Abstract
Introduction
Osteoarthritis (OA) is a progressively debilitating disease that affects mostly cartilage, with associated changes in the bone. The increasing incidence of OA and an ageing population, coupled with insufficient therapeutic choices, has led to focus on the potential of stem cells as a novel strategy for cartilage repair.
Methods
In this study, we used scaffold-free mesenchymal stem cells (MSCs) obtained from bone marrow in an experimental animal model of OA by direct intra-articular injection. MSCs were isolated from 2.8 kg white New Zealand rabbits. There were ten in the study group and ten in the control group. OA was induced by unilateral transection of the anterior cruciate ligament of the knee joint. At 12 weeks post-operatively, a single dose of 1 million cells suspended in 1 ml of medium was delivered to the injured knee by direct intra-articular injection. The control group received 1 ml of medium without cells. The knees were examined at 16 and 20 weeks following surgery. Repair was investigated radiologically, grossly and histologically using haematoxylin and eosin, Safranin-O and toluidine blue staining.
Results
Radiological assessment confirmed development of OA changes after 12 weeks. Rabbits receiving MSCs showed a lower degree of cartilage degeneration, osteophyte formation, and subchondral sclerosis than the control group at 20 weeks post-operatively. The quality of cartilage was significantly better in the cell-treated group compared with the control group after 20 weeks.
Conclusions
Bone marrow-derived MSCs could be promising cell sources for the treatment of OA. Neither stem cell culture nor scaffolds are absolutely necessary for a favourable outcome.
Cite this article: Bone Joint Res 2014;3:32–7.
Article focus
To find the role of stem cells in an OA knee
To find the role of scaffolds
To find the role of stem cell culture
Key messages
Stem cells definitely have role in early OA
Neither scaffolds nor culture are necessary for cartilage repair
Strengths and limitations
Strength: we have carried out a histopathological examination at one month intervals to see cartilage regeneration and no scaffolds were used for the delivery of stem cells.
Limitations: short sample size and follow-up.
Introduction
Osteoarthritis (OA) is a disease that limits the mobility of patients and is of considerable economic importance. In advanced stages, patients can experience severe pain and destruction of the joint surfaces, resulting in restriction of mobility.1-3 In many cases the consequence is an inability to work, often leading to the need for arthroplasty of the diseased joint.4 As cartilage tissue has only very limited capacity of self-renewal, the development of this disorder is chronic and progressive. The cause of OA is unknown, but several factors are involved in its aetiopathogenesis, including age, gender, body weight and metabolic activities.5-7 Chondrocytes, the formative cell type of hyaline cartilage, directly synthesise many of the degenerative enzymes (metalloproteinases) and inflammatory mediators responsible for its own destruction.8 Although total knee replacement has largely improved the pain and functional status in patients with end-stage OA of the knee, its use in patients with non-end-stage disease is limited by the expense of the procedure.1
Mesenchymal stem cells (MSCs) are multipotent cells present in adult bone marrow. They can replicate as undifferentiated cells and have the potential to differentiate to lineages of mesenchymal tissues, including cartilage, bone and fat.9,10 MSCs are therefore a promising cell source for the regeneration of cartilage, as they possess chondrogenic differentiation potential and are easy to obtain in high numbers. In order to explore a new treatment for OA patients suffering early degenerative lesions of hyaline cartilage, we proposed to transplant MSCs, obtained from bone marrow without scaffold, into an experimental animal model of OA. A rabbit model of OA induced by anterior cruciate ligament transection (ACLT) was selected.11,12 The therapeutic outcome of ACLT reconstruction by means of this strategy was evaluated in terms of radiology and histology.
Materials and Methods
The study was carried out in the Department of Orthopaedics, Institute of Medical Science, Banaras Hindu University in collaboration with Departments of Haematology (Blood Bank), Pathology and the Centre of Experimental Medicine and Surgery. The experiments were carried out at the Centre of Experimental Medicine and Surgery. The study was approved by the Institute’s Ethical Committee.
Specimens
Adult New Zealand white rabbits were acquired from the Department of Zoology (Banaras Hindu University, Varanasi, India). All animals (ten study and ten control) were aged > 16 weeks and weighed > 2 kg.
Operative technique
Only the right knees of the study animals were operated upon. Intramuscular (IM) ketamine (5 mg/kg) + midazolam (5 mg/kg) + 2% lignocaine with adrenaline at the operative site was used to anaesthetise the animals. The right hindleg was prepared using betadine and 70% alcohol after removing hairs with commercially available hair remover. With the animal placed supine, the knee joint was entered using a medial parapatellar approach. Incision was taken down to the anteromedial joint capsule, with knee flexion and extension helping to identify anatomical landmarks.
The joint capsule was incised obliquely between patellar tendon and medial collateral ligament and care was taken not to damage the tibial insertion of the lateral meniscus. The extensor mechanism was then dislocated laterally using atraumatic forceps. With valgus and anterior drawer forces on the flexed knee, the anterior ligaments were visualised and transected. Adequacy of the resection of the cruciate ligaments was checked with the Drawer’s test13 and perfect haemostasis was attained. The extensor mechanism was then relocated. After irrigation, the capsule was repaired medially with 2-0 vicryl absorbable suture and the skin closed with interrupted 3-0 mercerised silk sutures. The skin was then cleaned and dressed. Post-operatively, each rabbit was given ceftriaxzone (IM 50 mg/kg) with gentamycin (IM 2 mg/kg) daily for five days. Temperature was maintained at 25°C (± 2°C) with a dark–light schedule of 12 hours (± 1 hour). Humidity was maintained at between 55% and 65%. Bedding of paddy husk was provided on the floor. All the rabbits were properly fed with commercially available rabbit feed along with fresh leafy vegetables and water.
Animals were allowed unrestricted activity and were closely monitored regularly for signs of wound infection, wound breakdown and other complications.
Marrow harvesting
The contralateral proximal tibia was prepared using hair removal cream and twice painted with 10% povidine iodine. Again, rabbits were anaesthetised with ketamine (IM 5 mg/kg) + midazolam (5 mg/kg) and 2% Xylocaine locally. Around 10 ml of bone marrow was aspirated in a heparinised syringe with the hip of a bone marrow aspiration needle.
Stem cell isolation
Stem cells were isolated by the method of Pittenger et al.14,15 Ficoll-Hyopaque solution (1 ml at room temperature) (1.077 g/cm3; Sigma, St Louis, Missouri) was put in sterile centrifuge tubes and 10 ml marrow carefully layered over it. The mixture was put in centrifuge tube with spin speed of 1200 rpm for 30 minutes at 22°C. After centrifugation, the plasma layer was carefully aspirated and discarded without disturbing the plasma–Ficoll interface. Marrow aspirate concentrate was transferred into another sterile tube using a sterile pipette. Phosphate buffered saline was added to the cell suspension and washed thorougly to make a final suspension. When compared with other isolation and culture techniques, this method is more cost-effective.14
Stem cell implantation
Processed stem cells were transported back to the operating theatre (OT) using a specialised kit. After adequate painting and draping, processed stem cells were injected into the medial compartment of the operated joint aseptically at 12 weeks after the ACLT procedure. The study group was injected with approximately 1×106 cells suspended in 1 ml of medium into the medial compartment of the operated joint aseptically. The control group received 1 ml of medium without cells.
Radiological evaluation
Anteroposterior (AP) and lateral radiographs of the knee were performed at 12, 16 and 20 weeks after surgery. Radiographs were performed in the Department of Radiology at the Institute of Medicine, Banaras Hindu University, using a standardised protocol. Radiography was performed by the same operator with the same equipment (Siemens DC.12 m Japan (Munich, Germany), 42 kV, 250mA, 32 ms, 80 cm film–focus distance). For the AP view, rabbits were placed in dorsal recumbency, with legs extended caudally. Lateral radiographs were performed on semiflexed knee joints. Radiological OA was assessed by means of Kellgren and Lawrence’s16 grading system: radiological features including narrowing of joint space, presence of osteophytes, sclerosis of subchondral bone and deformity of bone ends are assessed, resulting in five grades of 0 (none), 1 (doubtful), 2 (minimal), 3 (moderate) and 4 (severe).
