Abstract
Objectives
The aim of this experimental study on New Zealand’s white rabbits was to investigate the transplantation of autogenous growth plate cells in order to treat the injured growth plate. They were assessed in terms of measurements of radiological tibial varus and histological characteristics.
Methods
An experimental model of plate growth medial partial resection of the tibia in 14 New Zealand white rabbits was created. During this surgical procedure the plate growth cells were collected and cultured. While the second surgery was being performed, the autologous cultured growth plate cells were grafted at the right tibia, whereas the left tibia was used as a control group.
Results
Histological examinations showed that the grafted right tibia presented the regular shape of the plate growth with hypertrophic maturation, chondrocyte columniation and endochondral calcification. Radiological study shows that the mean tibial deformity at the left angle was 20.29° (6.25 to 33) and 7.21° (5 to 10) in the right angle.
Conclusion
This study has demonstrated that grafting of autogenous cultured growth plate cells into a defect of the medial aspect of the proximal tibial physis can prevent bone bridge formation, growth arrest and the development of varus deformity.
Cite this article: Bone Joint Res 2014;3:310–16
Article focus
This article explores a radiological and histological investigation of the transplantation of autogenous cultured growth plate cells to treat the injured growth plate.
Could we obtain the morphology of the growth plate after autologous cultured growth plate cells?
Could this new plate growth present the growth impact?
Key messages
The grafting of autogenous cultured growth plate cells into a defect of the medial aspect of the proximal tibial physis can prevent bone bridge formation, growth arrest and the development of varus deformity.
Strengths and limitations
The cartilage cells were histologically examined after both removal of the growth plate and culture and at the end of study when the animals were killed. During this study, cartilage cells were discovered at each stage.
The growth impact of the rabbit tibia showed no graft rejection and the return of the ability to grow. The cells were transplanted after the creation of mechanical damage of the growth plate, but no other types of plate growth damage were made during this study.
Limitation: the number of attempts that were possible; the authors believe that the use of a greater number of individuals would give more reliable results, which would dispel any issues in the method.
Introduction
Chondrocyte hypertrophy has an important role in the longitudinal growth of the skeleton.1 Physeal injury can cause bone bridge formation between the epiphysis and the metaphysis, resulting in growth arrest and angular deformity.2,3 Damage of the physeal growth plate could be caused by trauma, infection, tumour and genetic causes. There have been numerous animal experiments to create, prevent and resect physeal bridges.4 The bone bridge formation leading to premature physeal fusion can be prevented by separating the epiphyseal and metaphyseal parts using an interpositional material placed in the resultant defect; this permits correction of the deformity by growth.2,5 Langenskiold6 filled osseous cavities in skeletally immature pigs with autogenous fat. Therefore, the use of fat can be effective for treating a physis with relatively little damage.5 In cases with large bone bridges, the interpositional material should give mechanical support to prevent subsidence in the resection site.5 In addition, the results with non-biological grafts (silastic, methylmethacrylate) for extensive growth plate injuries have been worse than with the biological grafts.2 Other authors have used iliac physeal plates, that is, cartilage from articulation, with modest success.3,7-9 Cultured chondrocytes grafted to injured growth plates provided evidence of correction of the bone deformity, but only in small physeal injury. In addition, the authors used the allogeneic chondrocytes, which are problematic in terms of their antigenicity. Autogenous chondrocyte growth plate grafting resolved this problem, and the cultured medium provided sufficient mechanical support.3,5
Materials and Methods
Surgical procedures
A total of 14 New Zealand white rabbits at the age of five weeks were selected for the experiment. Each animal was assigned a number from one to 14 (experimental rabbits in the subsequent part of the article will be referred to as samples). The surgery was performed twice on both the left and right tibias of each rabbit on two different days. Every animal of the group was operated on within one day. The surgery was performed under general anaesthetic with the implementation of subcutaneous atropine sulphate (0.1 mg). After ten minutes, an intramuscular injection of xylazine hydrochloride (0.2 mg/kg) was given. The puncture of the marginal ear vein cannulation, the cannula (0.7) and administration of a thiopental intravenous drip (25 mg/kg) were used, and both the respiratory rate and the heart rate were monitored (Datex Ohmeda Inc., Madison, Wisconsin). The skin over the knee was shaved and prepared using Skinsept Ecolab (Düsseldorf, Germany) and the area was draped. A 1.5 cm medial incision over the proximal part of the tibia was made in order to get to the right and the left physes. The periosteum was incised with a surgical scalpel blade and elevated gently from the medial aspect of the proximal part of the tibia. After a visual localisation of the growth plate at the right tibia, bone needles were used to remove the growth plate cartilage. A standard defect was thus created by removing a quarter of the proximal tibial physis, and inserting a standard drainage tube (Table I). The normal scale of the growth plates fuses at six to seven months of age.10 The culture medium is standard for chondrocyte culture as described by Peterson et al,11 however, this has since been modified.12
Table I
The course of work on the extraction, chondrocyte culture and chondrocyte transplantation of the right tibia
| Number | Excision of growth plate 22.08.2011 | Drainage tube in physis, 22.08.2011 | Chondrocyte culture, 22.08-12.09.2011 | Transplantation of autologous chondrocytes in physis, 12.09.2011 |
|---|---|---|---|---|
| 1 | + | + | + | + |
| 2 | + | + | + | + |
| 3 | + | + | + | + |
| 4 | + | + | + | + |
| 5 | + | + | + | + |
| 6 | + | + | + | + |
| 7 | + | + | + | + |
| 8 | + | + | + | + |
| 9 | + | + | + | + |
| 10 | + | + | + | + |
| 11 | + | + | + | + |
| 12 | + | + | + | + |
| 13 | + | + | + | + |
| 14 | + | + | + | + |
-
+ symbol indicates rabbits subjected to the procedure
At the left tibia, the same procedure of the physis excision was made (Table II). The left tibia, however, (samples 1 to 7) was filled with a drainage tube, (samples 8 to 11) the site of the physis resection was left without a drain, (samples 12 to 14) and no defect in the physis was created. The wound was closed with an interrupted suture using PDS 3-0 (Ethicon, Johnson & Johnson, Norderstedt, Germany). The mean time of the surgery was 25 minutes (12 to 37). The animals were allowed to bear weight and walk freely in the cages without a splint. A microscopic control (Olympus type BX51, Olympus America Inc., Melville, New York) was used in all cases to ensure that the physeal cartilage had been removed, completely exposing the epiphyseal and metaphyseal bone using haematoxylin and eosin staining. In all cases, the presence of the growth plate cartilage was confirmed.
