Abstract
Objectives
Osteochondral injuries, if not treated adequately, often lead to severe osteoarthritis. Possible treatment options include refixation of the fragment or replacement therapies such as Pridie drilling, microfracture or osteochondral grafts, all of which have certain disadvantages. Only refixation of the fragment can produce a smooth and resilient joint surface. The aim of this study was the evaluation of an ultrasound-activated bioresorbable pin for the refixation of osteochondral fragments under physiological conditions.
Methods
In 16 Merino sheep, specific osteochondral fragments of the medial femoral condyle were produced and refixed with one of conventional bioresorbable pins, titanium screws or ultrasound-activated pins. Macro- and microscopic scoring was undertaken after three months.
Results
The healing ratio with ultrasound-activated pins was higher than with conventional pins. No negative heat effect on cartilage has been shown.
Conclusion
As the material is bioresorbable, no further surgery is required to remove the implant. MRI imaging is not compromised, as it is with implanted screws. The use of bioresorbable pins using ultrasound is a promising technology for the refixation of osteochondral fractures.
Article focus
Can ultrasound-activated, resorbable pins refixate osteochondral fractures sufficient under physiological conditions?
Is there any effect on the surrounding cartilage by the induced temperature of the SonicPin system (Stryker GmbH)?
Is there an advantage to ultrasound activation compared with conventional pins?
Key messages
The SonicPin system can refixate osteochondral fractures in an ovine in vivo study
The healing ratio is higher than the conventional resorbable pins, but lower than the screw fixation
No cartilage damage could be observed by the application of the SonicPin system
Strengths and limitations
The ovine in vivo study concept can simulate physiological conditions
First examination of ultrasound-activated pins on osteochondral defect repairs
Full weightbearing might have a negative influence on healing ratio (no significant difference concerning resorbable implants)
Introduction
Osteochondral injuries, if not treated adequately, often lead to severe osteoarthritis of the affected joint.1 Osteochondral fractures, sometimes called ‘flakes’, occur as a completely detached or partially attached fragment. Without refixation of the osteochondral fragment, human cartilage can only repair these defects imperfectly. During healing of chondral defects, fibrous tissue is formed, which has inferior characteristics compared with intact cartilage in terms of weightbearing and surface structure.2 Most osteochondral injuries occur in knee lesions, especially if the patella is dislocated.3 Often, a fragment of the lateral condyle or a part of the rear side of the patella is detached.4
There are two methods of treating osteochondral fractures: 1) removal of the fragment followed by therapy to replace the chondral defect; and 2) refixation of the fragment.
Replacement therapy for osteochondral fractures
Pridie drilling or microfracture are common methods of treatment.5,6 However, the quality and quantity of the resultant developing tissue is unpredictable with either option. Normally a layer of fibrous tissue will cover the defect almost completely.7,8 Osteochondral grafts such as osteoarticular transfer system (OATS) and mosaicplasty can also be used,9 although these options risk donor-site morbidity. If the graft is not positioned precisely, chondrocyte death and fibrous tissue coverage of the gaps may occur and lead to pain and decreased range of movement.10,11 The autologous matrix-induced chondrogenesis (AMIC) procedure shows promising results for full thickness defects, but long-term results are still not yet available.12
Refixation
All existing refixation systems for chondral defects have specific disadvantages, and a gold-standard has not be defined.4 Non-resorbable implants include Kirschner (K)-wires, Smillie pins and cortical nails.4,13 The use of these implants can impair the quality of MRI investigations, and also necessitate further surgery in order to remove the implant. Other potential problems include loosening of the implant and the unresolved problem of rising metal ion levels in the peripheral blood.14,15 Metal screws can ensure better and more stable fixation of the fragment, but damage to the joint surface has been observed during removal of the implant.14,16,17
The use of resorbable implants would avoid some of these problems. More than 40 resorbable polymers have had their use described in orthopaedic implants. Polyglycolide (PGA), poly-L-lactide (PLLA), poly-D,L-lactide (PDLLA) and polydioxanone (PDA) are often used. By hydrolytic metabolism, these implants can be broken down into water and CO2 in the citric acid cycle.14,18,19 They do not require further surgery for their removal, including further morbidity or damage that may arise. However, resorbable implants often lack strong primary anchoring and long-term fixation.20 For example, stability against shear forces in PLLA pins has been seen to decrease by 25% to 50% after four weeks in situ.21 The Ethipin (Ethicon, Norderstedt, Germany) has been used as a resorbable implant for refixation of osteochondral fragments. The handling of the Ethipin was easy, the resorption was good, but the stability was only moderate.22-24
The ideal resorbable implant for the refixation of osteochondral fragments should have the mechanical characteristics of metal implants regarding compression force and stability against shear and pull-out forces. Most do not exhibit these characteristics.20 In order to address the problem of reduced axial stability with resorbable pins, implants have been developed that anchor in the bone using ultrasound. The use of a comparable device has shown promising results in craniofacial surgery.25
The aim of this study was to determine whether the SonicPin system (Stryker GmbH, Schönkirchen, Germany) has the potential to reliably refix osteochondral fragments. In order to evaluate this, we created osteochondral fragments in the knee joint of Merino sheep. Special attention was paid to the histological quality of the cartilage next to the implant, and to the heat caused by the application of ultrasound. In order to compare the SonicPin system with conventional methods, we also used the resorbable Ethipin system (Ethicon) and cannulated Asnis screws (Stryker GmbH). This breed of sheep have been used successfully in several investigations into cartilage.26-28 The anatomy of the sheep knee and the low flexion position of the knees are similar to those of the human knee, as are the biomechanical forces encountered in vivo.29
Materials and Methods
Implants
The SonicPin system (Stryker GmbH) comprises a polymer of poly(L-lactide-co-D,L-lactide) at a ratio of 70:30. It has a diameter of 2.2 mm and a length of either 22 mm or 26 mm. In preparation for use, the pin is connected to an ultrasound applicator. Then, an ultrasonic signal of high frequency and low amplitude is applied leading to a local welding of the pin tip, where the implant is in shear contact with bony structures. The SonicPin fills out the lacunes of the cancellous bone. Immediately after ceasing the ultrasound, the material solidifies and hardens, providing three-dimensional anchoring into the surrounding bone (Fig. 1). The ultrasound applicator can then be easily removed from the thread of the implanted pin.
