Abstract
Objectives
Recent studies have shown that modulating inflammation-related lipid signalling after a bone fracture can accelerate healing in animal models. Specifically, decreasing 5-lipoxygenase (5-LO) activity during fracture healing increases cyclooxygenase-2 (COX-2) expression in the fracture callus, accelerates chondrogenesis and decreases healing time. In this study, we test the hypothesis that 5-LO inhibition will increase direct osteogenesis.
Methods
Bilateral, unicortical femoral defects were used in rats to measure the effects of local 5-LO inhibition on direct osteogenesis. The defect sites were filled with a polycaprolactone (PCL) scaffold containing 5-LO inhibitor (A-79175) at three dose levels, scaffold with drug carrier, or scaffold only. Drug release was assessed in vitro. Osteogenesis was assessed by micro-CT and histology at two endpoints of ten and 30 days.
Results
Using micro-CT, we found that A-79175, a 5-LO inhibitor, increased bone formation in an apparent dose-related manner.
Conclusions
These results indicate that 5-LO inhibition could be used therapeutically to enhance treatments that require the direct formation of bone.
Article focus
Investigation into whether pharmacological inhibition of the 5-lipoxygenase (5-LO) pathway can enhance bone regeneration
Key messages
Inhibition of 5-LO using A-79175 increased bone formation in a dose-related manner
Strengths and limitations
The effects of A-79175 on osteogenesis were measured at multiple doses
The model of cortical defects in rat femora provides for proof of concept but lacks direct clinical applicability
The mechanism by which 5-LO inhibition increased osteogenesis was not analysed in these experiments
Introduction
Recent studies have shown that reducing 5-lipoxygenase (5-LO) activity accelerates fracture healing.1,2 In rats treated with oral doses of AA-861, a 5-LO inhibitor, femur fractures healed faster and had greater mechanical properties than placebo controls.1 Similarly, mice homozygous for a targeted mutation in Alox5, the 5-LO gene, were found to heal fractures of the femur faster and with greater mechanical integrity than controls matched by age and background genotype.2 Fracture healing was characterised by accelerated bone formation within the fracture callus, but it occurred concomitant with accelerated chondrogenesis in the 5-LO deficient animals. Thus, it is unclear whether loss or inhibition of 5-LO can enhance bone formation in the absence of endochondral ossification.
The principal function of 5-LO is to convert arachidonic acid into leukotriene A4, which is used to synthesise leukotriene B4 (LTB4) and the cysteinyl leukotrienes.3,4 LTB4 and the cysteinyl leukotrienes are lipid signalling molecules that affect many physiological processes, including inflammation, which is an early physiological response to fracture. Arachidonic acid is also the substrate for cyclooxygenase-1 and cyclooxygenase-2 (COX-2), which convert arachidonic acid into prostaglandin (PG)-H2. PGH2 is used to synthesise thromboxane A2, PGD2, PGE2, PGF2α, and PGI2. These prostaglandins also are lipid signalling molecules that affect many physiological processes including inflammation.5
Several studies have demonstrated that non-steroidal anti-inflammatory drugs can impair fracture healing and that this impairment is due to inhibition of COX-2.1,6-9 In other studies, reduced 5-LO activity was associated with enhanced fracture healing, including elevated callus chondrogenesis, increased bone formation and elevated COX-2 expression.1,2 These data were interpreted to be an acceleration of endochondral ossification that ultimately led to accelerated healing. Thus, 5-LO acts as a negative regulator of fracture healing while COX-2 is a positive regulator of fracture healing. However, many orthopaedic procedures rely upon direct bone formation, and not endochondral ossification, for healing. For example, stems used in arthroplasty often require the formation of bone in order to secure the implant in place.10-12 Also, fractures treated by rigid surgical fixation have reduced capacity for endochondral ossification, and rely more upon direct bone formation and remodelling to heal.13,14
The purpose of this study was to determine if a locally applied 5-LO inhibitor, A-79175, could enhance bone regeneration in an animal model of direct bone formation.15,16 For this study, a unicortical bone defect model was used in rat femora, in which the defect site was filled with a polycaprolactone (PCL) scaffold containing 5-LO inhibitor or carrier.17,18
Materials and Methods
Scaffold manufacture and sterilisation
The 5-LO inhibitor used was A-79175 (Accelalox Inc., Menlo Park, California).15,16 A custom-made sheet of Osteomesh (Osteopore International, Queenstown, Singapore) was used to make the PCL scaffolds. Analytical-grade calcium sulphate hemi-hydrate (CaSO4; J. T. Baker Chemicals, Deventer, The Netherlands) was used as a carrier for A-79175. The A-79175 was mixed into calcium sulphate to dosages of 0%, 0.01%, 0.05%, and 0.25% (weight/weight). Using a biopsy punch, 3 mm diameter × 3 mm tall scaffolds were cut from the Osteomesh sheet and placed in a cylindrical rubber mould. Saline was added to the CaSO4 and A-79175 mixtures (between 1 ml and 2 ml per gram) and the slurry was applied to the scaffolds. The PCL-CaSO4 scaffolds were air-dried and weighed in order to determine the amount of A-79175 present in each scaffold. PCL scaffolds without CaSO4 were also used as controls. The PCL-CaSO4 scaffolds weighed a mean of 0.015 g (sd 0.002), low-dose scaffolds weighed a mean 0.017 g (sd 0.002), medium-dose scaffolds weighed a mean 0.015 g (sd 0.002), and high-dose scaffolds weighed a mean 0.018 g (sd 0.001). Scaffolds were calculated to contain approximately 1.5 µg, 6.4 µg and 40.4 µg of A-79175 for the 0.01%, 0.05% and 0.25% doses, respectively. Before implantation all scaffolds were sterilised with 18 Gy of gamma irradiation using a Model 28-8 irradiator (JL Sheperd & Associates, San Fernando, California).
In vitro release of A-79175
Implants for each treatment group (six per group) were submerged in 1.5 ml of phosphate buffered saline (PBS) in a 2 ml tube. A 150 µl aliquot of the PBS was removed at days one, three, five, seven and nine in order to measure accumulated A-79175 release. On day ten, 2 ml of methanol was added to the remaining mixture and the samples were homogenised with 2.8 mm diameter stainless steel beads in a 2 ml centrifuge tube using a Precellys 24-DUAL Tissue Homogenizer (Bertin Technologies, Bordeaux, France) at 6500 rpm for 30 s twice. Particulates from all samples were removed by centrifugation and when necessary by filtration through a 0.4 µm membrane. The samples were diluted 1:1 with Solvent A (10 mM ammonium formate pH 9.1, 20% methanol) before liquid chromatography–mass spectrometry (LC-MS/MS) analysis.
A-79175 was detected by LC-MS/MS and quantified using an A-79175 standard curve. The LC-MS/MS system used included a FAMOS autosampler (LC Packings, San Francisco, California), an Agilent 1100 degasser and binary pump (Agilent Technologies, Inc., Santa Clara, California), and an AB SCIEX 2000 QTrap (AB SCIEX, Foster City, California). The A-79175 in vitro release samples and standards were separated by reverse-phase liquid chromatography using a Gemini C6-Phenyl column (150 mm × 2.00 mm, 3 µ; Phenomenex, Torrance, California) at room temperature using a flow rate of 300 µl/min. The C6-Phenyl column was developed using the following method: Solvent A for 3 mins; a linear gradient from 100% Solvent A to 100% Solvent B (100% methanol) between 3 and 5 mins; 100% Solvent B between 5 and 15 mins; a linear gradient from 100% Solvent B to 100% Solvent A between 15 and 18 mins; and 100% Solvent A from 18 to 20 mins.
