Abstract
Objectives
One commonly used rat fracture model for bone and mineral research is a closed mid-shaft femur fracture as described by Bonnarens in 1984. Initially, this model was believed to create very reproducible fractures. However, there have been frequent reports of comminution and varying rates of complication. Given the importance of precise anticipation of those characteristics in laboratory research, we aimed to precisely estimate the rate of comminution, its importance and its effect on the amount of soft callus created. Furthermore, we aimed to precisely report the rate of complications such as death and infection.
Methods
We tested a rat model of femoral fracture on 84 rats based on Bonnarens’ original description. We used a proximal approach with trochanterotomy to insert the pin, a drop tower to create the fracture and a high-resolution fluoroscopic imager to detect the comminution. We weighed the soft callus on day seven and compared the soft callus parameters with the comminution status.
Results
The mean operating time was 34.8 minutes (sd 9.8). The fracture was usable (transverse, mid-shaft, without significant comminution and with displacement < 1 mm) in 74 animals (88%). Of these 74 usable fractures, slight comminution was detected in 47 (63%). In 50 animals who underwent callus manipulation, slight comminution (n = 32) was statistically correlated to the amount of early callus created (r = 0.35, p = 0.015). Two complications occurred: one death and one deep infection.
Conclusions
We propose an accurate description of comminution and complications in order to improve experiments on rat femur fracture model in the field of laboratory research.
Cite this article: Bone Joint Res 2013;2:149–54.
Article focus
We aimed to estimate precisely the rate of comminution, its importance and its effect on the soft callus created along with the rate of complications of a rat model of femoral fracture for laboratory research
Key messages
12% of the fractures created were not usable for the research because of death, deep infection or a non-acceptable fracture. Of the remaining fractures, 63% displayed slight comminution. The slight comminution was related to a higher amount of soft callus created
Strengths and limitations
This study is the first to estimate the occurrence of comminution and its implication in a rat femur fracture model
We propose a classification of the comminution created and underline the importance of reporting it because of its implication for soft callus created in a rat model of femoral fracture
Introduction
Fracture models in rats are widely used as small-animal proof of concept in laboratory research. These models are of tremendous value, but the characteristics of an ideal model need to be analysed and its rate of complication well known. Therefore, an accurate knowledge of the chosen animal model is continuously useful from the conception of a study to the final interpretation of the results obtained.
One commonly used fracture model in the rat was described by Bonnarens and Einhorn1 in 1984 based on a previous 1970 design by Jackson et al.2 In this model, a fixed weight dropped onto a blunt guillotine creates a standardised closed midshaft fracture of the femur. The osteosynthesis is made before the fracture by using an intramedullary pin inserted through the knee in a retrograde fashion and subsequently bent in the trochanteric area.1 Recently, this model has been simplified by using a single-incision approach from the proximal direction and by monitoring the insertion of the pin with fluoroscopy.3-5 The main theoretical advantage of this model and its successive modifications is to create a standardised fracture with very low damage to the surrounding soft tissues.1
All authors do not universally agree on the simplicity of this rat fracture model. Some have reported recurrent complications including death, misplaced fracture, excess comminution and deep infection.6-13Moreover, it appears that comminution in different degrees has been particularly difficult to control for during analysis.14-17 As the degree of fracture comminution can affect the formation of callus, the relationship between the rat fracture model, the resultant comminution and the soft callus created should be characterised in order to increase the quality of laboratory research. To do so, we used a rat femur fracture model based on the Bonnarens description1 to estimate the occurrence of comminution with a new fluoroscopy device and its relationship to the amount of early callus formed. We also aimed to report a precise rate of complications such as death and infection.
Our hypothesis was that there is a correlation between comminution and the amount of soft callus created in a rat model of femoral fracture based on the Bonnarens model.1 The aim of our study was: a) to use a high-resolution fluoroscopy device to estimate the amount of comminution; b) to determine the relationship between comminution and the amount of early callus; and c) to estimate accurately the rate of complication.
Materials and Methods
After the Institutional Animal Care and Use Committee (IACUC) approved the design of the study, we acquired 84 female Sprague-Dawley rats (Harlan Laboratories, Indianapolis, Indiana). The animals were 12-weeks-old with a mean weight of 250.1 g (sd 8.1). They were housed and fed according to our national principles of laboratory animal care.
Pre-operatively, each rat was anaesthetised with inhalational anaesthetic (isofluorane 2.5% with oxygen 2 l/min; Baxter, Deerfield, Illinois), and a dose of general analgesia (buprenorphine at 0.01 mg/kg; American Regent Inc., Shirley, New York) was given intramuscularly. The rats were randomised to a left-sided or right-sided fracture by changing the side of the procedure every sixth rat. After depilating the rat’s leg with an electric razor and cleaning it with an alcohol wipe, we made a 1.5 cm incision over the greater trochanter under aseptic conditions. The gluteus maximus was divided to reveal the insertion of the gluteus medius onto the greater trochanter. With an osteotome, a trochanteric osteotomy was made in order to elevate the tip of the greater trochanter and visualise the insertion of the pelvitrochanteric muscles. A sterile 1.1 mm Kirschner (K-) wire was manually inserted through the gluteus medius, then through the trochanteric fossa into the femoral canal, and subsequently threaded down to the level of the femoral condyles. We used a high-definition fluoroscopic imager (LabScope; Glenbrook Technologies Inc., Randolph, New Jersey) to confirm the correct placement of the K-wire. We then cut the wire and bent it back onto itself. The spaces between both the gluteus medius and vastus lateralis and the incised gluteus maximus were closed with 5-0 absorbable sutures (Polysorb; Covidien Inc., Mansfield, Massachusetts). Finally, the skin was closed with surgical staples (Autoclip; Harvard Apparatus, Holliston, Massachusetts) and local anaesthetic (bupivacaine at 4 mg/kg, Hospira Inc., Lake Forest, Illinois) was injected at the incision site. Confirmatory fluoroscopy views of the femur with pin in place were taken in the lateral and anteroposterior (AP) views.