Histopathological examination
Animals were sacrificed with an overdose of propofol. A total of ten animals, five from the study and five from the control group were sacrificed at 16 weeks and 20 weeks respectively. Distal femora were cleared of all soft-tissue attachments and fixed using a 1:10 dilution of formalin solution. Sagittal sections of thickness between 3 mm and 4 mm were taken through the distal femur. Bone was decalcified in a solution comprising a mixture of hydrochloric acid, formic acid and formalin. The bone consistency was assessed every 24 hours and specimens were removed from the decalcifying solution once judged soft enough for cutting and further processing for light microscopy. The decalcified sections were embedded in paraffin and sectioned carefully at a thickness of 5 µ to 6 µ with a microtome and stained with haematoxylin and eosin (H& E), safranine O and fast green. Between four and eight sections from the distal femur and medial and lateral condyle of the articular surface of each rabbit were analysed.
The analysis of progression of cartilage lesions was quantified using a modification of the method proposed by Mankin et al.17 This widely used method of defining severity of lesions focusses on the quantitative evaluation of focal degenerative changes. The system assigns scores from each of four criteria, including structure (0 to 6), cells (0 to 3), Safranin O staining (0 to 4) and tidemark integrity (0 to 1), with higher total scores out of 14 indicating a higher grade of OA.
