Abstract
Background
Resveratrol is a polyphenolic compound commonly found in the skins of red grapes. Sirtuin 1 (SIRT1) is a human gene that is activated by resveratrol and has been shown to promote longevity and boost mitochondrial metabolism. We examined the effect of resveratrol on normal and osteoarthritic (OA) human chondrocytes.
Methods
Normal and OA chondrocytes were incubated with various concentrations of resveratrol (1 µM, 10 µM, 25 µM and 50 µM) and cultured for 24, 48 or 72 hours or for six weeks. Cell proliferation, gene expression, and senescence were evaluated.
Results
SIRT1 was significantly upregulated in normal chondrocytes with resveratrol concentrations of 25 µM and 50 µM on both two- (2D) (both p = 0.001) and three-dimensional (3D) cultures (p = 0.008 and 0.001, respectively). It was significantly upregulated in OA chondrocytes treated with 10 µM, 25 µM and 50 µM resveratrol on 2D cultures (p = 0.036, 0.002 and 0.001, respectively) and at 50 µM concentration on 3D cultures (p = 0.001). At 72 hours, the expression of collagen (COL)-10, aggrecan (AGG), and runt-related transcription factor 2 (RUNX2) was significantly greater in both 25 µM (p = 0.011, 0.006 and 0.015, respectively) and 50 µM (p = 0.019, 0.004 and 0.002, respectively) resveratrol-treated normal chondrocyte cultures. In OA chondrocytes, expression of COL10 and RUNX2 was significantly greater in 25 µM (p = 0.004 and 0.024) and 50 µM (p = 0.004 and 0.019) cultures at 72 hours on 3D cultures.
Conclusions
At concentrations of 25 µM and/or 50 µM, resveratrol treatment significantly upregulates SIRT1 gene expression in normal and osteoarthritic chondrocytes. Resveratrol induces chondrocytes into a hypertrophic state through upregulation of COL1, COL10, and RUNX2.
Cite this article: Bone Joint Res 2014;3:51–9.
Article focus
The effect of resveratrol on normal and osteoarthritic (OA) human chondrocytes
Key messages
At concentrations of 25 µM and/or 50 µM, resveratrol treatment significantly upregulates SIRT1 gene expression in normal and osteoarthritic (OA) chondrocytes
Strengths and limitations
Resveratrol treatment significantly upregulates SIRT1 gene expression in normal and osteoarthritic (OA) chondrocytes
Resveratrol induces chondrocytes into a hypertrophic state through upregulation of COL1, COL10, and RUNX2
Introduction
Resveratrol (trans-3,4',5-trihydroxystilbene) is a polyphenolic compound commonly found in the skins of red grapes and red wine. Resveratrol has anti-inflammatory, anti-oxidant, and anti-ageing properties, and numerous studies have documented its neuro-, cardio-, and chondroprotective effects.1-3 It has been suggested that the cardioprotective benefits of resveratrol are the cornerstone of the ‘French Paradox’, a diet consisting of high saturated fat and regular red wine consumption that has been associated with a low risk of coronary artery disease.4 Consumption or administration of resveratrol appears to mimic the effects of calorie restriction, which has long been identified as a mechanism of lifespan extension.5 Increased longevity due to calorie restriction has been documented in organisms such as yeast, worms, flies, and mammals, and credited largely to decreased activity of insulin-like growth factor (Igf) insulin and target of rapamycin (TOR) signalling pathways.6 Modulation of these pathways causes a variety of downstream effects, including altered metabolism7 and the decline of age-related diseases.8
The precise molecular mechanism, by which resveratrol acts, is still a subject of debate. However, one effect of resveratrol, activation of the sirtuin1 (SIRT1) gene, appears to improve cell survival and metabolism9-11 and increase longevity of mammalian cells under calorie restriction.11-15 Cartilage is avascular tissue with a limited potential for repair and regeneration in response to injury or disease. In particular, chondrocytes have both a low proliferative capacity and cellular metabolism.16 Because chondrocytes are solely responsible for maintaining cartilage tissue homeostasis, cell death and subsequent attrition of the extracellular matrix (ECM) can dramatically alter the structure and function of joints and result in the development of diseases such as osteoarthritis (OA).17 Processes such as cellular ageing, senescence and chondrocyte apoptosis have been largely implicated in the initiation and progression of cartilage degradation,18,19 which is characterised by mitochondrial dysfunction and depletion of cellular energy stores.
The purpose of our investigation was to examine the effect of resveratrol on osteoarthritic and normal human chondrocyte proliferation, senescence, and ECM gene activation. We hypothesised that resveratrol may prevent chondrocyte senescence and ageing, and increase cell proliferation and cartilage-specific gene expression.
Materials and Methods
All patients gave informed consent prior to inclusion. The study was authorised by the local ethical committee and was performed in accordance with the Ethical standards of the 1964 Declaration of Helsinki as revised in 2000.
Chondrocyte isolation and culture
Normal human chondrocytes were obtained from a commercially available non-transduced normal human chondrocyte cell system (Clonetics-Poietics, Walkersville, Maryland). Chondrocytes were passaged up to Passage 15 and were maintained at 37°C in humidified air containing 5% carbon dioxide during all experiments. Chondrocytes were grown in Dulbecco’s Modified Eagle Medium (DMEM)/F-12 supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic antimycotic (Life technologies, Carlsbad, California). Adult human osteoarthritic articular cartilage samples (n = 2) were obtained from donors with OA undergoing total knee replacement. The OA chondrocyte isolation procedure was carried out under institutional review board approval. Using ‘normal’ cartilage on the periphery of the joint, OA chondrocytes were released from the cartilage matrix following overnight collagenase treatment (type II and type IV, at a final concentration of 1 mg/ml each) (Worthington, Freehold, New York) at 37°C in 5% carbon dioxide in 15 ml of DMEM/F-12 containing 10% FBS and 1% antibiotic antimycotic. Single cells were filtered through a nylon mesh filter and were collected by centrifugation, then washed three times with 10 ml phosphate buffered saline (PBS). The collected OA chondrocytes were then plated at a density of 1 × 105 cells/cm2.
SIRT1 expression on two-dimensional culture
Normal and osteoarthritic chondrocytes were incubated in six-well plates (5 × 104 cells per well) for four days. Cells were then incubated with different concentrations of resveratrol (0, 1, 10, 25 and 50 µmol/l) (Sigma, St. Louis, Missouri) separately for 24 and 48 hours. Resveratrol liquid formulations were prepared using ethanol (Sigma) as a solvent. Concentrations were chosen to reflect the range of doses previously shown to induce differential cellular effects20 and all conditions were run in triplicate. The expression of SIRT1 was analysed by real-time-reverse transcription polymerase chain reaction (real time RT-PCR) (Applied Biosystems, Foster City, California).
