briefly, a lateral skin incision followed by splitting of omovertebral and deltoid muscles, exposed the SSP tendon at its insertion into the greater tuberosity. The tendon was transected close to its insertion, and any tendinous or fibrocartilaginous tissue still attached to the greater tuberosity was removed. There was no retraction of the tendon-muscle unit. The tendon was immediately reattached using the following technique: a 2 x 2 x 5 mm bony trough was made between the rim of the wall of the tuberosity and the articular cartilage using a burr. Three 1 mm holes were drilled from the lateral aspect of the greater tuberosity into the bony trough. Two nonabsorbable 3 - 0 prolene sutures were then placed as follows: the first thread was passed first through the most proximal drill hole, then through the tendon in a modified Mason-Allen fashion23 and finally exited through the middle drill hole. The second thread was passed first through the middle drill hole, then passed through the tendon in a similar fashion to the first suture and brought through the distal drill hole. Both sutures were tied over the lateral aspect of the cortex, thus adapting easily the tendon stump to the bony trough. The wound was closed in layers. The shoulder was not immobilised post-operatively; the rabbits could actively move their shoulder and they had free access to water and food. Pain control was achieved with transdermal fentanyl for four days and subcutaneous buprenorphine for three days. A total of 30 shoulders of 15 non-operated, weight and sex-matched rabbits, served as controls.
As the rabbits protected their operated forelimb for a few days, a fact that could have led to an overuse of the non-operated forelimb and thus may have influenced the muscle volume, we preferred to use both shoulders of 15 non-operated weight- and sex-matched rabbits as controls.
Specimen collection
The rabbits of the experimental group were killed with a pentobarbital overdose in three groups of 10, one group at one week and the other groups at two and six weeks after surgery. The shoulders were dissected, care being taken to remove the entire scapula in one piece including the SSP muscle, its tendon and the proximal part of the humerus. The SSP muscles were then carefully freed from the scapula, the extramuscular fat was removed and weight and volume were determined. Thereafter, the muscles were fixed for a minimum of one week in 4% paraformaldehyde. Three cross-sectional blocks 2 mm wide were cut at the proximal quarter, the mid-part and the distal quarter of the muscle belly. The blocks were placed in an osmium tetroxide solution on a rotating plate for one week. The solution was then changed and blocks left in the solution for another week. The tissues were then washed in a 50 ml centrifuge tube with water and placed thereafter in 60% alcohol. The blocks were embedded in paraffin in the vacuum oven, cut in 6 µm thin sections and counterstained with hematoxylin-eosin. The transverse sections were photographed using a Pixelink Megapixel FireWire camera (Vitana Corporation, Ottawa, Canada), mounted onto an Olympus SZ61 dissection microscope (Olympus Corporation, Tokyo, Japan). The magnification used was x 6.7. Entire SSP muscle cross-sectional areas were measured using ImageJ (1.34s, National Institutes of Health, Bethesda, Maryland).14 The area of fat, stained black, was measured the same way using the entire cross-sectional area.14 The percentage of the entire cross-sectional area occupied by fat was established.
The same post-mortem procedures were used for the 30 SSP muscles of the 15 control rabbits. All measurements were automated and performed randomly and blindly.
Statistical analysis
A database was built using SPSS statistical software version 20.0 (IBM, Armonk, New York). First we compared the muscle weight, volume and i-fat of the experimental rabbits at one, two and six weeks using a one-way ANOVA with post hoc Gabriel tests. We then compared the control rabbits with the experimental rabbits for each time point using a one-way ANOVA with post-hoc Hochberg’s tests, due to the uneven sample size. Finally, the distal quarter i-fat was compared with the mid-part and proximal quarters with 2-tailed paired t-tests. A p value of < 0.05 was considered statistically significant.
Ethics
This original model was approved by our University’s institutional Animal Care Committee on July 15, 2004 under protocol # ME-184 and renewed for this study.
Results
All surgical wounds healed primarily. At sacrifice, no dehiscence at the site of repair was observed. The mean weight of rabbits at harvest of the experimental group was not statistically different from that of 15 non-operated rabbits (3.9 kg (2.6 to 4.7) vs 4.1 kg (2.9 to 4.7) respectively).
Early loss of muscle weight was evident one and two weeks after detachment and immediate reattachment of the SSP muscle compared with controls (p = 0.004 and p = 0.000 respectively) (Fig. 1). By six weeks, SSP muscle weights had returned to control values.
