Abstract
Objectives
We aimed to examine the characteristics of deep venous flow in the leg in a cast and the effects of a wearable neuromuscular stimulator (geko; FirstKind Ltd) and also to explore the participants’ tolerance of the stimulator.
Methods
This is an open-label physiological study on ten healthy volunteers. Duplex ultrasonography of the superficial femoral vein measured normal flow and cross-sectional area in the standing and supine positions (with the lower limb initially horizontal and then elevated). Flow measurements were repeated during activation of the geko stimulator placed over the peroneal nerve. The process was repeated after the application of a below-knee cast. Participants evaluated discomfort using a questionnaire (verbal rating score) and a scoring index (visual analogue scale).
Results
The geko device was effective in significantly increasing venous blood flow in the lower limb both with a plaster cast (mean difference 11.5 cm/sec-1; p = 0.001 to 0.13) and without a plaster cast (mean difference 7.7 cm/sec-1; p = 0.001 to 0.75). Posture also had a significant effect on peak venous blood flow when the cast was on and the geko inactive (p = 0.003 to 0.69), although these differences were less pronounced than the effect of the geko (mean difference 3.1 cm/sec-1 (-6.5 to 10)). The geko device was well tolerated, with participants generally reporting only mild discomfort using the device.
Conclusion
The geko device increases venous blood flow in the lower limb, offering a potential mechanical thromboprolylaxis for patients in a cast.
Cite this article: Bone Joint Res 2013;2:179–85.
Article focus
Assessment of ultrasound flow characteristics of a new electrical stimulation device
Whether these characteristics are maintained in participants wearing a below-knee orthopaedic cast
Key messages
Thromboprophylaxis should be considered for some patients in casts
Traditional mechanical methods are unsuitable
A new portable electric stimulation device offers a potential pragmatic alternative
Strengths and limitations
Ultrasound data reliably confirm that the device significantly enhances flow
Further clinical validation will contribute to our understanding of its utility
Introduction
Deep-vein thrombosis (DVT) is a potential risk for patients undergoing treatment with casting.1 The elevated risk is likely to be due to a combination of hypercoagulability provoked by the injury and any subsequent surgery, together with venous stasis enforced by the plaster immobilisation.2
The formation of clots within the vein lumen is the result of interplay of various factors. The underlying pathophysiology was outlined by Virchow in 18563 and is still generally accepted.4 Thrombi usually form in areas of slow or disturbed flow in large venous sinuses and in the valve pockets. It has been shown that the majority of thrombi originate in the soleal veins and valve pockets following surgery.5-7 Thrombi also form in vein segments that have been subjected to direct trauma or inflammatory processes.
Pharmacological methods for the prevention of DVT target the third point of Virchow’s triad: blood coagulability.8 However, chemical agents are associated with a risk of bleeding9 and are therefore contraindicated for some patients.
Mechanical methods of prophylaxis address the second point of Virchow’s triad: stasis. Graduated compression stockings may be effective in some patient groups10,11 but depend on proper fitting,12 and are simply not practical in a plaster cast. Intermittent pneumatic compression (IPC) devices consist of an inflatable garment for the arm, leg or foot and a pump, which intermittently inflates and deflates the garment. Their effectiveness as thromboprophylactic devices has been demonstrated.13 Most pneumatic compression devices consist of plastic sleeves that enclose the foot or whole leg, causing problems with comfort and compliance. Furthermore, the size, weight and the need for and external power source, pump, and attached tubing further limit the application of IPCs.14
While the risk for clinically relevant thrombosis in a cast is not clearly established, imaging studies suggest a rate between 4.3% and 40%,15 so prophylaxis should be considered. Whether prophylaxis should be universal, or solely for those with identifiable risk factors, is controversial. The most suitable prophylaxis has not been established: a chemical agent can be given for the time in plaster, for which there is some evidence of effect.1,15 Mechanical methods would be more suitable for those with a risk of bleeding, particularly soon after injury or surgery; however, traditional mechanical methods are not practical for those in a plaster cast.
Electrical stimulation (ES) of the lower limb muscles has been shown to be effective in improving blood flow.16-19 There is also evidence to support the clinical effectiveness of ES in reducing the incidence of DVT.20,21 However, these studies of direct electrical muscle stimulation have not led to the adoption of effective and easy to use devices for blood flow enhancement. The failure to adopt ES may be explained by the level of discomfort experienced at high intensities of direct electrical stimulation to the muscle. There are a limited number of devices that use this technique, and they are generally used only under general anaesthetic. Such devices are also often rather cumbersome, require trailing leads and electrodes, and their use, as with IPC, is incompatible with normal ambulation or a plaster cast.