Statistical analysis
The mean histopathological and radiological scores were calculated with standard deviations. The difference between groups was analysed using the Mann–Whitney U test. Statistical analyses were performed using SPSS 16 (IBM, New York, United States) and a p-value < 0.05 was considered to indicate statistical significance.
Results
Macroscopic observations
In the MSC-treated group, articular cartilage at 16 weeks showed reduction in the severity of lesions (Fig. 1a). By 20 weeks, a good gross appearance was noticeable in the MSC-treated joints, which resembled normal articular cartilage (Fig. 1b).
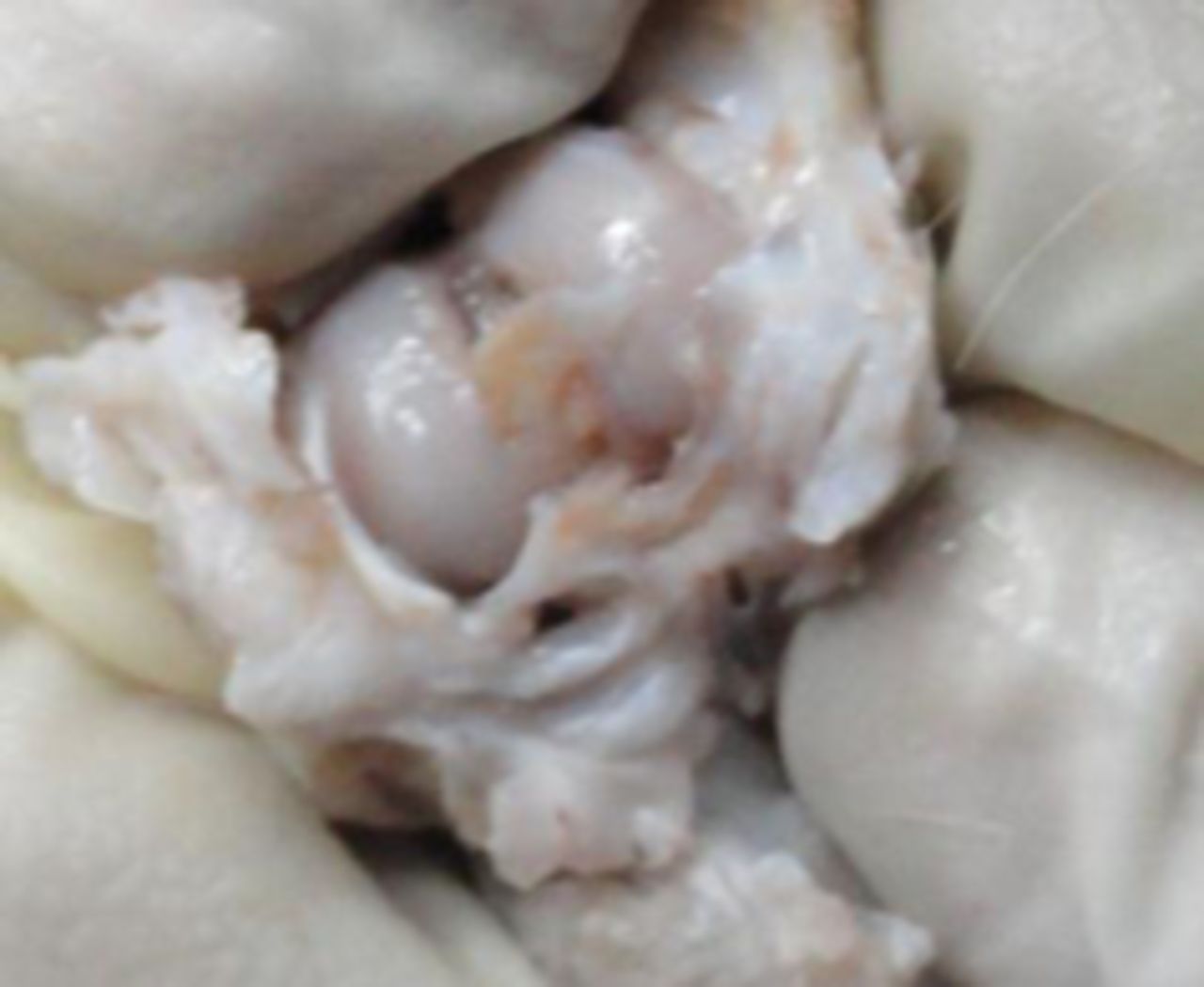
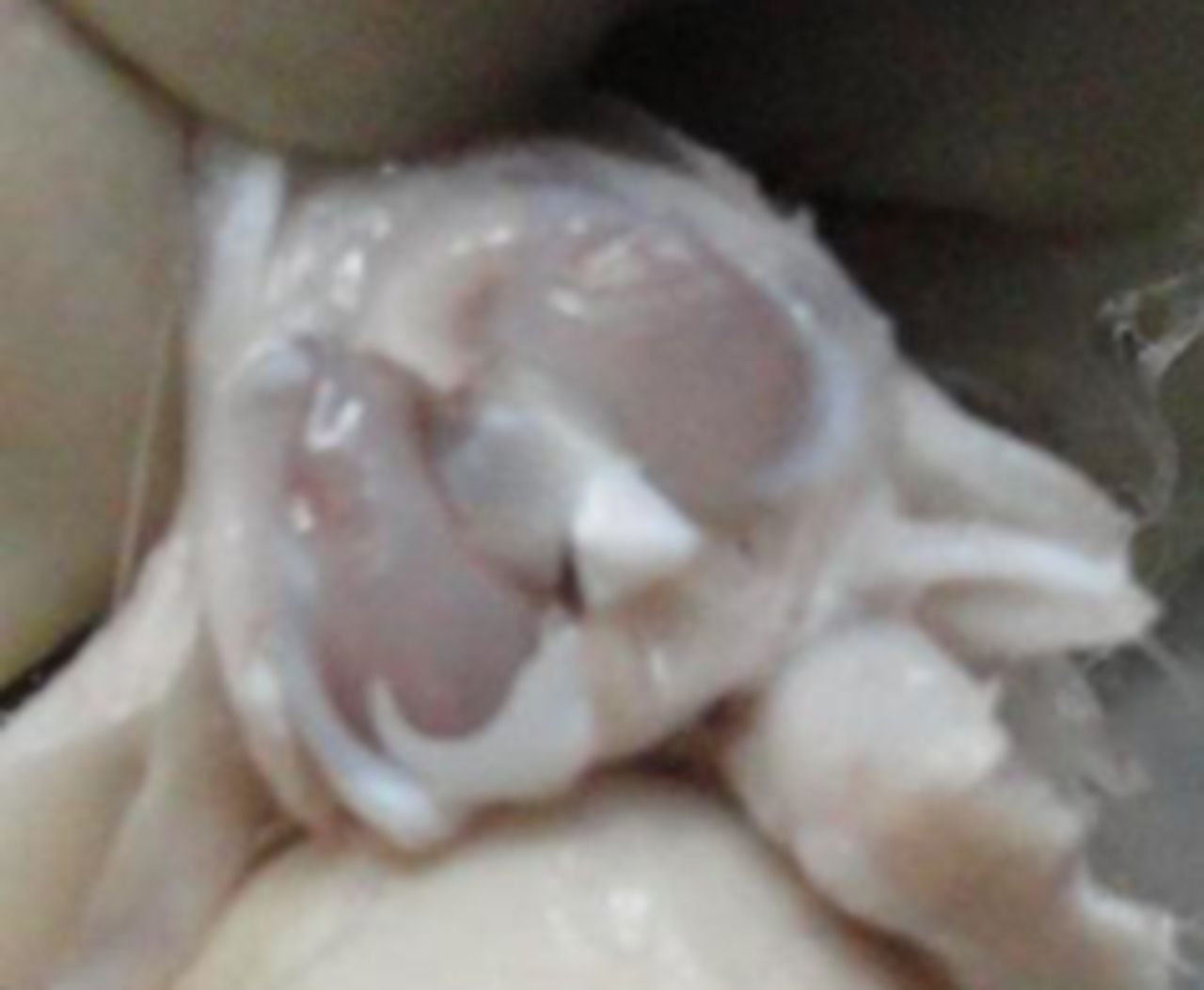
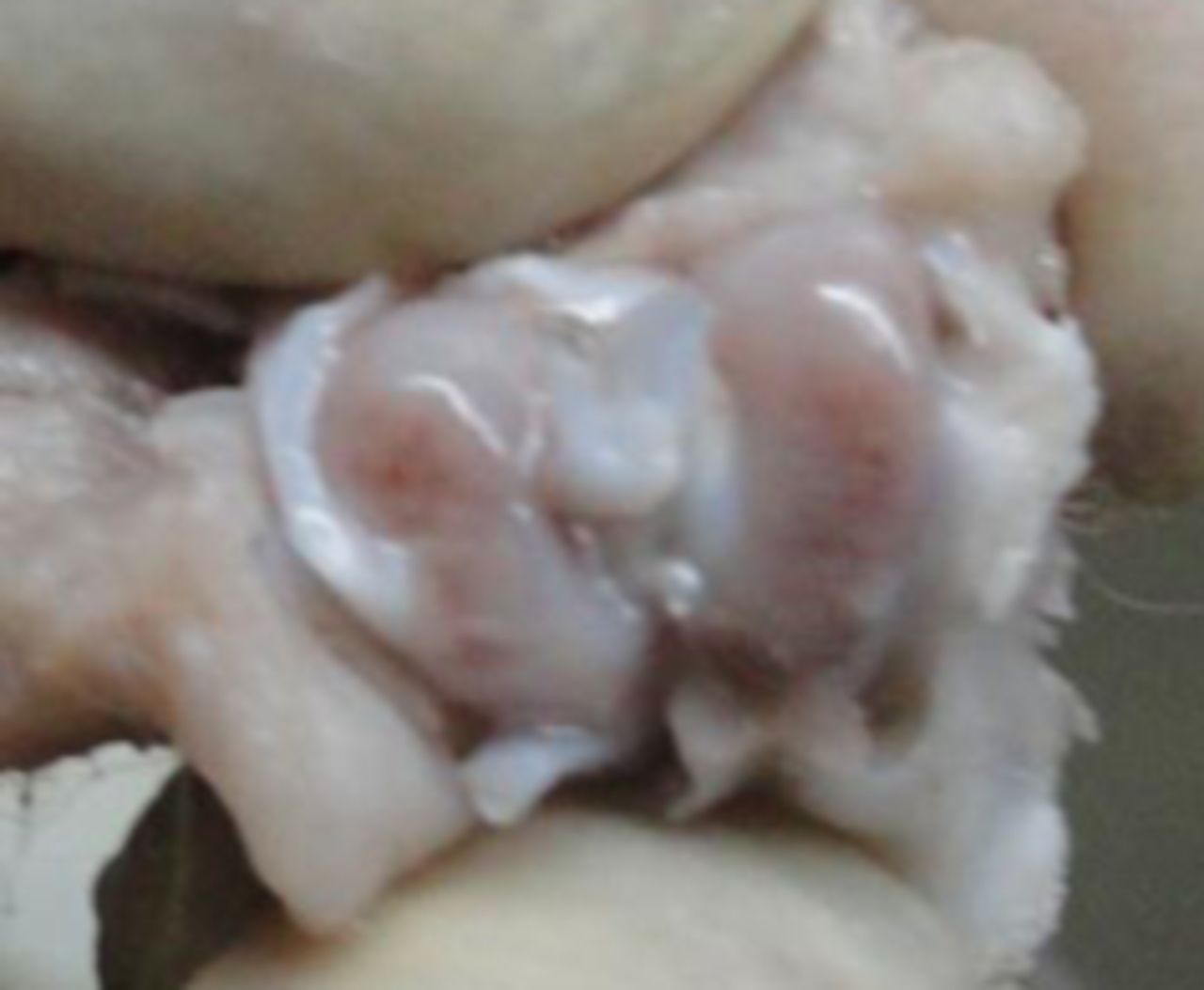
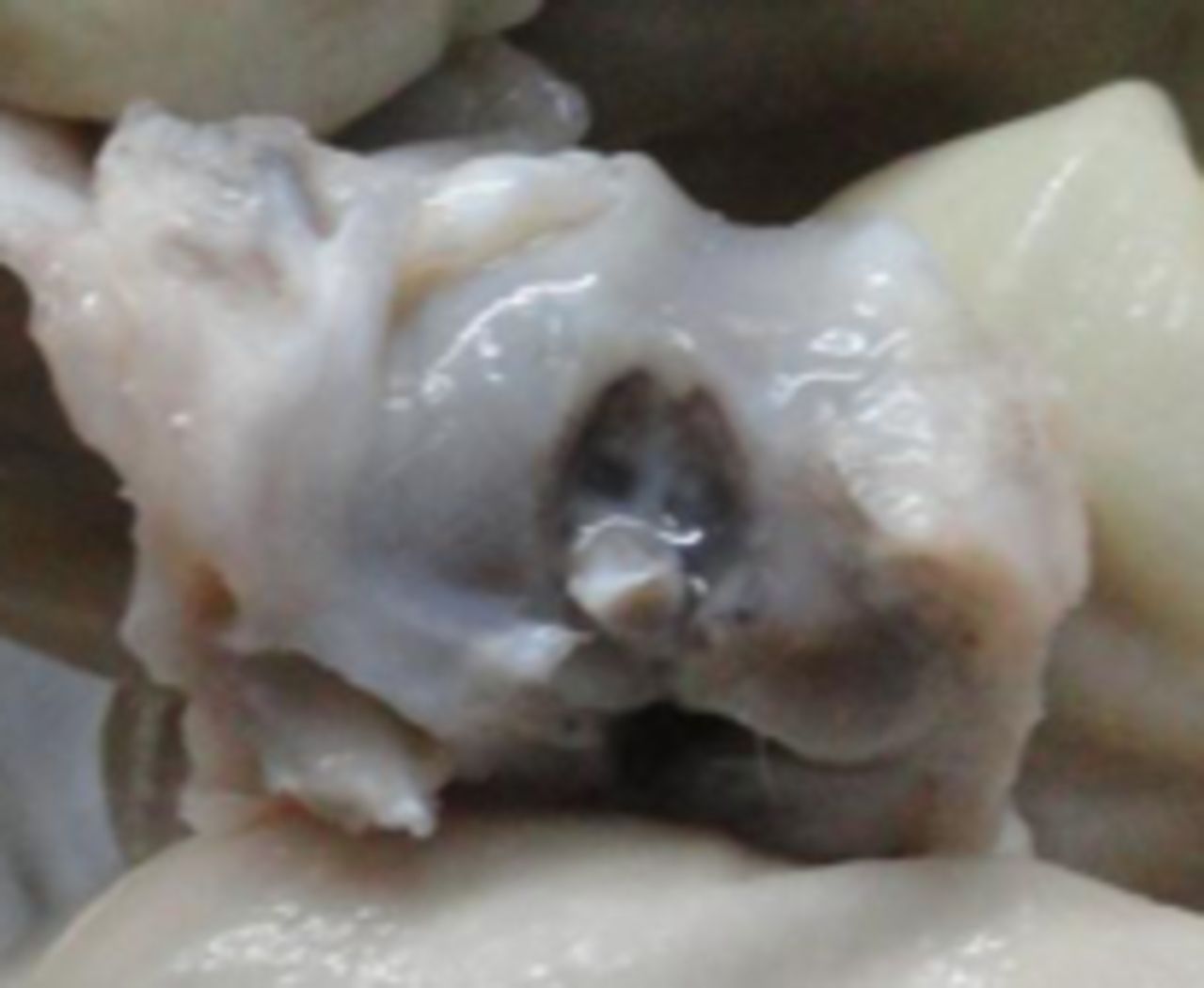
Figs. 1a - 1d
Figures 1a and 1b – gross photographs of femoral condyles in the mesenchymal stem cell (MSC)-treated group a) at 16 weeks post-operatively, showing a reduction in lesion severity and b) at 20 weeks post-operatively, showing further reduction in lesion severity. Figures 1c and 1d – gross photographs of femoral condyles in the control group at c) 16 and d) 20 weeks post-operatively, showing characteristics of osteoarthritis (OA) becoming more evident between time-points.
