Abstract
Aims
Osteoporosis and abnormal bone metabolism may prove to be significant factors influencing the outcome of arthroplasty surgery, predisposing to complications of aseptic loosening and peri-prosthetic fracture. We aimed to investigate baseline bone mineral density (BMD) and bone turnover in patients about to undergo arthroplasty of the hip and knee.
Methods
We prospectively measured bone mineral density of the hip and lumbar spine using dual-energy X-ray absorptiometry (DEXA) scans in a cohort of 194 patients awaiting hip or knee arthroplasty. We also assessed bone turnover using urinary deoxypyridinoline (DPD), a type I collagen crosslink, normalised to creatinine.
Results
The prevalence of DEXA proven hip osteoporosis (T-score ≤ -2.5) among hip and knee arthroplasty patients was found to be low at 2.8% (4 of 143). Spinal osteoporosis prevalence was higher at 6.9% (12 of 175). Sixty patients (42% (60 of 143)) had osteopenia or osteoporosis of either the hip or spine. The mean T-score for the hip was -0.34 (sd 1.23), which is within normal limits, and the mean hip Z-score was positive at 0.87 (sd 1.17), signifying higher-than-average BMD for age. The median urinary DPD/creatinine was raised in both female patients at 8.1 (interquartile range (IQR) 6.6 to 9.9) and male patients at 6.2 (IQR 4.8 to 7.5).
Conclusions
Our results indicate hip and knee arthroplasty patients have higher BMD of the hip and spine compared with an age-matched general population, and a lower prevalence of osteoporosis. However, untreated osteoporotic patients are undergoing arthroplasty, which may negatively impact their outcome. Raised DPD levels suggest abnormal bone turnover, requiring further investigation.
Cite this article: Bone Joint Res 2014;3:14–19.
Article focus
Bone quality in prospective hip and knee arthroplasty patients
Prevalence of osteoporosis and osteopenia in prospective hip and knee arthroplasty patients
Key messages
The prevalence of osteoporosis in patients undergoing hip and knee arthroplasty is low
Patients awaiting hip and knee arthroplasty have higher bone mineral density than average for their age
Markers of bone resorption are raised in hip and knee arthroplasty patients
Strengths and limitations
Unique study in the medical literature
Prospective study
Clinical significance of results requires further investigation
Introduction
The ninth annual report of the National Joint Registry for England and Wales reports that 71 672 primary total hip replacements (THRs) and 79 516 primary total knee replacements (TKRs) were performed in England and Wales in 2011.1 The available evidence suggests that the rate of hip and knee arthroplasty is set to increase in the United Kingdom,2 with significant cost implications for the NHS. Revision arthroplasty surgery has a poorer clinical outcome than primary joint surgery3 and is more costly.4 Between 2007 and 2011, the number of revision hip arthroplasties in England and Wales increased by 22.9%.1 Efforts to reduce the risk of arthroplasty failure are therefore urgently needed.
Much of the research in joint replacement surgery to date has focussed on implants and relatively little has focussed on the quality of bone into which the implants are inserted. The most common cause of revision arthroplasty is aseptic loosening,1 occurring when peri-prosthetic bone undergoes resorption. In 2011, aseptic loosening and lysis accounted for 55% of revision hip arthroplasties and 45% of revision knee arthroplasties in England and Wales.1 The authors feel that low bone mineral density (BMD) must be a risk factor for aseptic loosening. The loss of peri-prosthetic bone after arthroplasty of the hip and knee has been shown to adversely influence the longevity of implants.5-7 Despite this, bone quality is not routinely investigated during pre-operative planning in the United Kingdom.
The association between osteoarthritis and osteoporosis has been studied, with conflicting results. Although early studies suggested bone mass is increased in patients with OA,8,9 more recent cohort studies have suggested that there is in fact an increased risk of a fragility fracture in patients with OA.10-12 We have recently demonstrated in a retrospective population-based study of 41 995 patients that the use of bisphosphonates, which prevent bone resorption, significantly reduces the rates of revision surgery.13
In this study, which is part of the Clinical Outcomes in Arthroplasty Study (COASt), we have sought to prospectively characterise the bone quality of a cohort of patients awaiting primary and revision total hip and knee arthroplasty, and determine the prevalence of osteoporosis in the arthroplasty population. We recruited 194 patients listed for total hip or knee arthroplasty to measure BMD and levels of the bone turnover marker deoxypyridinoline (DPD).