The LC eluate was introduced into the 2000 Qtrap using the turbo electro-spray ion source in positive ion mode. A-79175 was detected using multiple reaction monitoring (MRM) by measuring the 305 to 206 m/z ion pair transition from Q1 to Q3. The following Qtrap parameters were used: CUR = 22 psi, CAD = 10, IS = 5000, TEM = 350°, GS1 = 30 psi, GS2 = 25 psi, IHE = ON, DP = 68 V, EP = 10 V, CE = 12 V, and CXP = 10 V.
A-79175 quantification was performed using Analyst Quantification Software (AB SCIEX). The peak area was determined for each sample using the Analyst Software quantitation wizard using a bunching factor of ten. Each peak was manually reviewed for accuracy. Data from the A-79175 standards were fit to a quadratic standard curve and this curve was used to calculate sample drug concentrations. The standard curve contained six standards ranging from 0 ng/µl to 1000 ng/µl and was done in triplicate. Quality control standards and blanks were used periodically throughout sampling to ensure accuracy.
Animal procedures
A total of 60 male Sprague-Dawley rats were obtained from Charles River Laboratory (Wilmington, Massachusetts). The rats weighed a mean of 520 g (sd 35) at the time of surgery and 533 g (sd 39) at death. All animal experiments were approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School.
Bilateral defects were made in the distal femora of each rat to assess local drug effects on bone formation. Rats were anaesthetised with an intraperitoneal injection of ketamine (50 mg/kg) and xylazine (10 mg/kg). Hind limbs were prepared for surgery by shaving the knee areas and scrubbing with 10% povidone-iodine solution. A lateral 1 cm incision was created above the knee on each thigh. The overlying muscle was bluntly dissected to the surface of the distal femur. A 2.7 mm diameter burr (ACE Surgical, Brockton, Massachusetts) and a variable speed drill (Dremel, Racine, Wisconsin) were used to create a 3 mm diameter unicortical defect in the distal femur. Each defect site was thoroughly irrigated with saline and sponged dry. A scaffold was placed within the defect using surgical tweezers. Wounds were closed in three layers using resorbable sutures. Radiographs of each animal were made to confirm and record the initial defect.
After the rats were numbered, those with even numbers had experimental scaffolds placed in their right femur and control scaffolds in their left femur, whereas odd-numbered rats had scaffolds placed in the opposite manner. Because of the bilateral defect study, the bone volume of each defect was compared with that of the contralateral defect in order to determine the percentage changes in bone formation.
The 60 rats were split into groups (each n = 6) between the two endpoints (days ten and 30) and the five treatment groups: 1) the scaffold group (empty defect and PCL-only scaffold); 2) CaSO4 group (PCL-only scaffold and CaSO4-only); 3) low dose (CaSO4-only and 0.01% A-79175); medium dose (CaSO4-only and 0.05% A-7915); and 5) high dose (CaSO4-only and 0.25% A-79175).18 The dose of A-79175 was calculated based on percentage dosage and the scaffold mass minus PCL scaffold mass. The rats were housed in pairs, maintained in a 12-hour light dark cycle, and provided food and water ad libitum. Rats were killed with an inhaled overdose of isoflurane. Each femur was excised and placed in formalin for five days, before being placed in 70% ethanol before micro-CT analysis.
Micro-CT analysis
Using a Skyscan 1172 (Bruker, Kontich, Belgium), three-dimensional (3D) micro-CT images were made in order to determine the bone volume (BV) of the defect site. Right and left femora were excised and scanned together as pairs in 70% ethanol to prevent drying. All samples were scanned using a 0.5 mm aluminum filter, at energy of 70 Kvp, intensity of 142 µA and with a voxel size of 12 µ isotropic. The micro-CT data were reconstructed with NRecon software (Bruker).
All reconstructed images were analysed using Bone J to determine bone and total volumes and with Skyscan software to determine bone mineral density.19,20 Cylindrical volume of interests with a mean diameter of 3.1 mm and mean height of 2.2 mm were created to isolate the defect. Each defect was analysed for bone volume, and the resulting ratio of bone volume to total volume (BV/TV) was calculated, based on the size of the cylinder isolated from the 3D reconstruction. The change in percentage bone volume was calculated by dividing the difference in experimental and control defect BV/TV values by the control BV/TV value and then multiplying by 100. Femora were scanned in a blinded fashion in that the operator (AM) was unaware of the treatments used in each femur.
Histological analysis
Following micro-CT scanning, samples were embedded in polymethylmethacrylate (PMMA).21 A single section was cut through the defect site perpendicular to the long axis of the femur. Sections were polished stained with van Gieson’s picrofuchsin to stain mineralised tissue red and Stevenel’s blue to stain cartilage deep blue.22 Digital images of the specimens were captured using an Olympus SZ40 microscope (Olympus America Inc., San Jose, California) and a Spot Idea Camera (Diagnostics Instruments Inc., Sterling Heights, Michigan) or a Nikon Diaphot 300 microscope with a Nikon DXM1200f digital camera (Nikon Instruments Inc., Melville, New York).
Data analysis
Data from CaSO4-only scaffold controls were compared between groups using analysis of variance (ANOVA). The comparison of the CaSO4-only controls ensured that each group was healing at similar rates, and that changes in bone volume could be attributed to A-79175 treatment. Data were compared between control and contralateral experimental femurs directly using a paired t-test.
Results
The surgical procedure was easily accomplished and well-tolerated by the rats. Slight variations in scaffold diameter sometimes necessitated creation of a burr hole slightly larger than 3 mm. This slight variation was accounted for in data analysis by creating a cylindrical volume of interest of ≥ 3 mm diameter, and was taken into account for the total volume of the cylinder when determining the bone volume to total volume ratio (BV/TV) and bone mineral density (BMD).
Disposition of animals and femur samples
Of the 60 rats (120 femora) used in the study, two rats died from the surgical anaesthesia before the surgical procedure and one died a day after surgery (two rats in the high-dose ten-day endpoint group and one rat in the scaffold-only 30-day endpoint group), one femur had a bi-cortical defect that made it unsuitable for data analysis (medium-dose ten-day endpoint group), and one rat developed an infection that prevented its use (medium-dose 30-day time point). An anticipated complication of the procedure was post-operative secondary fracture, which occurred in three rats of the high-dose 30-day endpoint group, one rat of the medium-dose 30-day group, one rat of the CaSO4-only 30-day endpoint group, and one rat of the medium-dose one-day group. Finally, sample handling errors caused the loss of one rat from the medium-dose 30-day group and one rat from the CaSO4-only 30-day group. The remaining animals were distributed as detailed in Tables I and II.