In the control group, gross characteristics of OA including fibrillation, erosion, and osteophyte formation were seen, especially in the medial femoral condyles, at 16 and 20 weeks post-operatively (Figs 1c and 1d). The severity of lesions was seen to worsen between specimens at 16 and 20 weeks.
Histopathological evaluation
Sample histological images for the MSC-treated and control groups at 16 and 20 weeks are provided in Figure 2. The histological appearance in the study group was characterised by appropriate thickness, normal distribution of the cells and consistent staining of the cartilage, with the surface layer showing very mild irregularity. Conversely, the control group showed structural disorganisation and severe hypocellularity of chondrocytes.
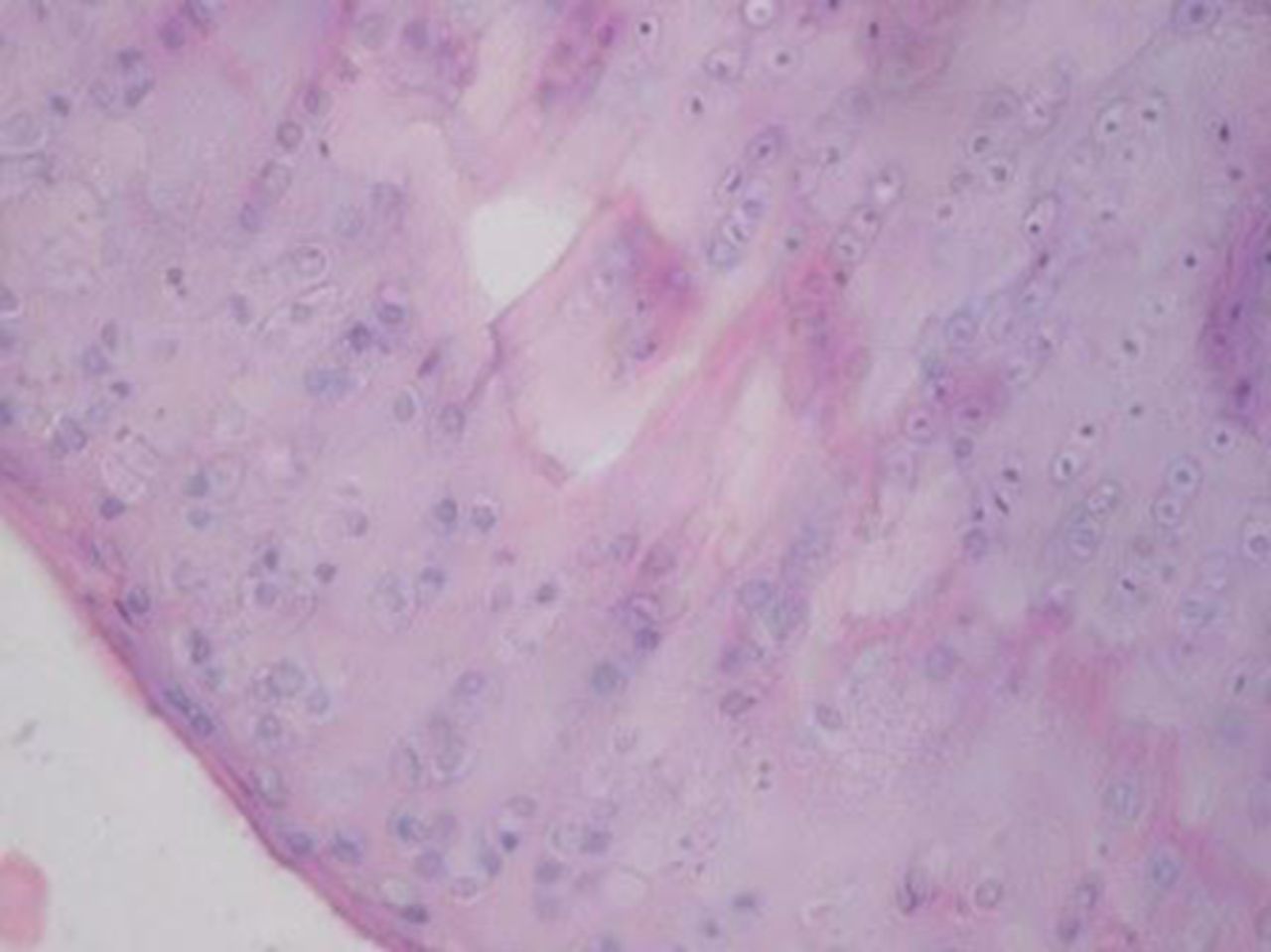
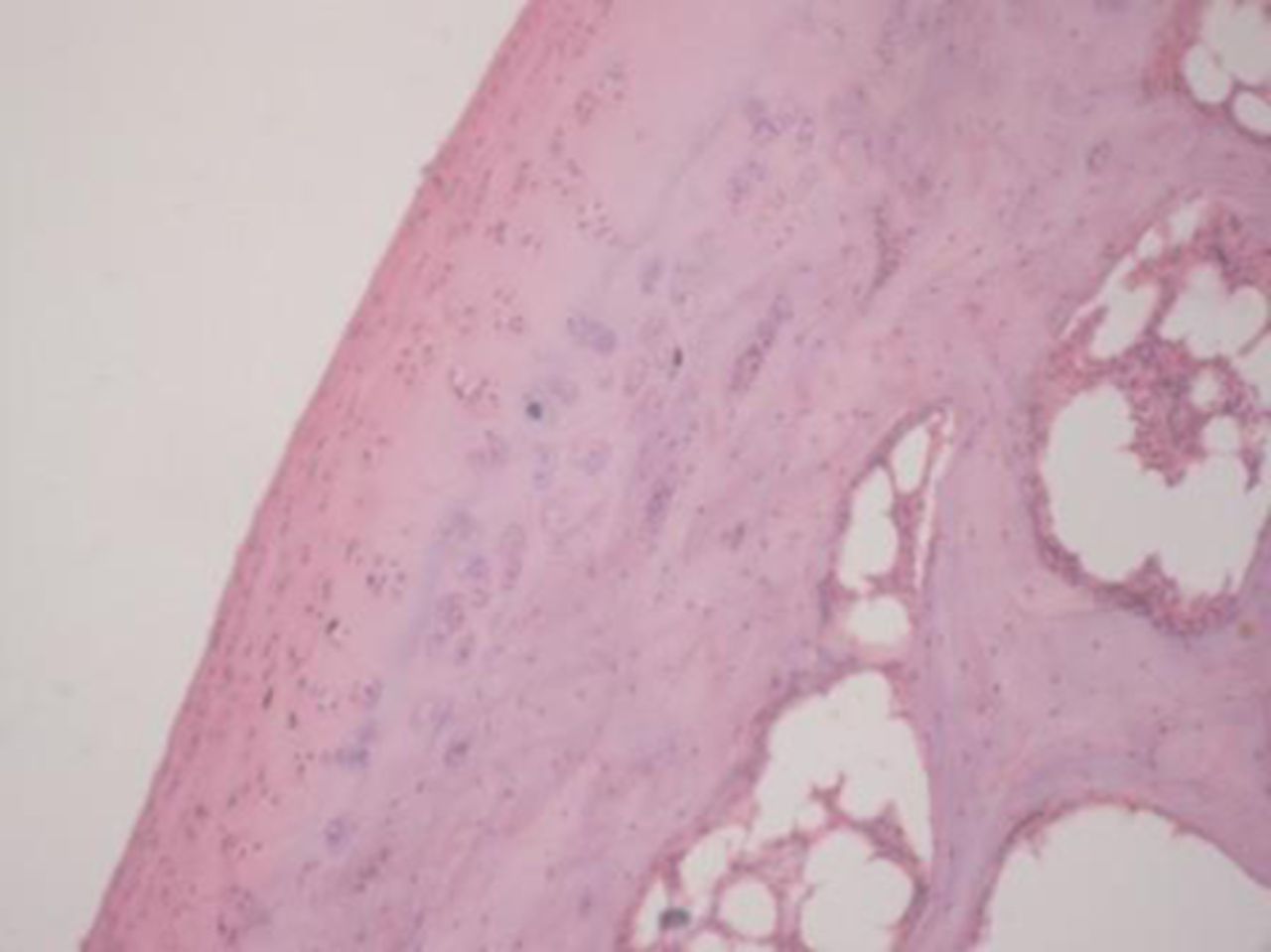
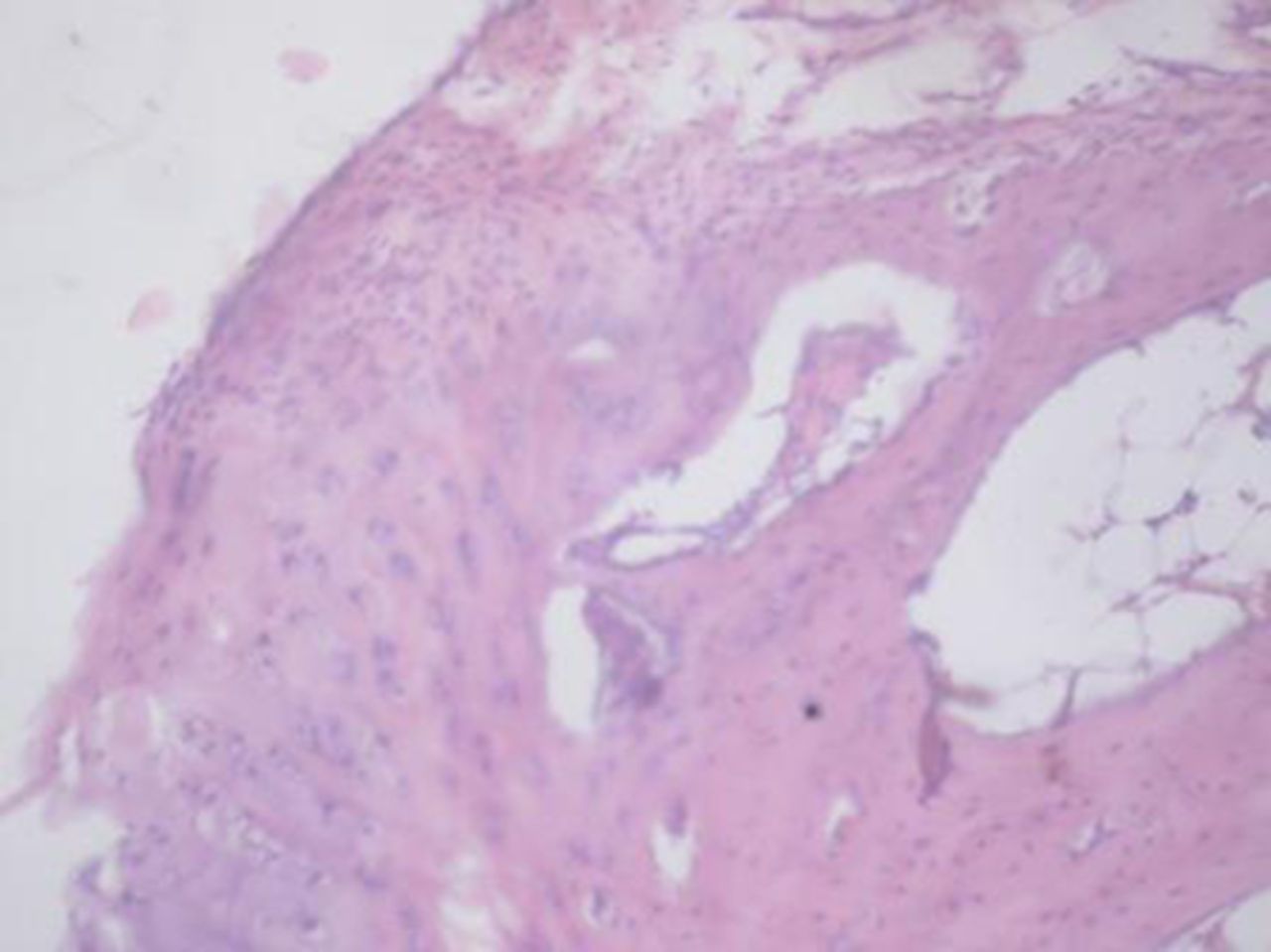
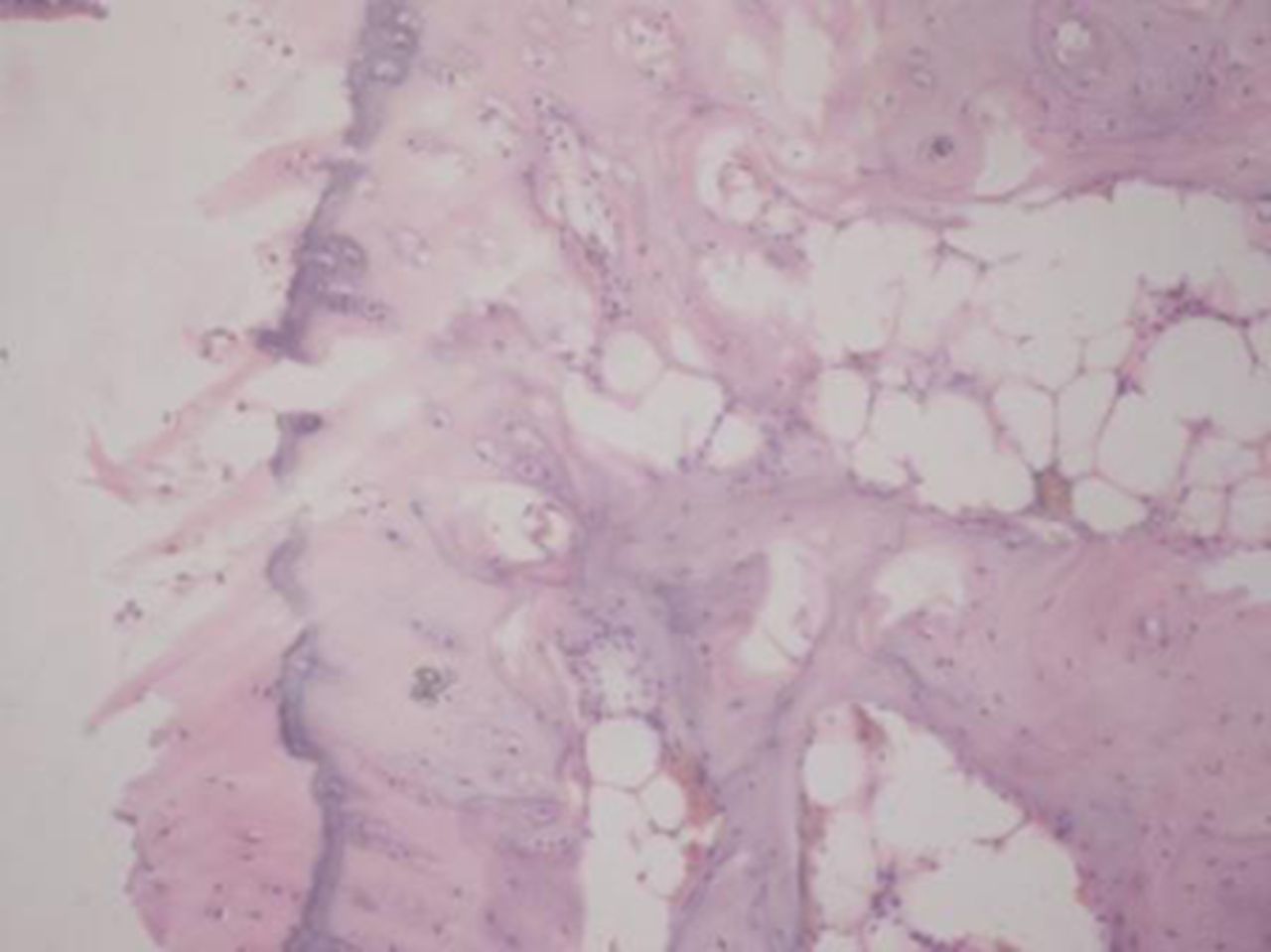
Figs. 2a - 2d
Figures 2a and 2b – histological images of articular cartilage in the mesenchymal stem cell (MSC)-treated group a) at 16 weeks and b) at 20 weeks post-operatively, showing appropriate thickness, normal distribution of the cells and consistent staining of the cartilage, with the surface layer showing very mild irregularity (haematoxylin and eosin, ×40). Figures 2c and 2d – histological images of articular cartilage in the control group c) at 16 weeks and d) at 20 weeks post-operatively, showing structural disorganisation and severe hypocellularity of chondrocytes.
The mean histological scores for the study and control specimens at 16 and 20 weeks are given in Table I. The study group had a significantly lower histological score than the controls at both 16 and 20 weeks post-operatively (p = 0.009 and p = 0.008, respectively).
Table I
Histopathological scores according to Mankin et al16
| Time-point | Study group | Control group | p-value |
|---|---|---|---|
| 16 weeks post-operatively | |||