Materials and Methods
Regional ethics committee approval was obtained prior to study commencement and all patients gave informed written consent. A total of 194 consecutive patients awaiting THR, TKR and planned revision THR and TKR were recruited prospectively from the orthopaedic outpatients department. Exclusion criteria were age < 40 years (those below 40 years would not be representative of the general arthroplasty population) and metal-on-metal revisions (owing to their atypical age and indications for revision).14 Each patient completed a questionnaire recording demographic data, past medical history and drug history. Body mass index (BMI) was measured and recorded during a clinic visit.
Bone mineral density
BMD was measured using dual energy x-ray absorptiometry (DEXA) using the Hologic DEXA system (Hologic, Waltham, Massachusetts). Pre-operative scans of the hip and spine were performed using the manufacturers’ recommended settings and patient positioning. The contralateral hip was scanned in normal scan mode on fast array (coefficient of variation 1%) as well as AP Spine (fast array) and whole body scan. Results were expressed as total hip and spine BMD, T-Score and Z-Score according to the criteria of the World Health Organization (WHO15). T-score represents the number of standard deviations from the mean young adult’s BMD, and Z-score represents the number of standard deviations from the age matched mean BMD. Where study subjects were found to be osteoporotic, their general practitioner was informed of the result.
Urinary deoxypyridinoline
DPD is a cross link for mature type I collagen, which is specific to bone. It is released into the circulation during the bone resorption process. It is unaffected by diet16 and is excreted unmetabolised in the urine, making it a sensitive and specific marker of bone resorption in osteoporosis and other metabolic bone diseases.17 The reference ranges for urinary DPD (normalised to creatinine level) have been established18 for adults aged > 25 years by a commonly available enzyme immunoassay Pyrilinks-D (Metra Biosystems Inc., Mountain View, California) and are as follows: females (3.0 to 7.4 nMol DPD/mMol Creatinine) and males (2.3 to 5.4 nM DPD/mMol Creatinine).
The Pyrilinks-D kit (Metra Biosystems Inc.) measures free DPD cross links in urine using competitive immunoassay in a microtitre plate format, using a monoclonal anti-D-Pyr antibody coated on the plate, to capture the DPD. The DPD results were read using the biorad microplate reader model Siemens Immulite 2000 XPi (Siemens, Munich, Germany) (coefficient of variation 9.5% at 98.8 nmol/l, 17.5% at 23.0 nmol/l) and corrected for urinary concentration by creatinine measured using a standard colorimetric method (coefficient of variation 8%).
The second urine void sample of the day was collected from study subjects during the pre-operative period and placed in a dark sterile plastic container and stored at < 20°C until the assay was processed. The second urinary void was chosen to standardise collection, as DPD has significant circadian variation with higher levels excreted at night than during the day.19
Statistical analysis
Statistical analysis was carried out using SAS v9.2 (SAS Institute Inc., Cary, North Carolina) and R (R Foundation for Statistical Computing, Vienna, Austria). Appropriate summary statistics were used to describe the data, depending on whether it showed evidence of approximate normal distribution. Non-parametric summary measures for the DPD/Creatinine ratio were presented, due to evidence of a right-skewed distribution for this variable. Comparisons between groups were made using t-tests except for DPD/Creatinine ratio, for which the Mann–Whitney U test was used. Pearson's rank correlation test was used to assess the linear correalation between DPD/Creatinine levels and hip T-scores. All tests were two-sided, with a 5% significance level.
Results
Between February 2011 and April 2012, 194 patients were recruited to the study. Of these, 104 were listed for primary THR, 23 for revision THR, 61 for primary TKR and six for revision TKR. Table I shows the demographics for the study group and each operative subgroup. There were 120 women and 74 men with a mean age of 68.3 years (sd 9.8). BMI data were available in 119 patients, with a mean BMI of 31.1 kg/m2 (sd 6.4). Indications for primary and revision surgery are displayed in Table II. Osteoarthritis was the most common indication for primary surgery and aseptic loosening was the predominant indication for revision surgery (Table II).