Table I
Effects of A-79175 on the bone volume/total volume (BV/TV) ratio at ten days post-operatively
| Mean (sd) BV/TV | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment group | Implant (mg) | Drug dose (w/w) (%) | Approximate dose (µg) | Group size (n) | Experimental | Control | p-value (t-test) | |
| Scaffold only | - | 0 | 0 | 6 | 0.32 (0.055) | 0.31 (0.054) | 0.812 | |
| CaSO4-only | 15.4 | 0 | 0 | 6 | 0.30 (0.088) | 0.29 (0.104) | 0.912 | |
| Low dose | 18.0 | 0.01 | 1.5 | 6 | 0.30 (0.086) | 0.27 (0.078) | 0.496 | |
| Medium dose | 14.8 | 0.05 | 6.4 | 5 | 0.37 (0.013) | 0.29 (0.047) | 0.011 | |
| High dose | 18.2 | 0.25 | 39.5 | 4 | 0.38 (0.049) | 0.30 (0.024) | 0.024 | |
In vitro release of A-79175 from the implants
There was an initial release (at day one) of A-79175 from the implants into the PBS (Fig. 1, Table III). However, after the initial release, the remaining A-79175 stayed within the implant with very little additional A-79175 released over the following eight days (Fig. 1). On day ten the implant was crushed in methanol in order to break apart the solidified calcium sulphate and extract the remaining A-79175. As drug release from solidified calcium sulphate depends upon surface erosion,23 it was unlikely that this procedure would extract all the remaining A-79175 still held in the small particles of solidified calcium sulphate. However, the large increase in surface area caused by the crushing enabled extraction of additional A-79175 from the implants, demonstrating that A-79175 was still present within the CaSO4 matrix even after nine days incubation in PBS (Table III).
Table III
Summary of A-79175 release in vitro (PBS, phosphate buffered saline)
| Treatment group | Mean drug per implant (µg) | Maximum total released into PBS (%) | Mean (sd) drug recovered from implant (µg) | Total recovered from implant on day ten (%) |
|---|---|---|---|---|
| Control | 0.0 | - | 0.0 (0.0) | - |
| Low dose (0.01%) | 1.5 | 66 | 0.30 (0.11) | 20 |
| Medium dose (0.05%) | 6.4 | 31 | 1.88 (0.88) | 29 |
| High dose (0.25%) | 40.4 | 12 | 6.89 (3.47) | 17 |
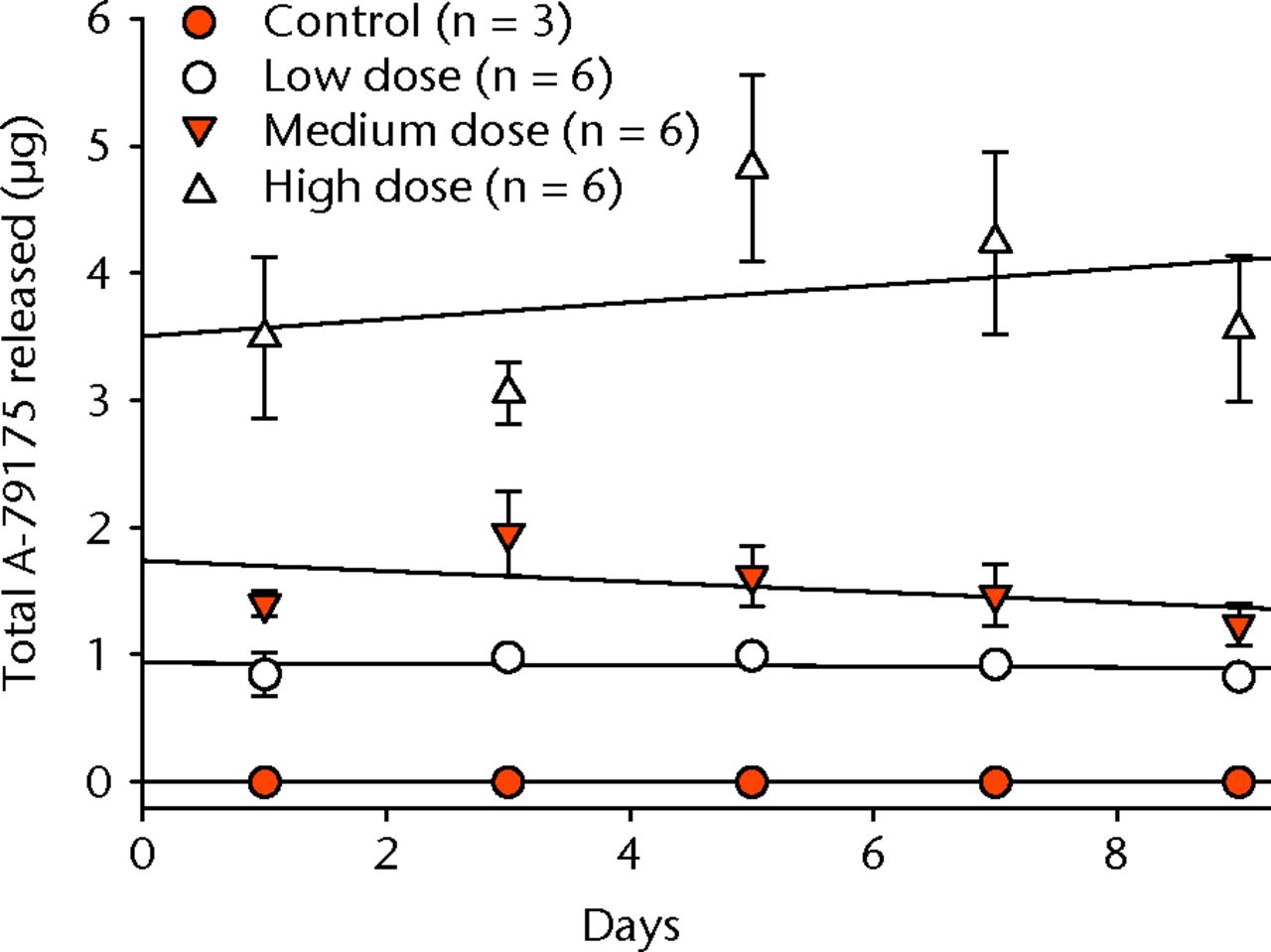
Fig. 1
Graph showing the mean cumulative A-79175 release from implants in vitro. Error bars show the standard deviation and lines of best fit are given for each group, including the controls (CaSO4-only).
Radiological analysis
Radiographs were made for each animal immediately after surgery in order to confirm defect site and placement. After death the excised femora were also examined by radiology. Visual evidence of healing was apparent in most of the radiographs compared with those made immediately post-operatively. Beyond the identification of complications, such as fractures or infections, the radiographs were not scored or in any other way correlated to the experimental treatments. Example radiographs of the defects in each group from the ten-day endpoint are shown in Figure 2.