| Specimens (n) | 5 | 5 | |
| Mean (sd) histopathological score | 4.20 (0.44) | 6.00 (0.70) | 0.009 |
| 20 weeks post-operatively | |||
| Specimens (n) | 5 | 5 | |
| Mean (sd) histopathological score | 5.80 (0.83) | 9.40 (0.54) | 0.008 |
Radiological findings
At 12 weeks after surgery all knees subjected to ACLT showed radiological signs of OA including marginal osteophytes, narrowing of joint space and subchondral bone sclerosis.
Sample radiological images for the MSC-treated and control groups at 16 and 20 weeks are provided in Figure 3. Assessment of radiographs at both time-points shows less severe radiological signs of OA (including osteophyte formation, subchondral bone sclerosis and articular surface irregularity) in the MSC group compared with the controls.
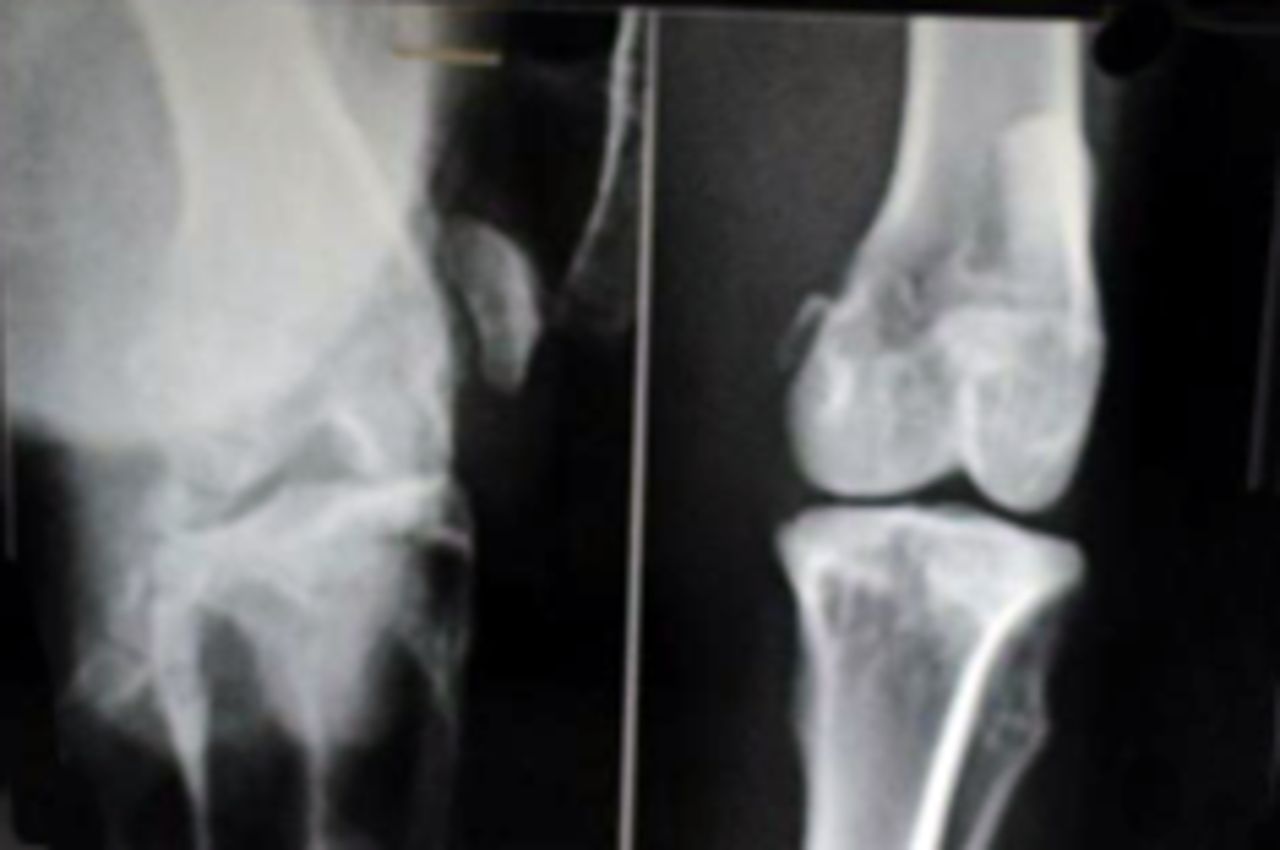
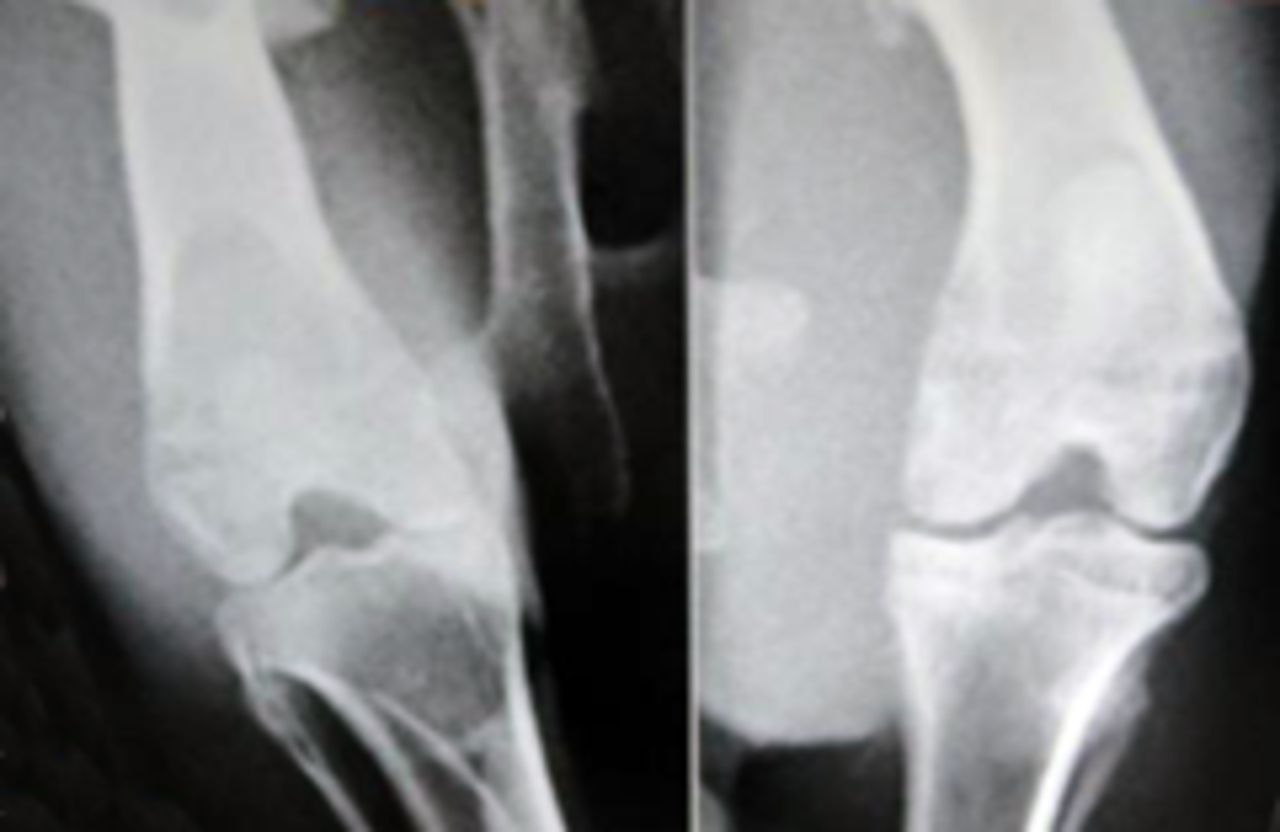
Figs. 3a - 3b
Figure 3a – anteroposterior (AP) radiographs at 16 weeks post-operatively in the mesenchymal stem cell (MSC)-treated group (left) and the control group (right). Figure 3b – AP radiographs at 20 weeks post-operatively in the MSC-treated group (left) and the control group (right). Radiological evaluation at both time-points shows less severe radiological signs of osteoarthritis (OA) (including osteophyte formation, subchondral bone sclerosis and articular surface irregularity) in the MSC group compared with the controls.
The mean radiological scores for the study and control specimens at 12, 16 and 20 weeks are given in Table II. Although there was no difference between the groups at 12 weeks (p = 0.481), the study group had a significantly lower mean score at both 16 and 20 weeks (p = 0.015 and p = 0.014, respectively).
Table II
Radiological scores for the severity of osteoarthritic lesions according to Kellgren et al16
| Time-point | Study group | Control group | p-value | |
|---|---|---|---|---|
| 12 weeks post-operatively | ||||
| Specimens (n) | 10 | 10 | ||