Table II
The course of work on the extraction, chondrocyte culture and chondrocyte transplantation of the left tibia
| Number | Excision of growth plate 22.08.2011 | Drainage tube in physis 22.08.2011 | Chondrocyte culture 22.08 to 12.09.2011 | Transplantation of autologous chondrocytes in physis 12.09.2011 |
|---|---|---|---|---|
| 1 | + | + | - | - |
| 2 | + | + | - | - |
| 3 | + | + | - | - |
| 4 | + | + | - | - |
| 5 | + | + | - | - |
| 6 | + | + | - | - |
| 7 | + | + | - | - |
| 8 | + | - | - | - |
| 9 | + | - | - | - |
| 10 | + | - | - | - |
| 11 | + | - | - | - |
| 12 | - | - | - | - |
| 13 | - | - | - | - |
| 14 | - | - | - | - |
-
+ symbol indicates rabbits subjected to the procedure - indicates no surgery
A total of 22 days after the first procedure caused damage to the physis, the second surgery, using the same method of anaesthetic and the same incision, was performed. A procedure was then developed to remove the drainage tube and to transplant autologous physeal cells into the focal defect in the medial part of the proximal right tibia. Each pre-implantation graft was controlled by surgeons in order to maintain accuracy of the numbering of the graft and the animal. After tube drain ablation, the shape of the graft was contoured to match the shape in the tibia’s defect. The graft was not fixed in situ, but a gentle periosteum suture was made using PDS 4-0 (Ethicon). Each graft was controlled for stability of fixation before the skin was sutured. At the left tibia, the graft of autologous growth plate cells was not used, however, the same type of incision was made and the wound closure was the same as at the right tibia. In samples 1 to 7 the drainage tubes were removed.
After the second surgery, the animals were allowed to walk freely in their cages. After 60 days the experimental animals were killed by intravenous administration of Morbital (200 mg/kg) (Biowet Ltd, Puławy, Poland).
Chondrocyte culture
The growth plate samples were placed in a sterile tube containing Ham's F12 (Mediatech Inc., Herndon, Virginia) with 50 g/ml gentamycin sulfate (PAA, Linz, Austria) and 2 g/ml amphotericin B (PAA). All samples were transported to the Cell Culture Laboratory Tissue Bank in Katowice, Poland. The next day a fragment of the cartilage was washed and cut into small pieces. The specimen was left in Ham's F12 (Mediatech) medium, supplemented as described above with 0.1% collagenase type II (Worthington, Lakewood, New Jersey), for six to seven hours. After digestion, the cell suspension was washed in the Ham’s F12 medium (Mediatech) once, and inoculated into flasks in a DMEM/HamF12 (PAA) medium containing 10% NCS (PAA), with 50 g/ml gentamycin sulfate (PAA), 2 g/ml amphotericin B (PAA) and 50 g/ml L-ascorbic acid (PLIVA, Kraków, Poland). Cells were cultured in an incubator at 37°C in a 5.5% CO2 environment. Within a few hours, seeding cells adhered to the bottom, and about three days later they started to divide. The culture medium was exchanged every 48 hours. Proliferation was monitored using inverted microscopy (Carl Zeiss, Oberkochen, Germany). When the cells were 80% confluent they were passaged to new culture flasks. The day before transplantation into the growth plate defect, cell grafts in fibrinogen were prepared. Human fibrinogen was prepared from Regional Blood Center voluntary donors. Plasma was frozen at -80°C and thawed twice in a water bath. Concentrated fibrinogen was centrifuged at 4°C at 1000 x g in a Heraeus Cryofuge (Thermo Scientific, Wilmington, Delaware) for 15 minutes. Supernatant was discarded and the pellet of concentrated fibrinogen was used for the preparation of fibrin glue. The donor’s blood (plasma) was then tested for the presence of infectious agents including HIV, Hepatitis B, Hepatitis C and TP-syphilis. Before surgery the chondrocyte suspension was mixed with fibrin glue to prepare the fibrograft. Cultured cells were trypsinised, and then washed twice in DMEM/Ham’s F12 (Mediatech) medium supplemented with 10% NCS serum. The cell pellet was mixed with fibrinogen and tranexamic acid, then thrombin dissolved in calcium chloride (10%) was added. The graft was adjusted to fit the size and thickness of the growth plate defect in the tibia. Prepared grafts were then transported to the operating room. The time of culture was 22 days, and 14 autologous chondrocyte growth plate cultures were made. In all 14 samples, the presence of growth plate chondrocytes was confirmed before implantation. Before grafting, each was controlled in a histological examination using S-100 (Fig. 1). When the cells were 80% confluent they were passaged to new culture flasks. The day before transplantation