The geko (FirstKind Ltd, High Wycombe, United Kingdom) is a compact, self-contained, self-adhesive, battery-powered neuromuscular electrical stimulation device (Fig. 1), which is applied to the skin over the common peroneal nerve, delivering charge-balanced short single pulse stimulation at 1 Hz at a point anatomically distant from the foot. A rhythmic dorsiflexion of the foot results from the contraction of a complex of muscles in the lower leg. This expresses blood from the superficial and deep venous systems by means of distension of the vessels within the calf muscle pump and foot pump. The technique has been shown to be effective in stimulating musculature contraction of the lower limb and in the enhancement of venous flow velocity and volume in the lower limb.22 Furthermore, the technique increases arterial and microcirculatory blood flow, together with a significant decrease in tissue plasminogen activator (tPA) antigen, indicating increased fibrinolytic activity.23
Fig. 1
Photograph of the geko electrostimulation device (FirstKind Ltd, High Wycombe, United Kingdom).
In this study, we aim to show whether this device can meet the unmet clinical need for mechanical prophylaxis for those in a plaster cast by examining the effect on blood flow for those wearing a below-knee cast. The primary objective of this study was to examine the characteristics of deep venous flow in the leg veins using Doppler ultrasound, with particular reference to how this flow is affected by the application of a cast and a wearable electrical stimulation device (geko). A further objective was to evaluate the participants’ tolerance of the device, using a visual analogue scale and verbal rating scoring index.
Patients and Methods
Ethics Committee approval was obtained from the National Research Ethics Service (NRES) Committee, London.
Ten healthy participants were recruited from the local university population. The subjects were provided with a full explanation of the nature, purpose and requirements of the study, including information sheets and consent forms. A screening assessment was conducted, including a medical history, physical examination and Doppler ultrasonography of the lower limbs to exclude pre-existing venous disease or dysfunction. Inclusion criteria were: good general health and fitness, age between 18 and 65 years and body mass index between 18 and 34 kg/m2. Exclusion criteria included: previous leg fracture, use of any medications in the past 30 days, history or signs of haematological disorders or peripheral arterial disease, family history of DVT, clinically significant varicose veins or ulceration of the lower limb, or venous dysfunction on Doppler screening.
The geko device was applied according to the manufacturer’s instructions. The skin was prepared by exfoliating with an abrasive pad and cleaning with an alcohol wipe. The backing strip was removed from the device, which was then applied to the skin over the common peroneal nerve, just above the crease of the knee. The on-button was lateral, with the electrode strip medial, and the arrowed marker line on the device aligned with the lateral hamstring tendon (Fig. 1). A charge-balanced short single pulse with a current of 27 mA delivered stimulation at 1 Hz. Pulse width was participant-specific in the range from 0 µs to 400 µs, producing a palpable twitch of the foot.
Baseline ultrasound
The participant lay supine for a stabilisation period of 30 minutes in a quiet and environmentally controlled assessment room.
Bilateral ultrasound measurements were made of the superficial femoral veins. Assessment of blood flow velocity, volume, and mean velocity using Doppler ultrasound, and vessel diameter by B-mode ultrasound imaging. Measurements were made in triplicate using an EsaoteMyLab 70 scanner (ESAOTE S.p.A., Genova, Italy) and digital video was recorded for validation at suitable probe settings. The measurements were made sequentially with the participant in the following positions: 1) supine, with back supported at 70° to 90° and lower limbs horizontal and resting; 2) lower limb elevated at 25° to 35° hip flexion with back of calf resting on a pillow and knee extended; 3) standing, non-weight-bearing (weight on contralateral leg); and 4) standing, weight-bearing with weight distributed on both legs.
Ultrasound with geko
The geko device was then switched on and then the stimulation level adjusted until it produced a palpable twitch of the foot, according to the manufacturer’s instructions. This took approximately one minute. The measurements in each position were then repeated with the geko device active. Each position was held for five minutes before measurement.
Ultrasound with geko and cast
A below-knee plaster cast was applied by a consultant orthopaedic surgeon (DJW) with three layers of plaster wool and a proprietory casting tape (Benecast; Benecaredirect, Manchester, United Kingdom) in approximately four layers. The measurements in each position were then repeated, with and without activation of the geko device, again having held the position for five minutes.
Participant rating
Participants were asked to evaluate acceptance and tolerability of the geko device using a verbal rating score (VRS): 1, no sensation; 2, minimal discomfort; 3, mild discomfort; 4, moderate discomfort; and 5, severe discomfort. Discomfort was related to normal measurement of blood pressure, measured on the upper arm using a standard sphygmomanometer cuff, which was standardised as rating 3. Participants also indicated the level of discomfort by marking a 10 cm visual analogue scale (VAS), with 0 denoting ‘no sensation’, and 10 indicating ‘severe discomfort’.
Statistical analysis
Statistical analysis was performed using MATLAB 2013b (MathWorks, Natick, Massachusetts). Shapiro–Wilk tests were performed to assess the distribution of the data. Descriptive statistics were used to summarise measurements (mean and standard error of the mean (sem)). Effects of the different experimental conditions (limb position, stimulation, plaster cast) were examined using a paired t-test. The Wilcoxon signed-rank test was used to analyse the scales that assessed tolerance of the geko device. A p-value < 0.05 was assumed to denote statistical significance.