| Mean (sd) radiological score | 2.20 (0.42) | 2.40 (0.51) | 0.481 | |
| 16 weeks post-operatively | ||||
| Specimens (n) | 10 | 10 | ||
| Mean (sd) radiological score | 2.80 (0.42) | 3.40 (0.51) | 0.015 | |
| 20 weeks post-operatively | ||||
| Specimens (n) | 5 | 5 | ||
| Mean (sd) radiological score | 3.00 (0.00) | 3.80 (0.44) | 0.014 |
Discussion
With the development of new techniques for exploring the various mechanisms in cellular and molecular biology, a better understanding of the basic events in cartilage damage is now possible. Several studies have demonstrated that the transplantation of mesenchymal progenitors found in bone marrow aspirates can be useful in the treatment of OA.18–20 Of the different types of adult stem cells, stem cells from the bone marrow are considered to have the highest multilineage potential and have been studied for therapeutic purposes.10 Bone marrow-derived MSCs in the treatment of musculoskeletal problems have been investigated in numerous pre-clinical studies in animals21-24 as well as some clinical studies.25-27 Promising results have been shown and the use of autologous cells enhances the safety of the treatment. In terms of ‘stemness’, MSCs possess the ability to regenerate cell types specific for different tissues, including adipose tissue, bone and cartilage.14
One major advantage of using human MSCs for in vivo therapy is that they are non-immunogenic. Several protocols have been developed for the isolation and expansion of MSCs from bone marrow, including the use of density-gradient centrifugation, fluorescence-activated cell sorting, specific cell surface antibody, selective adhesion to laminin-coated plates, Hoechest dye exclusion and size-sieved culture. Potential clinical disadvantages of these methods are the heterogeneity of cultured cells, high risk of contamination and the high cost of production.28-31