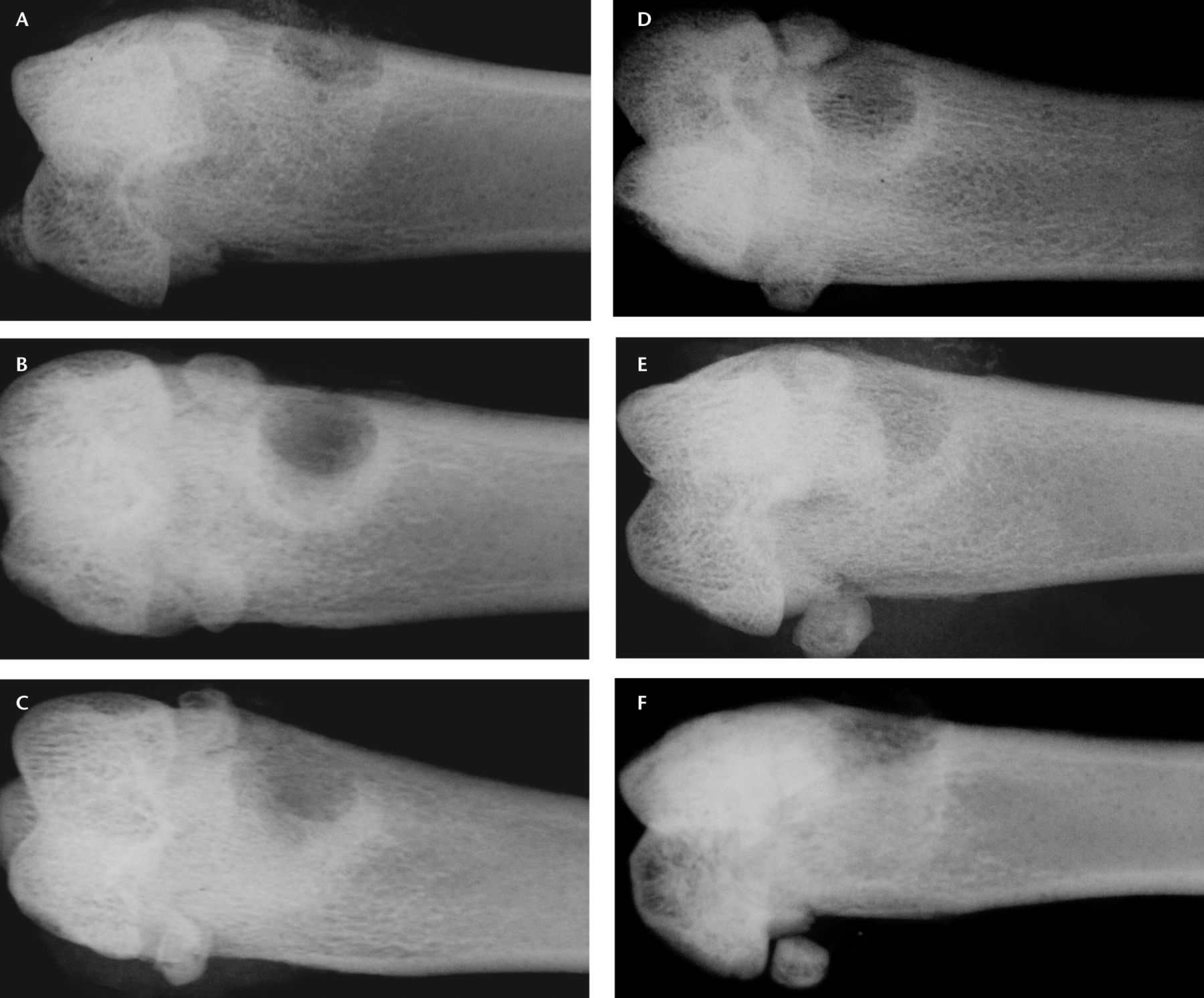
Fig. 2
Sample radiographs from each group after ten days of healing in a) an open defect, b) a polycaprolactone (PCL)-only defect, c) a CaSO4-only defect, d) a low-dose A-79175 defect, e) a medium-dose A-79175 defect, and f) a high-dose A-79175 defect.
Histological analysis of the healing defect
Histological examination of the defects sites showed bone deposition around and in the scaffold for control and treatment groups (Fig. 3). No cartilage was present in the sites suggesting that direct bone formation occurred in this model. Histomorphometry to determine the percentages of mineralised tissue was not conducted, as a previous study showed that histomorphometry and micro-CT analyses yield similar results in this bone defect model.18
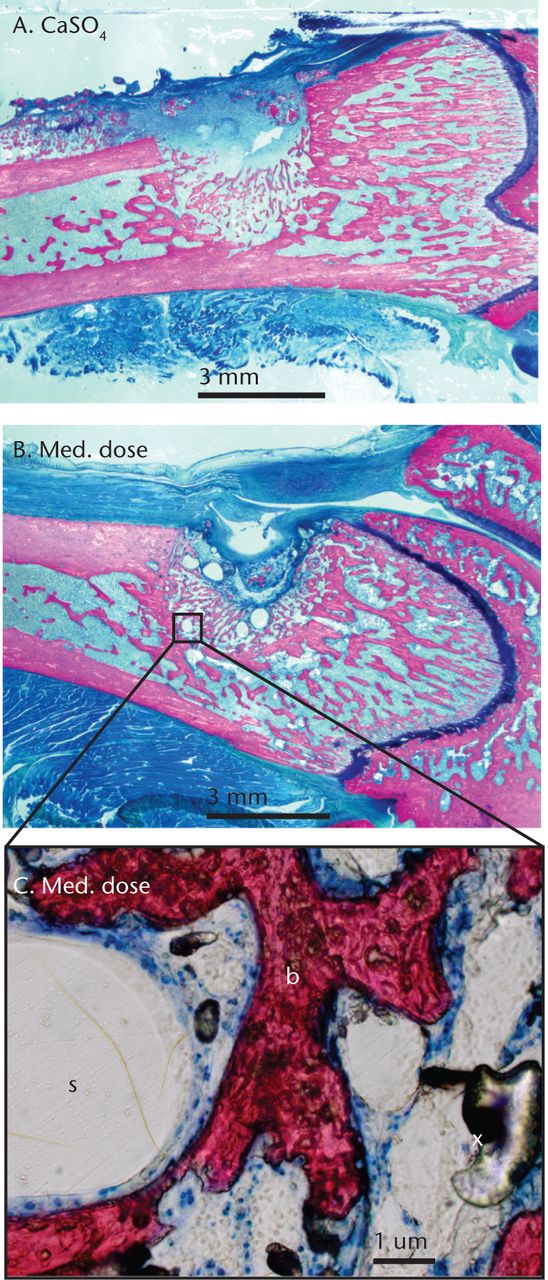
Fig. 3
Histological images of femoral defects at ten days post-operatively in a) the control group (CaSO4-only) and b) the medium-dose group treated with A-79175, and c) magnified to show newly formed bone (b), the void left by the polycaprolactone scaffold (s) and artifactual air bubbles from the embedding process (x).
Micro-CT analysis
New bone (BV/TV) and bone mineral density (BMD) in the defect site of each femur was measured by micro-CT. Representative 3D reconstructions of the defect from each of the treatment and control groups at the ten- and 30-day endpoints are shown in Figure 4. The CaSO4-only controls in each treatment group from the ten-day and 30-day time points were compared using ANOVA. No significant difference in BV/TV or BMD were found at either the ten-day (p = 0.84 and 0.19, respectively) or 30-day (p = 0.61 and 0.66, respectively) time points between the CaSO4 only scaffolds in each treatment group (Tables II, III, and IV). This indicates that no systemic drug effect or other healing variations occurred between groups. A two-way ANOVA showed a significant difference in BMD between the ten- and 30-day endpoints after accounting for differences in treatment (p < 0.001) (Table IV).