Table I
Baseline clinical and demographics characteristics (BMI, body mass index)
| Total hip replacement | Total knee replacement | ||||||
|---|---|---|---|---|---|---|---|
| Primary | Revision | Primary | Revision | All procedures | |||
| Patients (n) | 104 | 23 | 61 | 6 | 194 | ||
| Mean (sd) age (yrs) | 67.8 (11.0) | 68.1 (5.5) | 69.1 (9.1) | 71.0 (7.3) | 68.3 (9.8) | ||
| Female (n, %) | 66 (63.4) | 12 (52.2) | 39 (63.9) | 3 (50.0) | 120 (61.9) | ||
| BMI data available (n) | 66 | 15 | 36 | 2* | 119 | ||
| Mean (sd) BMI (kg/m2) | 30.5 (5.4) | 27.6 (3.3) | 33.0 (8.1) | n/a* | 31.0 (6.3) | ||
| Bisphosphonate therapy (n, %) | 12 (11.5) | 1 (4.3) | 3 (4.9) | 0 (0) | 16 (8.2) | ||
-
* number of subjects too small to report reliable estimates
Table II
Indications for Total Hip (THR) or total knee replacement (TKR)
| Primary procedure | Revision procedure | ||||
|---|---|---|---|---|---|
| Indication (n, %) | THR (n = 104) | TKR (n = 61) | THR (n = 23) | TKR (n = 6) | |
| PRIMARY PROCEDURE | |||||
| Primary osteoarthritis | 90 (86) | 59 (97) | |||
| Secondary osteoarthritis | 8 (8)* | - | |||
| Rheumatoid arthritis | 3 (3) | 1 (1.5) | |||
| Other inflammatory arthritis | 2 (2) | 1 (1.5) | |||
| Avascular necrosis | 1 (1) | - | |||
| REVISION PROCEDURE | |||||
| Aseptic loosening | 18 (78) | 3 (50) | |||
| Infection | 3 (13) | - | |||
| Dislocation | 2 (9) | - | |||
| Malalignment | - | 1 (17) | |||
| Instability | - | 1 (17) | |||
| Stiffness | - | 1 (17) | |||
-
* comprising developmental dysplasia of the hip (n = 3), slipped upper femoral epiphysis (n = 2), Perthes (n = 1) and acetabular fracture (n = 2)
Of the 194 patients, DEXA scans of both the hip and lumbar spine were performed on 143. A total of 32 patients underwent only spinal DEXA scan. Hip DEXA scans were unavailable in 28 of these patients as a result of having contralateral prostheses (making normal mode hip DEXA impossible) and four were not able to position appropriately for scanning. The remaining 19 patients had neither a hip nor spinal DEXA scan as they declined the investigation, but were included in the study as they gave urinary DPD samples.
The T-score for the hip was found to be normally distributed through the study population (Fig. 1). The mean T-score was -0.34 (sd 1.23) for the total hip and 0.03 (sd 1.79) for the lumbar spine, both of which were within the normal range (> -1.0) (Table III). The cohort’s mean hip Z-score and mean spine Z-score were positive at 0.87 (sd 1.17) and 1.52 (sd 1.66), respectively. Furthermore, the mean T- and Z-scores were within normal limits in each procedural subgroup. There was no significant difference between the mean hip T-scores of those awaiting primary THR versus revision THR (p = 0.58, unpaired t-test) or between primary TKR versus revision TKR (p = 0.14, unpaired t-test) (Table III).