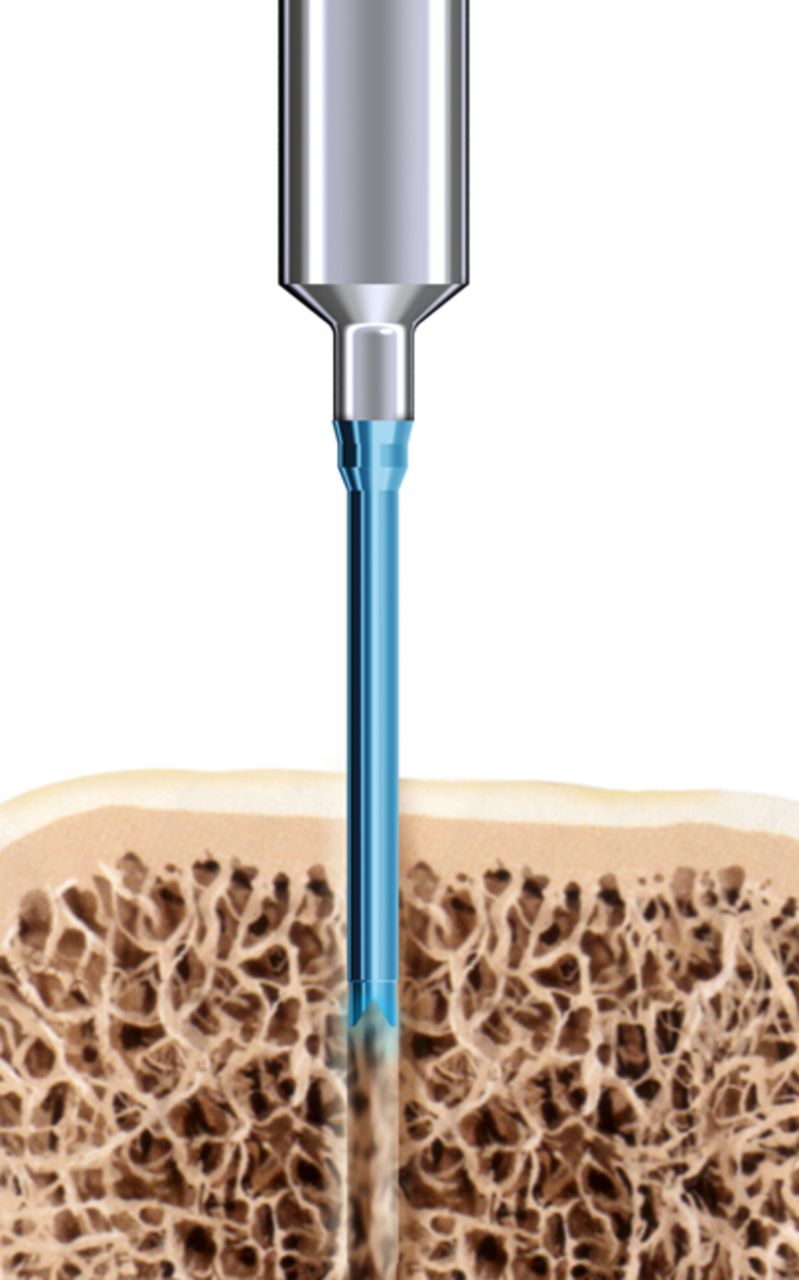
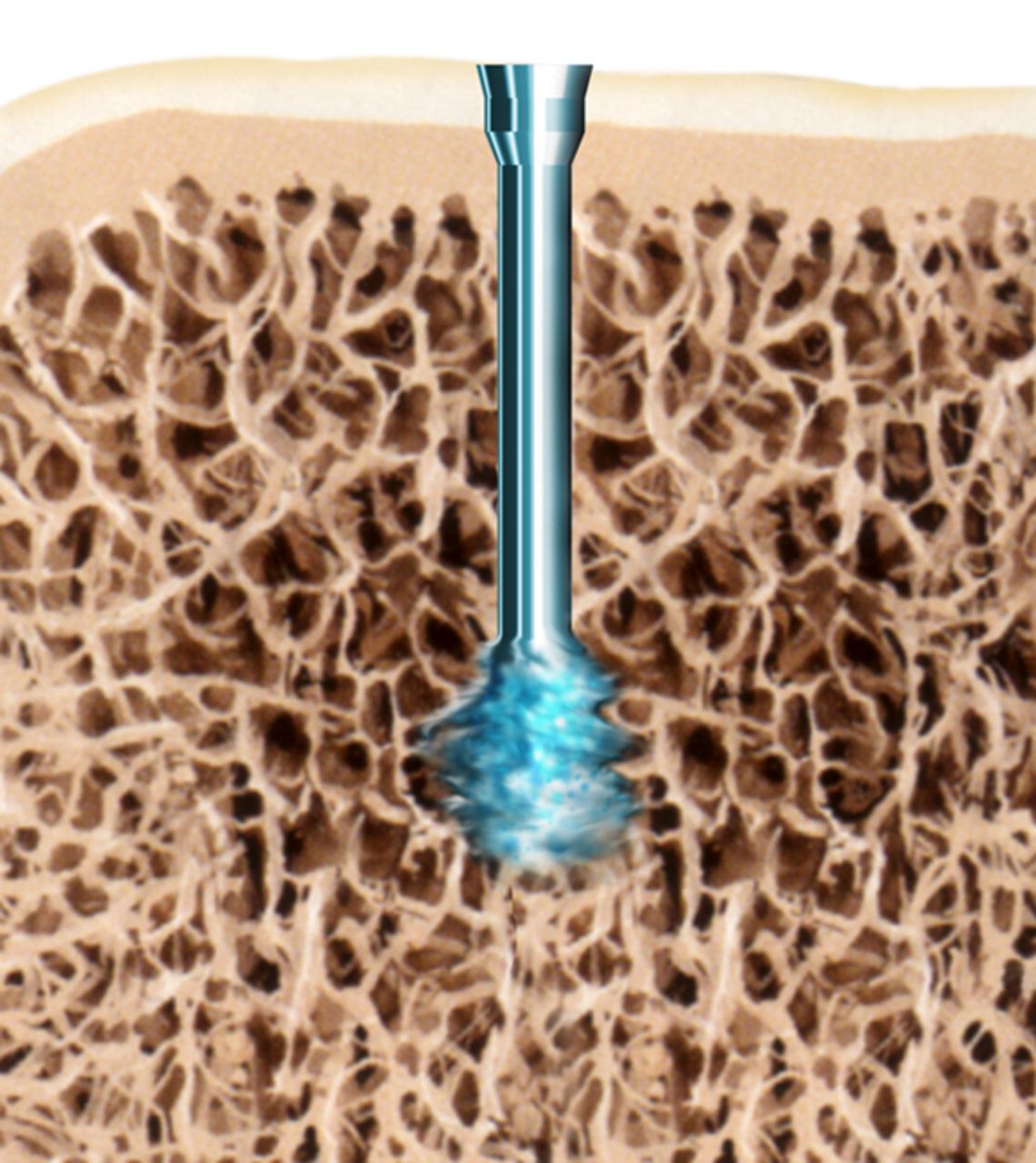

Figs. 1a - 1c
Diagrams showing the use of the SonicPin system (Stryker GmbH), a) at implantation, mounted on the ultrasound applicator, b) after ultrasound and removal of the applicator, and c) showing the ‘melting’ of the pin into the cancellous bone.
The Ethipin (Ethicon) consists of polydioxanone and has a length of 40 mm and a diameter of 1.3 mm. It is cylindrical with no change of diameter. For use, the osteochondral fragments are temporarily fixated using a K-wire and the Ethipin subsequently inserted using the application shell. If the Ethipin protrudes the surface of the joint cartilage, it can be cut away using a surgical knife at cartilage level.
The Asnis cannulated screws (Stryker) used had a length of 16 mm and a diameter of 2.0 mm. This screw has a self-cutting thread and is made of titanium. Like the Ethipin, the screw is inserted with the use of a previously positioned K-wire. Insertion of the entire screw below the level of the cartilage prevents damage to the corresponding joint surface.
Operation procedure
A total of 16 Merino sheep were used in this study. They had an approximate age of 1.5 years, at which age epiphyseal gaps have closed,30 and a mean weight of 55.4 kg (sd 4.7). Each hind leg of the sheep was used; therefore a total of 32 joints were operated upon. Prior biomechanical laboratory testing and a power analysis led to a distribution of 14 SonicPin applications and seven each of Ethipin and cannulated screws.