A blunt guillotine was used to create the fracture according to the concept previously described (Fig. 1).1,2 While still anaesthetised, the rat was placed in a lateral position, the pelvis against one anvil and the knee against the other anvil. The leg was maintained in this position with a self-locking 4.8 mm 12-inch zip-tie (Monoprice Inc., Rancho Cucamonga, California). As previously described, the rings were placed in a way that the motion of the blade was limited to 1.5 mm in order to avoid important soft-tissue damage.1 A weight of 1.1 kg was dropped from 15 cm, generating a theoretical impact force of 1079.10 N. The fracture was assessed by two authors (JCA and RMC) from lateral and AP fluoroscopic views. If no fracture was created, the procedure was repeated with the same weight dropped from 20 cm, which generated a theoretical impact force of 1438.80 N. If no fracture was created after two successive drops, the rat was excluded due to the expectation of significant soft-tissue trauma.
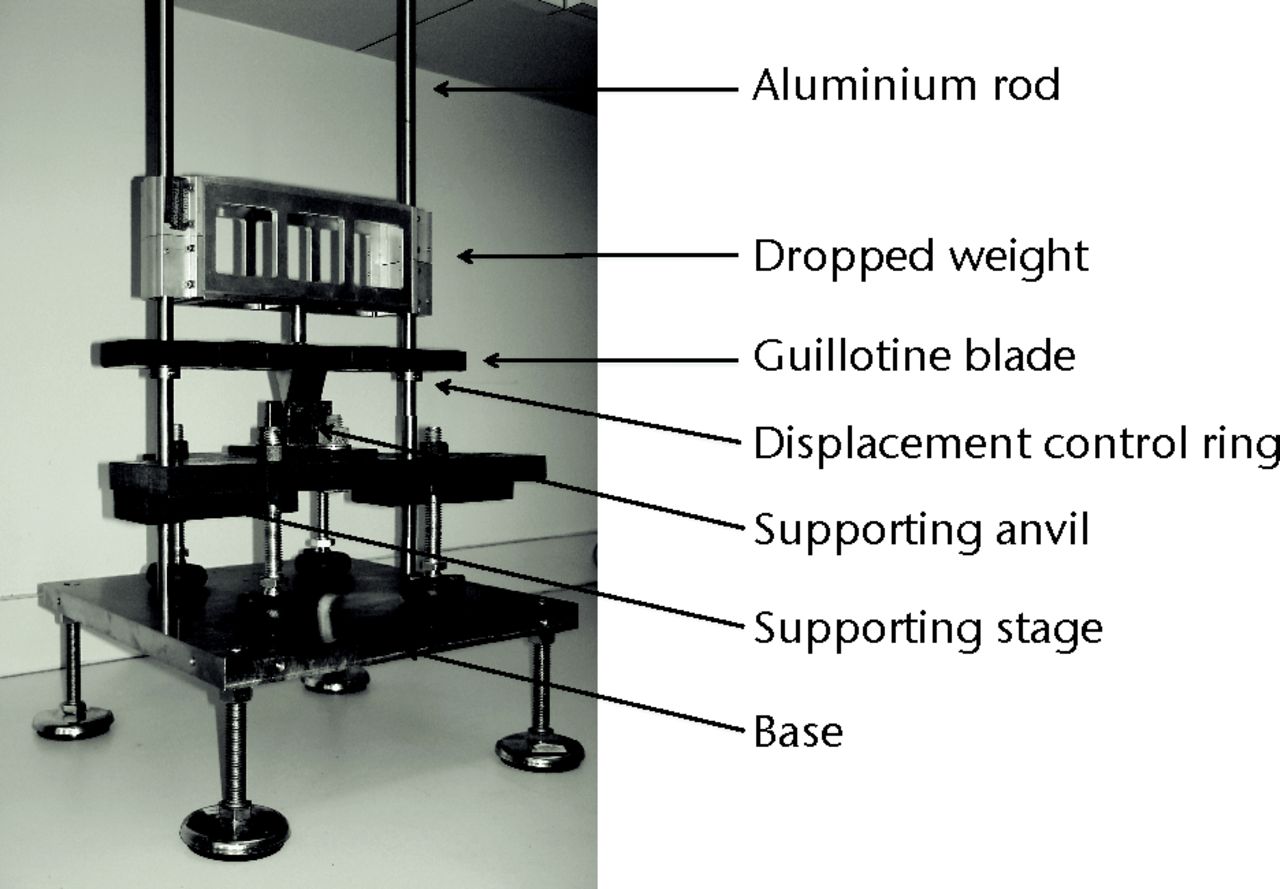
Fig. 1
Photograph showing the oblique view of the dropping tower.
After the surgeries, the rats were examined for bleeding, activity, feeding/drinking and leg weight-bearing twice daily. The rats were given systematic analgesia (buprenorphine at 0.01 mg/kg; American Regent Inc.) every 12 hours for a total of 48 hours coverage and further in case of pain.