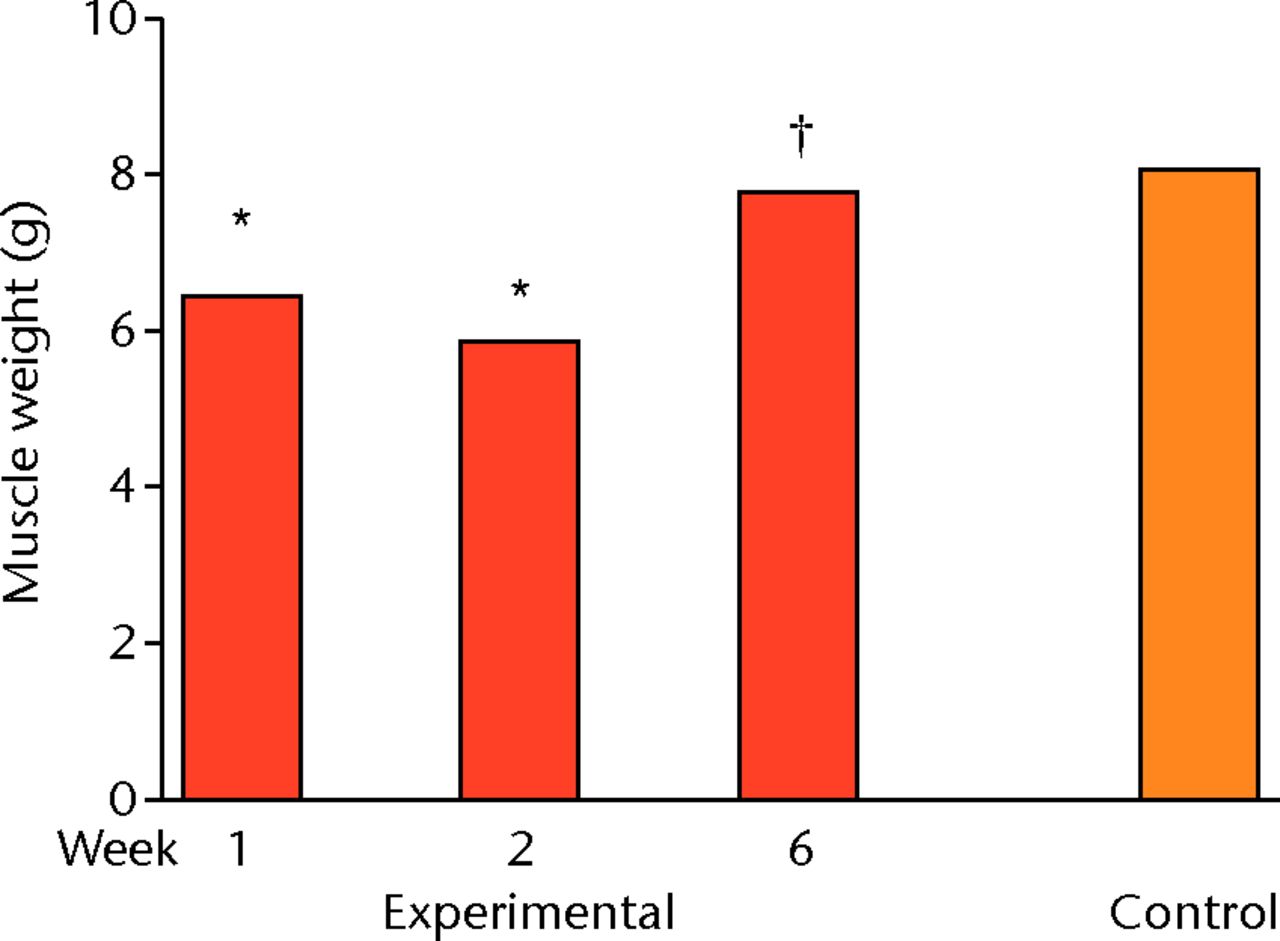
Fig. 1
Bar graph showing muscle weight one, two and six weeks after detachment and immediate repair of the supraspinatus (SSP) and in controls. Muscle weights in experimental shoulders are lower after one and two weeks but restored after six weeks. *p < 0.05 compared with control; † p < 0.05 compared with Week 2
The results for muscle volume were similar to those for weight, with mean volume in the experimental group at one week (6.4 ml, p = 0.003) and two weeks (5.9 ml, p < 0.0001) significantly less than the control value (8.0). By six weeks, SSP muscle volume had returned to control values (p = 0.989).