The operation was performed under combined anaesthesia with Rompun (Bayer Healthcare, Leverkusen, Germany) by intramuscular injection and spinal anaesthesia with Carbostesin (AstraZeneca, Wedel, Germany). Using medial arthrotomy, the medial condyle was exposed and a specific osteochondral fragment (1 cm diameter and 4 mm thickness) was created with a dedicated saw guide to simulate a typical osteochondral defect (International Cartilage Repair Society (ICRS) Grade 4).31
The fragment was then refixed using the previously randomised system of fixation. Two implants were used for each osteochondral fragment. The randomisation was done just before the refixation was performed in the operating theatre by opening opaque envelopes. Each hind joint of each animal was randomised, so different implants in the two hind knees of one animal could occur. The drilling process and the melting of the SonicPin were monitored by a FLIR ThermaCam E320 heat camera (FLIR Systems Inc., Wilsonville, Oregon). All implants used were implanted with a drill guide to ensure that the two implants were at an angle of 15° to each other. A soft cast and a sterile bandage were applied after the arthrotomy was completed. The soft cast was removed after five days. Full weightbearing was intended for the entire duration of the study for worst-case scenario testing.
At three months post-operatively the sheep were killed and the knees were removed. After opening the knees, the ratio of well-healed fragments were observed in addition to macroscopic scoring of the fragments and the corresponding cartilage surface by Outerbridge score (Table I).32,33 We defined a refixed fragment as ‘healed’ if it was in position and at least > 50% present.
Histological analysis
For histological assessment, the femoral probes only were fixated in 4% paraformaldehyde and decalcified by ethylenediaminetetraacetic acid (EDTA) over about eight weeks, because especially the interaction of the cartilage with the implant should be examined. For microscopic examination, haematoxylin & eosin and Giemsa staining was performed. The thickness of the cartilage, the clusters of chondrocytes found, the amount of fibrous tissue scars and the number of chondrocytes in each chondron were observed microscopically. In order to do this, a grid was projected behind the microscopic sample and the ten areas next to the implant and ten areas in the clinically unaffected cartilage area were counted and compared with each other. The amount of fibrous tissue and clustering of chondrons were also observed in this way. The orientation of the chondrons and the amount of fibrous tissue were both rated by two authors (HN and BK) on a scale of 1 to 5 points, with 5 points denoting no fibrous tissue and vertical orientation of the chondrones, and 1 point denoting only fibrous tissue, no cartilage left and circular clustering of chondrons (Table II). The thickness of the cartilage was measured by the same authors (HN and BK) in millimetres from the implant (macroscopically unaffected surface) and next to the implant by measuring the height from the surface to the subchondral plate.
Table II
Description of the scales used to assess orientation of chondrons and quantity of fibrous tissue
| Description of score | |||
|---|---|---|---|
| Orientation of chondrons | Fibrous tissue quantity | ||
| 1 | Only circular clustering of chondrons | Only fibrous tissue, no cartilage remaining | |
| 2 | 1/4 of the chondrons vertical orientated | 3/4 of the original cartilage height replaced by fibrous tissue | |
| 3 | 1/2 of the chondrons vertical orientated | 1/2 of the original cartilage height replaced by fibrous tissue | |
| 4 | 3/4 of the chondrons vertical orientated | 1/4 of the original cartilage height replaced by fibrous tissue | |
| 5 | All chondrons completely vertical orientated | No fibrous tissue | |
Statistical analysis
The statistical analyses were performed using the t-test and the Levene test. SPSS software v20 (IBM Germany, Ehningen, Germany) was used, and the level of significance was assumed to be p < 0.05.
Results
Two sheep died of pneumonia before the minimum survival time of three months. Therefore at three months a total of 14 knees with the SonicPin system, eight with the Asnis screw system and six knees with the Ethipin system were available to be assessed.
Temperature
The temperature data were available for all implants, including those in animals who died prematurely. The mean temperature observed during the drilling of the Ethipin system was 46.83°C (sd 1.47), significantly lower than that reached using during drilling of either the SonicPin (54.14°C (sd 2.41); p < 0.001) or the Asnis screw (54.88° (sd 2.94); p < 0.001). There was no statistically significant differences between the temperatures reached during drilling of the SonicPin and Asnis screw systems (p = 0.12). The mean temperature reached by the melting of the SonicPin implants was 50.57°C (sd 2.0), which was significantly lower compared with the drilling temperatures of the Asnis screw and SonicPin (p = 0.0002 and 0.0006, respectively), and significantly higher compared with the Ethipin drilling temperature (p = 0.0007).
Healing ratio
We defined a refixed fragment as properly healed if it was in position with > 50% of the original surface present. By these criteria, healing was seen to occur in nine of the 14 fractures treated using the SonicPin system, compared with three of six treated using the Ethipin and seven of eight treated with the Asnis cannulated screws, giving healing ratios of 64%, 50% and 87.5% for the SonicPin, Ethipin and Asnis screws, respectively. The Asnis screws performed significantly better than the SonicPin (p = 0.045) and the Ethipin (p = 0.23), although there was no significant difference between the healing ratios of the two resorbable implants (p = 0.18).
We observed that the failures in the two resorbable implant groups often exhibited a shearing mechanism in the original osteotomy gap with broken pins. Conversely, the single failure in the screw group showed the implants in their original position, while the fragment was lytic around the osteosynthesis.
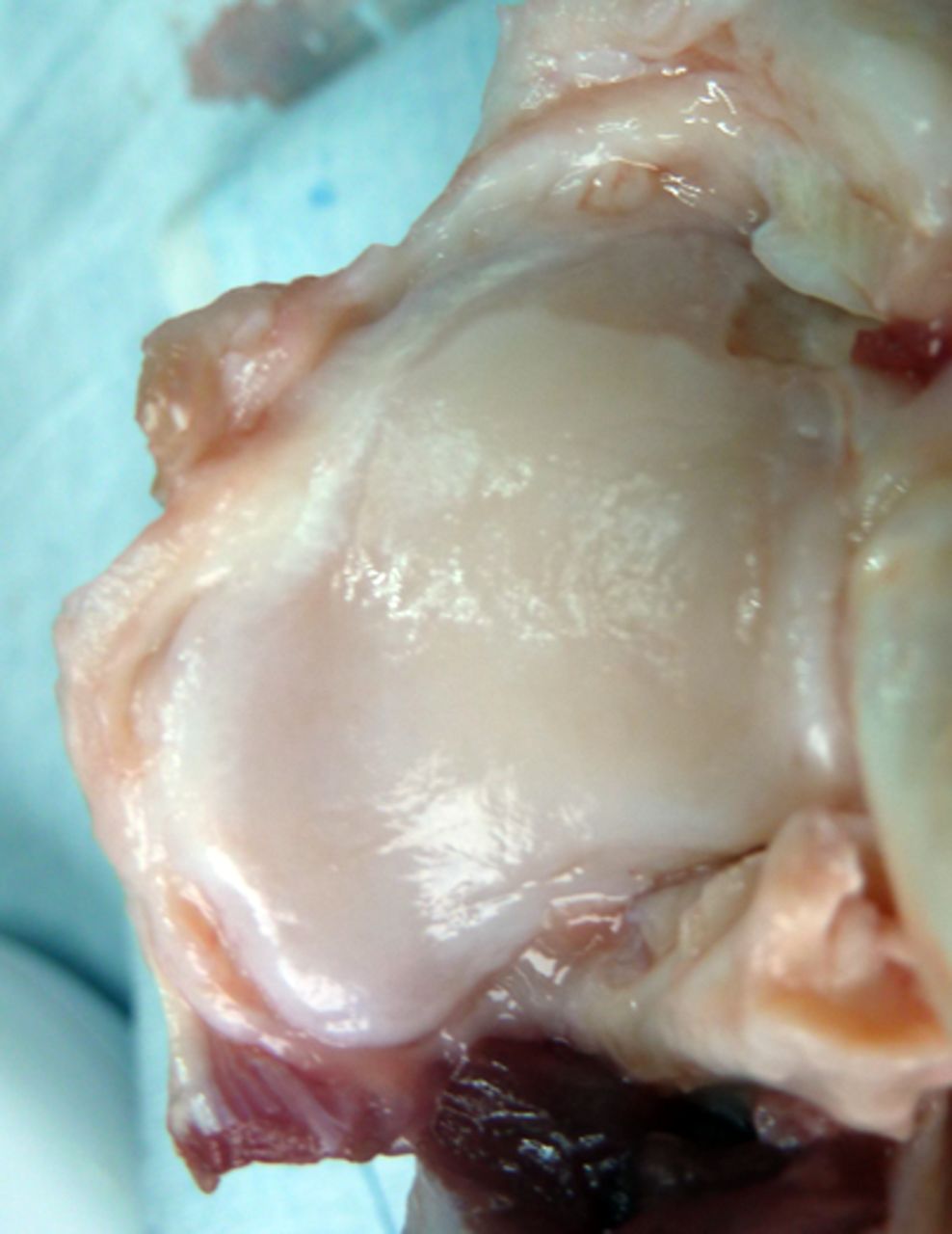
Macroscopic scoring
Macroscopic scoring of the chondral surfaces of the refixated fragment and the corresponding tibial plateau was performed according to the Outerbridge classification (Table I, Figs 2 and 3).32,33 Cases in which the fragment sheared away into the former osteotomy gap were rated as grade 4.