Table IV
Mean bone mineral densities at ten and 30 days post-operatively (PCL, polycaprolactone)
| Mean (sd) bone mineral density (mg/cm3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day ten | Day 30 | ||||||||||
| Treatment group | Experimental vs control | n | Experimental | Control | p-value | n | Experimental | Control | p-value | ||
| Scaffold | Empty vs PCL | 6 | 0.52 (0.10) | 0.52 (0.07) | 0.96 | 5 | 0.59 (0.10) | 0.58 (0.04) | 0.79 | ||
| CaSO4 | PCL vs PCL+CaSO4 | 6 | 0.54 (0.04) | 0.49 (0.05) | 0.07 | 3 | 0.54 (0.05) | 0.64 (0.12) | 0.30 | ||
| Low dose | A-79175 vs PCL+CaSO4 | 6 | 0.51 (0.05) | 0.54 (0.06) | 0.46 | 6 | 0.67 (0.04) | 0.70 (0.06) | 0.23 | ||
| Medium dose | A-79175 vs PCL+CaSO4 | 5 | 0.54 (0.04) | 0.55 (0.11) | 0.91 | 3 | 0.64 (0.08) | 0.66 (0.04) | 0.76 | ||
| High dose | A-79175 vs PCL+CaSO4 | 4 | 0.55 (0.06) | 0.53 (0.06) | 0.56 | 3 | 0.62 (0.02) | 0.67 (0.07) | 0.29 | ||
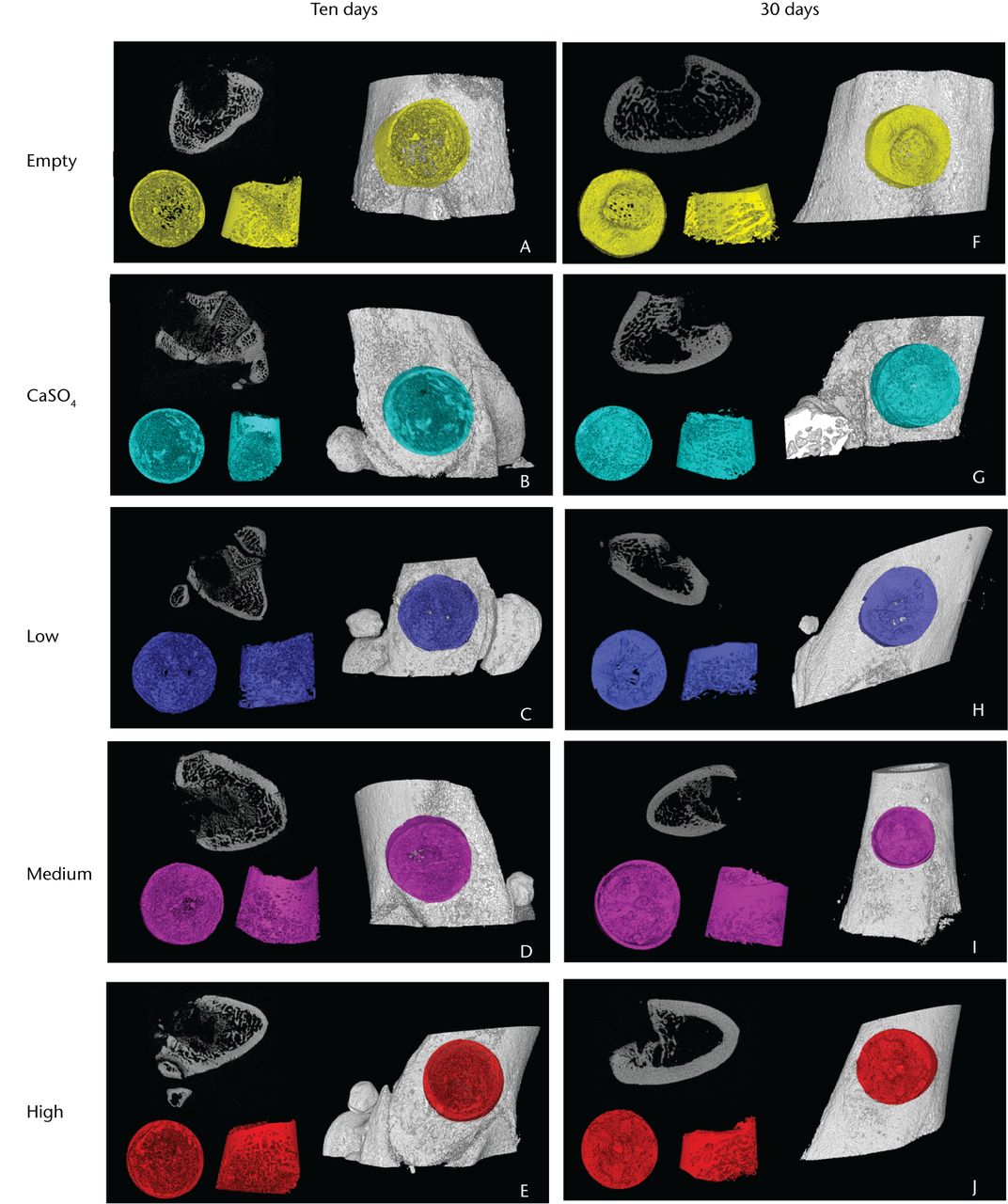
Fig. 4
Representative three-dimensional micro-CT reconstructions of the femoral defect sites, with the ten-day samples in the left column and the 30-day samples in the right. Within each panel are (top left) a two-dimensional slice of the defect, (bottom left) the analysed volume shown from top-down and side views, and (right) the highlighted defect within the femur.
The experimental design enabled a paired comparison of bone formation between experimental (scaffolds with CaSO4 and A-79175) and control (scaffolds with CaSO4 only) defects with Student’s t-tests. There was no significant difference in total volume (TV) between experimental and control defects for each group (data not shown). The data indicate that defects treated with medium and high doses of A-79175 had significantly more bone (BV/TV) after ten days of healing (Table I, Fig. 5). There was a mean increase in bone volume of 14.3% (sem 8.3) for the low-dose A-79175 treated defects compared with the CaSO4-only controls (6.6% (sem 8.4)) at the ten-day endpoint, although this was not statistically significant (p = 0.5). The mean change in BV/TV for the medium and high-dose groups was 27.5 (sem 8.3) and 28.3 (sem 8.6), respectively, both of which were significantly greater than the controls with CaSO4 only (p = 0.011 and p = 0.024, respectively). No statistically significant differences were found in the 30-day endpoint data (Table II). However, all A-79175 treated defects had a greater BV/TV ratio than their CaSO4-only controls at the 30-day time point.