The sample of cells may also be obtained after centrifugation, with mononuclear cells isolated or fractionated by conventional density-gradient centrifugation using Ficoll-Hypaque solution. The technique we used was according to the method described by Pittenger et al.14,15When compared with other isolation and culture techniques, this procedure is more cost-effective.14 In our study the total amount of bone marrow aspirate is 10 ml and that of stem cell is 1 ml, though the volume injected is less when compared with the bone marrow aspirate, however 1 million concentrated cells are present in 1 ml, which is injected into the stem cell group. On histological analysis, we had quantitatively calculated that around two to three million cells were injected. Various methods for the delivery of MSCs have been described, including cultured scaffolds, gel foams, autografts and systemic delivery.32,33 We preferred immediate isolation with percutaneous injection into the knee joint on the same day, which allows greater control over the site of application, as well as limiting further procedures to one injection.34 The technique is safe and minimally invasive. There were no incidences of haematoma or infection at the injection site. Currently, it is difficult to know the exact mechanism that follows once the injection is given. The mechanism by which stem cells act is a relevant matter for future studies.35-37
Most treatment options available for OA focus on managing the symptoms rather than addressing the cause.3,4 Our study using stems cell as a treatment option for OA has shown significant improvements histopathologically and radiologically, compared with control specimens at both 16 and 20 weeks of follow-up. We can conclude from our study that stem cells are a promising source for treatment of OA even without the use of scaffolds or stem cell culture, both of which add to the cost of treatment. However, studies with larger groups and longer follow-up are required to determine the use of stem cells as a therapeutic option.