into the growth plate defect, cell grafts in fibrinogen were prepared. Human fibrinogen was prepared from Regional Blood Center voluntary donors. Plasma was frozen at -80°C and thawed twice in a water bath. Concentrated fibrinogen was centrifuged at 4°C at 1000 x g in a Heraeus Cryofuge (Thermo Scientific, Wilmington, Delaware) for 15 minutes. Supernatant was discarded and the pellet of concentrated fibrinogen was used for the preparation of fibrin glue. The donor’s blood (plasma) was then tested for the presence of infectious agents including HIV, Hepatitis B, Hepatitis C and TP-syphilis. Before surgery the chondrocyte suspension was mixed with fibrin glue to prepare the fibrograft. Cultured cells were trypsinised, and then washed twice in DMEM/Ham’s F12 (Mediatech) medium supplemented with 10% NCS serum. The cell pellet was mixed with fibrinogen and tranexamic acid, then thrombin dissolved in calcium chloride (10%) was added. The graft was adjusted to fit the size and thickness of the growth plate defect in the tibia. Prepared grafts were then transported to the operating room. The time of culture was 22 days, and 14 autologous chondrocyte growth plate cultures were made. In all 14 samples, the presence of growth plate chondrocytes was confirmed before implantation. Before grafting, each was controlled in a histological examination using S-100 (Fig. 1) for 15 minutes. Supernatant was discarded and the pellet of concentrated fibrinogen was used for the preparation of fibrin glue. The donor’s blood (plasma) was then tested for the presence of infectious agents including HIV, Hepatitis B, Hepatitis C and TP-syphilis. Before surgery the chondrocyte suspension was mixed with fibrin glue to prepare the fibrograft. Cultured cells were trypsinised, and then washed twice in DMEM/Ham’s F12 (Mediatech) medium supplemented with 10% NCS serum. The cell pellet was mixed with fibrinogen and tranexamic acid, then thrombin dissolved in calcium chloride (10%) was added. The graft was adjusted to fit the size and thickness of the growth plate defect in the tibia. Prepared grafts were then transported to the operating room. The time of culture was 22 days, and 14 autologous chondrocyte growth plate cultures were made. In all 14 samples, the presence of growth plate chondrocytes was confirmed before implantation. Before grafting, each was controlled in a histological examination using S-100 (Fig. 1) was then tested for the presence of infectious agents including HIV, Hepatitis B, Hepatitis C and TP-syphilis. Before surgery the chondrocyte suspension was mixed with fibrin glue to prepare the fibrograft. Cultured cells were trypsinised, and then washed twice in DMEM/Ham’s F12 (Mediatech) medium supplemented with 10% NCS serum. The cell pellet was mixed with fibrinogen and tranexamic acid, then thrombin dissolved in calcium chloride (10%) was added. The graft was adjusted to fit the size and thickness of the growth plate defect in the tibia. Prepared grafts were then transported to the operating room. The time of culture was 22 days, and 14 autologous chondrocyte growth plate cultures were made. In all 14 samples, the presence of growth plate chondrocytes was confirmed before implantation. Before grafting, each was controlled in a histological examination using S-100 (Fig. 1) medium supplemented with 10% NCS serum. The cell pellet was mixed with fibrinogen and tranexamic acid, then thrombin dissolved in calcium chloride (10%) was added. The graft was adjusted to fit the size and thickness of the growth plate defect in the tibia. Prepared grafts were then transported to the operating room. The time of culture was 22 days, and 14 autologous chondrocyte growth plate cultures were made. In all 14 samples, the presence of growth plate chondrocytes was confirmed before implantation. Before grafting, each was controlled in a histological examination using S-100 (Fig. 1) was added. The graft was adjusted to fit the size and thickness of the growth plate defect in the tibia. Prepared grafts were then transported to the operating room. The time of culture was 22 days, and 14 autologous chondrocyte growth plate cultures were made. In all 14 samples, the presence of growth plate chondrocytes was confirmed before implantation. Before grafting, each was controlled in a histological examination using S-100 (Fig. 1). Articular chondrocytes are released from the surrounding matrix by enzyme isolation and then expanded during cell culture in a special cell medium as primary cells in culture are chondrocyte- differentiated somatic cells.
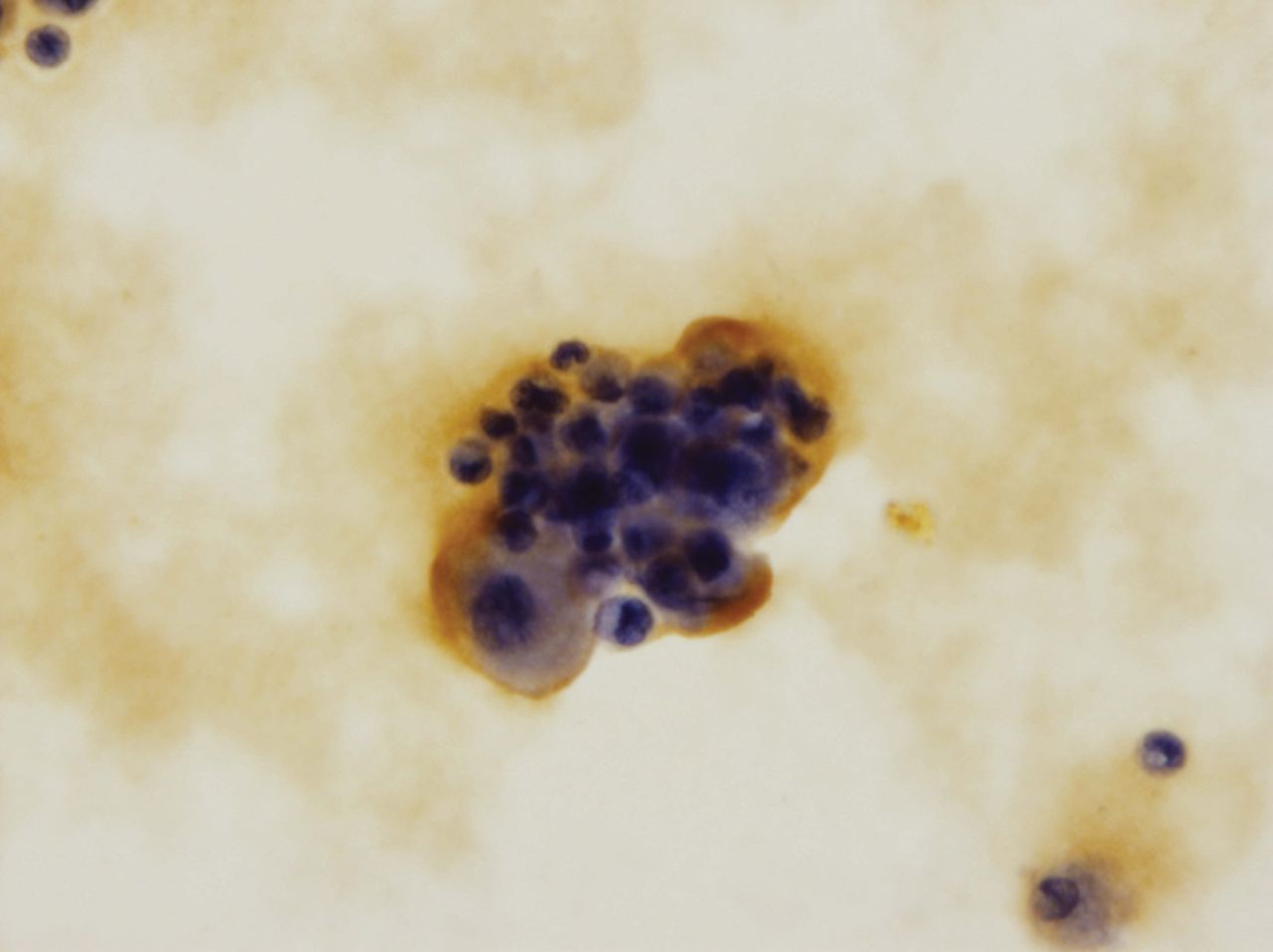
Fig. 1
Histological examination of the S-100 cultured chondrocytes (x 400).