Of the 84 rats were, a total of 57 were randomised to have callus manipulation at the seventh post-operative day. We decided to test the soft callus on the seventh day as this time point has been shown to be the peak timeframe of response to local trauma.18 In these animals we removed the callus under magnification (Leica Microsystems GmbH, Wetzlar, Germany) and subsequently weighed it with a precision scale (Torbal; Fulcrum Inc., Clifton, New Jersey).18
As endpoints, we reported the mean duration of each surgery and the number of drops needed to create the fracture. All fractures were analysed with two fluoroscopy views: the anatomical location, pattern, degree of pin bending in lateral and AP views and presence of comminution were reported for each fracture. For that step, three types of comminution were defined: 1) no comminution: no third fragment detected on the two fluoroscopy views; 2) slight comminution: small fragments all with a smaller size than the pin diameter (i.e. < 1.1 mm); and 3) important comminution: ≥ one wide fragment larger than the diameter of the pin (i.e. > 1.1 mm). By definition, we excluded from analysis every fracture meeting at least one exclusion criteria among the following: oblique, proximal, distal, important comminution or displacement > 1 mm. Finally, the mass of callus material extracted at day seven in the callus manipulation group was recorded and correlated afterward with the degree of comminution previously described.
Statistical analysis
Statistica software (StatSoft Inc., Tulsa, Oklahoma) was used to perform the analyses. We used a Mann–Whitney rank-sum order test to compare the group without comminution and the group with slight comminution. Any relationship between comminution and amount of callus was estimated by a Pearson correlation test. Standard errors were reported with standard deviation (sd) and p-values < 0.05 were considered to be statistically significant.
Results
A total of 84 fractures were created. The mean operating time was 34.8 minutes (sd 9.8). All but one of the fractures were obtained from the first drop and the remaining fracture from a second attempt. The fracture created was acceptable (i.e. transverse, midshaft, without significant comminution and with displacement < 1 mm) in 74 rats (88%). In ten rats (12%) the fracture was unacceptable and these were therefore excluded, including seven rats scheduled for callus manipulation.
In the 74 included fractures, the mean angulation of the pin was 2.4° (sd 4.7) and 6.8° (sd 8.9) in the lateral and AP planes, respectively. There was no fluoroscopic evidence of any degree of fracture comminution in 27 (37%) while the remaining 47 fractures (63%) exhibited slight comminution, as previously defined. Two complications occurred: one death and one deep infection. Both of the rats had a fracture without any comminution but were excluded from the further statistical analysis.
The fracture was unacceptable in seven of the 57 animals randomised to callus manipulation and were excluded, leaving 50 rats who underwent second surgery comprising removal of soft callus and weighing (Table I). The mean weight of removed callus was 0.077 g (sd 0.034). In the fractures with no comminution (n = 18) the mean weight of the removed callus was 0.060 g (sd 0.017), significantly less than that seen in the slightly comminuted fractures (0.079 g (sd 0.028)) (p = 0.015) (Fig. 2). The Pearson correlation coefficient between slight comminution on fluoroscopy and weight of callus gave an r-value of 0.35.
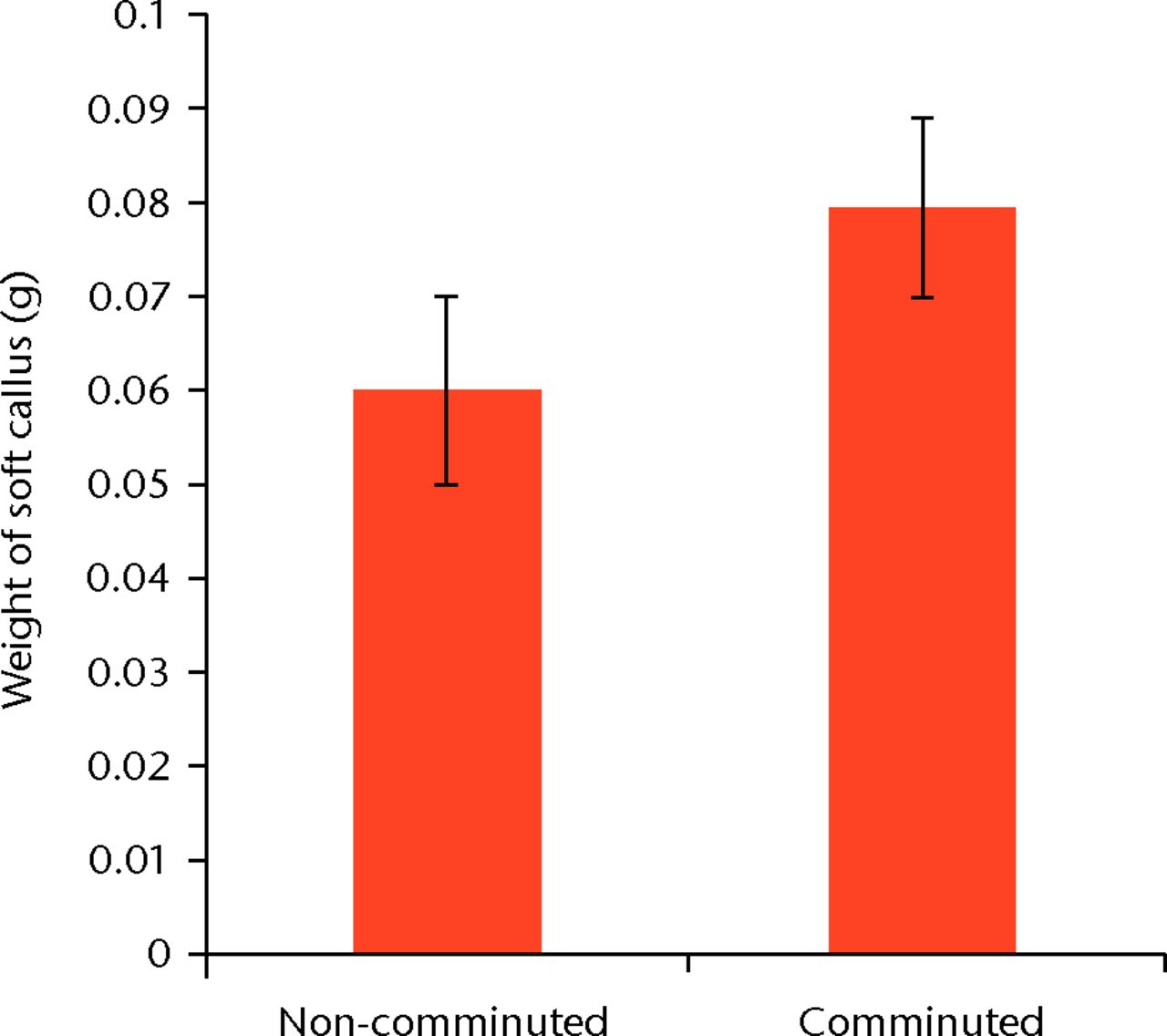
Fig. 2
Bar chart showing the mean weight of soft callus created by seven days in fractures with (n = 32) and without slight comminution (n = 18). The difference is statistically significant (p = 0.015). Error bars denote the standard deviation.