Results
Peak femoral vein velocity
Table I and Figure 2 shows the mean peak velocity measured by Doppler ultrasound at the femoral vein for all ten participants in each postural position with and without plaster cast, and with and without activation of the geko device. The peak venous velocity was higher in all postural positions when the geko device was active, both with a plaster cast (mean difference 11.5 cm/sec-1, p = 0.001 to 0.13) and without a plaster cast (mean difference 7.7 cm/sec-1, p = 0.001 to 0.75) (Table I, Fig. 2). This augmentation of blood flow was statistically significant regardless of plaster cast in all positions except elevation of the leg (no cast, p = 0.75; casting, p = 0.13) (Table I). Without the geko device active, posture had a significant effect on femoral venous flow when wearing a plaster cast, with flow being higher in the elevated limb than in the horizontal position (mean difference 4 cm/sec-1, p = 0.02), and lower in standing than the horizontal position (mean difference 4.8 cm/sec-1, p = 0.009), with no significant difference between weight-bearing and non-weight-bearing (p = 0.38). With activation of the geko device, wearing a plaster or postural position had no significant effect (p-values from 0.1 to 0.88).
Table I
Peak velocity and cross-sectional area (CSA) of the femoral vein as measured by Doppler and B-mode ultrasound for the ten participants, with respect to casting and geko device activation
| No plaster cast | Plaster cast applied | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean (sem) | Inactive geko device | Active geko device | p-value* | Inactive geko device | Active geko device | p-value* | ||
| Peak velocity femoral vein (cm/s) | ||||||||
| Supine | 15.3 (1.6) | 21.6 (2.2) | 0.04 | 15.5 (1.0) | 25.3 (3.1) | 0.03 | ||
| Leg elevated | 21.8 (3.4) | 23.4 (2.9) | 0.75 | 19.5 (2.1) | 24.6 (2.8) | 0.13 | ||
| Standing weight-bearing | 13.8 (1.9) | 21.8 (3.4) | 0.1 | 10.0 (1.6) | 22.3 (3.6) | 0.02 | ||
| Standing non-weight-bearing | 11.8 (1.0) | 26.8 (3.1) | < 0.001 | 10.7 (1.0) | 29.3 (2.8) | < 0.001 | ||
| CSA of femoral vein (mm2) | ||||||||
| Supine | 28.6 (5.9) | 34.2 (6.7) | 0.07 | 32.0 (8.2) | 31.7 (8.6) | 0.87 | ||
| Leg elevated | 32.2 (7.3) | 23.7 (4.2) | 0.08 | 27.6 (5.1) | 31.4 (7.0) | 0.32 | ||
| Standing weight-bearing | 50.5 (10.9) | 50.6 (10.5) | 0.89 | 38.1 (9.1) | 41.0 (7.1) | 0.65 | ||
| Standing non-weight-bearing | 52.4 (8.2) | 53.6 (7.7) | 0.5 | 46.6 (8.2) | 48.8 (9.0) | 0.49 | ||
-
* paired t-test
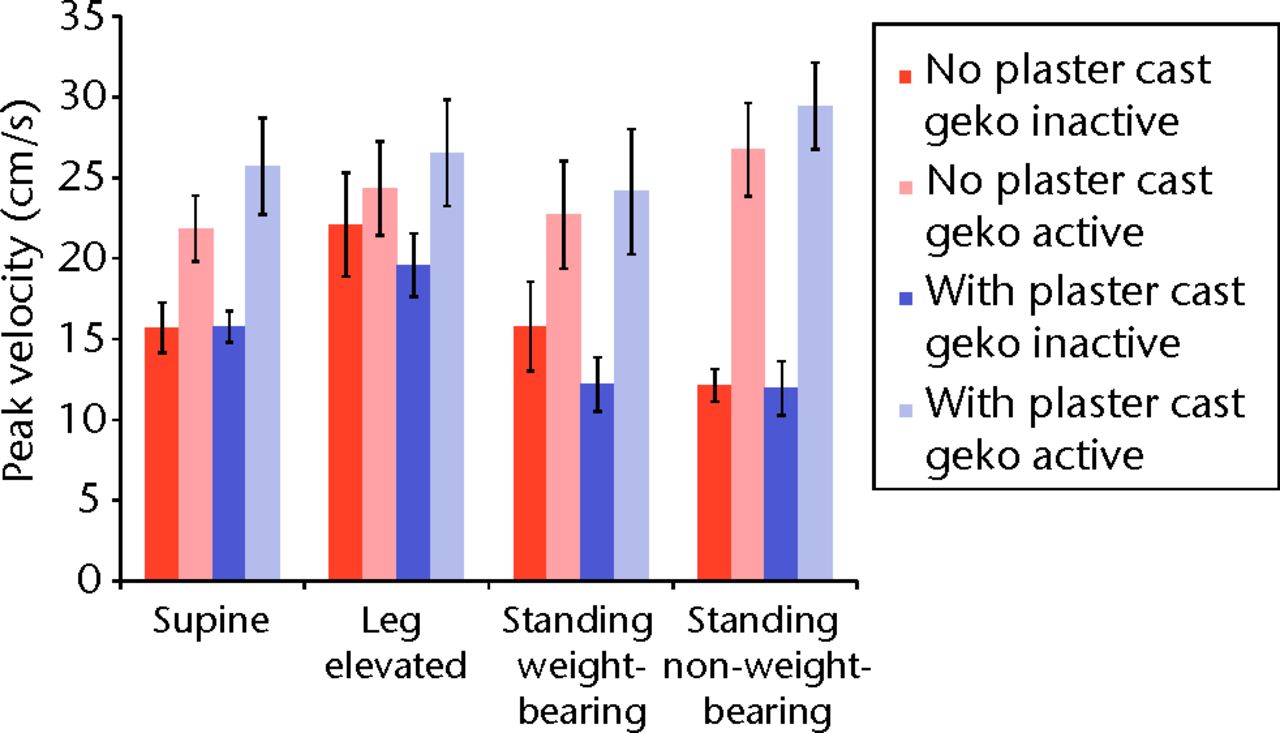
Fig. 2
Bar chart showing the mean peak velocity of the femoral vein according to activation of the geko device and posture. The error bars denote the standard error of the mean.