Table II
Effects of A-79175 on the bone volume/total volume (BV/TV) ratio at 30 days post-operatively
| Mean (sd) BV/TV | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment group | Implant (mg) | Drug dose (w/w) (%) | Approximate dose (µg) | Group size (n) | Experimental | Control | p-value (t-test) | |
| Scaffold | - | 0 | 0 | 5 | 0.33 (0.092) | 0.31 (0.057) | 0.630 | |
| CaSO4-only | 14.7 | 0 | 0 | 3 | 0.40 (0.138) | 0.38 (0.043) | 0.767 | |
| Low dose | 17.8 | 0.01 | 1.5 | 6 | 0.39 (0.101) | 0.34 (0.095) | 0.328 | |
| Medium dose | 14.6 | 0.05 | 6.3 | 3 | 0.43 (0.042) | 0.42 (0.046) | 0.372 | |
| High dose | 18.9 | 0.25 | 41.3 | 3 | 0.43 (0.103) | 0.38 (0.087) | 0.138 | |
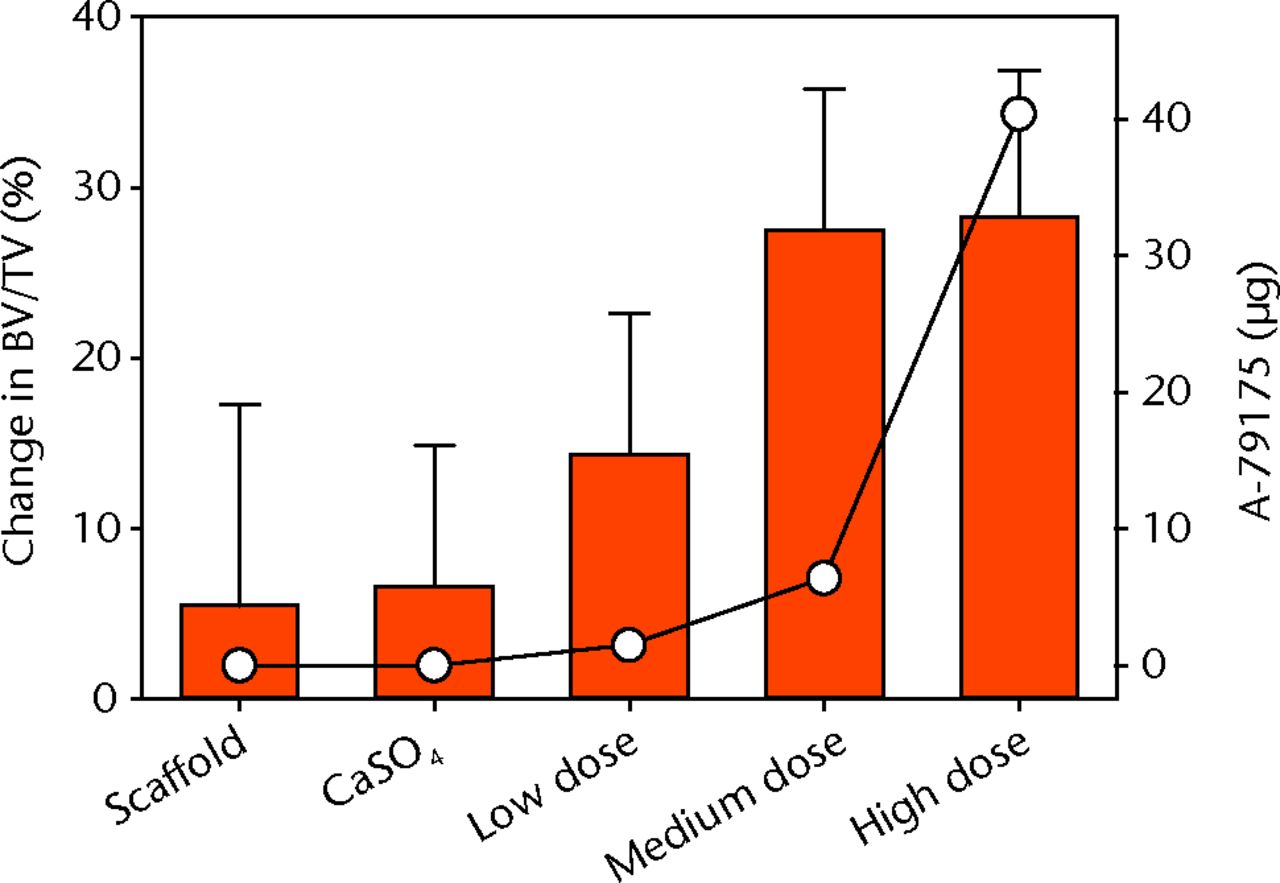
Fig. 5
Graph showing the mean percentage change in the bone volume/total volume (BV/TV) of the ten-day endpoint (as bar chart, with error bars denoting standard error) compared with the A-79175 dosage depicted by the open circles, for each of the groups.
Discussion
We found the local release of A-79175 from CaSO4 infused into PCL scaffolds to enhance bone formation in a cortical defect. The data demonstrated that local treatment with A-79175 at doses of 6.4 µg and 40.4 µg led to significantly more bone in the defect site by ten days post-operatively when compared with the contralateral femur defect treated with CaSO4-infused scaffolds without the drug. By 30 days after surgery, the differences in bone formed between controls and A-79175 treated defects were not significant, although the A-79175 treated defects had consistently higher BV/TV values. This result was expected as the control groups were expected to heal at a normal rate and achieve almost full healing by 30 days. The results support previous fracture healing studies, in which loss or inhibition of 5-LO was associated with accelerated bone regeneration.1,2
Differences in BV/TV between the A-79175 dose groups at the ten-day endpoint were compared using ANOVA, but no significant difference was found between the three doses (p = 0.125). However, the experiment was designed to allow paired comparisons between defects treated with A-79175 and their corresponding contralateral CaSO4-only controls, and, as a consequence, the ANOVA comparison between the dosage groups was statistically underpowered (power = 0.249). Given the large observed differences between the A-79175 low-dose group and the medium- and high-dose groups, larger sample sizes would be likely to show a significant dose-dependent effect. This potential difference between the low-, medium- and high-dose groups may reflect the in vitro burst release of over half of the A-79175 from the low-dose implants (Table III). In contrast, the medium- and high-dose groups retained over 40% of the initial A-79175 after nine days of incubation in vitro, suggesting that these doses provided for sustained release of A-79175 in vivo as the calcium sulphate matrix is released.
Any osteoconductive effects of the PCL scaffold or the infused CaSO4 was controlled for in the scaffold group, in which an empty defect was compared with a PCL scaffold containing no CaSO4, and the CaSO4-only group in which a PCL scaffold was compared with a PCL scaffold infused with CaSO4. The micro-CT analysis found no significant differences between the PCL scaffold and empty defect groups at ten days (p = 0.836) or at 30 days (p = 0.630). Additionally, no significant differences were found when the PCL scaffold was compared with PCL infused with CaSO4 at ten days (p = 0.745) or at 30 days (p = 0.767).
The paired design of this study controlled for local effects of A-79175 within each rats. However, a risk associated with this design is that systemic distribution of A-79175 could influence bone formation in the control defect. As the absolute dose of A-79175 per animal was very low and was further tempered by nominally slow-release from CaSO4, the potential for confounding effects of systemic A-79175 was considered to be low. A comparison of all treatment groups failed to identify any significant effects of drug treatment on BV/TV in the defects filled with CaSO4-only scaffolds (ten-day endpoint, p = 0.19; 30-day endpoint, p = 0.66; both ANOVA) suggesting that, at the dose used and in the CaSO4 carrier, the A-79175 had no systemic effect on bone formation.
Local treatment with A-79175 increased direct bone formation in this rat femur defect model. Bone healing in this model occurs by direct formation and not endochondral ossification, and therefore suggests that 5-LO inhibition can stimulate osteoblasts to synthesise bone.18 Previous studies have shown accelerated bone formation during fracture repair when 5-LO was absent in mice homozygous for a targeted mutation in Alox5 or in rats treated with oral doses of AA-861, a 5-LO inhibitor.1,2 The accelerated fracture repair was associated with enhanced chondrogenesis, suggesting that inhibition of 5-LO accelerates endochondral bone formation. However, these previous studies also showed early increases in peripheral fracture site bone formation, which is initially mediated by direct bone formation from the periosteum and would be consistent with the current findings.1,2
Bone healing by direct bone formation occurs by osteoclast resorption of the damaged bone and subsequent new bone formation by osteoblasts.24 Studies have shown that 5-LO inhibition can inhibit the activity of osteoclasts and osteoblasts.25,26 However, cathepsin K and osteocalcin mRNA expression, indicators of osteoclast and osteoblast activity, respectively, were elevated during fracture repair in animals treated with 5-LO inhibitor.1 Similarly, others have found that 5-LO metabolites can inhibit osteoblast or osteosarcoma cell line proliferation and function.27,28 The inconsistent findings between studies likely relates to differences between in vitro and in vivo findings, differences in cell preparations, and differences in outcome measurements. Therefore, whether A-71975 stimulation of bone formation is occurring by mediating osteoclast activity, osteoblast activity, a combination of osteoclast and osteoblast activity, or is mediated through another cell type remains to be resolved.