Table I
| Outerbridge grade | Description |
|---|---|
| 0 | Normal cartilage |
| 1 | Cartilage with softening and swelling |
| 2 | Partial-thickness defect with fissures on the surface that do not reach subchondral bone or exceed 1.5 cm in diameter |
| 3 | Fissuring to the level of subchondral bone in an area with diameter > 1.5 cm |
| 4 | Exposed subchondral bone |
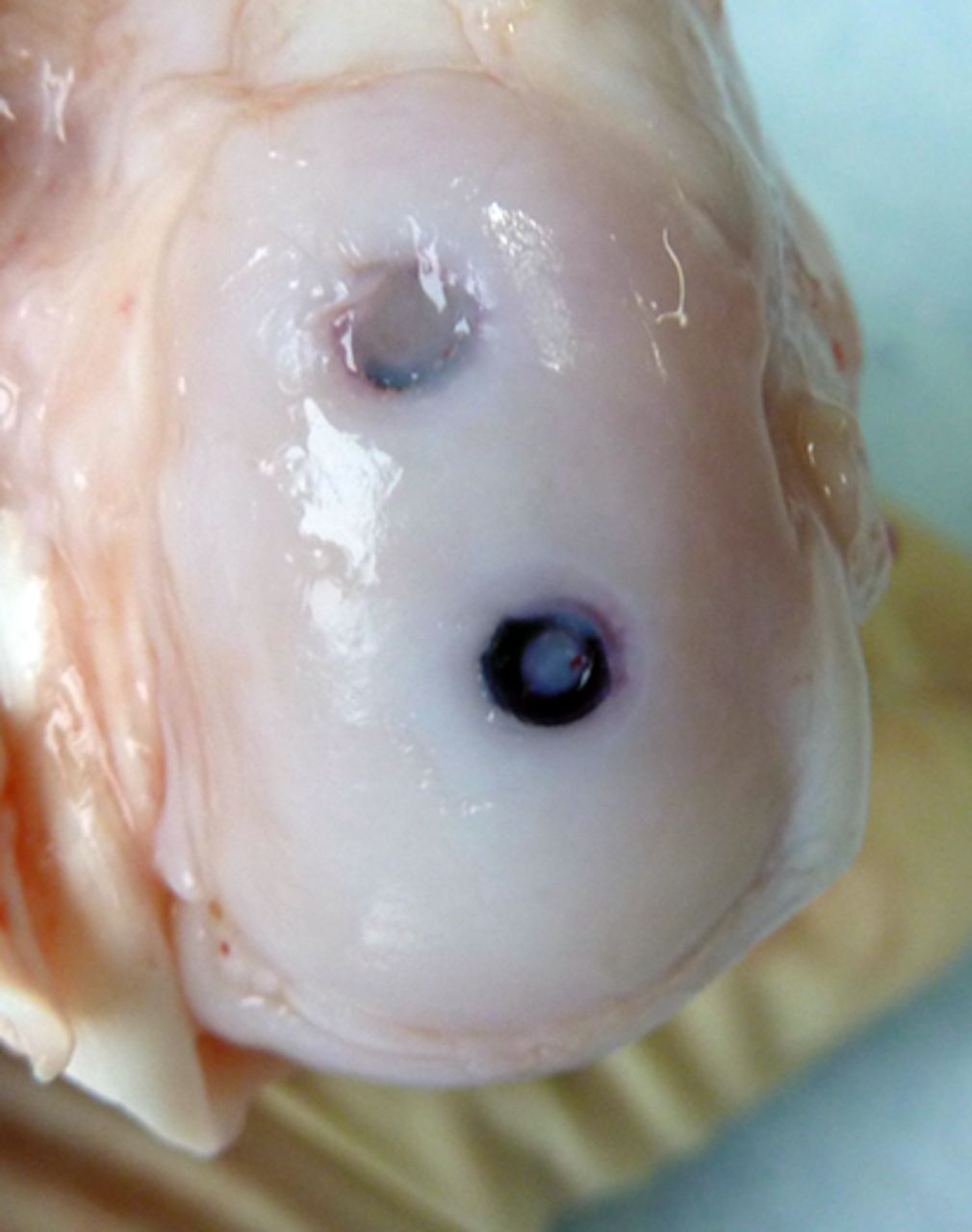
Figs. 2a - 2b
Photograph at macroscopic assessment showing the SonicPin (Stryker GmbH) fixation a) in the femoral surface and b) the corresponding tibial surface, showing minimal damage.