Tissue examination
The tissues were taken for histological and radiological examination. The tibia and femur were excised and fixed in 10% formalin. The radiograph was performed on the day the animals were killed using an X-ray machine (Siemens type Multix Top, Munich, Germany). Both an anteroposterior and lateral view allowed for the bone bridge’s appearance and angular deformation evaluation. The angle between the longitudinal axis of the tibia and a line parallel to the tibial condyles was measured for each (n = 28). All downloaded bone material was decalcified using TBD2 (Shandon, Thermo Scientific, Waltham, Massachusetts). Sections 5 µ thick were frontally cut through the grafted area of the tibia before visual localisation of the grafted area was established. The histological examination was undertaken by three pathologists (RT, AG, AW) and haematoxylin and eosin staining was made.
Results
Histological findings
Following the grafting of the autologous physeal cartilage (samples 1 to 5, 11, 13), the right tibia presented a regular shape of plate growth with hypertrophic maturation, chondrocyte columniation and endochondral calcification. No inflammatory reaction was observed.
Following the grafting of the autologous physeal cartilage (samples 7 to 9), the right tibia presented an irregular physeal shape with the lacunae but with normal morphological changes during the process of cartilage differentiation and no bone bridge formation. After the grafting of the autologous physeal cartilage (sample 14), the right tibia presented with bone bridge formation at the operated medial side of approximately 15% of the growth plate width, however, the remaining grafted cartilage on another 10% of the growth plate width showed an irregular growth plate with the lacunae.
In those samples without grafting of the autologous physeal cartilage (1 to 11), the left tibia displayed bone bridge formation over growth plate resection. However, the left tibia in samples 12 to 14 (no surgery) presented no changes in plate growth (Fig. 2), the left tibia displayed bone bridge formation over growth plate resection. However, the left tibia in samples 12 to 14 (no surgery) presented no changes in plate growth (Fig. 2) presented no changes in plate growth (Fig. 2). The difference between the mean values of the number of cells in both the right tibias and the left was 475.17. In the right tibia there were 66% more cells in 1 mm2 of the plate than there were in the left tibia.
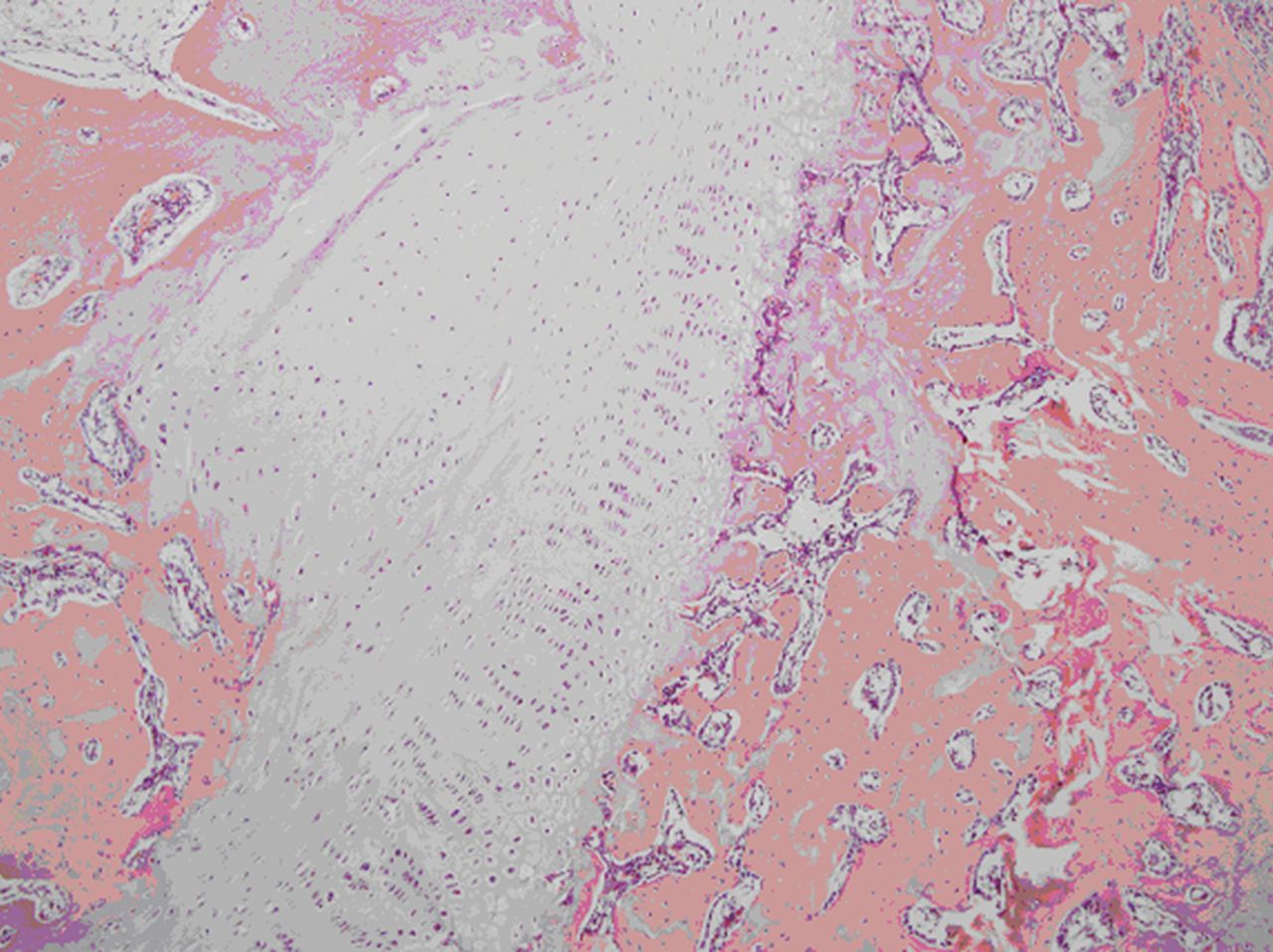
Fig. 2
Microscopic image of a tibia after grafting of an autologous cultured growth chondrocyte.