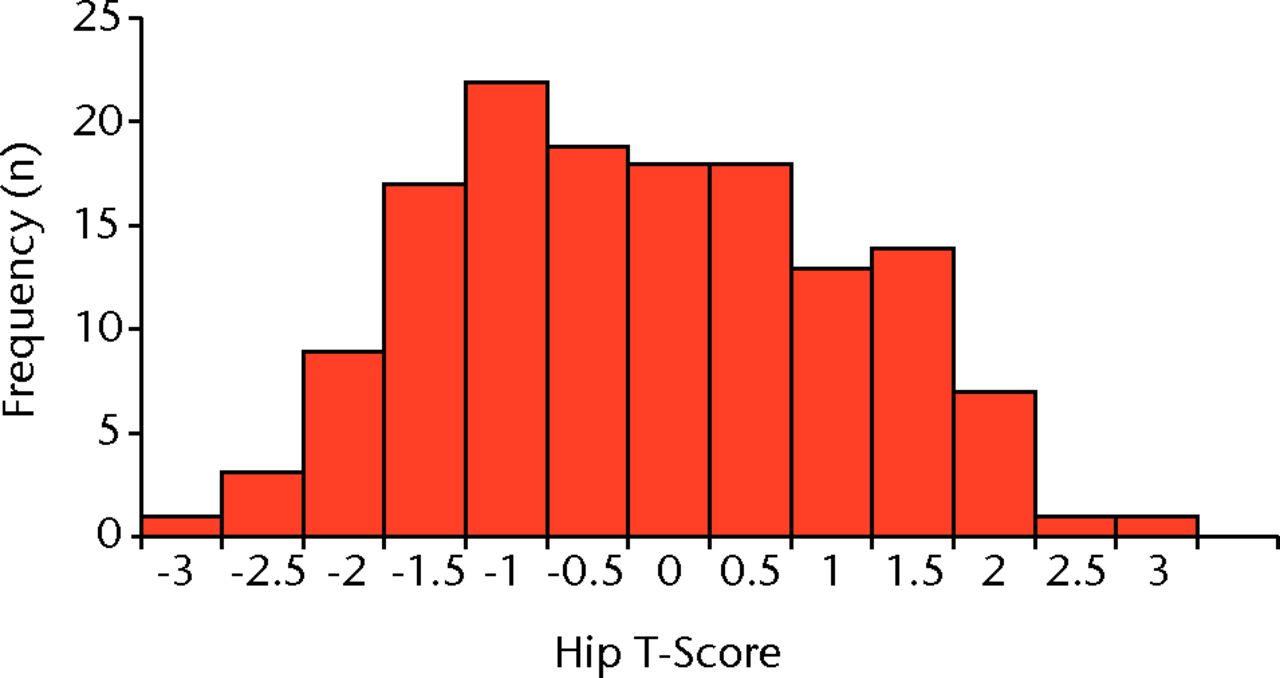
Fig. 1
Histogram showing the distribution of total hip T-scores (n = 143)
Table III
Baseline bone mineral density (BMD) results
| Total hip replacement | Total knee replacement | ||||||
|---|---|---|---|---|---|---|---|
| Mean (sd) BMD results [no. patients] | Primary | Revision | Primary | Revision | All procedures | ||
| Hip | |||||||
| BMD (g/cm2) | 0.92 (0.17) [n = 73] | 0.97 (0.18) [n = 11] | 0.94 (0.16) [n = 53] | 0.86 (0.20) [n = 6] | 0.93 (0.17) [n = 143] | ||
| T-score | -0.40 (1.25) [n = 73] | -0.16 (1.36) [n = 11] | -0.21 (1.20) [n = 53] | -1.07 (1.18) [n = 6] | -0.34 (1.23) [n = 143] | ||
| Z-score | 0.72 (1.09) [n = 71] | 0.87 (1.36) [n = 11] | 0.98 (1.23) [n = 51] | 0.08 (1.08) [n = 6] | 0.80 (1.13) [n = 139] | ||
| Spine | |||||||
| BMD (g/cm2) | 1.02 (0.18) [n = 87] | 1.10 (0.27) [n = 23] | 1.08 (0.20) [n = 59] | 1.01 (0.22) [n = 6] | 1.06 (0.20) [n = 175] | ||
| T-score | -0.25 (1.54) [n = 87] | 0.43 (2.51) [n = 23] | 0.32 (1.76) [n = 59] | -0.48 (1.86) [n = 6] | 0.03 (1.79) [n = 175] | ||
| Z-score | 1.25 (1.35) [n = 85] | 1.81 (2.47) [n = 23] | 1.83 (1.98) [n = 57] | 0.98 (1.56) [n = 6] | 1.51 (1.77) [n = 171] | ||
Prevalence of osteoporosis (T-score ≤ 2.5) among the cohort was low (Table IV). The rate of DEXA proven osteoporosis was 2.8% (4 of 143) for the hip, 6.9% (12 of 175) for the spine and 8.4% (12 of 143) for either the hip or spine. The rate of osteopenia (T-score from -1.0 to -2.5) was much higher however, with 42% (60 of 143) having osteoporosis or osteopenia of either the hip or the spine. Of those with DEXA-defined osteoporosis (hip or spinal), only five of 12 (42%) were on bisphosphonate therapy.