Measurement of i-fat showed early fat accumulation compared with controls one week (statistically significant (p = 0.005) at mid-part), two weeks (mid-part (p = 0.001), distal quarter (p = 0.016)), and six weeks (p = 0.000 at all sites) after detachment and immediate reattachment of the SSP tendon (Figs 2 and 3). Contrary to muscle weight and volume, i-fat continued to accumulate six weeks after repair (Fig. 3). Fat deposition occurred almost exclusively between muscle bundles. The fat accumulation occurred in an increasing proximal-to-distal gradient and was statistically significant (p = 0.012 and p = 0.019 respectively) at two and six weeks (Fig. 3). No gradient was observed in control SSP muscles.
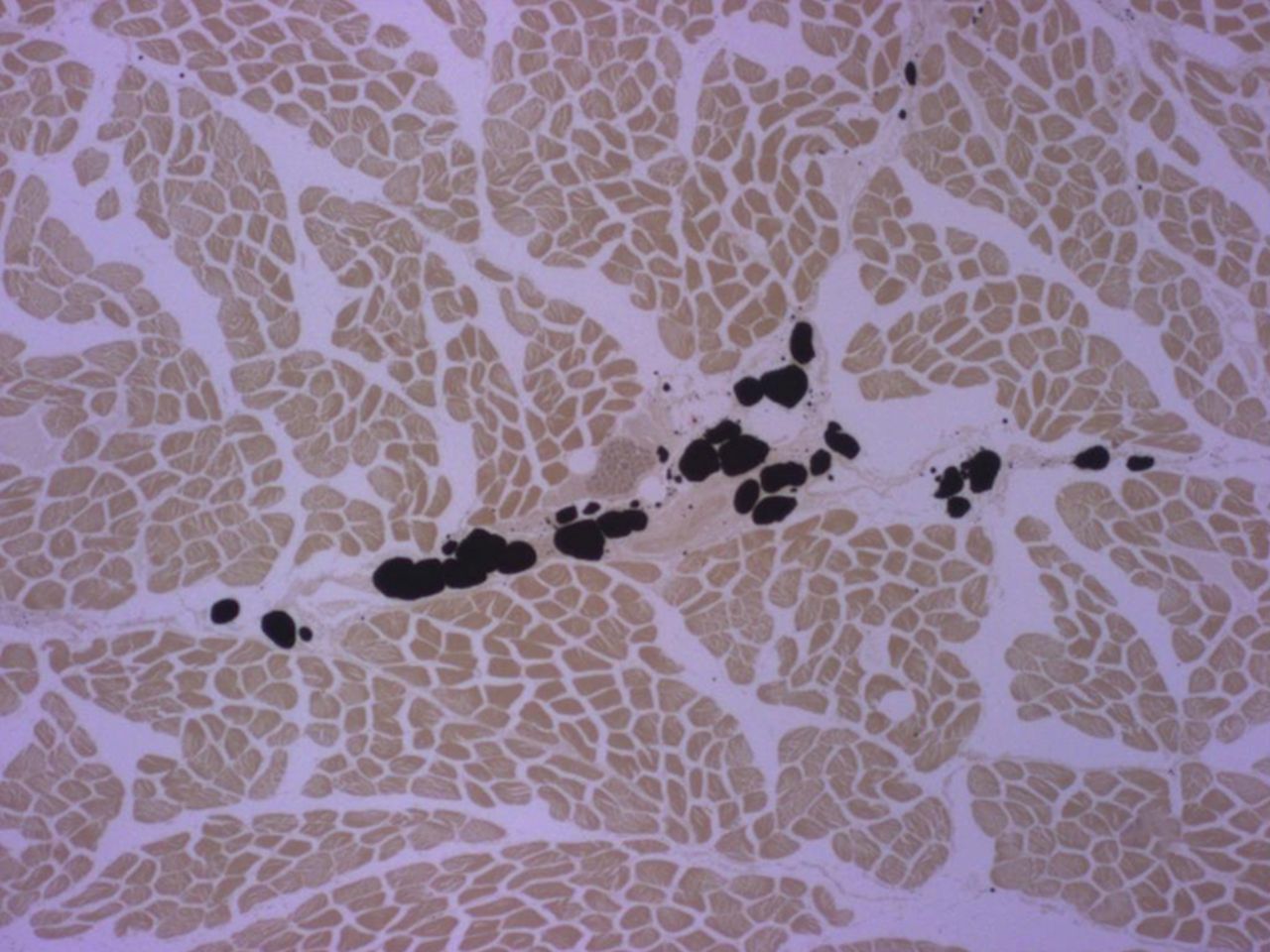
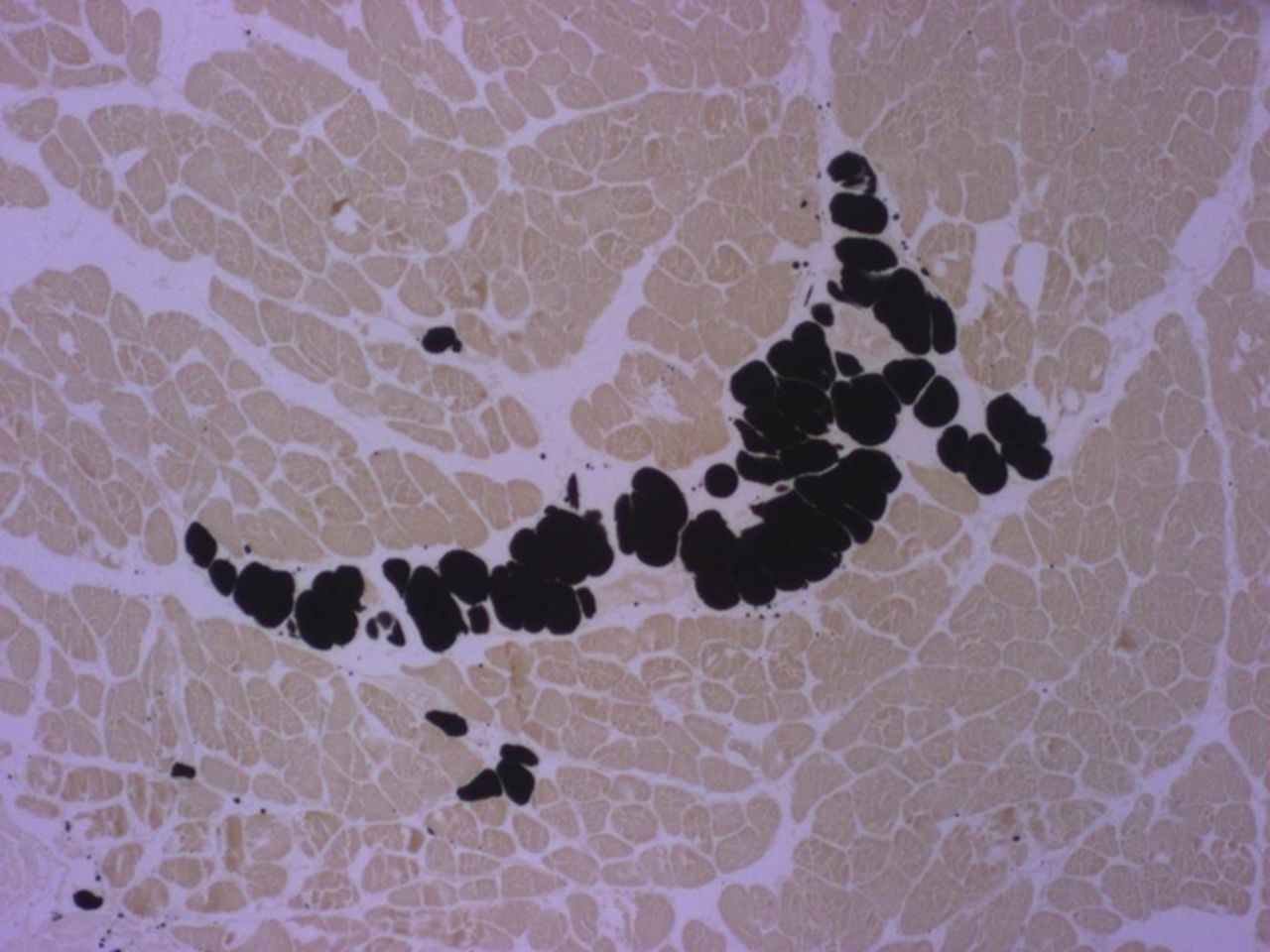
Figs. 2a - 2b
Micrographs of cross-sections at the mid-part of rabbit supraspinati (SSP); the black staining represents fat vacuoles within adipocytes called ‘intramuscular fat’ (i-fat). a) Control specimen. b) Experimental specimen six weeks after detachment and immediate reattachment of the SSP tendon. I-fat accumulated between the muscle bundles. Osmium tetroxide; x 25.