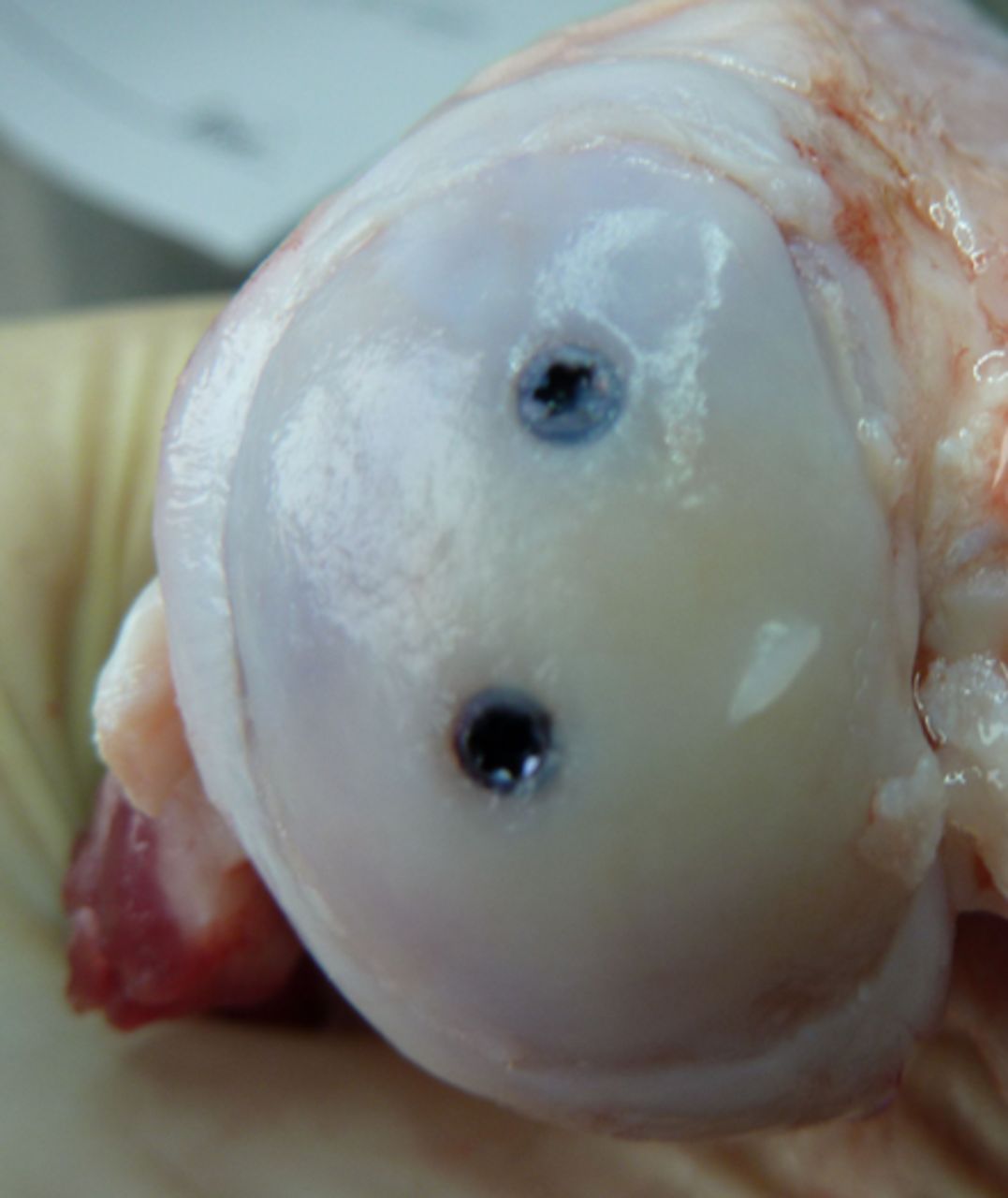
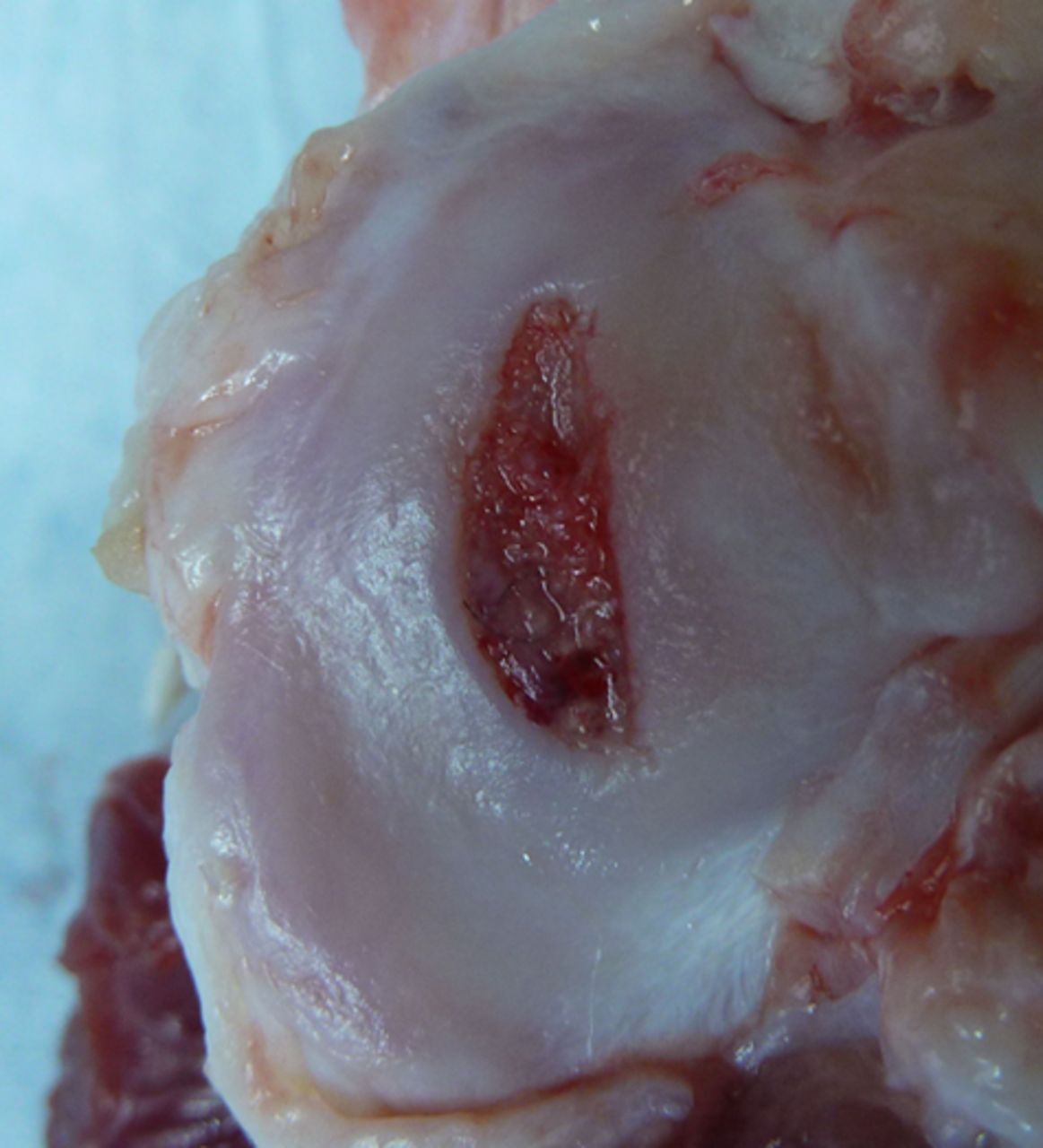
Figs. 3a - 3b
Photograph at macroscopic assessment showing the Asnis screw (Stryker GmbH) fixation a) in the femoral surface and b) the corresponding tibial surface, showing marked and deep chondral damage.
Table III details the mean Outerbridge scores for the femora and tibiae for each group. Although there were no significant differences between the implants on either the femoral or tibial side, the majority of the documented tibial defects were markedly deep.
Table III
Outerbridge scoring for the femora and tibiae
| p-values | |||||||
|---|---|---|---|---|---|---|---|
| Mean Outerbridge score | SonicPin | Ethipin | Asnis | SonicPin vs Ethipin | SonicPin vs Asnis | Ethipin vs Asnis | |
| Femur | 1.7 | 2.0 | 0.9 | 0.191 | 0.119 | 0.123 | |
| Tibia | 2.1 | 1.3 | 2.9 | 0.130 | 0.609 | 0.203 | |
Histological scoring
The mean measured thickness of the femoral cartilage next to the implant in the SonicPin group was 88% (sd 19) of the original height of the cartilage. The mean thickness was also 88% (sd 25) in the Asnis group, and 74% (sd 32) in the Ethipin group. These results did not differ significantly (SonicPin vs Ethipin, p = 0.205; SonicPin vs Asnis, p = 0.314; Asnis vs Ethipin, p = 0.115).
The cell count in each chondron near and distant from the implant showed a higher number of cells nearer the implant in all cases (Table IV). This difference was statistically significant in the SonicPin group (p = 0.006), but not in either the Ethipin or Asnis groups (p = 0.184 and p = 0.179, respectively).