Table IV
Prevalence of osteoporosis and osteopenia
| Osteoporosis (n, %) | Osteopenia (n, %) | Osteoporosis or osteopenia (n, %) | |
|---|---|---|---|
| Hip (n = 143) | 4 (2.8) | 44 (30.7) | 48 (33.6) |
| Spine (n = 175) | 12 (6.9) | 48 (27.4) | 60 (34.3) |
| Hip or spine (n = 143) | 12 (8.4) | 48 (33.5) | 60 (41.9) |
Urinary DPD analysis was performed in 190 subjects. Four subjects did not provide a urine sample. The results are displayed in Table V. Median DPD/Creatinine was raised in males at 6.2 nMol/mMol (interquartile range (IQR) 4.8 to 7.5) compared with the normal range (reference range 2.3 to 5.4) and also in females at 8.1 nMol/mMol (IQR 6.6 to 9.9; reference range 3.0 to 7.4). The histogram of DPD/Creatinine (Fig. 2) shows that the distribution is skewed to the right. DPD/creatinine ratio was raised above the upper bound of the reference range in 54.2% (103 of 190) of the study population, and more than twice the upper bound in 5.7% (11 of 190) of the study population. In females, median DPD/Creatinine was higher in the revision THR group than the THR group (9.2 vs 7.9), although this did not reach significance (p = 0.056, Mann–Whitney U test). There was no difference in males between the revision THR and THR groups (p = 0.73, Mann–Whitney U test). Correlations between the continuous variables, hip T-score and DPD/creatinine, were assessed using Pearson’s rank correlation. Pearson’s test showed a very weak negative correlation between Hip T-score and DPD/creatinine (rho = -0.136, p = 0.11).
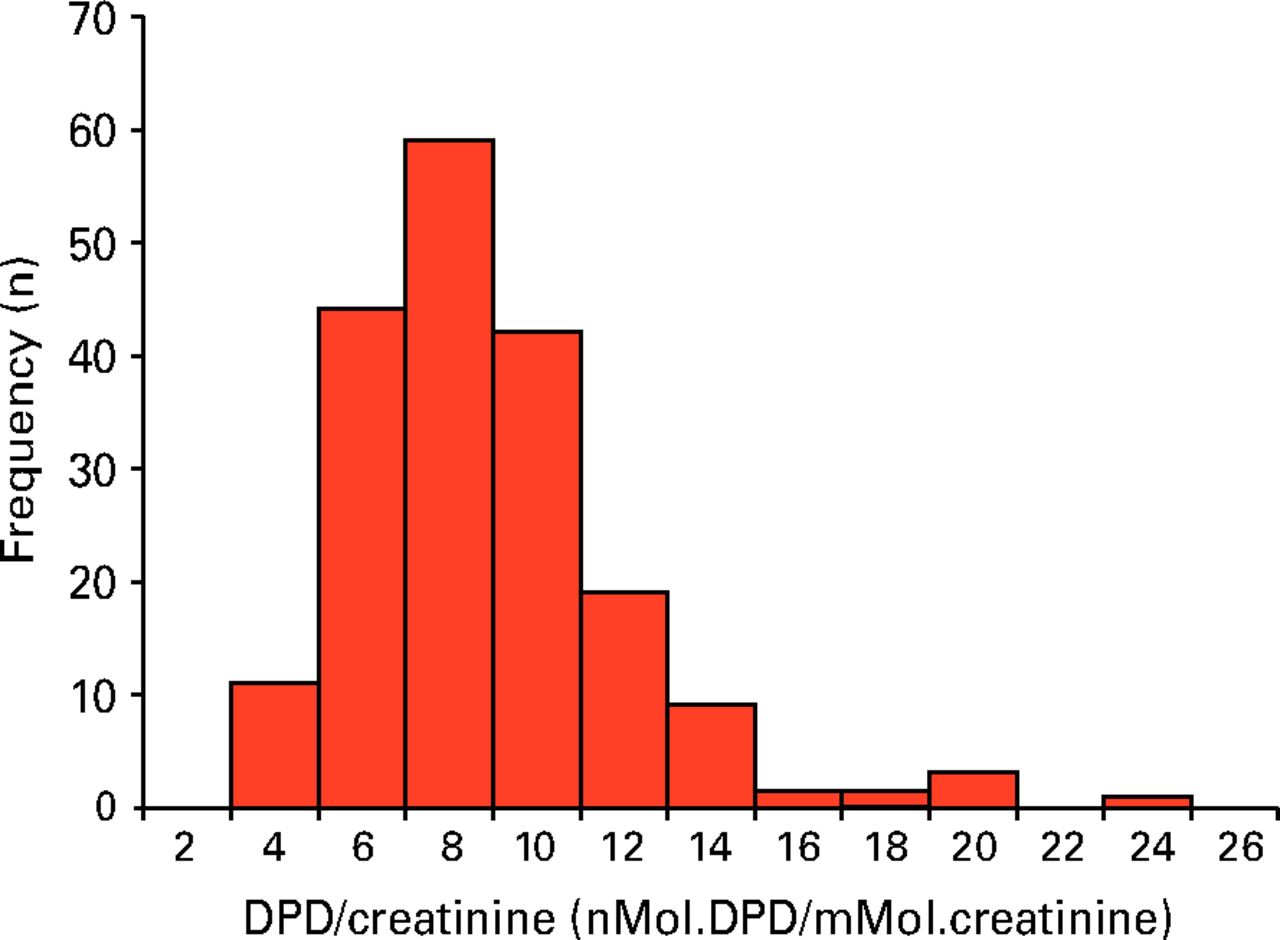
Fig. 2
Histogram showing the distribution of deoxypyridinoline (DPD)/creatinine ratios (n = 190)
Table V