Cell proliferation assay
The effect of resveratrol on chondrocyte proliferation was determined using alamarBlue cell viability reagent assay (Invitrogen Life Technologies, Grand Valley, New York). AlamarBlue is a non-toxic aqueous fluorescent dye that does not affect cell phenotype, viability or cell proliferation.21 Cells were incubated in 96-well plates (5 × 104 cells/well) for four days, to which resveratrol was added (to make a series of final concentrations: 1, 10, 25 and 50 μmol/l). Each concentration was applied to eight wells, whereas media supplemented with ethanol served as a control. After both a 24 hour and 48 hour culture, chondrocytes were supplemented with 10% (v/v) alamarBlue reagent and incubated for another three hours. Then, 100 µl of supernatant was read at 570/585 nm in a SpectraMax/M2 microplate reader (Molecular Devices, Sunnyvale, California). Cell numbers were determined from the standard curve.
Senescence-associated β-galactosidase activity
Senescence-associated β-galactosidase (SA-β-gal) activity was measured with a β-galactosidase staining kit (BioVision, Palo Alto, California). The protocol was conducted according to the manufacturer’s instructions. Briefly, chondrocytes at day seven were treated with different concentrations of resveratrol (0, 1, 10, 25 or 50 μM) separately for 24 hours and 48 hours. Then chondrocytes were washed in PBS, fixed for ten to 15 minutes at room temperature with 0.5 ml of fixative solution, washed and incubated overnight at 37°C with the staining solution mix. Cells were observed under a microscope for development of blue colour (total magnification × 200) and eight photomicrographs were obtained from each culture condition. A blue cell was defined as one having ≥ 50% area stained blue. In each image, two blinded observers quantified total cells, normal, and blue-stained cells. Cell counts from the two observers were averaged for analysis.22 For both normal and OA chondrocytes, the percentage of senescent cells in 1 µM, 10 µM, 25 µM, and 50 µM resveratrol-treated groups were compared with the proportion of senescent cells in the control group.
Preparation and cell seeding of scaffolds (three-dimensional culture)
Aqueous-derived silk fibroin scaffolds were prepared by adding 4 g of granular NaCl (particle size approximately 600 µm to 710 µm) into 2 ml of 6 wt% silk fibroin solution in disk-shaped Teflon (Savillex, Eden Prairie, Minnesota) containers. The containers were covered and left at room temperature for 24 hours, then immersed in water and the NaCl extracted for two days. The porosity of the aqueous-derived silk scaffolds was approximately 97% and the compressive strength and modulus were 60 KPa (sd 5) and 770 (sd 50) KPa, respectively.23
Chondrocytes (1×106 cells/scaffold) were seeded onto pre-wetted (DMEM/F12, overnight) scaffolds (5 mm diameter × 3 mm thick). The constructs were placed into 12-well plates. Cells were allowed to attach for 1.5 to 2 hours. The constructs were placed and incubated with different concentrations of resveratrol (0, 25 µmol/l and 50 µmol/l) in a humidified incubator at 37°C and 5% CO2. All conditions were run in triplicate. Media were replaced every two to three days for six weeks.
Real-time RT-PCR
Total RNA from constructs (n = 3 per concentration) were extracted using Trizol reagent (Invitrogen, Carlsbad, California) and the isolated RNA concentration and quality was determined using a spectrophotometer. Quantitative real-time PCR assays for SIRT1, aggrecan (AGG), type X collagen (COL10), type I collagen (COL1), type II collagen (COL2), SOX9, runt-related transcription factor 2 (Runx2), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcripts were carried out using gene-specific double-fluorescence-labeled probes in 7900 Sequence Detector (PE Applied Biosystems, Foster City, California). These probes and primers related to chondrogenesis and chondrocyte hypertrophy were obtained from Applied Biosystems as assays-on-demand gene expression products. Briefly, the RNA samples were reverse transcribed into cDNA using oligo (dT)-selection according to the manufacturer’s protocol (High-Capacity cDNA Archive Kit; Applied Biosystems). Real-time PCR amplification was performed in a 384-well plate with a 13 ml reaction mixture. The thermal cycling conditions were 2 minutes at 50°C and 10 minutes at 95°C, followed by 45 cycles of 15 seconds of denaturation at 95°C and one minute of annealing and extension at 60°C. The comparative threshold (Ct) PCR cycle detection method (∆∆Ct method) that compares the differences in Ct values and treatment groups, was used to calculate the relative fold change in gene expression.
Statistical analysis
Data analysis was performed with SPSS Version 19 (SPSS Inc., Chicago, Illinois). The Shapiro–Wilk test was used to assess data normality. Normally distributed data were evaluated with a one-way analysis of variance (ANOVA) and Dunnett’s two-sided post-hoc comparison. Data having a non-normal distribution were evaluated non-parametrically using Kruskal–Wallis and Bonferroni-corrected Mann–Whitney U tests. Significance was set at p < 0.05.
Results
Baseline gene expression
Expression of untreated chondrocytes after 72 hours in culture was used as the baseline gene expression; the mean (sd) is presented in Table I.
Table I
RT-PCR results for gene expression for normal and osteoarthritic (OA) chondrocytes following 72 hours of culture without resveratrol treatment. Type I collagen (COL1), type II collagen (COL2), type X collagen (COL10), Sox-9 (SOX9), and aggrecan (AGG) were chosen for their roles in chondrocyte metabolism and chondrocyte hypertrophy. Data is expressed as mean (sd).
| COL1 | COL2 | COL10 | SOX9 | AGG | |
|---|---|---|---|---|---|
| Normal | 1.2 (0.80) | 11.8 (27.2) | 1.1 (0.50) | 1.3 (1.00) | 1.4 (1.00) |
| OA | 1.0 (0.30) | 1.0 (0.13) | 1.0 (0.22) | 1.0 (0.16) | 1.0 (0.15) |
SIRT1 expression in 2D culture
In normal chondrocytes, there were no significant differences in SIRT1 expression when comparing control with resveratrol-treated groups at 24 hours (p = 0.282). However after 48 hours culture, a significant increase in SIRT1 expression was observed in groups treated with 25 µM and 50 µM resveratrol when compared with controls (both p = 0.001). There was no significant difference in SIRT1 expression between 1 µM and 10 µM resveratrol-treated groups and controls at 48 hours (p = 0.997 and p = 0.892, respectively) (Fig. 1).