Radiological findings
A radiograph of the tibia at both the anteroposterior and the lateral view presented a regular visible growth plate at the site of grafting at the right tibia (samples 1 to 6, 11, 13). The growth plate was irregular with a folded shape (samples 7 to 9). The growth plate in sample 14 presented the appearance of an irregular growth plate (Fig. 3). The growth plate was irregular with a folded shape (samples 7 to 9). The growth plate in sample 14 presented the appearance of an irregular growth plate (Fig. 3). The growth plate in sample 14 presented the appearance of an irregular growth plate (Fig. 3) with bone bridge formation at the medial side covering about 15% of the growth cartilage (Fig. 4).
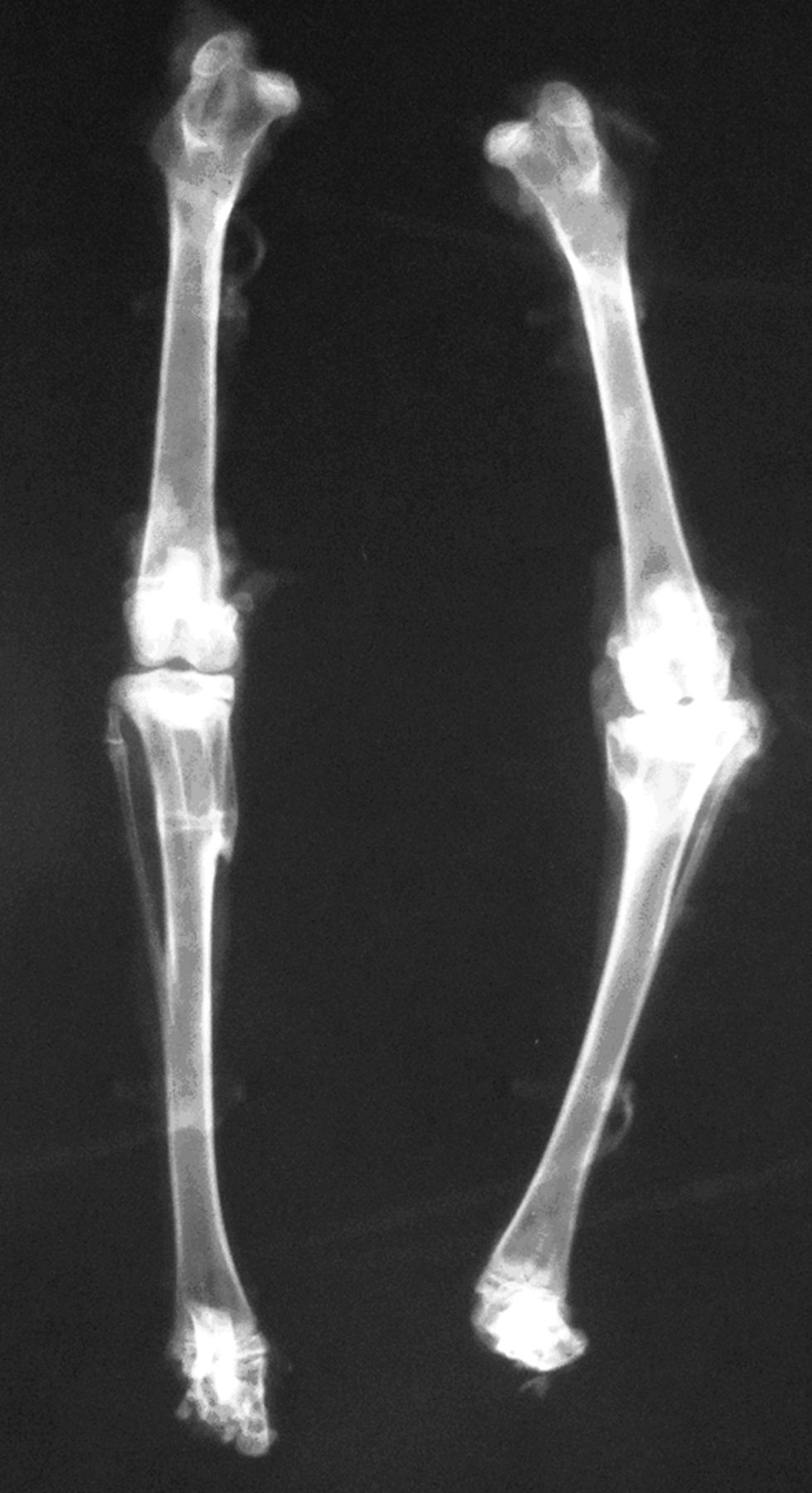
Fig. 3
Radiograph showing the right tibia after autologous cultured growth chondrocytes with no angular deformity, and the left tibia after partial resection of the plate growth without grafting varus deformity.