Baseline urinary deoxypyridinoline (DPD)/creatinine results (units: nMol.DPD/mMol.creatinine). Reference ranges are 3.0 to 7.4 for females and 2.3 to 5.4 for males (IQR, interquartile range)
| Total hip replacement | Total knee replacement | ||||||
|---|---|---|---|---|---|---|---|
| Median ratio (IQR) [range] | Primary (n = 100) | Revision (n = 23) | Primary (n = 61) | Revision (n = 6) | All procedures (n = 190) | ||
| Females (n = 117) | 7.9 (6.5 to 9.9) [7.2 to 16] | 9.2 (7.7 to 13.6) [6.5 to 23.4] | 8.0 (6.4 to 10) [2.5 to 20] | n/a* | 8.1 (6.6 to 9.9) [2.5 to 23.4] | ||
| Males (n = 73) | 6.2 (4.3 to 7.5) [3.1 to 18] | 6.4 (4.7 to 9.1) [4.1 to 13.6] | 6.5 (5.3 to 7.4) [3.6 to 13.4] | n/a* | 6.2 (4.8 to 7.5) [3.1 to 18] | ||
-
* number of subjects too small to report sensible estimates
Discussion
In this unique cohort we have shown that the prevalence of DEXA-defined osteoporosis amongst hip and knee arthroplasty patients is low, but that 42% of patients have osteopenia or osteoporosis in either the hip or spine. We have shown that median DPD/creatinine ratios are raised above normal ranges in both males and females, and that there is large variation in DPD/creatinine ratios, with abnormally raised levels in over 54% of patients. Of those patients with DEXA-defined osteoporosis, fewer than half were on bisphosphonate therapy.20
The authors acknowledge two limitations of the study. Firstly, the small size of the revision knee subgroup limits the reliability of the data for this subgroup and the conclusions and comparisons which can be drawn from the data. Secondly, instances of selection bias occurred during data collection, the effects of which are considered further below.