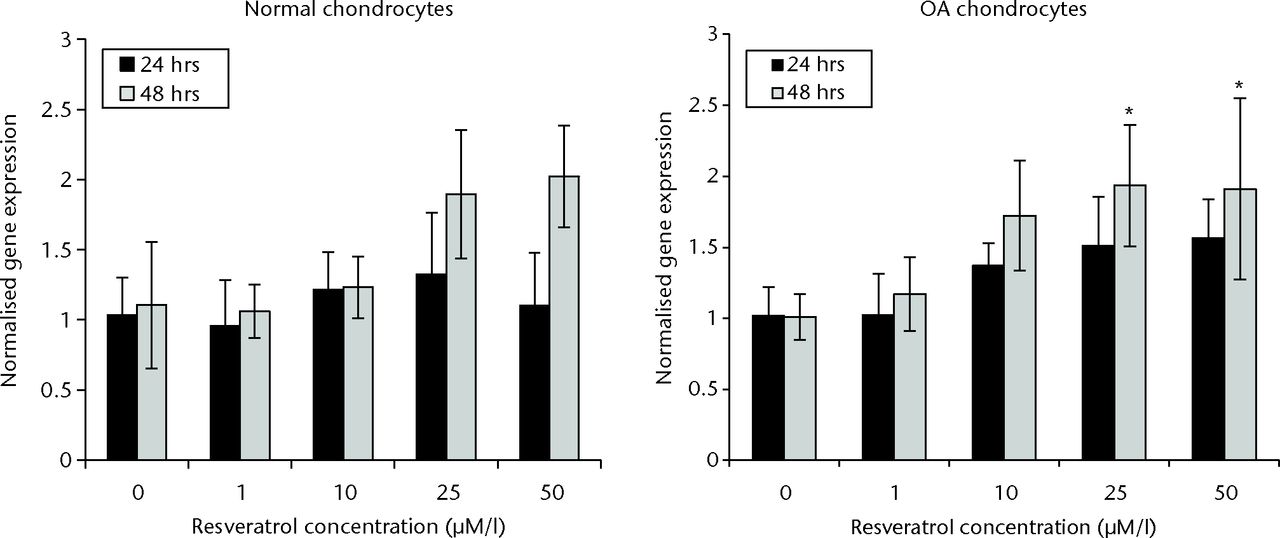
Fig. 1
Bar charts showing sirtuin 1 (SIRT1) expression in normal (left) and osteoarthritic (OA) chondrocytes (right) at 24 and 48 hours. Chondrocytes were incubated in six-well plates with different concentrations of resveratrol (0, 1 µM/l, 10 µM/l, 25 µM/l and 50 µM/l). Data are shown as the mean from eight samples with error bars denoting the standard deviation. * represents statistically significant differences compared with the control (0 µM) samples at the same incubation time. Transcript level was normalized to GAPDH (glyceraldehyde 3-phosphate dehydrogenase) and untreated control within the linear range of amplication.
In OA chondrocytes, a significant increase in SIRT1 expression was observed in cultures treated with 10 µM, 25 µM, and 50 µM resveratrol when compared with control groups after 24 hours (p = 0.036, 0.002 and 0.001, respectively). No significant difference was observed between 1 µM resveratrol-treated cultures and control cultures (p = 1.00). At 48 hours, SIRT1 expression was significantly elevated in 25 µM and 50 µM resveratrol-treated groups compared with control groups (p = 0.011 and p = 0.014, respectively). A significant difference was not observed in SIRT1 expression when comparing 1 µM and 10 µM resveratrol-treated cultures with control cultures at 48 hours (p = 0.915 and p = 0.058, respectively) (Fig. 1).
Cell proliferation
No significant difference in cell proliferation was observed between treatment conditions in normal chondrocytes at either 24 or 48 hours (p = 0.497 and p = 0.104, respectively) (Fig. 2). In OA chondrocytes, cell proliferation was significantly less in cultures treated with 10 µM or 50 µM resveratrol compared with controls at 24 hours (p = 0.001 and p = 0.003, respectively). However a significant difference between cultures was not observed at 48 hours (p = 0.058) (Fig. 2).
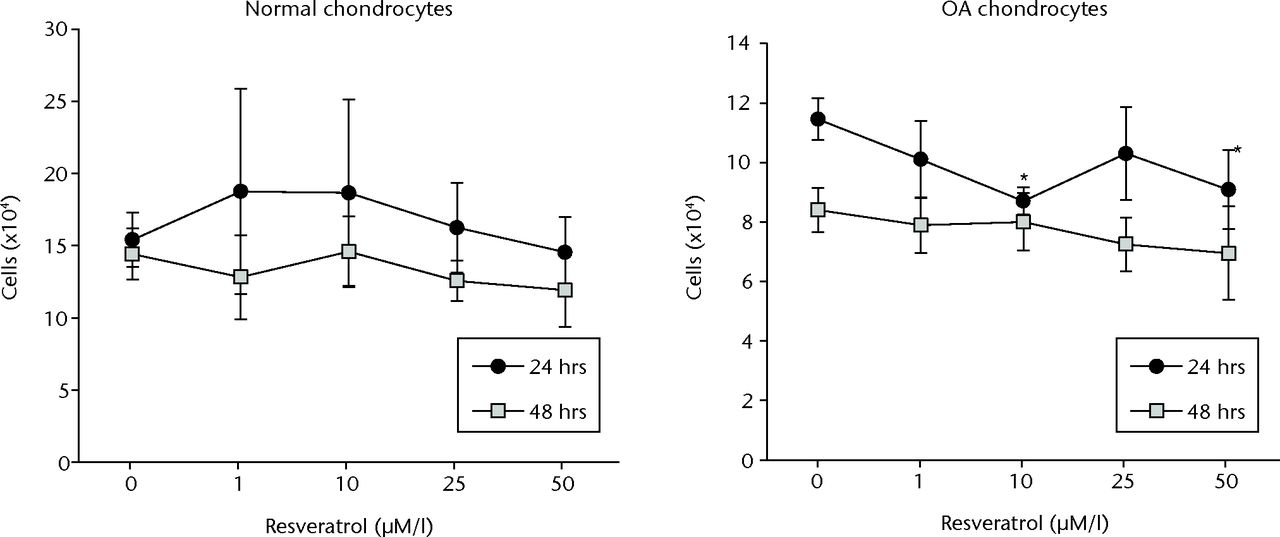
Fig. 2
Graphs of the results of alamar Blue cell proliferation assay, showing mean cell counts in each treatment group for normal (left) and osteoarthritic (OA) chondrocytes (right) at 24 and 48 hours. Cell counts were determined from the standard curve. Data are shown as the mean from eight samples, with error bars denoting the standard deviation. * represents statistically significant differences compared with the control (0 µM) samples at the same incubation time.