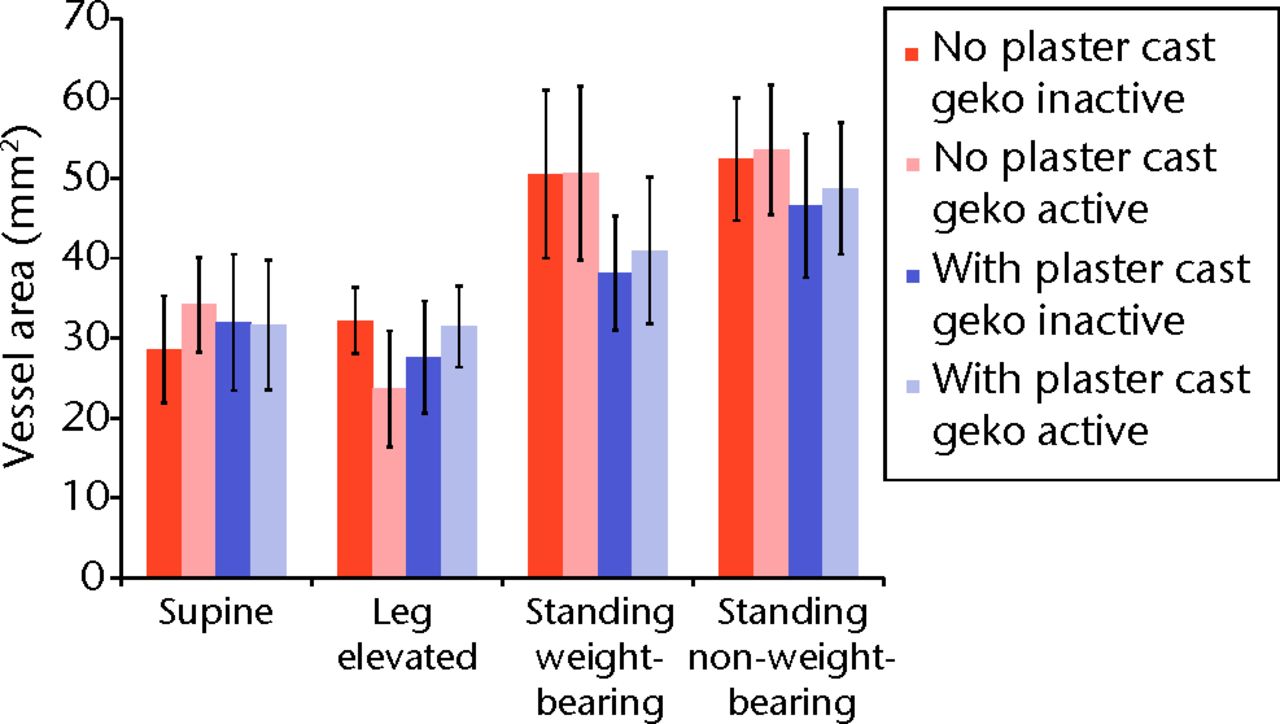
Fig. 3
Bar chart showing the mean cross-sectional area of the femoral vein according to activation of the geko device and posture. The error bars denote the standard error of the mean.
Cross-sectional area of the femoral vein
Table I and Figure 3 shows the cross-sectional area of the femoral vein for all postural positions, with and without a plaster cast, and with and without activation of the geko device. Although no statistically significant differences were observed (Table I), the data generally suggested a trend to greater vein area in the standing position, subject to greater hydrostatic effects, and that this hydrostatic effect may be mitigated by the presence of the plaster cast.
Patient-rated discomfort
The median level of discomfort measured on the VRS across all postural positions using the geko device was 2, denoting ‘minimal discomfort’. Table II and Figure 4 show that the median level of discomfort was slightly higher without casting compared with discomfort when casting was applied. The VRS showed that in the supine and leg-elevated positions participants were significantly less comfortable without casting compared with the same position with casting (median difference 1, p = 0.008 to 0.02).