Table I
Comparison of comminuted and non-comminuted fractures in callus manipulation group (n = 50). P-values calculated for null hypothesis that comminuted and non-comminuted fractures have same true values using a two-sided Mann-Whitney rank sum test
| Comminuted | Non-comminuted | p-value* | |
|---|---|---|---|
| Fractures (n) | 32 | 18 | |
| Right-sided fractures (n, %) | 13 (41) | 11 (61) | 0.17 |
| Mean (sd) weight (g) | 249.7 (9.0) | 251.1 (11.0) | 0.66 |
| Mean (sd) procedure time (mins) | 35.2 (9.3) | 32.9 (6.5) | 0.35 |
| Mean (sd) weight of removed callus (g) | 0.079 (0.028) | 0.060 (0.017) | 0.015 |
| Mean (sd) pin bending (°) | |||
| Anteroposterior | 6.7 (8.3) | 4.1 (5.7) | 0.22 |
| Lateral | 2.4 (4.7) | 1.2 (3.8) | 0.45 |
-
* calculated for null hypothesis that comminuted and non-comminuted fractures have same true values, using a two-sided Mann-Whitney rank sum test
Discussion
The rat model of fracture described by Bonnarens and Einhorn1 is a commonly used model in the literature. It has several appealing characteristics. First, the rat femur has proven be the most suitable bone for biomechanical testing in a small animal.19Secondly, the fracture is created in a closed fashion with very low damage to the surrounding soft tissues.1 This characteristic allows the preservation of the early biological response to trauma, which is not the case of other fracture models that use an open midshaft femoral osteotomy.1,20 Thirdly, the model is very effective; all of our fractures but one were created on the first drop, and the remaining animal showed no apparent consequences in term of soft-tissue damage, comminution or ambulatory restriction. Finally, the intramedullar pinning provides a rigid fixation and preserves a nearly normal ability for the rat to walk, which makes it preferable to other options for fracture fixation, such as an external fixator.21,22
Some authors in the literature have used other devices to create a closed midshaft fracture. The two main alternate options are the manual creation of the fracture, or a three-point bending device.22-25 However, those present two important differences from the blunt guillotine. First, they create a progressive bending that crushes the soft tissues surrounding the fracture site, potentially jeopardising the initial inflammatory response to the fracture.1,18 Secondly, the energy needed to create the fracture is not perfectly controlled when considered in comparison with a dropping tower, which delivers a highly standardised amount of energy.1,23,24 For these reasons, we recommend the use of a blunt guillotine as designed by Bonnarens and Einhorn.1
Originally, two surgical approaches were used.1 A knee approach was made to insert the K-wire in the femur, followed by the trochanteric approach to pull out and bend the K-wire. Some authors reported later that the trochanteric approach alone could be sufficient to perform the entire process, with advantages of limiting the occurrence of complications related to the knee approach such as condyle fracture, knee infection and pain.3,4 However, the proximal entry point for the K-wire is hard to estimate because of the peculiar bending of the femur in the AP plane (Fig. 3). In order to place the intramedullary pin accurately, we used a partial trochanterotomy to expose the trochanteric fossa and accurately locate the exact entry point for the K-wire. This approach proved to be fast and safe, with only one deep infection reported. Moreover, all the rats of the study ambulated within 24 hours after awakening. Finally, to the best of our knowledge, there is no evidence showing any correlation between trochanteric approach and bone healing of the femur diaphysis. These two parts are distant and never operated on in the same time.