Cell senescence
In normal chondrocytes at 24 hours, the proportion of senescent cells was significantly greater in cultures treated with 25 µM resveratrol compared with control cultures (56.4% (sd 5.63) vs 41.0% (sd 5.8), p = 0.003). Significant differences were not observed at any other concentrations. At 48 hours, the percentage of senescent cells did not differ significantly between control cultures and any of the treatment groups (Table II).
Table II
The proportion of senescent cells in normal and osteoarthritic (OA) chondrocyte cultures at 24 and 48 hours. Chondrocytes were incubated in 12-well plates with different concentrations of resveratrol (0, 1 µmol/l, 10 µmol/l, 25 µmol/l and 50 µmol/l) in four samples for each experiment
| Resveratrol concentration (µM) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (sd) proportion of senescent cells (%) | 0 | 1 | p-value* | 10 | p-value* | 25 | p-value* | 50 | p-value* | |||||||||
| 24 HOURS | ||||||||||||||||||
| Normal chondrocytes | 41.0 (5.8) | 33.0 (6.9) | 0.148 | 36.1 (1.9) | 0.509 | 56.4 (5.6) | 0.003 | 40.5 (6.1) | 1.00 | |||||||||
| OA chondrocytes | 48.8 (8.2) | 48.0 (4.9) | 0.999 | 51.2 (5.3) | 0.908 | 49.7 (3.0) | 0.996 | 42.5 (2.8) | 0.300 | |||||||||
| p-value | 0.171 | 0.012 | 0.002 | 0.048 | 0.565 | |||||||||||||
| 48 HOURS | ||||||||||||||||||
| Normal chondrocytes | 30.6 (14.0) | 34.9 (7.0) | 0.844 | 37.9 (4.5) | 0.503 | 44.4 (5.3) | 0.076 | 42.9 (3.2) | 0.123 | |||||||||
| OA chondrocytes | 31.5 (2.2) | 35.0 (4.3) | 0.533 | 42.3 (3.7) | 0.004 | 39.8 (3.6) | 0.028 | 44.2 (5.0) | 0.001 | |||||||||
| p-value | 0.898 | 0.987 | 0.179 | 0.197 | 0.691 | |||||||||||||
-
* compared with control cultures (0 µM concentration). Analysed using independent t-test
In OA chondrocytes at 24 hours, the percentage of senescent cells was in control cultures was not significantly different from the percentage of senescent cells in groups treated with 1 µM, 10 µM, 25 µM, or 50 µM resveratrol (Table I). However, compared with control cultures at 48 hours (31.52% (sd 2.15)), the percentage of senescent cells was significantly greater in cultures treated with 10 µM (42.29% (sd 3.66), p = 0.004), 25 µM (39.75% (sd 3.60), p = 0.028) and 50 µM (44.18% (sd 5.0), p = 0.001) (Table II).
Gene expression in 3D culture
In normal chondrocytes at 72 hours, expression of COL10 (p = 0.011), AGG (p = 0.006), and RUNX2 (p = 0.015) was significantly greater in cultures treated with 25 µM resveratrol when compared with control cultures. In 50 µM resveratrol-treated cultures, expression of SITR1 (p = 0.001), COL1 (p = 0.004), COL10 (p = 0.019), SOX9 (p = 0.010), AGG (p = 0.004) and RUNX2 (p = 0.002) was significantly greater than in control cultures; however, a difference was not observed with COL2 (p = 0.522).
At six weeks, expression of SIRT1 (p = 0.008), COL1 (p = 0.004), COL10 (p = 0.004), AGG (p = 0.010), and RUNX2 (p < 0.01) was significantly greater in cultures treated with 25 µM resveratrol when compared with control cultures but no difference was observed for COL2 (p = 0.150) or SOX9 (p = 0.150). In 50 µM resveratrol-treated cultures, expression of SIRT1 (p = 0.001), COL1 (p = 0.004), COL10 (p = 0.004), AGG (p = 0.037) and RUNX2 (p = 0.012) was significantly greater than in control cultures, but differences were not observed for COL2 or SOX9 (both p = 0.337) (Fig. 3).
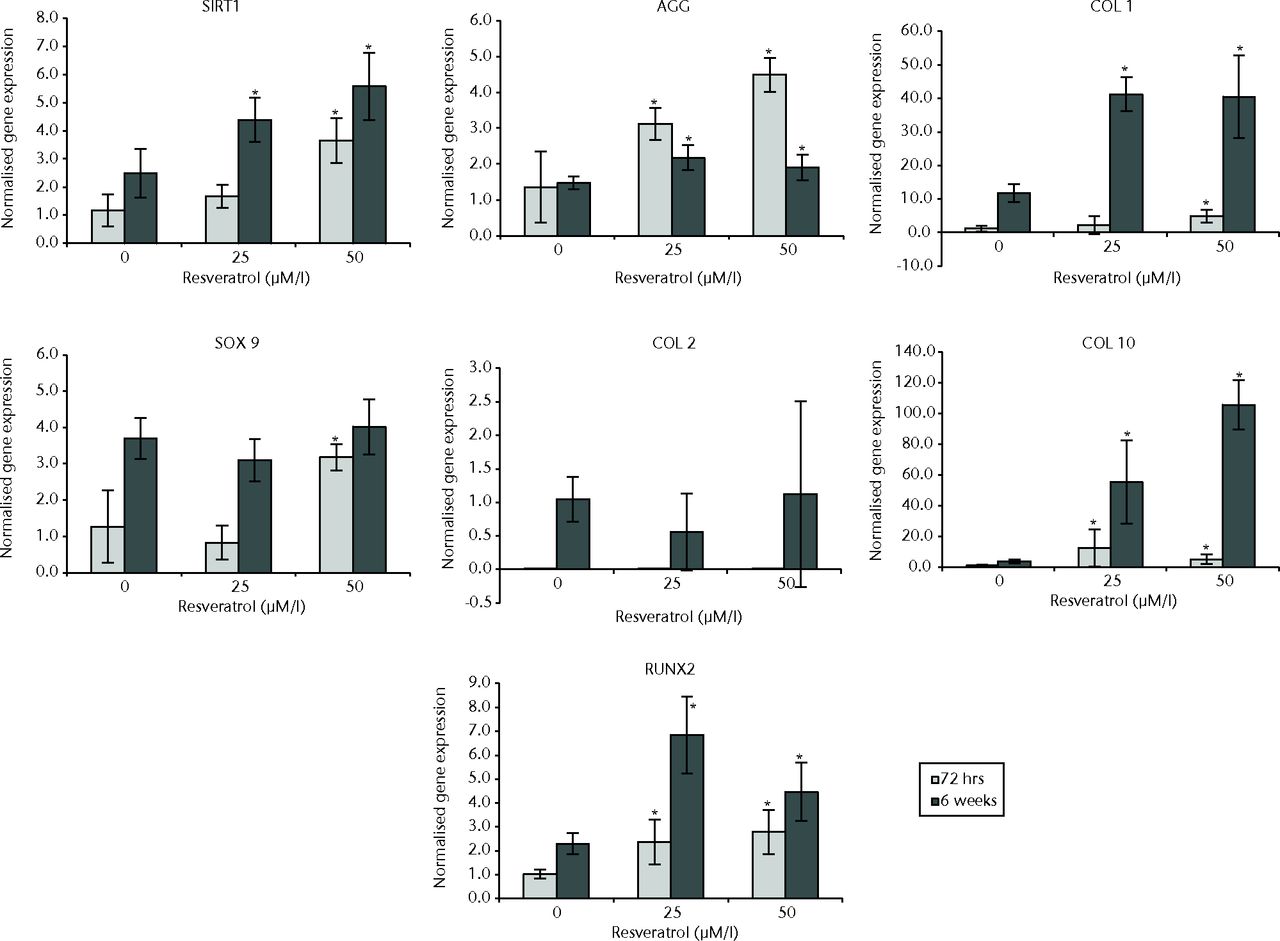
Fig. 3
Bar charts showing the expression of sirtuin 1 (SIRT1) and chondrogenesis-related genes by concentration of resveratrol in normal chondrocytes. Chondrocytes were cultured on scaffolds with different concentrations of resveratrol (0, 25 µM/l and 50 µM/l) up to six weeks. Data are shown as the mean from six samples with error bars denoting the standard deviation. * represents statistically significant differences compared with the control (0 µM) samples at the same incubation time. Transcript levels were normalized to GAPDH and untreated controls within the linear range of amplification. (AGG, aggrecan; COL, collagen; RUNX2, runt-related transcription factor 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase).