Table II
Results of participant-related discomfort with the use of the geko electrostimulation device with and without plaster casting (IQR, interquartile range)
| Discomfort assessment | No plaster cast | Plaster cast | p-value* |
|---|---|---|---|
| Median VRS (IQR)† | |||
| Supine | 3.0 (2 to 3) | 2.0 (1 to 2) | 0.008 |
| Leg elevated | 3.0 (2 to 3) | 2.0 (1 to 2) | 0.02 |
| Standing weight-bearing | 2.0 (2 to 3) | 2.0 (1 to 2) | 0.28 |
| Standing non-weight-bearing | 2.0 (2 to 2) | 2.0 (1 to 2) | 0.38 |
| Median VAS (IQR)‡ | |||
| Supine | 3.5 (2.7 to 4.0) | 2.4 (2.1 to 2.5) | 0.002 |
| Leg elevated | 3.0 (2.5 to 4.1) | 2.2 (2.1 to 2.6) | 0.004 |
| Standing weight-bearing | 2.5 (2.5 to 2.7) | 2.3 (1.7 to 2.5) | 0.06 |
| Standing non-weight-bearing | 2.4 (2.3 to 2.7) | 1.8 (1.4 to 2.4) | 0.05 |
-
* Wilcoxon signed-rank test † VRS, verbal rating scale from 1 (no sensation) to 5 (severe discomfort) ‡ VAS, visual analogue scale from 0 (no sensation) to 10 (severe discomfort)
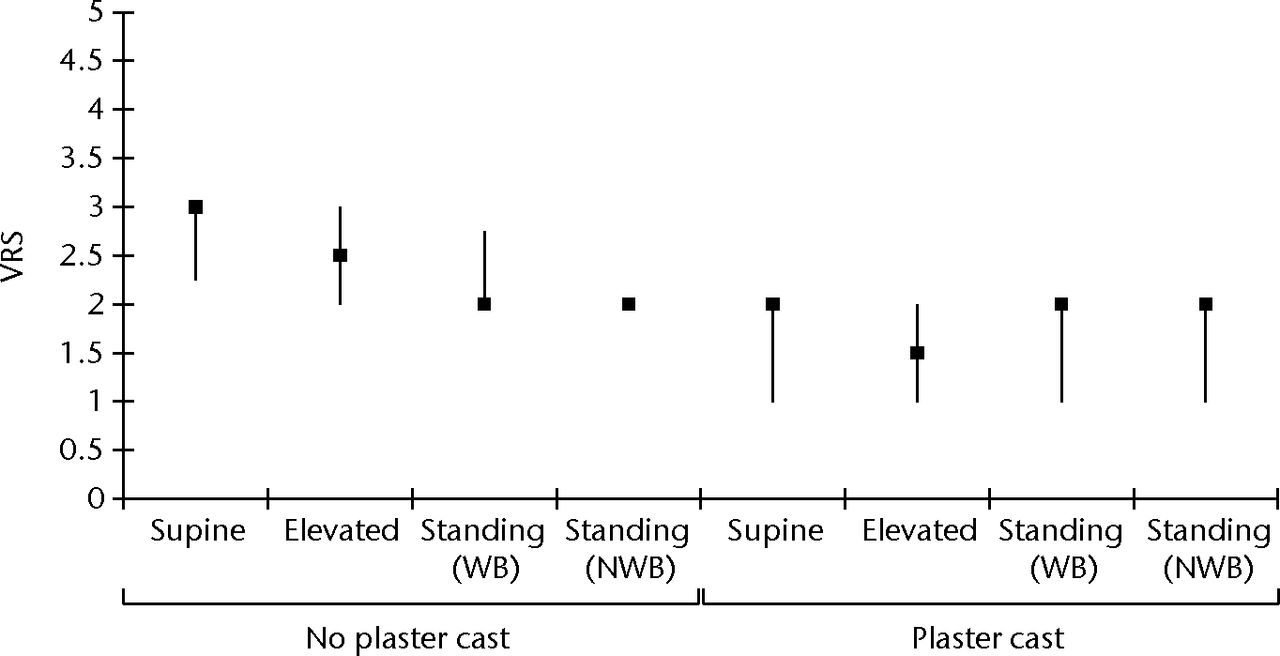
Fig. 4
Box and whisker plot of the verbal rating score (VRS) for discomfort using the geko device in all postural positions with and without plaster cast. The VRS ranges from 1 (no sensation) to 5 (severe discomfort). The error bars denote the interquartile range (WB, weight-bearing; NWB, non-weight-bearing).
Table II and Figure 5 show the results of the VAS for discomfort with the use of the geko device. The median VAS across all positions was 2.5 (0.9 to 6.5). Again, a lower level of discomfort was reported in the supine and leg-elevated positions with casting compared with no casting (median differences 1, p = 0.002 to 0.004).