This study did not explore the mechanisms by which 5-LO inhibition is enhancing osteogenesis. Although this model predominantly works through primary bone formation, it is unclear whether A-79175 is increasing bone formation or decreasing bone turnover. Future research will focus on understanding the mechanisms by which 5-LO and its downstream products effect the major cell types involved in primary and secondary bone healing. Our histological examination was consistent with primary bone formation as expected suggesting that 5-LO inhibition can affect primary bone formation as well as endochondral ossification as previously published.1,2 Additional measures of bone formation were not attempted, as our previous studies have found a high degree of correlation between micro-CT measured bone volumes and bone areas measured by histomorphometry.18
The present study had some limitations. Differences in surgical creation of the defect sites required optimisation of micro-CT volume measurements for each defect. In order to account for these small differences, the amount of bone formed was normalised to the volume assayed (BV/TV). Surgical limitations were not limited to defect size variations. The implants used were PCL scaffolds infused with CaSO4 and A-79175. The implants would crumble under light pressure or dissolve when exposed to any aqueous substance, such as saline or blood. Efforts were made to ensure the defect site was of adequate size and dry before attempting to insert the implant. However, scaffolds did break on occasion. In that event, the pieces of the scaffold were sufficiently moldable that the scaffold pieces were pushed into the defect site, much like a paste, rather than inserted as an entire solid scaffold. The paired experimental design allowed for statistical comparisons between an experimental scaffold and the control scaffold inserted in the defect in the contralateral femur. However, this also reduced the required number of animals in each group and reduced the statistical power for across group comparisons.
We are unaware of any approved pharmaceutical treatments to promote cortical bone defect healing or to promote direct bone formation into an arthroplastic device. Bone morphogenetic protein-2 is used to treat severe tibial fractures and is used in other application such as spinal arthrodesis.29-31 Platelet derived growth factor is used in dental application to promote bone formation.32-34 Presently, cortical bone defects are left to heal without intervention if the defect is small enough or may be filled with graft materials including autograft, allograft, xenograft, or synthetic materials to promote healing. Synthetic graft materials, such as calcium sulphate pellets and β-tricalcium phosphate particles, rely upon osteoconduction to promote healing.35,36 Similarly, implants used in arthroplasty are sometimes coated with hydroxyapatite to promote osteoconduction.37 However, no small molecule drug therapy is currently available as a systemic or local therapy for promoting bone formation. Several pharmaceutical approaches are being developed to address this clinical need. Potential therapies include use of locally applied bisphosphonates,19,38 other inhibitors of osteoclast development or function,18,39 use of parathyroid hormone or analogues,40,41 Wnt pathway modifiers,42,43 bone morphogenetic protein analogues,44 prostaglandins analogues,45-47 statins,48,49 and as described here, drugs that modify the 5-lipoxygenase pathway. As there is no set standard pharmaceutical treatment for this model, and because the goal of the study was determine whether A-79175 would promote bone healing in a model of direct bone formation, no comparisons with other developing therapies was performed. Future studies that directly compare the efficacy of A-79175 therapy with other therapies in more clinically relevant models would aid in cost-benefit and clinical decisions regarding use of A-79175 or other 5-LO inhibitor therapies. However, additional experiments are needed to optimise drug-carrier, drug-release profiles and 5-LO inhibitor choice, in order to maximise bone formation before such comparisons are undertaken.
Conclusions
Local application of A-79175, a second-generation 5-LO inhibitor, effectively enhanced bone formation in a rat femoral defect model. Future studies with A-79175 or other 5-LO inhibitors are required in order to ascertain the ideal dose, carrier, and release profile for each clinical application. Studies involving larger mammals are currently underway to test a similar drug for efficacy as a cost-effective method for achieving accelerated bone healing.
The authors would like to thank K. Uhrich (Rutgers University, New Jersey) and J. Ricci (New York University College of Dentistry, New York) for their comments on the manuscript.
1 Cottrell JA , O’ConnorJP. Pharmacological inhibition of 5-lipoxygenase accelerates and enhances fracture-healing. J Bone Joint Surg [Am]2009;91-A:2653–2665.CrossrefPubMed Google Scholar
2 Manigrasso MB , O’ConnorJP. Accelerated fracture healing in mice lacking the 5-lipoxygenase gene. Acta Orthop2010;81:748–755.CrossrefPubMed Google Scholar
3 Gijón MA , ZariniS, MurphyRC. Biosynthesis of eicosanoids and transcellular metabolism of leukotrienes in murine bone marrow cells. J Lipid Res2007;48:716–725.CrossrefPubMed Google Scholar
4 Murphy RC , GijónMA. Biosynthesis and metabolism of leukotrienes. Biochem J2007;405:379–395.CrossrefPubMed Google Scholar
5 Funk CD . Prostaglandins and leukotrienes: advances in eicosanoid biology. Science2001;294:1871–1875.CrossrefPubMed Google Scholar
6 Simon AM , ManigrassoMB, O’ConnorJP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res2002;17:963–976.CrossrefPubMed Google Scholar
7 Simon AM , O’ConnorJP. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg [Am]2007;89-A:500–511.CrossrefPubMed Google Scholar
8 Cottrell J , O’ConnorJP. Effect of non-steroidal anti-inflammatory drugs on bone healing. Pharmaceuticals2010;3:1668–1693.CrossrefPubMed Google Scholar
9 O’Connor JP , CapoJT, TanV, et al.A comparison of the effects of ibuprofen and rofecoxib on rabbit fibula osteotomy healing. Acta Orthop2009;80:597–605.CrossrefPubMed Google Scholar
10 Holt G , MurnaghanC, ReillyJ, MeekRM. The biology of aseptic osteolysis. Clin Orthop Relat Res2007;460:240–252.CrossrefPubMed Google Scholar
11 Lee K , GoodmanSB. Current state and future of joint replacements in the hip and knee. Expert Rev Med Devices2008;5:383–393.CrossrefPubMed Google Scholar
12 Otani T , WhitesideLA. Failure of cementless fixation of the femoral component in total hip arthroplasty. Orthop Clin North Am1992;23:335–346.PubMed Google Scholar
13 Schenk RK. Biology of fracture repair. In: Browner BD, Jupiter JB, Levine AM, Trafton PG, eds. Skeletal trauma. Philadelphia: WB Saunders Company, 1992:31–75. Google Scholar
14 Ueno M , UrabeK, NaruseK, et al.Influence of internal fixator stiffness on murine fracture healing: two types of fracture healing lead to two distinct cellular events and FGF-2 expressions. Exp Anim2011;60:79–87.CrossrefPubMed Google Scholar
15 Carter GW , BellRL, MarshK, et al.Stereoselective metabolism of the 5-lipoxygenase inhibitor A-78773. Ann N Y Acad Sci1994;744:262–273.CrossrefPubMed Google Scholar