In OA chondrocytes at 72 hours, cultures treated with 25 µM resveratrol exhibited expression of COL1 (p = 0.004), COL10 (p = 0.004), and RUNX2 (p = 0.024) that was significantly greater than control cultures, while expression of COL2 (p = 0.025) and SOX9 (p = 0.004) was significantly less when compared with control cultures. No difference was observed for SIRT1 (p = 0.820) and AGG expression (p = 0.262). In 50 µM resveratrol-treated cultures, expression of SIRT1 (P = 0.004), COL10 (p = 0.004) and RUNX2 (p = 0.019) was significantly greater compared with control cultures, while expression of COL2 (p = 0.004) and AGG (p = 0.037) was significantly less when compared with controls. Though not statistically significant, similar trends for COL1 and SOX9 were observed: expression of COL1 was greater compared with control cultures (p = 0.055) and SOX9 expression was less (p = 0.055).
At six weeks, in cultures treated with 25 µM resveratrol, expression of COL10 (p = 0.004) was significantly greater than control cultures while expression of COL2 (p = 0.004), SOX9 (p = 0.025), and RUNX2 (p = 0.004) was significantly less compared with controls. No differences were observed for SIRT1 (p = 0.728), COL1 (p = 0.873) and AGG (p = 0.262). In 50 µM resveratrol cultures, expression of SIRT1 (p = 0.001) and COL10 (p = 0.004) were significantly greater than control cultures while expression of COL1 (p = 0.016), COL2 (p = 0.004) and RUNX2 (p = 0.004) was significantly less compared with controls. Significant differences were not observed for SOX9 (p = 0.337) or AGG (p = 0.150) (Fig. 4).
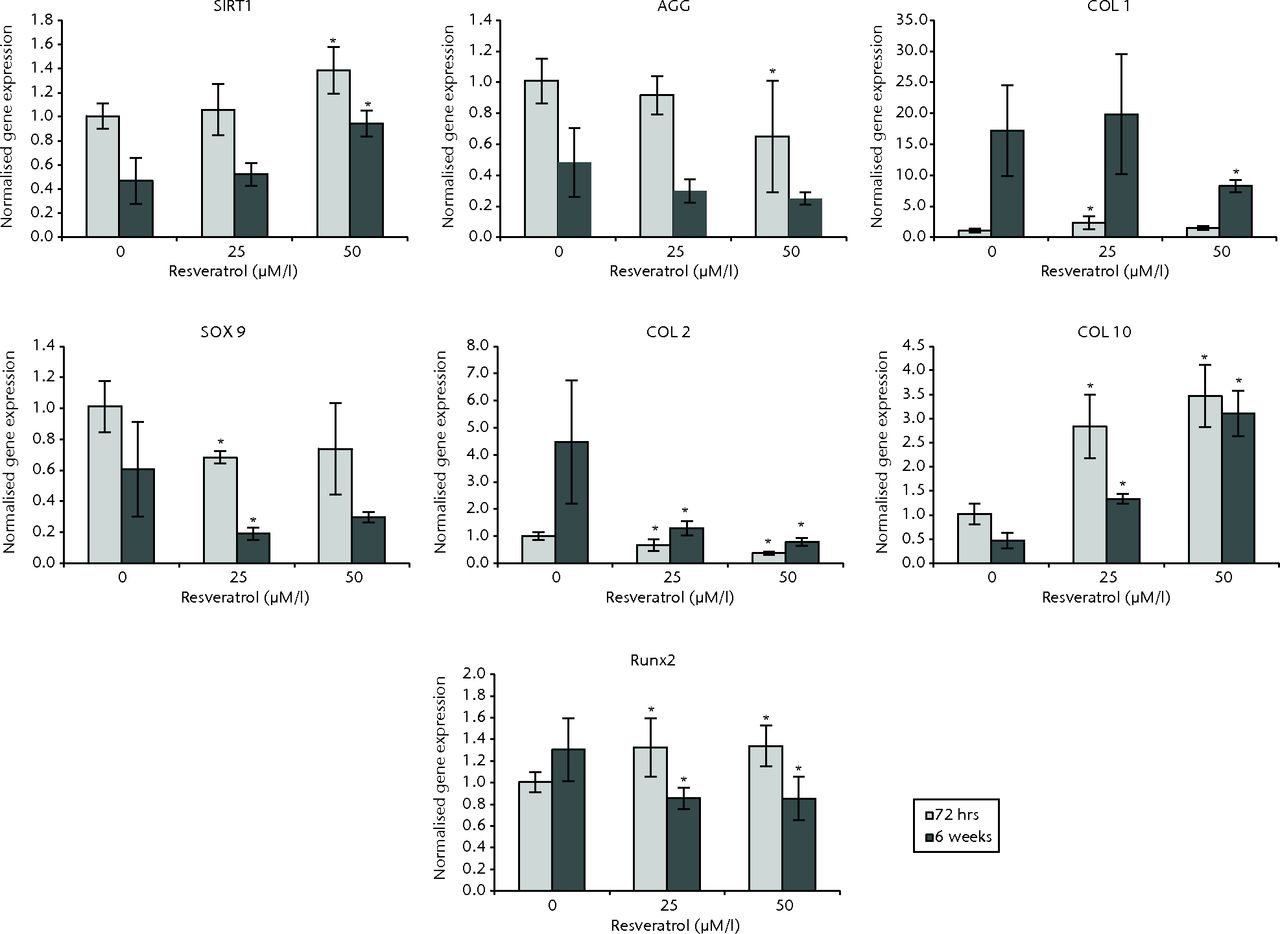
Fig. 4
Bar charts showing the expression of sirtuin 1 (SIRT1) and chondrogenesis-related genes by concentration of resveratrol in osteoarthritic chondrocytes. Chondrocytes were cultured on scaffolds with different concentrations of resveratrol (0, 25 and 50 µM/l) up to six weeks. Data are shown as the mean from six samples with error bars denoting the standard deviation. * represents statistically significant differences compared with the control (0 µM) samples at the same incubation time Transcript levels were normalized to GAPDH and untreated controls within the linear range of amplification. (AGG, aggrecan; COL, collagen; RUNX2, runt-related transcription factor 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase).
Discussion
Resveratrol (trans-3,4',5-trihydroxystilbene) is a polyphenolic compound found in the skins of red grapes and red wine that has been shown to have anti-oxidant, anti-inflammatory, metabolic and anti-ageing properties.24 However, it is increasingly understood that the specific effects of resveratrol are dependent on both dosage and cell type. In vitro resveratrol treatment of chondrocytes has been shown to suppress various IL-1β-induced inflammatory signaling cascades,25-27 protect cells against apoptosis26,28 and decrease advanced glycation end products and matrix metalloproteinase production.29In vivo intra-articular injections of resveratrol have demonstrated potential chondroprotective effects, particularly in animal models of OA.30,31 The precise molecular mechanism by which resveratrol acts is not firmly established, but it is generally believed that the beneficial effects of resveratrol are due to the direct or indirect activation of SIRT1, a human gene shown to improve cell survival and increase longevity.12,13
SIRT1 activation has been correlated with the upregulation of the cartilage-specific genes COL2 and AGG,9 while SIRT1 inhibition has been shown to result in gene expression characteristic of chondrocyte hypertrophy, including upregulation of COL10 in normal human chondrocytes.10 This evidence led to our hypothesis that treatment with resveratrol might activate SIRT1 in normal and osteoarthritic chondrocytes, resulting in increased cellular proliferation, upregulation of cartilage-specific genes, and decreased cellular senescence.