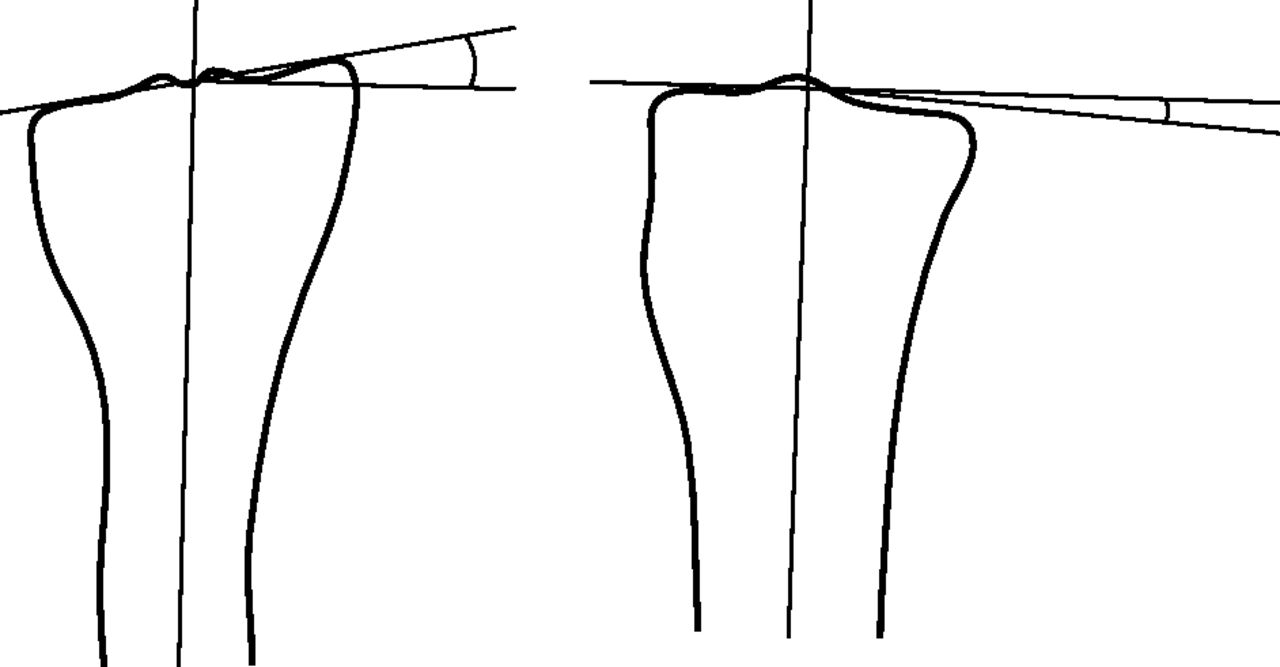
Fig. 4
Diagram showing how the angular deformity is measured between the long axis of the tibia and the tibial plateau (anteroposterior view on the left).
In samples 1 to 11 at the left tibia a bone bridge was presented and it filled a mean of 45% (35% to 70%) of the growth plate. The growth plate in samples 12 to 14 was observed without abnormality. The results of the right and left tibial angular deformity are listed in Table III, and differences between them in Figure 5.
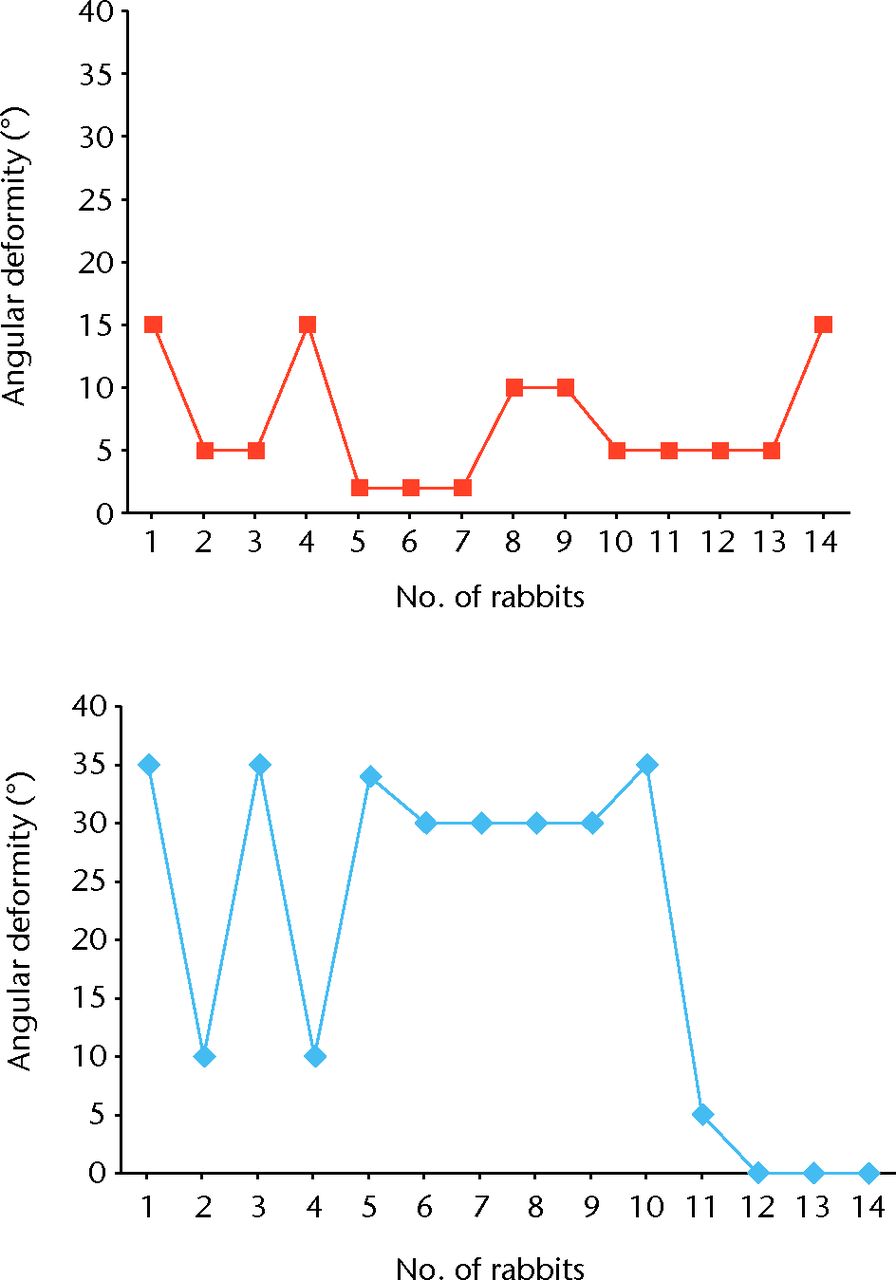
Fig. 5
Diagrams of the results of right (top graph) and left (bottom graph) tibial angular deformity
Table III
The results of right and left tibial angular deformity
| Number of rabbits | Tibial angular deformity | ||
|---|---|---|---|
| Right | Left | ||
| 1 | 15° | 35° | |
| 2 | 5° | 10° | |
| 3 | 5° | 35° | |
| 4 | 15° | 10° | |
| 5 | 2° | 34° | |
| 6 | 2° | 30° | |
| 7 | 2° | 30° | |
| 8 | 10° | 30° | |
| 9 | 10° | 30° | |
| 10 | 5° | 35° | |
| 11 | 5° | 5° | |
| 12 | 5° | 0° | |
| 13 | 5° | 0° | |
| 14 | 15° | 0° | |
-
Five animals developed an angular deformity of 10° to 15°, which is not significant in this examination.