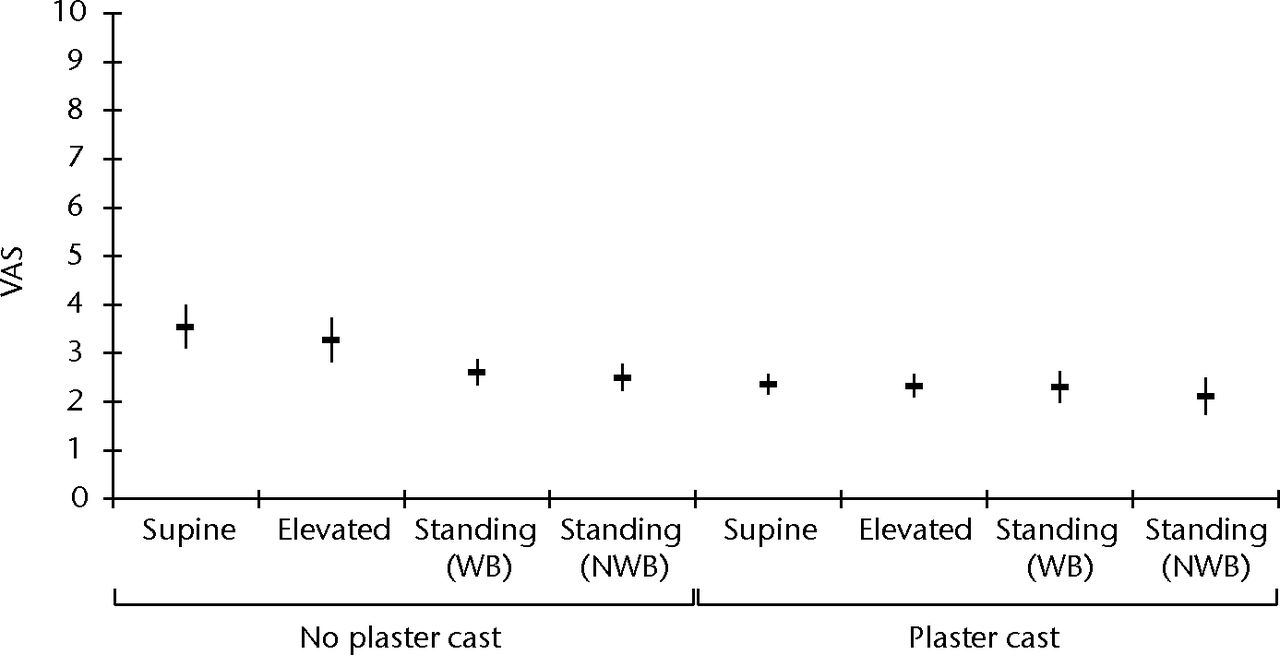
Fig. 5
Box and whisker plot of the visual analogue scale (VAS) feedback for discomfort using the geko device in all postural positions with and without plaster cast. The VAS ranges from 0 (no sensation) to 10 (severe discomfort) (WB, weight-bearing; NWB, non-weight-bearing).
Discussion
Patients in a plaster cast following injury and surgery are at risk of thromboembolism but the majority receive no DVT prophylaxis.24 Chemical prophylaxis is traditionally used for those at highest risk, but administration is either awkward (heparin injections or monitored warfarin) or expensive and off-label (anti Xa or direct thrombin inhibitors). Traditional mechanical devices are impractical as they cannot be used in tandem with plaster casting. A small, portable neuromuscular stimulation device fitted above the cast would be a practical solution.
The efficacy of medical devices which enhance lower limb blood flow and thus potentially reduce thrombosis risk are conventionally examined with Doppler ultrasound. Increased flow is assumed to correlate with benefit against thrombosis.25,26
The present results demonstrate that the geko electrical stimulation device is able to generate an effect in an individual wearing a plaster cast, increasing flow in the femoral vein by between 18% and 131%. This increase compares favourably with the reported haemodynamic efficacies of devices such as intermittent pneumatic compression which cannot be used in conjunction with a plaster cast.25,26
Furthermore, the geko device consistently increased flow regardless of posture of the leg aside apart from elevation, during which flow is already high. This confirms previously published data for the device.22 The practical clinical application is that the device will continue to have an effect whenever it is worn – whether the patient is ambulating with or without weight-bearing or whether they are recumbent in bed or sitting in a chair.
The geko device was well tolerated, with most participants reporting only ‘mild discomfort’ while using the device. Interestingly, visual analogue scores for discomfort were significantly lower (supine and leg-elevated positions, p = 0.008 to 0.02) when wearing a plaster cast than when not. Perhaps the plaster cast in some way mitigates any discomfort caused by the electrical stimulus by reducing or substituting the physiological sensation, or perhaps the effect is psychological, attributable to a shift in tolerance.