The present study revealed three significant findings: 1) resveratrol concentrations of 25 µM and/or 50 µM significantly upregulated SIRT1 mRNA in both normal and osteoarthritic chondrocytes; 2) treatment with resveratrol induced gene expression characteristic of chondrocyte hypertrophy, suggesting that chondrocytes were progressing down an endochondral bone pathway in response to resveratrol treatment; and 3) OA chondrocytes treated with 10 µM, 25 µM, or 50 µM resveratrol for 48 hours showed significantly more senescence compared with control cultures. No significant difference in cell proliferation was observed in either normal or OA chondrocytes.
The upregulation of SIRT1 mRNA observed in both normal and OA chondrocytes following treatment with 25 µM and/or 50 µM resveratrol (Figs 1, 3 and 4) is consistent with previous studies in which resveratrol was identified as a small molecule activator of SIRT1.32,33 Though the precise molecular link between SIRT1 and resveratrol is still being explored, the results of our investigation support the theory that resveratrol upregulates the expression of SIRT1 and may act in a dose-dependent manner.
Recent investigations9,11 have suggested that SIRT1 activation may protect cartilage from OA-like degeneration via inhibition of apoptosis and upregulation of cartilage-specific genes. Accordingly, consistent with the observed SIRT1 upregulation, we expected to see increased expression of SOX9, COL2, and AGG and decreased expression of COL1 and COL10. However, we found no evidence that resveratrol stimulated chondrogenic gene expression.
In normal chondrocytes treated with 25 µM and 50 µM resveratrol, there was significant upregulation of COL1, COL10, and RUNX2, all markers of hypertrophic progression in chondrocytes. This effect was seen at 72 hours and six weeks, with a more profound effect at the longer period (Fig. 3). The significant upregulation of hypertropic chondrocyte markers, including terminal differentiation marker RUNX2,34 suggests that treatment with resveratrol induces normal chondrocytes to progress down the hypertropic chondrocyte lineage.
Similar trends were observed in OA chondrocytes. At 25 µM and 50 µM concentrations of resveratrol, expression of COL1, COL10, and RUNX2 was significantly greater, while expression of COL2, SOX9, and AGG was significantly lower at 72 hours. At six weeks, OA chondrocytes also showed significantly greater expression of COL10 and significantly less COL2, SOX9, and RUNX2 expression, which is consistent with reported SOX9 suppression indicative of chondrocyte hypertrophy.35 As was the case with normal chondrocytes, the decrease in expression of SOX9, COL2, and AGG and the increase in expression of COL1 and COL10 in OA chondrocytes suggest a progression towards endochondral ossification and osteogenesis. However, unlike normal chondrocytes, RUNX2 expression in OA chondrocytes was significantly less in both 25 µM and 50 µM cultures when compared with controls (Fig. 4), possibly suggesting that OA chondrocytes were slower in progressing to the hypertrophic chondrocyte stage or had lost the capacity for osteogenic differentiation. The aggregate of these results indicates that both OA and normal chondrocytes treated with 25 µM and 50 µM resveratrol exhibit changes in gene expression characteristic of osteochondral ossification, in which cellular hypertrophy eventually progresses to mineralisation and bone formation.36
Previous investigations have shown that resveratrol stimulates and promotes osteogenic and osteoblastic differentiation in human periodontal ligament cells,37 murine-induced pluripotent stem cells38 and mesenchymal stem cells (MSCs).39,40 In particular, it has been demonstrated that activation of SIRT1 via resveratrol results in upregulation of RUNX2 in human MSCs,37 and that resveratrol may antagonise osteoclast differentiation41,42 and promote osteoblast formation.43 These findings, coupled with our results, suggest there may be future roles for resveratrol in fracture healing.