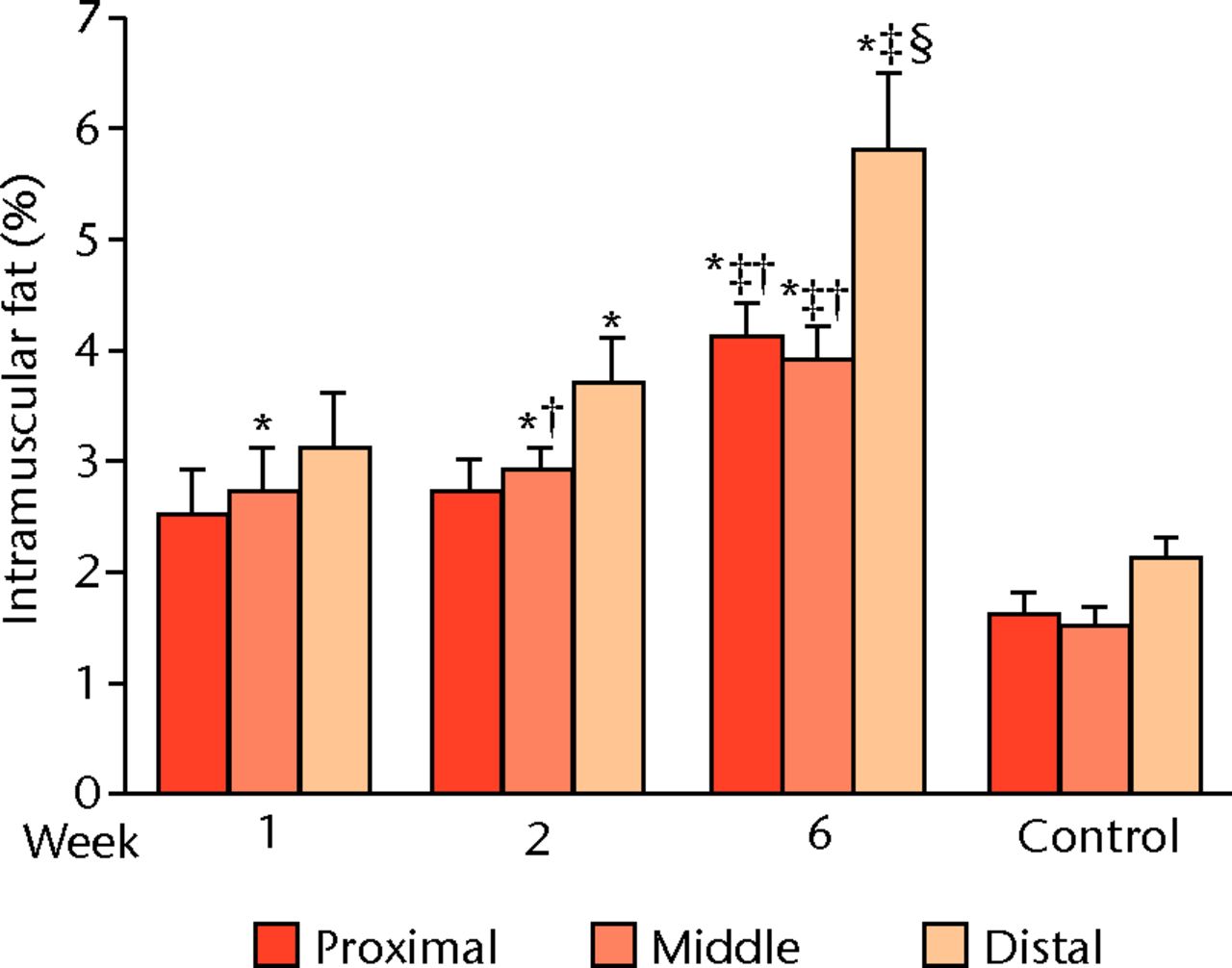
Fig. 3
Bar graph of intramuscular fat (i-fat) at the proximal quarter, the mid-part and the distal quarter showed a proximal-to-distal gradient with the greatest accumulation close to the musculotendinous junction (distal quarter). Control supraspinati (SSP) did not display this gradient. *p < 0.05 compared with control; † p < 0.05 compared with distal ‡ p < 0.05 compared with Week 1; § p < 0.05 compared with Week 2.
Discussion
Our study demonstrates a statistically significant loss of SSP muscle weight and volume, one and two weeks after immediate repair. By six weeks, muscle weight and volume had returned to control values. Our investigation also shows that SSP tendon transection, followed by an immediate reattachment, leads to early i-fat accumulation. Compared with controls, there was already more i-fat by one week in the reattached SSP muscle than in controls. Contrary to muscle weight and volume, i-fat accumulation did not return to normal levels. It continued to accumulate to reach a more than twofold increase at six weeks. I-fat accumulated in a proximal-to-distal gradient.