The device presents a number of advantages over previous ES devices. Indirect muscle stimulation via the nerve allows muscle contraction to be effected using a much lower level of stimulus than direct muscle stimulation. This means that a given level of contraction is considerably more tolerable to the patient. The branching of the common peroneal nerve distal to the knee results in contraction of a whole complex of lower leg muscles, including those muscles responsible for foot dorsiflexion, and stabilisers. Dorsiflexion has been shown to provide more effective blood pumping than plantar flexion.27 This gives a more effective evacuation (by distension) of the valved vessels of the calf than by contraction of the gastrocnemius muscle.
Previous ES devices delivered a train of multiple electrical pulses to the muscle, to give a tetanic contraction over a period of approximately 0.5 to 1 second. This was then relaxed and repeated at regular intervals. A dose-ranging study have found that prolonged tetanic contraction produced no further venous flow after the initial momentary contraction, as the vein was already empty.22 This led to the adoption of a charge-balanced stimulus comprising a single pulse with duration < 1 ms delivered every second. As well as providing optimal blood pumping, this proved much more tolerable to the user, and consumed substantially less power. The short duration of muscle contractions places very little metabolic demand on the musculature, and so prolonged use does not lead to muscle fatigue.
The reductions in power consumption enable the device to be powered from a single button-cell battery; this allows the design of a miniature, integral device comprising battery, control circuit, and adhesive electrodes which can be directly applied to the patient with no wires or separate box (Fig. 1). This has obvious advantages in terms of convenience, electrical safety and patient ambulation. Furthermore, the application of the device to the common peroneal nerve, remote from the muscle pumps, permits its use in a cast.
Formal validation that the geko actually reduces the prevalence of VTE would require a randomised study with objective imaging outcome. A control group with no prophylaxis would be unethical, so a potential comparator would most likely be a low-molecular-weight heparin. Such studies are exceedingly expensive; the duration of prophylaxis and thus the optimal moment for outcome assessment is uncertain. Therefore, reliance on a venous flow surrogate is generally regarded as reasonable proof of concept.25,26,28
Limitations of the study include the use on volunteers rather than on those with a cast for recent injury or surgery which might influence flow characteristics. Further studies should validate the device in these groups. A further issue to be studied will be the tolerance of the device over a longer period of time both from a patient tolerance perspective. In addition, physiological data should be acquired on the neuromuscular effects from prolonged stimulation.
Conclusions
Stimulation of the common peroneal nerve using the geko device increased blood flow in the femoral vein, including when the lower leg was immobilised in a cast, thus potentially offering a viable means of thromboprophylaxis for patients in a plaster cast for whom other modes of mechanical prophylaxis are unsuitable.
The authors would like to thank the participants for giving their time. This research was conducted within the multidisciplinary Southampton Musculoskeletal Research Unit (University Hospital Southampton and University of Southampton).
1 Roberts C . Towards evidence-based emergency medicine: best BETs from the Manchester Royal Infirmary: BET 3: thromboprophylaxis significantly reduces venous thromboembolism rate in ambulatory patients immobilised in below-knee plaster cast. Emerg Med J2012;29:424–425. Google Scholar
2 Healy B , BeasleyR, WeatherallM. Venous thromboembolism following prolonged cast immobilisation for injury to the tendo Achillis. J Bone Joint Surg [Br]2010;92-B:646–650.CrossrefPubMed Google Scholar
3 Virchow R. Cellular pathology. London: Churchill, 1860. Google Scholar
4 Malone PC , AgutterPS. The aetiology of deep venous thrombosis. QJM2006; 99:581–593.CrossrefPubMed Google Scholar
5 Nicolaides AN , KakkarVV, FieldES, RenneyJT. The origin of deep vein thrombosis: a venographic study. Br J Radiol1971;44:653–663.CrossrefPubMed Google Scholar
6 Nicolaides AN , KakkarVV, FieldES, FishP. Venous stasis and deep vein thrombosis. Br J Surg1972;59:713–717. Google Scholar
7 Sevitt S. Pathology and pathogenesis of deep vein thrombosis. In: Poller L, ed. Recent advances in thrombosis. Edinburgh: Churchill Livingstone, 1973:17–38. Google Scholar
8 Kujath P , SpannagelU, HabscheidW. Incidence and prophylaxis of deep venous thrombosis in outpatients with injury of the lower limb. Haemostasis1993;23(Suppl 1):20–26.CrossrefPubMed Google Scholar
9 Bounameaux H , RighiniM, PerrierA. Venous thromboembolism: contemporary diagnostic and therapeutic aspects. Vasa2008;37:211–226.CrossrefPubMed Google Scholar