Although we expected to see increased proliferation following resveratrol administration, no significant difference in cell proliferation was observed for normal chondrocytes treated with resveratrol, and OA chondrocytes treated with resveratrol demonstrated significantly less cell proliferation after 24 hours (Fig. 2). Previous studies have demonstrated that resveratrol inhibits cell proliferation in synoviocytes,44 retinal pigment epithelial cells45 and endothelial cells,46 while other investigations reported increased cell proliferation in endothelial progenitor cells,47 human bone marrow-derived MSCs48 and osteoblastic MC3T3-E1 cells.49 In the present study, resveratrol did not enhance or increase cell proliferation in normal or OA chondrocytes, suggesting that the proliferative effects of resveratrol may be dependent on cell type. Similarly, despite the increase in SIRT1 upregulation, we observed that cell senescence in OA chondrocytes was significantly increased in cultures treated with 10 µM, 25 µM, or 50 µM resveratrol (Table I). Increased senescence following resveratrol treatment has been recently observed in multiple strains of primary human fibroblasts and is thought to be mediated by p38 activity.50 However, given that SIRT1 activation has been shown to prevent growth arrest and senescence51 it is was particularly interesting that OA chondrocytes simultaneously demonstrated both SIRT1 upregulation and cellular senescence. Our results showed that normal chondrocytes had greater upregulation of SIRT1 compared with OA chondrocytes, a finding that had been previously documented.52 It is plausible then, that abnormal cellular metabolism characteristic of OA chondrocytes resulted in irreversible damage that could not be repaired, even after SIRT1 upregulation.
As with any laboratory investigation, this study was subject to inherent limitations. A 3D cell scaffold was used in attempt to mimic the environment present in en bloc cartilage; however, these conditions are not equivalent to the in vivo joint environment. Our observations of significant SIRT1 upregulation and expression of markers of chondrocyte hypertrophy following treatment with resveratrol, suggest the potential for resveratrol to be used in bone healing models.
At 25 µM and/or 50 µM concentrations, resveratrol treatment significantly upregulates SIRT1 gene expression in both normal and osteoarthritic chondrocytes. It appears that resveratrol also induces normal and osteoarthritic chondrocytes into a hypertrophic state through upregulation of COL1, COL10, and RUNX2. In osteoarthritic chondrocytes, 10 µM, 25 µM, and 50 µM concentrations of resveratrol increased cellular senescence. The addition of resveratrol to chondrocyte cultures had minimal effect on cell proliferation.
Acknowledgements: The authors would like to thank A. Sox-Harris, PhD, for assistance with statistical analysis.
1 Delmas D , LançonA, ColinD, JanninB, LatruffeN. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets2006;7:423–442.CrossrefPubMed Google Scholar
2 Fulda S , DebatinKM. Resveratrol modulation of signal transduction in apoptosis and cell survival: a mini-review. Cancer Detect Prev2006;30:217–223.CrossrefPubMed Google Scholar
3 Csaki C , KeshishzadehN, FischerK, ShakibaeiM. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem Pharmacol2008;75:677–687.CrossrefPubMed Google Scholar
4 Sun AY , SimonyiA, SunGY. The “French Paradox” and beyond: neuroprotective effects of polyphenols. Free Radic Biol Med2002;32:314–318. Google Scholar
5 Brandl A , HartmannA, BechmannV, et al.Oxidative stress induces senescence in chondrocytes. J Orthop Res2011;29:1114–1120.CrossrefPubMed Google Scholar
6 Fontana L , PartridgeL, LongoVD. Extending healthy life span: from yeast to humans. Science2010;328:321–326. Google Scholar
7 Broughton SJ , PiperMD, IkeyaT, et al.Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands. Proc Natl Acad Sci U S A2005;102:3105–3110.CrossrefPubMed Google Scholar
8 Selman C , TulletJM, WieserD, et al.Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science2009;326:140–144.CrossrefPubMed Google Scholar
9 Dvir-Ginzberg M , GagarinaV, LeeEJ, HallDJ. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J Biol Chem2008;283:36300–36310.CrossrefPubMed Google Scholar
10 Fujita N , MatsushitaT, IshidaK, et al.Potential involvement of SIRT1 in the pathogenesis of osteoarthritis through the modulation of chondrocyte gene expressions. J Orthop Res2011;29:511–515.CrossrefPubMed Google Scholar
11 Takayama K , IshidaK, MatsushitaT, et al.SIRT1 regulation of apoptosis of human chondrocytes. Arthritis Rheum2009;60:2731–2740.CrossrefPubMed Google Scholar
12 Alcendor RR , GaoS, ZhaiP, et al.Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res2007;100:1512–1521.CrossrefPubMed Google Scholar
13 Tang BL . SIRT1, neuronal cell survival and the insulin/IGF-1 aging paradox. Neurobiol Aging2006;27:501–505.CrossrefPubMed Google Scholar
14 Uchiumi F , WatanabeT, HasegawaS, et al.The effect of resveratrol on the Werner syndrome RecQ helicase gene and telomerase activity. Curr Aging Sci2011;4:1–7.CrossrefPubMed Google Scholar
15 Kao CL , ChenLK, ChangYL, et al.Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J Atheroscler Thromb2010;17:970–979. Google Scholar
16 Loughlin J , IrvenC, AthanasouN, CarrA, SykesB. Differential allelic expression of the type II collagen gene (COL2A1) in osteoarthritic cartilage. Am J Hum Genet1995;56:1186–1193.PubMed Google Scholar
17 Grogan SP , D'LimaDD. Joint aging and chondrocyte cell death. Int J Clin Rheumtol2010;5:199–214.CrossrefPubMed Google Scholar
18 Martin JA , KlingelhutzAJ, Moussavi-HaramiF, BuckwalterJA. Effects of oxidative damage and telomerase activity on human articular cartilage chondrocyte senescence. J Gerontol A Biol Sci Med Sci2004;59:324–337.CrossrefPubMed Google Scholar
19 von Zglinicki T . Oxidative stress shortens telomeres. Trends Biochem Sci2002;27:339–344.CrossrefPubMed Google Scholar
20 Gu J , WangCQ, FanHH, et al.Effects of resveratrol on endothelial progenitor cells and their contribution to reendothelialization in intima-injured rats. J Cardiovasc Pharmacol2006;47:711–721. Google Scholar