Statistical analysis
The means and standard deviations were calculated for tibial right and left varus deviation and angular deformity at the lateral view. The values were statistically analysed using STATISTICA version 10 (StatSoft Inc., Tulsa, Oklahoma). A brief analysis of the data reveals some significant results (Table IV).
Table IV
Angular deformity. CI, confidence interval
| Tibia | Mean (range) | Median (IQR) | Standard deviation | Minimum | Maximum | 95% CI |
|---|---|---|---|---|---|---|
| Right (n = 14) | 7.21°(5 to 10) | 5° (5) | 4.87 | 2° | 15° | 4.03 to 10.39 |
| Left (n = 14) | 20.29° (6.25 to 33.00) | 30° (26.75) | 14.92 | 0° | 35° | 4.74 to 34.05 |
The mean tibial deformity of the left angle was 20.29° and the right was 7.21°. The median value in the left tibia was 30º, which indicates that 50% of the results were above this value, and the other half was below. The median of the right tibial angle was 5° when compared with the angular deformity of the left tibia. The high rate of standard deviation shows differentiation of the obtained results. Of the samples of the left tibia, 67% show the reduction in the plateau in different parts of the value of 50°. The right reduction occurred in 78% of the tested samples and showed a considerably lower value, with an average of 12°. Results are presented in Table V.
Table V
Plateau deformity. CI, confidence interval
| Tibia | Mean (range) | Median (IQR) | Standard deviation | Minimum | Maximum | 95% CI |
|---|---|---|---|---|---|---|
| Right (n = 9) | 0.12° (0.1 to 0.1) | 0.1° (0) | 0.057° | 0.1° | 0.25° | 0.024 to 0.1 |
| Left (n = 9) | 0.5° (0.00 to 0.5) | 0.5° (0.5) | 0° | 0.5° | 0.5° | 0.00 to 0.5 |
Discussion
The growth plate is responsible for longitudinal growth through chondrocyte proliferation, hypertrophy, apoptosis, cartilage matrix synthesis, mineralisation and vascularisation. The growth plate cartilage is being replaced by bone, a process called endochondral ossification.3,7 Disruption of plate growth cartilage maturation leads to development of axial and length disturbance, which can lead to the rapid appearance of bone bridge formation.2,9,13 Osseous bridging of the physis may occur after any injury to the physis, the most common cause being physeal injury. Partial physeal arrest may also be due to infection, tumour, therapeutic irradiation, burn, frostbite, electrical injury, metabolic or haematological abnormality, sensory neuropathy, microvascular ischaemia or insertion of metal.2,3,10,14-16 The size and location of the physeal bridge eventually determines the clinical deformity. When partial physeal arrest is located peripherally, the remainder of the physis usually continues to grow, producing an angular deformity.10 Partial physeal arrest is particularly apt to produce progressive angular deformation when a bridge of sclerotic bone forms eccentrically between the epiphyseal ossification centre and the metaphyseal bone, replacing a segment of the physis and the zone of Ranvier.10 There are three basic patterns of partial physeal arrest: peripheral, linear and central.10 Peripheral creates a very severe angular deformation which was shown in our control tibia group. There was no tendency for the remaining physeal cartilage to grow laterally into the defect7,17,18 and a bone bridge had been formed between the epiphysis and the metaphysis. There appears to be a relationship between the size and time of physeal defect and bridge formation.2 A variety of procedures evolved to treat the length of angular discrepancies. In an effort to restore normal growth, well-localised focal bone bridges have been resected and an artificial filling has been inserted; this is called the Langenskiold method6,7,14,19 The materials used for infill could be biological (fat, autogenous rib, sternal and iliac cartilage with or without an associated vascular supply) or prosthetic (methylmethacrylate, silicone rubber) MSC allogeneic and autogenous.3,14 Embryonic stem cells7 have been inserted to prevent re-formation.9,10,13 Other surgical procedures have also been recommended such as the use of distraction epiphysiolysis to break the osseous bridge, simultaneously addressing the correction of longitudinal and angular deformities.16 Allogeneic chondrocytes are problematic not only in terms of their antigenicity9,17,18 which is responsible for poor results, but also because of the risk of transmissible diseases.5,7,18 Lee et al20 used the cultured chondrocytes from iliac apophyseal bone and showed the superiority of this graft over silastic and fat, as also demonstrated by Campbell et al,9 but this procedure can lead to deformity of the ilium.3,18 Use of autologous chondrocytes avoids immuno-response-related problems but permits the chondrocyte cultures to start a small quantity of chondrocyte cells,4 which could be obtained using a needle puncture from the same growth plate for grafting. The disadvantage of the methods using the cultured autologous chondrocytes is the time taken to collect, expand and re-implant the chondrocytes, which can be up to three weeks. During this time, in case of growth plate injury, a bone bridge has already started to develop.3,7,15 The total regeneration of the growth plate was not observed but it does not seem to be important to normal growth if bridge formation can be prevented.14 We realise that the bone bridge resection must be performed as soon as possible, even before grafting, and the place of resection should be protected. In our study this was done by use of a tube drain implantation. Chondrocytes embedded in atelocollagen gel may, with time, give better mechanical support than fat to prevent a collapse. It is uncertain whether chondrocytes containing gel acquire sufficient mechanical stiffness at transplantation, but transplanted chondrocytes can proliferate and synthesise the extracellular matrix some time after transplantation, thereby providing a stiffer interposition material. Finally, transplanted chondrocytes can be replaced with bone and united by ossification at the uninjured growth plate.5 Chondrocytes derived from the growth plate were embedded in a gel–cell carrier based on the fibrinogen obtained from human plasma. Fibrinogen, the main component of fibrin glue, is widely used in clinical applications. Its polymerisation is achieved by the addition of thrombin and calcium chloride. Applied fibrinogen was obtained by cryoprecipitation; the freezing and thawing of plasma. Cryoprecipitation allows for fibrinogen enriched with other plasma proteins, hormones and growth factors, fibronectin and binding proteins.20-22 A fibrinogen carrier prepared at the Regional Blood Centre in combination with cells constituted highly plastic and adhesive grafts. This facilitates and accelerates implantation, and arthroscopy can be applied, which is a great advantage. The cartilaginous physis has a dual epiphyseal and metaphyseal blood supply. From the epiphyseal vessels, nutrients are carried by diffusion through the cartilaginous extracellular matrix to chondrocytes; the metaphyseal vasculature serves as a source of osteoprogenitor cells that lay down bone on the calcified cartilage matrix to complete the endochondral sequence. A basic requirement for the survival and normal function of free physeal transplants is that this dual blood supply is preserved at the graft site, and that the free physeal graft is not covered by tissues that impede the diffusion of nutrients from blood vessels into the extracellular matrix of the physis.13
This study has demonstrated that grafting of autologous cultured growth plate cells into a defect of the medial aspect of the proximal tibial physis can prevent bone bridge formation, growth arrest and the development of varus deformity.