10 Amarigiri SV , LeesTA. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev2000;3:CD001484.CrossrefPubMed Google Scholar
11 CLOTS Trials Collaboration; Dennis M , SandercockPA, ReidJ, et al.Effectiveness of thigh-length graduated compression stockings to reduce the risk of deep vein thrombosis after stroke (CLOTS trial 1): a multicentre, randomised controlled trial. Lancet2009;373:1958–1965.CrossrefPubMed Google Scholar
12 Best AJ , WilliamsS, CrozierA, et al.Graded compression stockings in elective orthopaedic surgery: an assessment of the in vivo performance of commercially available stockings in patients having hip and knee arthroplasty. J Bone Joint Surg [Br]2000;82-B:116–118. Google Scholar
13 Roderick P , FerrisG, WilsonK, et al.Towards evidence based guidelines for the prevention of venous thromboembolism: systematic review of mechanical methods, oral anticoagulation, dextran and regional anaesthesia as thromboprophylaxis. Health Technol Assess2005;9:1–78. Google Scholar
14 Comerota AJ , KatzML, WhiteJV. Why does prophylaxis with external pneumatic compression for deep vein thrombosis fail?Am J Surg1992;164:265–268.CrossrefPubMed Google Scholar
15 Testroote M , StigterW, de VisserDC, JanzingH. Low molecular weight heparin for prevention of venous thromboembolism in patients with lower-leg immobilization. Cochrane Database Syst Rev2008;4:CD006681.CrossrefPubMed Google Scholar
16 Currier DP , PetrilliCR, ThrelkeldAJ. Effect of graded electrical stimulation on blood flow to healthy muscle. Phys Ther1986;66:937–943.CrossrefPubMed Google Scholar
17 Liu HI , CurrierDP, ThrelkeldAJ. Circulatory response of digital arteries associated with electrical stimulation of calf muscle in healthy subjects. Phys Ther1987;67:340–345.CrossrefPubMed Google Scholar
18 Faghri PD , Van MeerdervortHF, GlaserRM, FigoniSF. Electrical stimulation-induced contraction to reduce blood stasis during arthroplasty. IEEE Trans Rehabil Eng1997;5:62–69.CrossrefPubMed Google Scholar
19 Kaplan RE , CzyrnyJJ, FungTS, UnsworthJD, HirshJ. Electrical foot stimulation and implications for the prevention of venous thromboembolic disease. Thromb Haemost2002;88:200–204.PubMed Google Scholar
20 Browse NL , NegusD. Prevention of postoperative leg vein thrombosis by electrical muscle stimulation: an evaluation with 125I-labelled fibrinogen. Br Med J1970;3:615–618. Google Scholar
21 Lindström B , HolmdahlC, JonssonO, et al.Prediction and prophylaxis of postoperative thromboembolism: a comparison between peroperative calf muscle stimulation with groups of impulses and dextran 40. Br J Surg1982;69:633–637. Google Scholar
22 Tucker AT , MaassA, BainDS, et al.Augmentation of venous, arterial and microvascular blood supply in the leg by isometric neuromuscular stimulation via the peroneal nerve. Int J Angiol2010;19:31–37.CrossrefPubMed Google Scholar
23 Jawad H, Bain DS, Dawson H, et al. The effect of OnPulse in improving lower limb blood flow in healthy volunteers. Presented at Union Internationale de Phlebologie, Prague, Czech Republic; September 2011. Google Scholar
24 Iqbal HJ , DahabR, BarnesS. UK national survey of venous thromboembolism prophylaxis in ankle fracture patients treated with plaster casts. Foot Ankle Surg2012;18:157–159.CrossrefPubMed Google Scholar
25 Warwick DJ , MartinA, GlewD, BannisterGC. Measurement of femoral vein blood flow during total hip replacement: Duplex ultrasound with and without the use of a foot-pump. J Bone Joint Surg [Br]1994;76-B:918–921. Google Scholar
26 Westrich GH , SpechtLM, SharrockNE, et al.Venous haemodynamics after total knee arthroplasty: evaluation of active dorsal to plantar flexion and several mechanical devices. J Bone Joint Surg [Br]1998;80-B:1057–1066. Google Scholar
27 Panny M , AmmerK, KundiM, KatzenschlagerR, HirschlM. Severity of chronic venous disorders and its relationship to the calf muscle pump. Vasa2009;38:171–176.CrossrefPubMed Google Scholar
28 Warwick DJ , DewburyK, ForresterA. Intermittent pneumatic compression: a comparison of femoral vein velocity with five different devices. Int Angiol2013;32:404–409. Google Scholar
Funding statement:
Funding for this study was provided by FirstKind Medical to cover University Hospital Southampton R& D overhead, ultrasonography equipment hire, ultrasonogaphy costs and nominal PI costs.
Author contributions:
D. J. Warwick: Study design, Study co-ordination, Write-up and editing of paper, Data collection
A. Shaikh: Data collection, Data analysis, Write-up of paper
S. Gadola: Data collection
M. Stokes: Study design, Data analysis, Writing of paper
P. Worsley: Protocol development, Data analysis
D. Bain: Data analysis, Writing of paper
A. T. Tucker: Study design, Write-up and editing of paper
S. D. Gadola: Study co-ordination and design, Write-up and editing of paper
ICMJE Conflict of Interest:
Dr Tucker ad Dr Bain have paid consultancy agreements with FirstKind. Fees were received by SG for performance of ultrasonography. Nominal reimbursement at established R& D rates were provided for PI time to the Institution. The PI and all other authors received no personal reimbursement or reward in kind.
©2013 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