Table IV
Histological cell counts
| SonicPin | Ethipin | Asnis | |
|---|---|---|---|
| Number of distant cells/chondrons | 1.74 | 1.85 | 1.99 |
| Number of cells/chondrons near implant | 2.63 | 2.21 | 2.04 |
| Difference | +33% | +17% | +3% |
| p-value | 0.006 | 0.184 | 0.179 |
The best vertical orientation of all implants was observed in the screw group, compared with both both resorbable implants (p = 0.143 and p = 0.253 for SonicPin and Ethipin, respectively) (Fig. 4, Table V). Similarly, the resorbable implants exhibited a little more fibrous tissue reaction in comparison with the screw fixation, but these did not reach statistical significance.
Table V
Mean histological scores
| p-values | ||||||
|---|---|---|---|---|---|---|
| Mean histological score* | SonicPin | Ethipin | Asnis | SonicPin vs Ethipin | SonicPin vs Asnis | Ethipin vs Asnis |
| Chondron orientation | 3.4 | 3.5 | 4.1 | 0.314 | 0.143 | 0.253 |
| Fibrous tissue | 3.9 | 3.5 | 4.4 | 0.298 | 0.324 | 0.192 |
-
* scored from 1 (circular clustering of chondrons/only fibrous tissue) to 5 (wholly vertical orientation/no fibrous tissue); see Table II
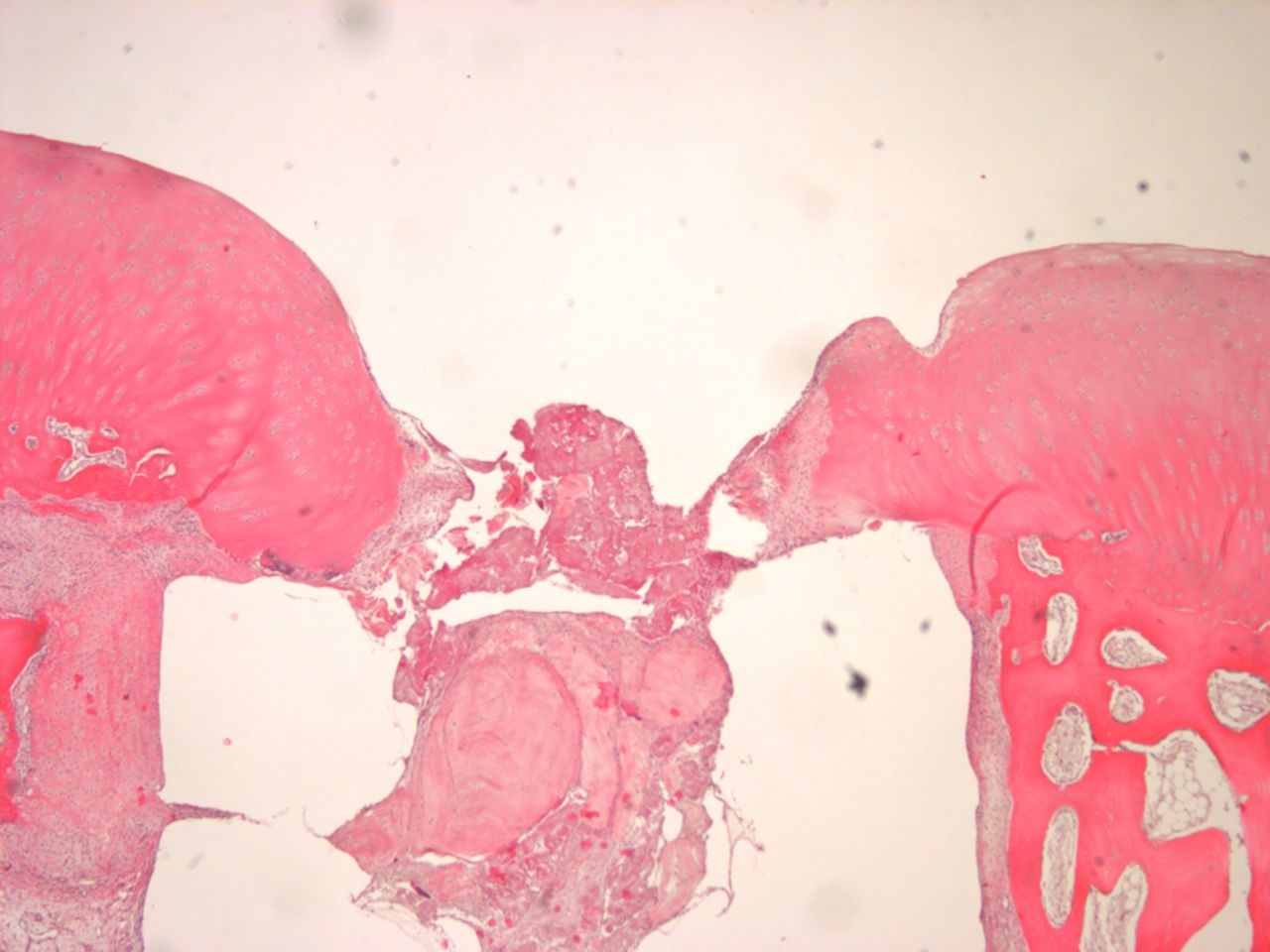
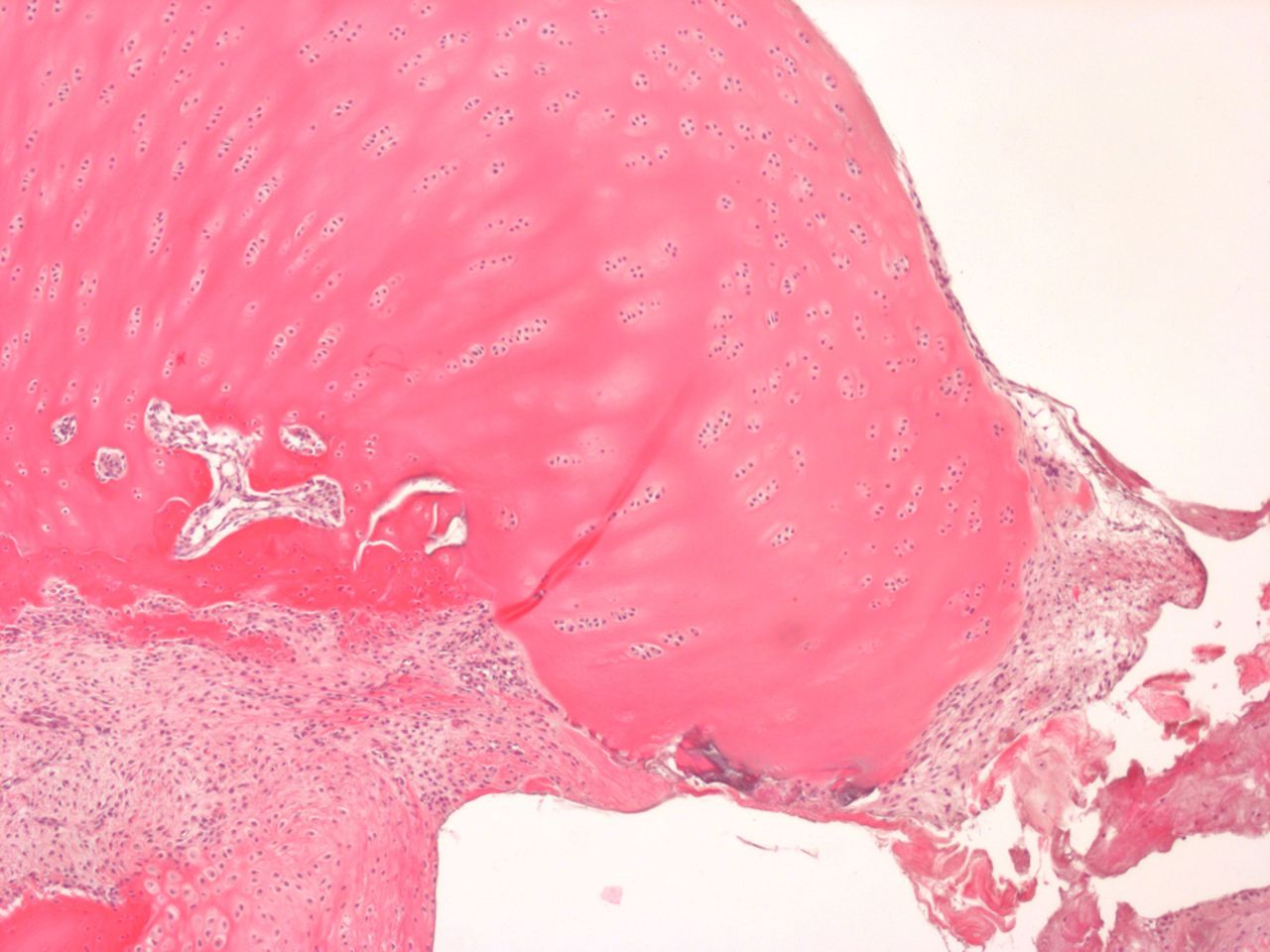
Figs. 4a - 4b
Histological images of cartilage after removal of the SonicPin system (Stryker GmbH), a) at × 25 magnification, showing the cartilage surface without major damage after removing the SonicPin, and b) at × 40 magnification, showing the cartilage next to the removed SonicPin.