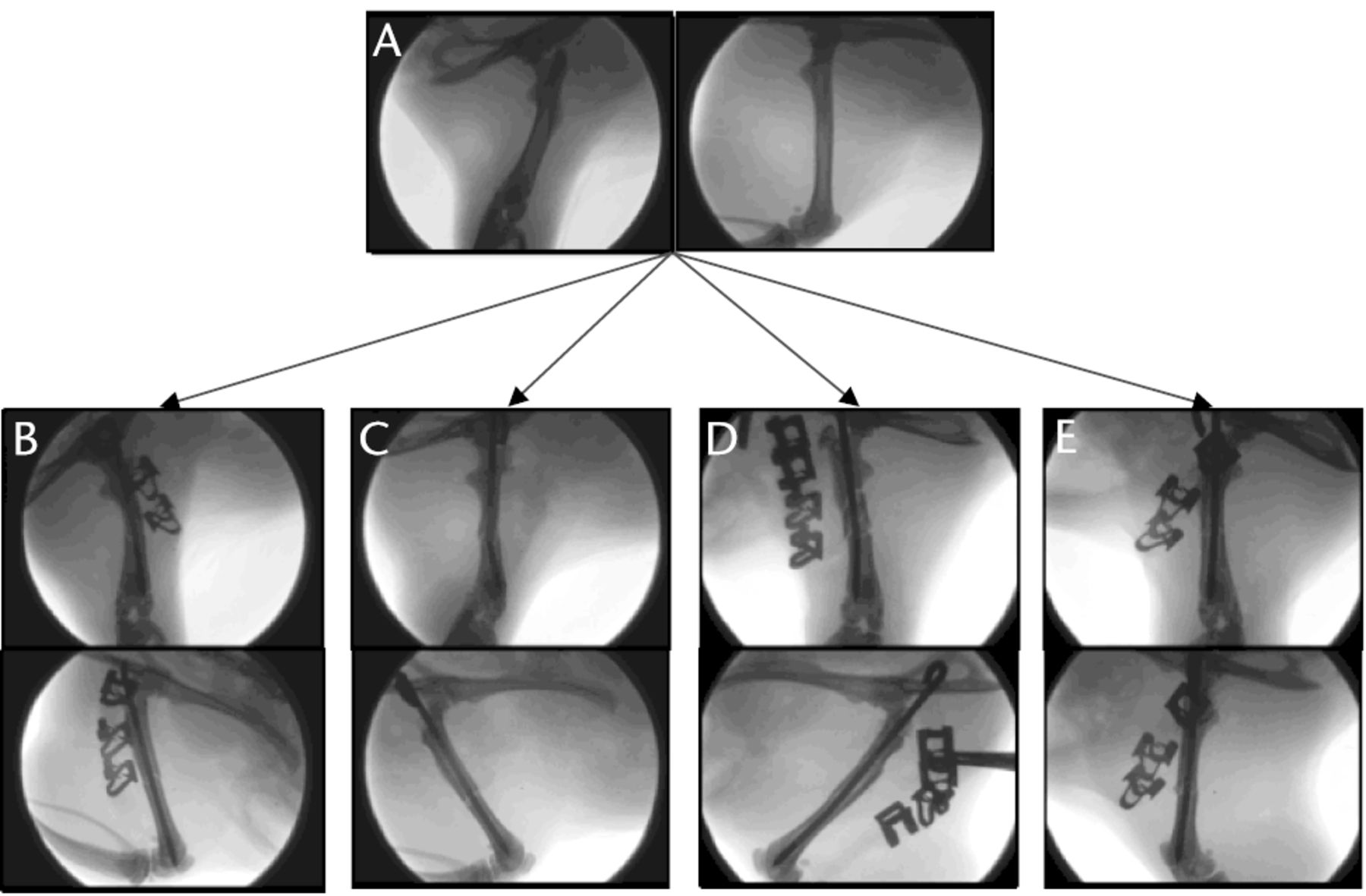
Fig. 3
Fluoroscopic images showing the range of fractures created with a dropping tower: a) normal rat femur, b) perfect fracture, c) fracture with no bending in the lateral plane but marked bending in the anteroposterior plane, d) fracture with slight comminution, and e) fracture with excessive comminution.
The quality criteria used to assess a fracture created from an animal model are usually its anatomical location (proximal, midshaft or distal), its pattern (transverse, oblique or spiroid), the K-wire placement (intramedullar or misplaced), the resultant bending of the K-wire and the absence of important comminution. In our series, 74 rats (88%) met all those criteria while the ten remaining animals (12%) exhibited at least one of the unacceptable criteria. However, we reported a significantly higher mean bending of the K-wire among the acceptable fractures than the original description.1 In fact, Bonnarens and Einhorn1 reported an average bending of 1.8° (sd 2.8°) but the angulation was calculated only in the lateral plane. Our more prominent bending in the AP plane (6.8° (sd 8.9)) could be explained by the lateral impact of the blunt guillotine on the thigh. In fact, we observed numerous fractures without any bending in the lateral plane but a significant one in the AP plane (Fig. 3). Given the fact that significant bending could jeopardise the safe removal of the K-wire and any further biomechanical testing, we recommend that the bending should be systematically assessed in both planes.