1 Ballock RT , O'KeefeRJ. The biology of the growth plate. J Bone Joint Surg [Am]2003; 85-A:715–726.PubMed Google Scholar
2 Martiana K , LowCK, TanSK, PangMW. Comparison of various interpositional materials in the prevention of transphyseal bone bridge formation. Clin Orthop Relat Re s 1996;325:218–224.CrossrefPubMed Google Scholar
3 Foster BK , HansenAL, GibsonGJ, et al.Reimplantation of growth plate chondrocytes into growth plate defects in sheep. J Orthop Res1990;8:555–564.CrossrefPubMed Google Scholar
4 Olin A , CreasmanC, ShapiroF. Free physeal transplantation in the rabbit. An experimental approach to focal lesions. J Bone Joint Surg [Am]1984;66-A:7–20.PubMed Google Scholar
5 Tobita M , OchiM, UchioY, et al.Treatment of growth plate injury with autogenous chondrocytes: a study in rabbits. Acta Orthop Scand2002;73:352–358.CrossrefPubMed Google Scholar
6 Langenskiold A . An operation for partial closure of an epiphysial plate in children, and its experimental basis. J Bone Joint Surg [Br]1975;57-B:325–330.PubMed Google Scholar
7 Chung R , FosterBK, XianCJ. Preclinical studies on mesenchymal stem cell-based therapy for growth plate cartilage injury repair. Stem Cells Int2011;570125.CrossrefPubMed Google Scholar
8 Planka L , GalP, KecovaH, et al.Allogeneic and autogenous transplantations of MSCs in treatment of the physeal bone bridge in rabbits. BMC Biotechnol2008;8:70.CrossrefPubMed Google Scholar
9 Campbell CJ , GrisoliaA, ZanconatoG. The effects produced in the cartilaginous epiphyseal plate of immature dogs by experimental surgical traumata. J Bone Joint Surg [Am]1959;41-A:1221–1242.PubMed Google Scholar
10 Ogden JA . The evaluation and treatment of partial physeal arrest. J Bone Joint Surg [Am]1987;69-A:1297–1302.PubMed Google Scholar
11 Peterson L , MinasT, BrittbergM, et al.Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res2000;374:212–234.CrossrefPubMed Google Scholar
12 Wysocka A , MannK, BursigH, DecJ, GaździkTS. Chondrocyte suspension in fibrin glue. Cell Tissue Bank2010;11:209–215.CrossrefPubMed Google Scholar
13 Broughton NS , DickensDRY, ColeWG, MenelausMB. Epiphyseolysis for partial growth plate arrest: results after four years at maturity. J Bone Joint Surg [Br]1989;71-B:13–16. Google Scholar
14 Osterman K . Healing of large surgical defects of the epiphysial plate: an experimental study. Clin Orthop Relat Res1994;300:264–268. Google Scholar
15 McCarty RC , XianCJ, GronthosS, ZannettinoAC, FosterBK. Application of Autologous Bone Marrow Derived Mesenchymal Stem Cells to an Ovine Model of Growth Plate Cartilage Injury. Open Orthop J2010;4:204–210.CrossrefPubMed Google Scholar
16 Son SM , ParkIH, OhCW, et al.A histomorphometric study of cellular layers after hemiepiphyseal stapling on the physeal plate in rabbits. J Orthop Sci2013;8:152–158.CrossrefPubMed Google Scholar
17 Bright RW . Operative correction of partial epiphyseal plate closure by osseous-bridge resection and silicone-rubber implant. J Bone Joint Surg [Am]1974;56-A:655–664.PubMed Google Scholar
18 Canadell J , de PablosJ. Correction of angular deformities by physeal distraction. Clin Orthop Relat Re s 1992;283:98–105.PubMed Google Scholar
19 Gotfried Y , YaremchukMJ, RandolphMA, WeilandAJ. Histological characteristics of acute rejection in vascularized allografts of bone. J Bone Joint Surg [Am]1987;69-A:410–425.PubMed Google Scholar
20 Lee EH , ChenF, ChanJ, BoseK. Treatment of growth arrest by transfer of cultured chondrocytes into physeal defects. J Pediatr Orthop1998;18:155–160.PubMed Google Scholar
21 Eyrich D , GöpferichA, BlunkT. Fibrin in tissue engineering. Adv Exp Med Biol2006;585:379–392. Google Scholar
22 Homminga GN , BumaP, KootHW, van der KraanPM, van den BergWB. Chondrocyte behavior in fibrin glue in vitro. Acta Orthop Scand1993;64:441–445.CrossrefPubMed Google Scholar
Funding statement:
None declared
Author contributions:
R. Tomaszewski: Data collection, Data analysis, Performed surgeries, Writing the paper
J. Bohosiewicz: Data analysis, Writing the paper
A. Gap: Data collection, Data analysis, Performed surgeries, Writing the paper
H. Bursig: Data collection, Data analysis, Writing the paper
A. Wysocka: Data collection, Data analysis, Writing the paper
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