Discussion
The SonicPin was successfully implanted in all procedures. The handling of the system appears to be reliable and intuitive. The study was sufficiently statistically powered, and the two animals that were excluded did not limit this power. Clinically, a limitation of this study was the full weightbearing of the animals, which would not be allowed in patients with similar injuries that are treated surgically. However, our results could be seen to demonstrate the results of a worst case scenario of full weightbearing.
Temperature measurement showed significant differences, but the absolute numbers differed across a range of only 8°C. Lower temperatures occurred with the Ethipin, as drilling was performed exclusively by K-wire and not by a larger drill. Superficial temperatures using the SonicPin system were lower than the temperature generated by the drilling itself. Therefore, a higher risk for cartilage damage compared with conventional drilling is not expected from the ultrasound-activated implant. Furthermore, Arnoldi et al34 reported that no significant tissue damage was caused by the heat generated at the welded region at the tip of the pin, even when the ultrasound energy level was increased by 30%.
In terms of the healing ratio, the Asnis cannulated screws showed the best results, followed by the SonicPin and then the Ethipin systems. The healing ratios of the SonicPin and Ethipin groups did not differ significantly, but the screws were significantly better than both resorbable implants. The mechanism of failure of the implants differed between the bioresorbable systems and the cannulated screws. Due to the greater compression caused by the screws, synovial fluid cannot flow into the osteotomy gap.35 This might be the reason for the most common type of failure exhibited in the resorbable implant group. In these cases the complete fragment did not heal and the osteotomy gap was still visible. Ferguson et al36 demonstrated greater anchoring of ultrasound-activated pins, compared with non-ultrasound-activated, resorbable implants. But their biomechanical tests were performed without the effect of synovial fluid and direct shear forces. Although the screws in our study showed the best rate of healing, these implants have to be removed in a second operation, potentially incurring significant cartilage damage during the procedure.37
Macroscopic scoring showed no significant difference or advantage of any particular systems. Although the statistical differences were not significant, the clinical impression of tibial cartilage damage in the screw group should at least cast doubt on this ovine testing scenario. The clinical impression of the depth of the tibial cartilage damage in the screw group should lead to critical challenging of the treatment of osteochondral fractures with conventional screws in patients.
Histological scoring of the femur showed that the height of the cartilage in the Ethipin groups is about 74% of the original height of the cartilage. Cartilage height in the screw and SonicPin group was slightly better, at about 88% of the original height. Missing cartilage or bare subchondral membrane was not observed in any of the groups. The difference in cartilage thickness does not indicate the possibility of heat damage, as the Ethipin is implanted without generating any heat, and yet has the same cartilage thickness as the SonicPin.
With regard to the number of cells per chondron and the orientation of the chondrons, both resorbable implants cause a rise in the number of cells and cluster development, which is assumed to be a sign of inferior cartilage quality.38 In this comparison the SonicPin produces the most cluster development of all the implants. If a press-fit chondral autograft is not properly positioned and a gap results between the graft and the original cartilage, cluster formation and chondrocyte death are observed, as has been shown by Kock at al.39 Cluster development cannot be easily associated with the heat generated by the SonicPin system, particularly as we did not observe more fibrous tissue scars from heat damage. However, the reaction of chondral tissue to physical or chemical damage would result in replacement fibrous tissue.40 The clustering of chondrocytes in the resorbable implants groups in this study results from degradation of the implant itself. After three months, the resorbable implants have absorbed fluid and are at least partially resorbed, causing a gap or hole to occur in the cartilage. This may explain the cluster development. The screws do not present this problem, as they are not hydrophilic and do not change their form during the implantation period. The clustering in the SonicPin group compared with the Ethipin group can be explained by the different sizes of the drilled holes.
Conclusions
In a sheep study, the SonicPin system has been successfully used to for the refixation of osteochondral fragments. The healing ratio is higher than with the Ethipin system. A negative effect on the cartilage by the heat generated by the SonicPin system cannot be proven. No further surgery is necessary for removal of the implant, and MRI imaging is not compromised.