These results confirmed our first hypothesis that both, muscle atrophy and i-fat accumulation occur very early, one week after transection of the SSP tendon. Our findings improve on the results of our previous study from a different group of rabbits,14 where tendons detached for durations ranging from four to 12 weeks, caused muscle atrophy and i-fat accumulation in the SSP muscle and of Rowshan et al17 who did not detect any loss of muscle weight nor any increase in i-fat two weeks after transection of the subscapularis tendon in rabbits. Our results also demonstrate the ability of the muscle to recover normal weight and volume after an immediate SSP tendon reattachment, confirming a beneficial effect of early surgical repair. Late SSP tendon repair (12 weeks) did not lead to a recovery of muscle atrophy.7
The current study also confirms our second hypothesis, by identifying i-fat accumulation in the absence of SSP muscle retraction. SSP tendon tear causing SSP muscle retraction and fat accumulation has been documented by us and others.14,24 Rabbit SSP tendon transection covered with an inert membrane to prevent post-operative adhesions, allowed retraction of the SSP muscle and caused fat accumulation.14 The current study contributes new knowledge that fat accumulated, in spite of an immediate repair, and consequently in the absence of muscle retraction. The complete tear and repair of the SSP tendon, in isolation, led to fat accumulation. These results show that despite the earliest possible repair of a SSP tendon tear, fat accumulation was not prevented. After repair, fat did not reverse, but rather, continued to accumulate in the SSP muscle up to six weeks in this study and up to 12 weeks in a previous study.7 These experimental findings correlate with two large clinical follow-up studies that also failed to detect reversal of fat accumulation after repair and even showed continued accumulation.1,2 In contrast with the recovery of SSP muscle atrophy that benefited from early repair of the SSP tendon, there appears to be no clinical benefit to an immediate SSP tendon repair in improving i-fat accumulation.
SSP muscle atrophy and muscle fat accumulation show contrasting features after SSP tendon transection and SSP tendon repair. The peak muscle weight and volume at one and two weeks contrasted with the peak i-fat at six weeks. In addition, the reversible muscle atrophy contrasted with the irreversible i-fat accumulation following its repair. These experimental data support the possibility that uncoupled pathophysiological mechanisms may guide muscle loss and fat accumulation. The contrasting response of SSP muscle and i-fat to tendon tear/repair could be explained by rapid muscle atrophy when tensile strength is removed and rapid recovery when anatomic continuity and mechanical tension are restored.18 On the other hand, the trigger for fat accumulation may reside in biological processes related to tendon-bone healing. At repair, the surgery does not immediately restore the enthesis’ biologic continuity. The formation of a new enthesis as measured by cellular counts, metachromasia of the extracellular matrix and spatial arrangement of collagen takes more than 24 weeks in rabbits.25 The very incomplete enthesis reformation at six weeks could explain the progressing fat accumulation that we measured in this study and that was observed clinically.1,2
Another possibility for i-fat accumulation involves SSP muscle retraction and damage to the SSN that could be tethered at the level of the scapular notch.12,17,21 Such a notch is absent in rabbits as documented in the study of Crumet et al.26 Still, the SSN may be stretched by muscle retraction. However, the current experimental design of immediate repair prevented SSP muscle retraction. Therefore, fat accumulated in our model in the absence of both a scapular notch and SSP muscle retraction. The current study clearly supports the idea that tethering of the SSN does not constitute a prerequisite for occurrence of fat accumulation. Our findings confirm those of Gayton et al,16 who concluded from their histologic and neurophysiologic study in rabbits that: “In the rabbit fatty infiltration can occur in the absence of denervation of the supraspinatus”.16
The irregular distribution of i-fat7,9,10 along a proximal-to-distal gradient14,27 is reported in this study at its earliest onset, two weeks after SSP tendon tear and immediate repair.