To the best of our knowledge, this study is the first to assess the fracture created from an animal model with a high-resolution fluoroscopy device. In the authors’ opinion, comminution is hard to estimate with the use of a low-resolution imaging device. With subsequent improvements in available technology, digital fluoroscopy with high-resolution images has become available, making the description of the comminution created more accurate. As no comminution classification is reported in the literature about small animal model of fracture, we decided to separate it into three categories: no comminution, slight comminution and important comminution (Fig. 3). Unfortunately, this description is highly subjective. While important comminution is obvious to detect and generally leads to the exclusion of the subject (Fig. 3), slight comminution requires an attentive examination of all the planes and questions the potential consequences on the results of the experiment (Fig. 4). In fact, we noticed several recent studies that reported an increasing observation rate of slight comminution in their subject and some studies that displayed histological slides showing fractures with slight comminution.6-16
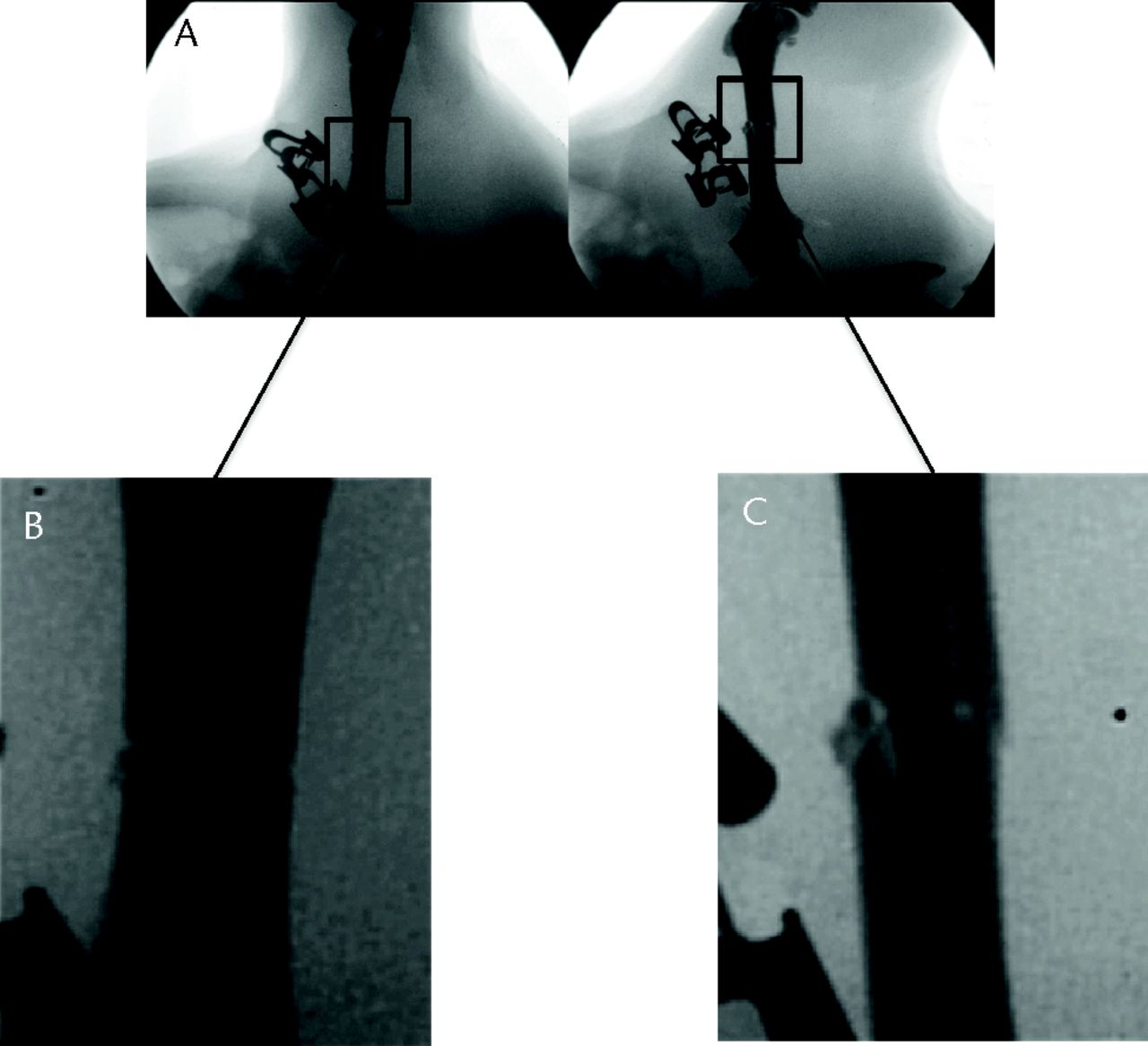
Fig. 4
Fluoroscopic images showing an example of slight comminution in an acceptable fracture with fracture sites magnified.