1 Eckstein F , MilzS, AnetzbergerH, PutzR. Thickness of the subchondral mineralised tissue zone (SMZ) in normal male and female and pathological human patellae. J Anat1998;192:81–90.CrossrefPubMed Google Scholar
2 Otte P. Biology of articular cartilage with special reference to transplantation. Z Orthop Ihre Grenzgeb 1972;110:677–685 (in German). Google Scholar
3 Greve H, Holste J. Refixation of osteochondral fragments using absorbable plastic pins. Aktuelle Traumatol 1985;15:145–149 (in German). Google Scholar
4 Hammerle CP , JacobRP. Chondral and osteochondral fractures after luxation of the patella and their treatment. Arch Orthop Trauma Surg1980;97:207–211.CrossrefPubMed Google Scholar
5 Beiser IH , KanatIO. Subchondral bone drilling: a treatment for cartilage defects. J Foot Surg1990;29:595–601.PubMed Google Scholar
6 Sledge SL . Microfracture techniques in the treatment of osteochondral injuries. Clin Sports Med2001;20:365–377.CrossrefPubMed Google Scholar
7 Hunt SA , JazrawiLM, ShermanOH. Arthroscopic management of osteoarthritis of the knee. J Am Acad Orthop Surg2002;10:356–363.PubMed Google Scholar
8 Steadman JR , BriggsKK, RodrigoJJ, et al.Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy2003;19:477–484.CrossrefPubMed Google Scholar
9 Duchow J , HessT, KohnD. Primary stability of press-fit-implanted osteochondral grafts. Influence of graft size, repeated insertion, and harvesting technique. Am J Sports Med2000;28:24–27.CrossrefPubMed Google Scholar
10 Redman SN , OldfieldSF, ArcherCW. Current strategies for articular cartilage repair. Eur Cell Mater2005;9:23–32.CrossrefPubMed Google Scholar
11 Ahmad CS , GuineyWB, DrinkwaterCJ. Evaluation of donor site intrinsic healing response in autologous osteochondral grafting of the knee. Arthroscopy2002;18:95–98.CrossrefPubMed Google Scholar
12 Gille J , SchuseilE, WimmerJ, et al.Mid-term results of Autologous Matrix-Induced Chondrogenesis for treatment of focal cartilage defects in the knee. Knee Surg Sports Traumatol Arthrosc2010;18:1456–1464.CrossrefPubMed Google Scholar
13 Gäde EA. About early operative treatment of osteochondrosis dissecans of the knee (author's transl). Z Orthop Ihre Grenzgeb 1976;114:233–240 (in German). Google Scholar
14 Weiler A , HoffmannRF, StähelinAC, HellingHJ, SüdkampNP. Biodegradable implants in sports medicine: the biological base. Arthroscopy2000;16:305–321.CrossrefPubMed Google Scholar
15 Sirlin CB , BoutinRD, BrossmannJ, et al.Polydioxanone biodegradable pins in the knee: MR imaging. AJR Am J Roentgenol2001;176:83–90.CrossrefPubMed Google Scholar
16 Wouters DB , BosRR, van HornJR, van LuynMJ. Should in the treatment of osteochondritis dissecans biodegradable or metallic fixation devices be used?: a comparative study in goat knees. J Biomed Mater Res B Appl Biomater2008;84:154–164. Google Scholar
17 Lewis PL , FosterBK. Herbert screw fixation of osteochondral fractures about the knee. Aust N Z J Surg1990;60:511–513.CrossrefPubMed Google Scholar
18 Hollinger JO , BattistoneGC. Biodegradable bone repair materials: synthetic polymers and ceramics. Clin Orthop Relat Res1986;207:290–305. Google Scholar
19 Lam KH , SchakenraadJM, EsselbruggeH, FeijenJ, NieuwenhuisP. The effect of phagocytosis of poly(L-lactic acid) fragments on cellular morphology and viability. J Biomed Mater Res1993;27:1569–1577.CrossrefPubMed Google Scholar
20 Daniels AU , ChangMK, AndrianoKP. Mechanical properties of biodegradable polymers and composites proposed for internal fixation of bone. J Appl Biomater1990;1:57–78.CrossrefPubMed Google Scholar
21 Gerlach KL, Eitenmüller J, Schmitz H. In vivo study of the strength properties of biodegradable polymers for application as osteosynthesis materials. Dtsch Z Mund Kiefer Gesichtschir 1987;11:211–216 (in German). Google Scholar
22 Jerosch J , HoffstetterI, ReerR. Current treatment modalities of osteochondritis dissecans of the knee joint: results of a nation-wide German survey. Acta Orthop Belg1996;62:83–89.PubMed Google Scholar
23 Beck E. Fractures of the talus. Orthopade 1991;20:33–42 (in German). Google Scholar
24 Hoffmann R, Krettek C, Haas N, Tscherne H. Distal radius fracture: fracture stabilization with biodegradable osteosynthesis pins (Biofix): experimental studies and initial clinical experiences. Unfallchirurg 1989;92:430–434 (in German). Google Scholar
25 Pilling E , MaiR, TheissigF, et al.An experimental in vivo analysis of the resorption to ultrasound activated pins (Sonic weld) and standard biodegradable screws (ResorbX) in sheep. Br J Oral Maxillofac Surg2007;45:447–450.CrossrefPubMed Google Scholar
26 Rudert M . Histological evaluation of osteochondral defects: consideration of animal models with emphasis on the rabbit, experimental setup, follow-up and applied methods. Cells Tissues Organs2002;171:229–240.CrossrefPubMed Google Scholar
27 Bruns J , Meyer-PannwittU, SilbermannM. The rib perichondrium: an anatomical study in sheep of a tissue used as transplant in the treatment of hyaline-cartilage defects. Acta Anat (Basel)1992;144:258–266. Google Scholar
28 Willie BM , BloebaumRD, BireleyWR, BachusKN, HofmannAA. Determining relevance of a weight-bearing ovine model for bone ingrowth assessment. J Biomed Mater Res A2004;69:567–576.CrossrefPubMed Google Scholar
29 Allen MJ , HoultonJE, AdamsSB, RushtonN. The surgical anatomy of the stifle joint in sheep. Vet Surg1998;27:596–605.CrossrefPubMed Google Scholar
30 Hollinger JO . Preliminary report on the osteogenic potential of a biodegradable copolymer of polyactide (PLA) and polyglycolide (PGA). J Biomed Mater Res1983;17:71–82.CrossrefPubMed Google Scholar
31 van den Borne MP , RaijmakersNJ, VanlauweJ, et al.International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthritis Cartilage2007;15:1397–1402.CrossrefPubMed Google Scholar
32 Outerbridge RE . The etiology of chondromalacia patellae. J Bone Joint Surg [Br]1961;43-B:752–757.CrossrefPubMed Google Scholar
33 Rodríguez-Merchán EC , Gómez-CarderoP. The Outerbridge classification predicts the need for patellar resurfacing in TKA. Clin Orthop Relat Res2010;468:1254–1257.CrossrefPubMed Google Scholar
34 Arnoldi J , HenryP, ProcterP, RobioneckB, JönssonA. In vivo tissue response to ultrasound assisted application of biodegradable pins into cortical and cancellous bone structures: a histological and densitometric analysis in rabbits. J Biomater Sci Polym Ed2012;23:663–676.CrossrefPubMed Google Scholar
35 Schmalzried TP , AkizukiKH, FedenkoAN, MirraJ. The role of access of joint fluid to bone in periarticular osteolysis: a report of four cases. J Bone Joint Surg [Am]1997;79-A:447–452. Google Scholar
36 Ferguson SJ , WeberU, von RechenbergB, MayerJ. Enhancing the mechanical integrity of the implant-bone interface with BoneWelding technology: determination of quasi-static interfacial strength and fatigue resistance. J Biomed Mater Res B Appl Biomater2006;77:13–20.CrossrefPubMed Google Scholar
37 Nuzzo MS , PosnerM, WarmeWJ, et al.Compression force and pullout strength comparison of bioabsorbable implants for osteochondral lesion fixation. Am J Orthop (Belle Mead NJ)2011;40:E61–E63.PubMed Google Scholar
38 Meinhardt M , LückS, MartinP, et al.Modeling chondrocyte patterns by elliptical cluster processes. J Struct Biol2012;177:447–458.CrossrefPubMed Google Scholar
39 Kock NB , HanninkG, van KampenA, et al.Evaluation of subsidence, chondrocyte survival and graft incorporation following autologous osteochondral transplantation. Knee Surg Sports Traumatol Arthrosc2011;19:1962–1970.CrossrefPubMed Google Scholar
40 Ochs BG , Müller-HorvatC, AlbrechtD, et al.Remodeling of articular cartilage and subchondral bone after bone grafting and matrix-associated autologous chondrocyte implantation for osteochondritis dissecans of the knee. Am J Sports Med2011;39:764–773.CrossrefPubMed Google Scholar
Funding statement:
This study undertaken by the University of Lübeck, Lübeck, Germany, was partially funded by Stryker Trauma GmbH, Schönkirchen, Germany.
Author contributions:
H. Neumann: Conception, execution and design of the study
C. Jürgens: Data interpretation, Revision of manuscript
B. Kienast: Conception, execution and design of the study
A. P. Schulz: Manuscript revision, Data analysis
J. Gille: Assistance in execution of the animal trial
M. Klinger: Preparation and interpretation of histological samples
N. Reimers: Technical support
ICMJE Conflict of Interest:
None declared
©2013 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