Only 2.8% (4 of 143) of the study cohort who underwent hip DEXA scanning had hip osteoporosis defined by a total hip T-score ≤ -2.5. Two of these patients were on bisphosphonate therapy. Of the remaining 14 patients on bisphosphonate therapy, five had hip T-scores which did not meet the threshold for osteoporosis (> -2.5), and nine did not undergo the hip DEXA scan (declined or bilateral hip prostheses in situ). If the assumption is made that all patients on bisphosphonate therapy had hip osteoporosis prior to treatment initiation (which is neither a requirement nor likely), the overall prevalence of hip osteoporosis may be up to 9.3% (18 of 194). This prevalence is lower than in the general population of a similar age. Kanis and Johnell21 estimated the prevalence of femoral neck osteoporosis in the Swedish population aged between 65 and 69 years to be 7.4% in males and 20.2% in females. Given that our study population was 64% female with a mean age of 66.7 years, one could expect a prevalence of hip osteoporosis of around 15.6%, based on the estimates of Kanis and Johnell.21
BMD in the study cohort was found to be normally distributed. The mean hip T-score was within normal limits. Mean Z-score was positive, which indicates that hip and knee arthroplasty patients have, on average, higher BMD compared with the general population of a similar age. Standard deviations are large however, indicating a broad range of results.
The prevalence of spinal osteoporosis in the cohort was higher than total hip osteoporosis (6.9% vs 2.8%). It could be argued that even in the contralateral side, an arthritic process at the head of the femur could cause a local increase in BMD in the neck and trochanteric region of the femur, and is therefore misrepresentative of the skeletal bone mineral density as a whole. However, the fact that the mean hip T-score was lower in the THR than the TKR group, contradicts this argument.
The mean DPD/Creatinine levels for the study group were raised in men, and 54% of the study population had raised DPD/creatinine ratios, while nearly 6% had more than twice normal ratios. This suggests abnormal bone turnover and raised resorption in a large proportion of arthroplasty patients. Furthermore, since bisphosphonate therapy is known to reduce DPD/creatinine levels significantly, and no adjustment was made for this, our results may underestimate abnormal bone resorption.
In post-menopausal women, resorption markers have been shown to predict the degree of bone loss.22 Ross and Knowlton22 monitored post-menopausal women for 13 years, and found that baseline markers of bone resorption (including DPD) were significant predictors of the rate of bone loss. They also found that the odds of rapid bone loss increased 1.8- to two-fold with each standard deviation increase in DPD.22 Arthroplasty patients with raised DPD/Creatinine may therefore be at a higher risk of peri-prosthetic bone loss and aseptic loosening. However, our results did not show a strong negative correlation between DPD/Creatinine and baseline hip T-scores.
The results did not show a significant difference between the hip T-scores or DPD/creatinine ratios of those awaiting primary compared with revision surgery. This might suggest that osteoporosis is not a major contributory factor to arthroplasty failure and the need for revision surgery. However, the small numbers in the revision group may have resulted in a type II error. Furthermore, bilateral prostheses precluded 52% (12 of 23) of revision THR patients from having hip DEXA scans, introducing selection bias.
This research has provided important information into the baseline bone quality of arthroplasty patients. Further work is now required to determine the significance of BMD and/or DPD on arthroplasty outcome, and whether these parameters should be routinely measured prior to surgery.
Acknowledgements
The authors would like to thank all the participants of the COASt Study and Professor N. Arden, S.Garden, and the COASt Team for their time and dedication and the NIHR for their funding support to the study.
1 No authors listed. National Joint Registry 9th Annual Report. National Joint Registry for England and Wales, 2012. http://www.njrcentre.org.uk/njrcentre/Reports,PublicationsandMinutes/tabid/85/Default.aspx (date last accessed 4 October 2013). Google Scholar
2 Culliford DJ , MaskellJ, BeardDJ, et al.Temporal trends in hip and knee replacement in the United Kingdom: 1991 to 2006. J Bone Joint Surg [Br]2010;92-B:130–135.CrossrefPubMed Google Scholar