Clinically, atrophy of the rotator cuff muscles and i-fat accumulation are disease markers of rotator cuff tendon tears, yet they remain incompletely characterised. Rotator cuff tears are often treated surgically, with the goals of re-establishing anatomy, relieving symptoms, restoring function and reversing muscle atrophy and fat accumulation. The current study in an animal model of a rotator cuff tear, suggests that the complications of muscle atrophy and fat accumulation start as soon as the tear takes place, which is earlier than previously demonstrated. These complications worsen with time after onset of the tear. Early timing of surgical repair can be beneficial in recovering muscle mass and volume. These experiments showed that i-fat will continue to accumulate, despite a successful repair, and its presence and intensification do not indicate a failure of the surgical repair. The differential diagnosis of the etiology of muscle atrophy and fat accumulation includes a SSN compression (tethering) or stretch injury. This study demonstrated that both muscle atrophy and fat accumulation proceeded without any retraction of the SSP muscle. Therefore, surgeons should not need to investigate patients with a rotator cuff tear, muscle atrophy and fat accumulation neurophysiologically for the possibility of SSN neuropathy, nor should they routinely explore this nerve during reparative surgery.
Limitations
The current results obtained in an animal model cannot readily be generalised to clinical situations. We and others used surgical tear models to study fat accumulation and muscle alterations, and the effects of retraction, delayed repair or suprascapular neuropathy.14,16-18,27 Could the surgical tendon repair in isolation have contributed to the fat accumulation? This is not known since neither we nor previous investigators compared data with a sham surgery in an unoperated animal.16-19 However, SSP tendon tear repair, rather than just a surgical intervention, may constitute a likely reason for the SSP i-fat accumulation for a number of reasons: the amount of fat accumulation in this study is proportional to results of our publications with tear, retraction and delayed repair14,27; we found no published evidence of SSP muscle fat accumulation after surgical repair of non-SSP-related shoulder conditions neither in animal models nor clinically e.g. in capsuloplasty or labral repair; and a spontaneous SSP tendon tear has been clinically documented to cause fat accumulation in the SSP muscle without shoulder surgery.15
Conclusion
Our data confirm that both muscle atrophy and i-fat accumulation occur in SSP muscles within one week of a SSP tendon tear. Muscle atrophy recovered six weeks after repair but not i-fat accumulation. These changes happened in the absence of muscle retraction.
Acknowledgements: We thank J. Courchesne and P. Poitras for the surgeries, Y. Nie for tissue processing, M. Aubé for intramuscular fat measurement and G. Baker for editing.
1 Gladstone JN , BishopJY, LoIK, FlatowEL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med2007;35:719–728. Google Scholar
2 Mellado JM , CalmetJ, OlonaM, et al.Surgically repaired massive rotator cuff tears: MRI of tendon integrity, muscle fatty degeneration, and muscle atrophy correlated with intraoperative and clinical findings. AJR Am J Roentgenol2005;184:1456–1463.CrossrefPubMed Google Scholar
3 Barton ER , GimbelJA, WilliamsGR, SoslowskyLJ. Rat supraspinatus muscle atrophy after tendon detachment. J Orthop Res2005;23:259–265.CrossrefPubMed Google Scholar
4 Coleman SH , FealyS, EhteshamiJR, et al.Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg [Am]2003;85-A:2391–2402.CrossrefPubMed Google Scholar
5 Gerber C , MeyerDC, FreyE, et al.Neer Award 2007: Reversion of structural muscle changes caused by chronic rotator cuff tears using continuous musculotendinous traction. An experimental study in sheep. J Shoulder Elbow Surg2009;18:163–171.CrossrefPubMed Google Scholar
6 Gerber C , MeyerDC, SchneebergerAG, HoppelerH, von RechenbergB. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg [Am]2004;86-A:1973–1982.CrossrefPubMed Google Scholar
7 Matsumoto F , UhthoffHK, TrudelG, LoehrJF. Delayed tendon reattachment does not reverse atrophy and fat accumulation of the supraspinatus–an experimental study in rabbits. J Orthop Res2002;20:357–363. Google Scholar
8 Rubino LJ , SprottDC, StillsHF Jr, CrosbyLA. Fatty infiltration does not progress after rotator cuff repair in a rabbit model. Arthroscopy2008;24:936–940. Google Scholar