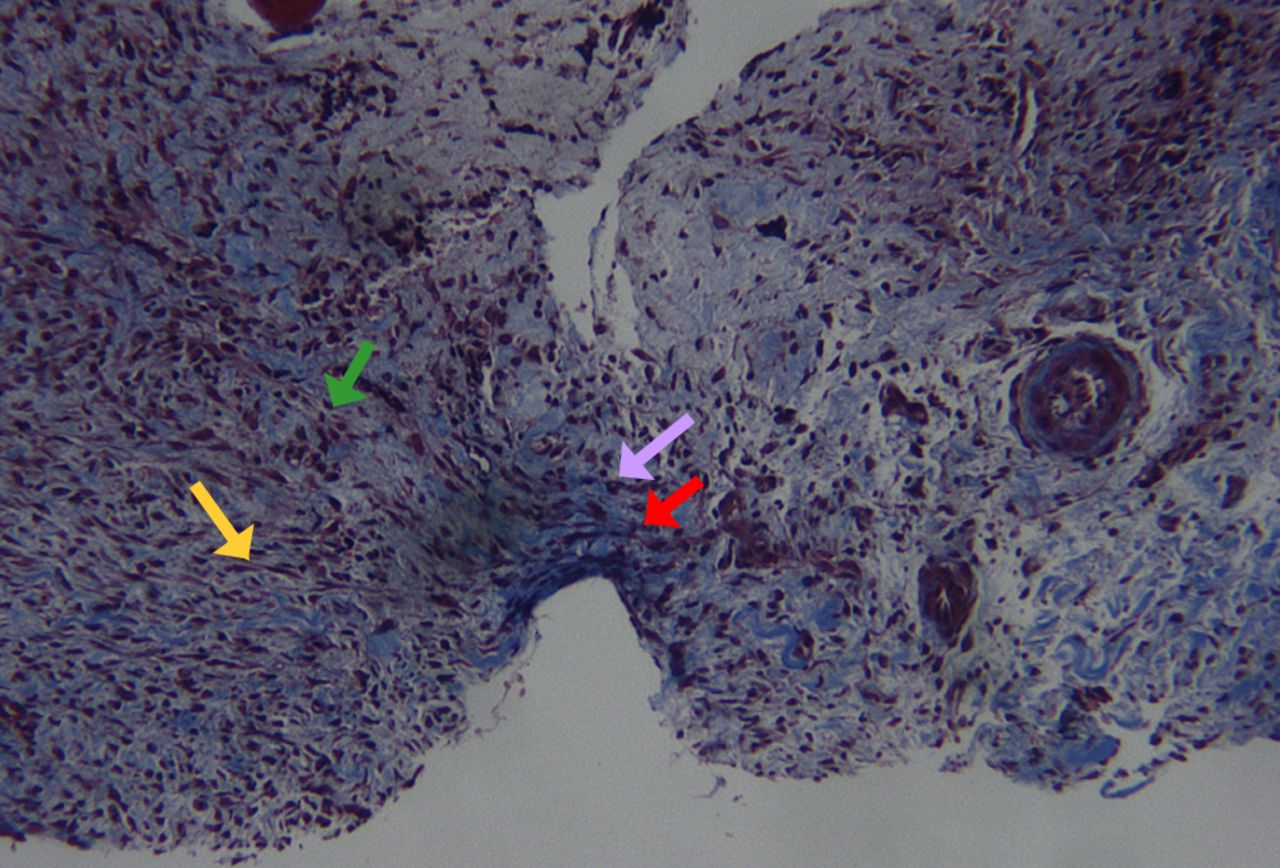
Fig. 5
Histological image of soft callus harvesting at day seven (Masson’s trichrome staining). Note the presence of mesenchymal stem cells (green arrow), fibroblasts (yellow arrow), collagen fibers (red arrow) along with hypertrophic and proliferating chondrocytes (pink arrow). Note also the absence of any surrounding soft tissues (original magnification ×25).
Finally, we tried to evaluate the potential influence of slight comminution on the bone healing process in a small animal model of fracture. We estimated that the later the chosen endpoint would be, the smaller the differences we would be able to detect. With the growing importance of research about drugs testing the bone formation early after fracture, we decided to evaluate the relationship between the slight comminution and the amount of early callus created at its peak of response to the trauma, which is believed to be seven days after the initial trauma.18 Using a Mann-Whitney rank sum order test, we found a statistically significant difference between the amount of callus produced after a slightly comminuted and a non-comminuted fracture (p = 0.015). This finding suggests that slight comminution could have an influence on the early phase of fracture healing, but its influence of the final healing is still unclear. We did not find any statistical link between comminution and sidedness of the procedure, AP or lateral pin bending, duration of surgery, or the weight of the animal.
One limitation of our study is the removal of the soft callus with magnification. While there is no way to be sure that the entire soft callus was removed from the surrounding soft tissues, we dissected the callus in each rat in this group in a blinded fashion, and a subsequent histological analysis was randomly performed in each group to control the quality of the harvesting (Fig. 5), as shown previously.18
In summary, we used the most common rat femoral fracture model to detail the comminution expected after the creation of the fracture and accurately estimate the rate of complications. We also reported a statistical relationship between the presence of slight comminution and the amount of early callus created. Finally, we suggest that an accurate estimate of the comminution should be considered when an animal fracture model is used to estimate the early post-fracture healing process in laboratory research.