3 Hossain F , PatelS, HaddadFS. Midterm assessment of causes and results of revision total knee arthroplasty. Clin Orthop Relat Res2010;468:1221–1228.CrossrefPubMed Google Scholar
4 Bozic KJ , KurtzSM, LauE, et al.The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg [Am]2009;91-A:128–133.CrossrefPubMed Google Scholar
5 Malchau H , HerbertsP, AhnfeltL. Prognosis of total hip replacement in Sweden: follow-up of 92,675 operations performed 1978-1990. Acta Orthop Scand1993;64:497–506. Google Scholar
6 Haddad FS , MasriBA, GarbuzDS, DuncanCP. The prevention of periprosthetic fractures in total hip and knee arthroplasty. Orthop Clin North Am1999;30:191–207.CrossrefPubMed Google Scholar
7 van Loon CJ , de Waal MalefijtMC, BumaP, VerdonschotN, VethRP. Femoral bone loss in total knee arthroplasty: a review. Acta Orthop Belg1999;65:154–163. Google Scholar
8 Nevitt MC , LaneNE, ScottJC, et al.Radiographic osteoarthritis of the hip and bone mineral density: The Study of Osteoporotic Fractures Research Group. Arthritis Rheum1995;38:907–916. Google Scholar
9 Hannan MT , AndersonJJ, ZhangY, LevyD, FelsonDT. Bone mineral density and knee osteoarthritis in elderly men and women: The Framingham Study. Arthritis Rheum1993;36:1671–1680. Google Scholar
10 Arden NK , GriffithsGO, HartDJ, DoyleDV, SpectorTD. The association between osteoarthritis and osteoporotic fracture: the Chingford Study. Br J Rheumatol1996;35:1299–1304.CrossrefPubMed Google Scholar
11 Arden NK , CrozierS, SmithH, et al.Knee pain, knee osteoarthritis, and the risk of fracture. Arthritis Rheum2006;55:610–615.CrossrefPubMed Google Scholar
12 Bergink AP , van der KliftM, HofmanA, et al.Osteoarthritis of the knee is associated with vertebral and nonvertebral fractures in the elderly: the Rotterdam Study. Arthritis Rheum2003;49:648–657.CrossrefPubMed Google Scholar
13 Prieto-Alhambra D , JavaidMK, JudgeA, et al.Association between bisphosphonate use and implant survival after primary total arthroplasty of the knee or hip: population based retrospective cohort study. BMJ2011;343:7222.CrossrefPubMed Google Scholar
14 Liddle AD , SatchithanandaK, HenckelJ, et al.Revision of metal-on-metal hip arthroplasty in a tertiary center. Acta Orthop2013;84:237–245.CrossrefPubMed Google Scholar
15 No authors listed. World Health Organization: prevention and management of osteoporosis. http://whqlibdoc.who.int/trs/who_trs_921.pdf (date last accessed 9 December 2013). Google Scholar
16 Delmas PD. Biochemical markers for the assessment of bone turnover. In: Riggs BL, Melton L, eds. Osteoporosis: etiology, diagnosis and management. Philadelphia: Lippincott-Raven, 1995:319–333. Google Scholar
17 Delmas PD , GineytsE, BertholinA, GarneroP, MarchandF. Immunoassay of pyridinoline crosslink excretion in normal adults and in Paget’s disease. J Bone Miner Res1993;8:643–648. Google Scholar
18 No authors listed. Siemens Immulite. http://www.medical.siemens.com/siemens/en_GLOBAL/gg_diag_FBAs/files/package_inserts/immulite/Bone_Metabolism_n/pilkpd-11.pdf (date last accessed 9 December 2013). Google Scholar
19 No authors listed. Clinical Chemistry. http://www.clinchem.org/content/42/12/2037.full.pdf+html (date last accessed 9 December 2013). Google Scholar
20 Yu SL , HoLM, LimBC, SimML. Urinary deoxypyridinoline is a useful biochemical bone marker for the management of postmenopausal osteoporosis. Ann Acad Med Singapore1998;27:527–529.PubMed Google Scholar
21 Kanis JA , JohnellO. Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone2000;27:585–590.CrossrefPubMed Google Scholar
22 Ross PD , KnowltonW. Rapid bone loss is associated with increased levels of biochemical markers. J Bone Miner Res1998;13:297–302.CrossrefPubMed Google Scholar
Funding statement:
This article presents independent research commissioned by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research funding scheme (RP-PG-0407-10064). The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Support was also received from the Oxford NIHR Musculoskeletal Biomedical Research Unit, Nuffield Orthopaedic Centre and University of Oxford.
Author contributions:
S. J. James: Wrote the paper, Data analysis
S. B. Mirza: Data Collection, Data Analysis, Contributed to writing of paper
D. J. Culliford: Data Analysis, Contributed to writing of paper
P. A. Taylor: Data Collection, Data Analysis
A. J. Carr: Contributed to writing of paper and design of study
N. K. Arden: Study design, Data analysis, Contributed to writing of paper
ICMJE Conflict of Interest:
None declared
©2014 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