1 Brooks PM . Impact of osteoarthritis on individuals and society: how much disability?: social consequences and health economic implications. Curr Opin Rheumatol2002;14:573–577. Google Scholar
2 Dennison E, Cooper C. Osteoarthritis: epidemiology and classification. In: Hochberg MC, Silman AJ, Smolen JS, et al, eds. Rheumatology. London: Mosby, 2003:1781–1791. Google Scholar
3 Lapsley HM , MarchLM, TribeKL, CrossMJ, BrooksPM. Living with osteoarthritis: patient expenditures, health status, and social impact. Arthritis Rheum2001;45:301–306.CrossrefPubMed Google Scholar
4 Mapel DW , ShainlineM, PaezK, GunterM. Hospital, pharmacy, and outpatient costs for osteoarthritis and chronic back pain. J Rheumatol2004;31:573–583.PubMed Google Scholar
5 Brandt KD , DieppeP, RadinEL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am2008;34:531–559.CrossrefPubMed Google Scholar
6 Loeser RF Jr . Aging and the etiopathogenesis and treatment of osteoarthritis. Rheum Dis Clin North Am2000;26:547–567.CrossrefPubMed Google Scholar
7 Hunter DJ . Osteoarthritis. Best Pract Res Clin Rheumatol2011;25:801–814.CrossrefPubMed Google Scholar
8 McIlwraith CW. General pathobiology of the joint and response to injury. In: McIlwraith CW, Trotter GW, eds. Joint disease in the horse. Philadelphia: WB Saunders, 1996:40–70. Google Scholar
9 De Bari C , Dell’AccioF. Mesenchymal stem cells in rheumatology: a regenerative approach to joint repair. Clin Sci (Lond)2007;113:339–348.CrossrefPubMed Google Scholar
10 Kolf CM , ChoE, TuanRS. Mesenchymal stromal cells: biology of adult mesenchymal stem cells: regulation of niche, self-renewal and differentiation. Arthritis Res Ther2007;9:204. Google Scholar
11 Yoshioka M , CouttsRD, AmielD, HackerSA. Characterization of a model of osteoarthritis in the rabbit knee. Osteoarthritis Cartilage1996;4:87–98.CrossrefPubMed Google Scholar
12 Vignon E , BejuiJ, MathieuP, et al.Histological cartilage changes in a rabbit model of osteoarthritis. J Rheumatol1987;14:104–106.PubMed Google Scholar
13 van Eck CF , van den BekeromMP, FuFH, PoolmanRW, KerkhoffsGM. Methods to diagnose acute anterior cruciate ligament rupture: a meta-analysis of physical examinations with and without anaesthesia. Knee Surg Sports Traumatol Arthrosc2013;21:1895–1903.CrossrefPubMed Google Scholar
14 Pittenger MF , MackayAM, BeckSC, et al.Multilineage potential of adult human mesenchymal stem cells. Science1999;284:143–147.CrossrefPubMed Google Scholar
15 Aggarwal S , PittengerMF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood2005;105:1815–1822.CrossrefPubMed Google Scholar
16 Kellgren JH , LawrenceJS. Radiological assessment of rheumatoid arthritis. Ann Rheum Dis1957;16:485–493.CrossrefPubMed Google Scholar
17 Mankin HJ , DorfmanH, LippielloL, ZarinsA. Biochemical andmetabolic abnormalities in articular cartilage from osteoarthritic human hips: II: correlation of morphology with biochemical and metabolic data. J Bone Joint Surg [Am]1971;53-A:523–537. Google Scholar
18 Wakitani S , ImotoK, YamamotoT, et al.Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage2002;10:199–206.CrossrefPubMed Google Scholar
19 Murphy JM , FinkDJ, HunzikerEB, BarryFP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum2003;48:3464–3474.CrossrefPubMed Google Scholar
20 Grigolo B , LisignoliG, DesandoG, et al.Osteoarthritis treated with mesenchymal stem cells on hyaluronan-based scaffold in rabbit. Tissue Eng Part C2009;15:647–658.CrossrefPubMed Google Scholar
21 Chong A , AngD, GohJ, et al.Bone marrow-derived mesenchymal stem cells influence early tendon-healing in a rabbit achilles tendon model. J Bone Joint Surg [Am]2007;89-A:74–81.CrossrefPubMed Google Scholar
22 Ricco S , RenziS, Del BueM, et al.Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int J Immunopathol Pharmacol2013;26(Suppl):61–68.CrossrefPubMed Google Scholar
23 Anderson DG , MarkovaD, AnHS, et al.Human umbilical cord blood-derived mesenchymal stem cells in the cultured rabbit intervertebral disc: a novel cellsource for disc repair. Am J Phys Med Rehabil2013;92:420–429. Google Scholar
24 Li Z , LiaoW, ZhaoQ, et al.Angiogenesis and bone regeneration by allogeneic mesenchymal stem cell intravenous transplantation in rabbit model of avascular necrotic femoral head. J Surg Res2013;183:193–203.CrossrefPubMed Google Scholar
25 Barry FP. Mesenchymal stem cell therapy in joint disease. Novartis Found Symp 2003;249:86-96; discussion 96-102, 170-4, 239-41. Google Scholar
26 Kobayashi T , OchiM, YanadaS, et al.A novel cell delivery system using magnetically labeled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthroscopy2008;24:69–76.CrossrefPubMed Google Scholar
27 Kuroda R , IshidaK, MatsumotoT, et al.Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage2007;15:226–231.CrossrefPubMed Google Scholar
28 Pasut A , OleynikP, RudnickiMA. Isolation of muscle stem cells by fluorescence activated cell sorting cytometry. Methods Mol Biol2012;798:53–64.CrossrefPubMed Google Scholar
29 Williams EL , WhiteK, OreffoRO. Isolation and enrichment of Stro-1 immunoselected mesenchymal stem cells from adult human bone marrow. Methods Mol Biol2013;1035:67–73.CrossrefPubMed Google Scholar
30 Stern MM , TygrettLT, WaldschmidtTJ, BickenbachJR. Cells isolated from the epidermis by Hoechst dye exclusion, small size, and negative selection for hematopoietic markers can generate B lymphocyte precursors. J Invest Dermatol2008;128:1386–1396.CrossrefPubMed Google Scholar
31 Shihabuddin LS . Adult rodent spinal cord-derived neural stem cells: isolation and characterization. Methods Mol Biol2008;438:55–66.CrossrefPubMed Google Scholar
32 Koga H , ShimayaM, MunetaT, et al.Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res Ther2008;10:R84.CrossrefPubMed Google Scholar
33 Guo X , ParkH, LiuG, et al.In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials2009;30:2741–2752.CrossrefPubMed Google Scholar
34 Toghraie FS , ChenariN, GholipourMA, et al.Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in rabbit. Knee2011;18:71–75.CrossrefPubMed Google Scholar
35 Uccelli A , PistoiaV, MorettaL. Mesenchymal stem cells: a new strategy for immunosuppression. Trends Immunol2007;28:219–226.CrossrefPubMed Google Scholar
36 Chen X , ArmstrongMA, LiG. Mesenchymal stemcells in immunoregulation. Immunol Cell Biol2006;84:413–421. Google Scholar
37 Caplan AI , DennisJE. Mesenchymal stem cells as trophic mediators. J Cell Biochem2006;98:1076–1084.CrossrefPubMed Google Scholar
Funding statement:
None declared
Author contributions:
A. Singh: Writing the paper, Data collection, Data analysis
S. C. Goel: Data analysis
K. K. Gupta: Stem cell isolation
M. Kumar: Histopathological analysis
G. R. Arun: Data collection
H. Patil: Data collection
V. Kumaraswamy: Data analysis
S. Jha: Histopathalogical analysis
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