1 Bonnarens F , EinhornT. Production of a standard closed fracture in laboratory animal bone. J Orthop Res1984;2:97–101.CrossrefPubMed Google Scholar
2 Jackson RW , ReedCA, IsraelJA, Abou-KeerFK, GarsideH. Production of a standard experimental fracture. Can J Surg1970;13:415–420.PubMed Google Scholar
3 Olmedo ML , WeissAP. An experimental rat model allowing controlled delivery of substances to evaluate fracture healing. J Orthop Trauma1994;8:490–493.PubMed Google Scholar
4 Pelker RR , FriedlaenderGE. The Nicolas Andry Award-1995: fracture healing: radiation induced alterations. Clin Orthop Relat Res1997;341:267–282. Google Scholar
5 Bhandari M , ShaughnessyS. A minimally invasive percutaneous technique of intramedullary nail insertion in an animal model of fracture healing. Arch Orthop Trauma Surg2001;121:591–593.CrossrefPubMed Google Scholar
6 Azuma Y , ItoM, HaradaY, et al.Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J Bone Miner Res2001;16:671–680.CrossrefPubMed Google Scholar
7 Gerstenfeld LC , ThiedeM, SeibertK, et al.Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res2003;21:670–675.CrossrefPubMed Google Scholar
8 Makino T , HakDJ, HazelwoodSJ, CurtissS, ReddiAH. Prevention of atrophic nonunion development by recombinant human bone morphogenetic protein-7. J Orthop Res2005;23:632–638.CrossrefPubMed Google Scholar
9 Hak DJ , StewartRL, HazelwoodSJ. Effect of low molecular weight heparin on fracture healing in a stabilized rat femur fracture model. J Orthop Res2006;24:645–652.CrossrefPubMed Google Scholar
10 Zhou XZ , ZhangG, DongQR, et al.Low-dose X-irradiation promotes mineralization of fracture callus in a rat model. Arch Orthop Trauma Surg2009;129:125–132.CrossrefPubMed Google Scholar
11 McDonald MM , DulaiS, GodfreyC, et al.Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone2008;43:653–662. Google Scholar
12 Kidder LS , ChenX, SchmidtAH, LewWD. Osteogenic protein-1 overcomes inhibition of fracture healing in the diabetic rat: a pilot study. Clin Orthop Relat Res2009;467:3249–3256.CrossrefPubMed Google Scholar
13 Tägil M , McDonaldMM, MorseA, et al.Intermittent PTH(1-34) does not increase union rates in open rat femoral fractures and exhibits attenuated anabolic effects compared to closed fractures. Bone2010;46:852–859. Google Scholar
14 Hausman MR , SchafflerMB, MajeskaRJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone2001;29:560–564.CrossrefPubMed Google Scholar
15 Kokubu T , HakDJ, HazelwoodSJ, ReddiAH. Development of an atrophic nonunion model and comparison to a closed healing fracture in rat femur. J Orthop Res2003;21:503–510.CrossrefPubMed Google Scholar
16 Amanat N , McDonaldM, GodfreyC, BilstonL, LittleD. Optimal timing of a single dose of zoledronic acid to increase strength in rat fracture repair. J Bone Miner Res2007;22:867–876.CrossrefPubMed Google Scholar
17 Pei Y , FuQ. Yeast-incorporated gallium promotes fracture healing by increasing callus bony area and improving trabecular microstructure on ovariectomized osteopenic rats. Biol Trace Elem Res2011;141:207–215.CrossrefPubMed Google Scholar
18 Claes L , Maurer-KleinN, HenkeT, et al.Moderate soft tissue trauma delays new bone formation only in the early phase of fracture healing. J Orthop Res2006;24:1178–1185.CrossrefPubMed Google Scholar
19 Ritchie RO , KoesterKJ, IonovaS, et al.Measurement of the toughness of bone: a tutorial with special reference to small animal studies. Bone2008;43:798–812.CrossrefPubMed Google Scholar
20 Markbreiter LA , PelkerRR, FriedlaenderGE, PeschelR, PanjabiMM. The effect of radiation on the fracture repair process: a biomechanical evaluation of a closed fracture in a rat model. J Orthop Res1989;7:178–183. Google Scholar
21 Smith-Adaline EA , VolkmanSK, IgnelziMA Jr, et al.Mechanical environment alters tissue formation patterns during fracture repair. J Orthop Res2004;22:1079–1085.CrossrefPubMed Google Scholar
22 Willie B , AdkinsK, ZhengX, SimonU, ClaesL. Mechanical characterization of external fixator stiffness for a rat femoral fracture model. J Orthop Res2009;27:687–693.CrossrefPubMed Google Scholar
23 Halıcı M , ÖnerM, GüneyA, et al.Melatonin promotes fracture healing in the rat model. Eklem Hastalik Cerrahisi2010;21:172–177.PubMed Google Scholar
24 Cottrell JA , MeyenhoferM, MedicherlaS, HigginsL, O’ConnorJP. Analgesic effects of p38 kinase inhibitor treatment on bone fracture healing. Pain2009;142:116–126.CrossrefPubMed Google Scholar
25 Ekeland A , EngesaeterLB, LangelandN. Mechanical properties of fractured and intact rat femora evaluated by bending, torsional and tensile tests. Acta Orthop Scand1981;52:605–613.CrossrefPubMed Google Scholar
Funding statement:
This work was supported by the Orthopaedic Scientific Research Foundation (New York, NY, United States) and the Osteosynthesis and Trauma Care Foundation (Bern, Switzerland, Grant number 2011-JDMR).
Author contributions:
J-C. Aurégan: Study conduct, Data collection and interpretation, Manuscript draft, Final approval of manuscript
R. M. Coyle: Study conduct, Data collection, analysis and interpretation, Manuscript draft, Final approval of manuscript
J. R. Danoff: Study design, Study conduct, Data collection and interpretation, Manuscript draft, Final approval of manuscript
R. E. Burky: Study conduct, Manuscript draft, Final approval of manuscript
Y. Akelina: Study conduct, Manuscript draft, Final approval of manuscript
M. P. Rosenwasser: Study design, Manuscript draft, Final approval of manuscript
ICMJE Conflict of Interest:
None declared
©2013 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