21 Ahmed SA , GogalRM Jr, WalshJE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods1994;170:211–224.CrossrefPubMed Google Scholar
22 Tsujimoto RN , HamiltonM, BergerDE. Averaging multiple judges to improve validity: aid to planning cost-effective clinical research. Psychol Assess1990;2:432–437. Google Scholar
23 Kim UJ , ParkJ, KimHJ, WadaM, KaplanDL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials2005;26:2775–2785.CrossrefPubMed Google Scholar
24 Csaki C , KeshishzadehN, FischerK, ShakibaeiM. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem Pharmacol2008;75:677–687.CrossrefPubMed Google Scholar
25 Csaki C , MobasheriA, ShakibaeiM. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1beta-induced NF-kappaB-mediated inflammation and apoptosis. Arthritis Res Ther2009;11:R165.CrossrefPubMed Google Scholar
26 Shakibaei M , CsakiC, NebrichS, MobasheriA. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem Pharmacol2008;76:1426–1439.CrossrefPubMed Google Scholar
27 Shakibaei M , JohnT, SeifarthC, MobasheriA. Resveratrol inhibits IL-1 beta-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann N Y Acad Sci2007;1095:554–563.CrossrefPubMed Google Scholar
28 Dave M , AtturM, PalmerG, et al.The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum2008;58:2786–2797.CrossrefPubMed Google Scholar
29 Liu FC , HungLF, WuWL, et al.Chondroprotective effects and mechanisms of resveratrol in advanced glycation end products-stimulated chondrocytes. Arthritis Res Ther2010;12:R167.CrossrefPubMed Google Scholar
30 Elmali N , EsenkayaI, HarmaA, et al.Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm Res2005;54:158–162.CrossrefPubMed Google Scholar
31 Wang J , GaoJS, ChenJW, LiF, TianJ. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol Int2012;32:1541–1548.CrossrefPubMed Google Scholar
32 Borra MT , SmithBC, DenuJM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem2005;280:17187–17195.CrossrefPubMed Google Scholar
33 Howitz KT , BittermanKJ, CohenHY, et al.Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature2003;425:191–196.CrossrefPubMed Google Scholar
34 Komori T . Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res2010;339:189–195.CrossrefPubMed Google Scholar
35 Grassel S , AhmedN. Influence of cellular microenvironment and paracrine signals on chondrogenic differentiation. Front Biosci2007;12:4946–4956.CrossrefPubMed Google Scholar
36 Saito T , FukaiA, MabuchiA, et al.Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med2010;16:678–686.CrossrefPubMed Google Scholar
37 Lee YM , ShinSI, ShinKS, LeeYR, ParkBH, KimEC. The role of sirtuin 1 in osteoblastic differentiation in human periodontal ligament cells. J Periodontal Res2011;46:712–721.CrossrefPubMed Google Scholar
38 Kao CL , TaiLK, ChiouSH, et al.Resveratrol promotes osteogenic differentiation and protects against dexamethasone damage in murine induced pluripotent stem cells. Stem Cells Dev2010;19:247–258.CrossrefPubMed Google Scholar
39 Tseng PC , HouSM, ChenRJ, et al.Resveratrol promotes osteogenesis of human mesenchymal stem cells by up-regulating RUNX2 gene expression via SIRT1/FOXO3A axis. J Bone Miner Res2011;26:2552–2563. Google Scholar
40 Bäckesjö CM , LiY, LindgrenU, HaldosénLA. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. Cells Tissues Organs2009;189:93–97.CrossrefPubMed Google Scholar
41 He X , AnderssonG, LindgrenU, LiY. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem Biophys Res Commun2010;401:356–362.CrossrefPubMed Google Scholar
42 Shakibaei M , BuhrmannC, MobasheriA. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kappaB ligand (RANKL) activation of NF-kappaB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem2011;286:11492–11505.CrossrefPubMed Google Scholar
43 Zhou H , ShangL, LiX, et al.Resveratrol augments the canonical Wnt signaling pathway in promoting osteoblastic differentiation of multipotent mesenchymal cells. Exp Cell Res2009;315:2953–2962. Google Scholar
44 Tang LL, Gao JS, Chen XR, Xie X. Inhibitory effect of resveratrol on the proliferation of synoviocytes in rheumatoid arthritis and its mechanism in vitro. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2006;31:528–533 (in Chinese). Google Scholar
45 King RE , KentKD, BomserJA. Resveratrol reduces oxidation and proliferation of human retinal pigment epithelial cells via extracellular signal-regulated kinase inhibition. Chem Biol Interact2005;151:143–149.CrossrefPubMed Google Scholar
46 Szende B , TyihakE, Kiraly-VeghelyZ. Dose-dependent effect of resveratrol on proliferation and apoptosis in endothelial and tumor cell cultures. Exp Mol Med2000;32:88–92.CrossrefPubMed Google Scholar
47 Wang XB , HuangJ, ZouJG, et al.Effects of resveratrol on number and activity of endothelial progenitor cells from human peripheral blood. Clin Exp Pharmacol Physiol2007;34:1109–1115.CrossrefPubMed Google Scholar
48 Dai Z , LiY, QuarlesLD, SongT, et al.Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine2007;14:806–814.CrossrefPubMed Google Scholar
49 Mizutani K , IkedaK, KawaiY, YamoriY. Resveratrol stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun1998;253:859–863.CrossrefPubMed Google Scholar
50 Faragher RG , BurtonDG, MajechaP, et al.Resveratrol, but not dihydroresveratrol, induces premature senescence in primary human fibroblasts. Age (Dordr)2011;33:555–564. Google Scholar
51 Guarente L . Diverse and dynamic functions of the Sir silencing complex. Nat Genet1999;23:281–285.CrossrefPubMed Google Scholar
52 Brandl A , HartmannA, BechmannV, et al.Oxidative stress induces senescence in chondrocytes. J Orthop Res2011;29:1114–1120.CrossrefPubMed Google Scholar
Funding statement:
Funding for the study was received from the Department of Orthopaedic Surgery
Author contributions:
H. J. Kim: Data collection, Data analysis, Writing the paper
H. J. Braun: Data analysis, Writing the paper, Revising the paper
J. L. Dragoo: Creation of study, Management of trials, Editing the paper
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