9 Rubino LJ , StillsHF Jr, SprottDC, CrosbyLA. Fatty infiltration of the torn rotator cuff worsens over time in a rabbit model. Arthroscopy2007;23:717–722.CrossrefPubMed Google Scholar
10 Uhthoff HK , MatsumotoF, TrudelG, HimoriK. Early reattachment does not reverse atrophy and fat accumulation of the supraspinatus–an experimental study in rabbits. J Orthop Res2003;21:386–392. Google Scholar
11 Fuchs B , WeishauptD, ZanettiM, HodlerJ, GerberC. Fatty degeneration of the muscles of the rotator cuff: assessment by computed tomography versus magnetic resonance imaging. J Shoulder Elbow Surg1999;8:599–605.CrossrefPubMed Google Scholar
12 Goutallier D , PostelJM, BernageauJ, LavauL, VoisinMC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res1994;304:78–83.PubMed Google Scholar
13 Meyer DC , PirklC, PfirrmannCW, ZanettiM, GerberC. Asymmetric atrophy of the supraspinatus muscle following tendon tear. J Orthop Res2005;23:254–258.CrossrefPubMed Google Scholar
14 Trudel G , RyanSE, RakhraK, UhthoffHK. Does computed tomography reliably parallel extramuscular and intramuscular fat accumulation early after rabbit supraspinatus tendon division?Radiology2010;255:434–441. Google Scholar
15 Melis B , DeFrancoMJ, ChuinardC, WalchG. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Relat Res2010;468: 1498-505.CrossrefPubMed Google Scholar
16 Gayton JC , RubinoLJ, RichMM, et al.Rabbit supraspinatus motor endplates are unaffected by a rotator cuff tear. J Orthop Res2013;31:99–104.CrossrefPubMed Google Scholar
17 Rowshan K , HadleyS, PhamK, et al.Development of fatty atrophy after neurologic and rotator cuff injuries in an animal model of rotator cuff pathology. J Bone Joint Surg [Am]2010;92-A:2270–2278.CrossrefPubMed Google Scholar
18 Frey E , RegenfelderF, SussmannP, et al.Adipogenic and Myogenic Gene Expression in Rotator Cuff Muscle of the Sheep after Tendon Tear. J Orthop Res2009;27:504–509.CrossrefPubMed Google Scholar
19 Soslowsky LJ , ThomopoulosS, EsmailA. , et al. Rotator cuff tendinosis in an animal model: role of extrinsic and overuse factors. Ann Biomed Eng2002;30:1057–1063. Google Scholar
20 Van Dyke JM , BainJLW, RileyDA. Preserving sarcomere number after tenotomy requires stretch and contraction. Muscle Nerve2012;45:367–375.CrossrefPubMed Google Scholar
21 Albritton MJ , GrahamRD, RichardsRS 2nd, BasamaniaCJ. An anatomic study of the effects on the suprascapular nerve due to retraction of the supraspinatus muscle after a rotator cuff tear. J Shoulder Elbow Surg2003;12:497–500.CrossrefPubMed Google Scholar
22 Meyer DC , WieserK, FarshadM, GerberC. Retraction of supraspinatus muscle and tendon as predictors of success of rotator cuff repair. Am J Sports Med2012;40:2242–2247.CrossrefPubMed Google Scholar
23 Gerber C , SchneebergerAG, BeckM, SchlegelU. Mechanical strength of repairs of the rotator cuff. J Bone Joint Surg [Br]1994;76-B:371–380.PubMed Google Scholar
24 Meyer DC , HoppelerH, von RechenbergG, GerberC. A pathomechanical concept explains muscle loss and fatty muscular changes following surgical tendon release. J Orthop Res2004;22:1004–1007.CrossrefPubMed Google Scholar
25 Koike Y , TrudelG, UhthoffHK. Formation of a new enthesis after attachment of the supraspinatus tendon: A quantitative histologic study in rabbits. J Orthop Res2005;23:1433–1440.CrossrefPubMed Google Scholar
26 Crumet RC , HadleyS, DiltzMV, LeeTQ, GuptaR. Development of a new model for rotator cuff pathology: the rabbit subscapularis muscle. Acta Orthop2009;80:97–103.CrossrefPubMed Google Scholar
27 Trudel G , RyanSE, RakhraK, UhthoffHK. Muscle tissue atrophy, extramuscular and intramuscular fat accumulation and fat gradient after delayed repair of the supraspinatus tendon, a comparative study in the rabbit. J Orthop Res2012;30:781–786. Google Scholar
Funding statement:
This work was supported in part by the Workplace Safety and Insurance Board of Ontario (grant # 04031) and the Canadian Institutes of Health Research (grant # MOP110995)
Author contributions:
H. K. Uhthoff: Experimental design, Interpretation and discussion of results, Writing the manuscript, Approved final version of manuscript
E. Coletta: Data management, Data analysis, Discussion and interpretation of results, Approved final version of manuscript
G. Trudel: Experimental design, Guided data analysis, Interpretation and discussion of results, Assisted with writing manuscript, Critical review of manuscript, Approved final version of manuscript
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