16 Bell RL , BouskaJB, MaloPE, et al.Optimization of the potency and duration of action of N-hydroxyurea 5-lipoxygenase inhibitors. J Pharmacol Exp Ther1995;272:724–731.PubMed Google Scholar
17 Artham T , DobleM. Biodegradation of aliphatic and aromatic polycarbonates. Macromol Biosci2008;8:14–24.CrossrefPubMed Google Scholar
18 Cottrell JA , ValesFM, SchachterD, et al.Osteogenic activity of locally applied small molecule drugs in a rat femur defect model. J Biomed Biotechnol2010;2010:597641.CrossrefPubMed Google Scholar
19 Doube M , KłosowskiMM, Arganda-CarrerasI, et al.BoneJ: free and extensible bone image analysis in ImageJ. Bone2010;47:1076–1079.CrossrefPubMed Google Scholar
20 Schneider CA , RasbandWS, EliceiriKW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods2012;9:671–675.CrossrefPubMed Google Scholar
21 Baron R, Vignery A, Neff L, Silverglate A, Santa Maria A. Processing of undecalcified bone specimens for bone histomorphometry. In: Recker RR, ed. Bone histomorphometry: techniques and interpretation. Boca Raton, CRC Press, 1983:13–35. Google Scholar
22 Maniatopoulos C , RodriguezA, DeporterDA, MelcherAH. An improved method for preparing histological sections of metallic implants. Int J Oral Maxillofac Implants1986;1:31–37.PubMed Google Scholar
23 Rosenblum SF , FrenkelS, RicciJR, AlexanderH. Diffusion of fibroblast growth factor from a plaster of Paris carrier. J Appl Biomater1993;4:67–72.CrossrefPubMed Google Scholar
24 Frost HM . The biology of fracture healing: an overview for clinicians: Part I. Clin Orthop Relat Res1989;248:283–293. Google Scholar
25 Franchi-Miller C , SaffarJL. The 5-lipoxygenase inhibitor BWA4C impairs osteoclastic resorption in a synchronized model of bone remodeling. Bone1995;17:185–191.CrossrefPubMed Google Scholar
26 Paredes Y , MassicotteF, PelletierJP, et al.Study of the role of leukotriene B()4 in abnormal function of human subchondral osteoarthritis osteoblasts: effects of cyclooxygenase and/or 5-lipoxygenase inhibition. Arthritis Rheum , 2002;46:1804–1812. Google Scholar
27 Ren W , DziakR. Effects of leukotrienes on osteoblastic cell proliferation. Calcif Tissue Int1991;49:197–201.CrossrefPubMed Google Scholar
28 Traianedes K , DallasMR, GarrettIR, MundyGR, BonewaldLF. 5-Lipoxygenase metabolites inhibit bone formation in vitro. Endocrinology1998;139:3178–3184.CrossrefPubMed Google Scholar
29 Glassman SD , CarreonL, DjurasovicM, et al.Posterolateral lumbar spine fusion with INFUSE bone graft. Spine J2007;7:44–49.CrossrefPubMed Google Scholar
30 Boden SD , KangJ, SandhuH, HellerJG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976)2002;27:2662–2673.CrossrefPubMed Google Scholar
31 Govender S , CsimmaC, GenantHK, et al.Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg [Am]2002;84-A:2123–2134.CrossrefPubMed Google Scholar
32 Graham S , LeonidouA, LesterM, et al.Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin Investig Drugs2009;18:1633–1654.CrossrefPubMed Google Scholar
33 Jayakumar A , RajababuP, RohiniS, et al.Multi-centre, randomized clinical trial on the efficacy and safety of recombinant human platelet-derived growth factor with beta-tricalcium phosphate in human intra-osseous periodontal defects. J Clin Periodontol2011;38:163–172. Google Scholar
34 Nevins M, Kao RT, McGuire MK, et al. PDGF promotes periodontal regeneration in localized osseous defects: 36 month extension results from a randomized, controlled, double-masked clinical Trial. J Periodontol 2012:Epub ahead of print. Google Scholar
35 Petruskevicius J , NielsenS, KaalundS, KnudsenPR, OvergaardS. No effect of Osteoset, a bone graft substitute, on bone healing in humans: a prospective randomized double-blind study. Acta Orthop Scand2002;73:575–578.CrossrefPubMed Google Scholar
36 Altermatt S , SchwöbelM, PochonJP. Operative treatment of solitary bone cysts with tricalcium phosphate ceramic: a 1 to 7 year follow-up. Eur J Pediatr Surg1992;2:180–182. Google Scholar
37 Hamadouche M , WitvoetJ, PorcherR, et al.Hydroxyapatite-coated versus grit-blasted femoral stems: a prospective, randomised study using EBRA-FCA. J Bone Joint Surg [Br]2001;83-B:979–987. Google Scholar
38 Back DA , PaulyS, RommelL, et al.Effect of local zoledronate on implant osseointegration in a rat model. BMC Musculoskelet Disord2012;13:42.CrossrefPubMed Google Scholar
39 Jerome C , MissbachM, GamseR. Balicatib, a cathepsin K inhibitor, stimulates periosteal bone formation in monkeys. Osteoporos Int2012;23:339–349.CrossrefPubMed Google Scholar
40 Alkhiary YM , GerstenfeldLC, KrallE, et al.Enhancement of experimental fracture-healing by systemic administration of recombinant human parathyroid hormone (PTH 1-34). J Bone Joint Surg [Am]2005;87-A:731–741.CrossrefPubMed Google Scholar
41 Arrighi I , MarkS, AlvisiM, et al.Bone healing induced by local delivery of an engineered parathyroid hormone prodrug. Biomaterials2009;30:1763–1771.CrossrefPubMed Google Scholar
42 Rey JP , ElliesDL. Wnt modulators in the biotech pipeline. Dev Dyn2010;239:102–114.CrossrefPubMed Google Scholar
43 Gamie Z , KorresN, LeonidouA, GrayAC, TsiridisE. Sclerostin monoclonal antibodies on bone metabolism and fracture healing. Expert Opin Investig Drugs2012;21:1523–1534.CrossrefPubMed Google Scholar
44 Zouani OF , CholletC, GuillotinB, DurrieuMC. Differentiation of pre-osteoblast cells on poly(ethylene terephthalate) grafted with RGD and/or BMPs mimetic peptides. Biomaterials2010;31:8245–8253.CrossrefPubMed Google Scholar
45 Paralkar VM , BoroveckiF, KeHZ, et al.An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc Natl Acad Sci U S A2003;100:6736–6740.CrossrefPubMed Google Scholar
46 Li M , KeHZ, QiH, et al.A novel, non-prostanoid EP2 receptor-selective prostaglandin E2 agonist stimulates local bone formation and enhances fracture healing. J Bone Miner Res2003;18:2033–2042.CrossrefPubMed Google Scholar
47 Tanaka M , SakaiA, UchidaS, et al.Prostaglandin E2 receptor (EP4) selective agonist (ONO-4819.CD) accelerates bone repair of femoral cortex after drill-hole injury associated with local upregulation of bone turnover in mature rats. Bone2004;34:940–948.CrossrefPubMed Google Scholar
48 Mundy G , GarrettR, HarrisS, et al.Stimulation of bone formation in vitro and in rodents by statins. Science1999;286:1946–1949.CrossrefPubMed Google Scholar
49 Gutierrez GE , EdwardsJR, GarrettIR, et al.Transdermal lovastatin enhances fracture repair in rats. J Bone Miner Res2008;23:1722–1730.CrossrefPubMed Google Scholar
Funding statement:
This research was supported by National Institute of Dental & Craniofacial Research (Award Number R01DE019926). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Health.
Author contributions:
J. A. Cottrell: Data collection, Data analysis, Writing the paper, Experimental design
A. Mitchell: Data collection, Data analysis
J. P. O’Connor: Experimental design, Data analysis, Writing the paper
V. Keshav: Performed surgeries, Data collection, Data analysis, Writing the paper
ICMJE Conflict of Interest:
JPOC is founder and owner of Accelalox Inc. (Menlo Park, California). However, this research was not supported, approved or reviewed by Accelalox, nor were any funds received relevant to this study.
©2013 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










