Abstract
Objectives
To conduct a pilot randomised controlled trial to evaluate the feasibility of conducting a larger trial to evaluate the difference in Victorian Institute of Sports Assessment-Achilles (VISA-A) scores at six months between patients with Achilles tendinopathy treated with a platelet-rich plasma (PRP) injection compared with an eccentric loading programme.
Methods
Two groups of patients with mid-substance Achilles tendinopathy were randomised to receive a PRP injection or an eccentric loading programme. A total of 20 patients were randomised, with a mean age of 49 years (35 to 66). All outcome measures were recorded at baseline, six weeks, three months and six months.
Results
The mean VISA-A score for the injection group at the primary endpoint of six months was 76.0 (95% confidence interval (CI) 58.3 to 93.7) and for the exercise group was 57.4 (95% CI 38.1 to 76.7). There was no statistically significant difference between these scores (p = 0.171), which was expected from such a pilot study.
Conclusions
This pilot study has been key to providing data to inform a larger study and shows that the methodology is feasible.
Cite this article: Bone Joint Res 2013;2:227–32.
Article focus
Is it feasible to conduct a large randomised controlled trial answering the question: ‘In patients who have failed previous non-operative management, is there a difference in Victorian Institute of Sports Assessment-Achilles (VISA-A) scores at six months between those managed with a platelet-rich plasma (PRP) injection and those managed with an eccentric loading programme’
Key messages
In this study patients are prepared to forego surgery in favour of a final programme of non-operative management, with only two patients out of 22 approached refusing to take part
The data from this pilot study was used to estimate a sample size for a full trial. The sample size calculation provided further evidence in support of future feasibility
Strengths and limitations
This was a randomised design, using validated outcome measures at pre-defined follow-up time-points
The main limitation is that definitive conclusions regarding the effectiveness of PRP for this indication cannot be drawn from this pilot trial
Introduction
Soft-tissue disorders related to tendons are termed ‘tendinopathies’.1 Tendinopathy in the mid-substance of the Achilles tendon occurs as a result of failure to mediate the repair and degeneration processes, causing pain and disability.2 The general population has an incidence of 2.35 per 1000 people, equivalent to more than 150 000 people in the United Kingdom every year.3
The management options for patients with mid-substance Achilles tendinopathy range from initial advice and modification of activities through to electrotherapy modalities, exercise programmes and surgical procedures.4 Recent guidelines on the management of Achilles tendinopathy4 and a recent meta-analysis5 have both advocated that eccentric exercises should be the ‘gold standard’ of management. This is in keeping with previous reviews on the topic.6,7
Patients who do not improve after initial non-operative management are referred to an orthopaedic consultant for a surgical opinion. Operative treatment is the final treatment option offered, as it is associated with a failure rate of up to 30%.2 There is also a small but serious risk of worsening the patient outcome through development of associated complications, such as deep-vein thrombosis, infection and nerve injury.8-10
Before undertaking a surgical procedure an orthopaedic consultant will advocate a final period of non-operative management to prevent a patient undertaking any unnecessary risks. Subsequently, a further programme of eccentric exercises is advocated at this point. However, problems related to motivation and compliance in this chronic subgroup of patients has led to the investigation of alternative modalities.1,11
Injections of platelet-rich plasma (PRP) are one such alternative that have been reviewed recently by the National Institute for Health and Clinical Excellence (NICE).12 Their report concluded that the evidence for such injections lacked in both quantity and quality. Consequently, the safety and efficacy of the intervention was unclear. Therefore their key recommendation was for further high-quality randomised controlled trials (RCTs) to be completed in this new and emerging area.
Since the publication of the NICE review there has been a subsequent high-profile RCT published on this topic.13 The authors of this paper compared PRP injection with placebo injection, with both groups also undergoing an eccentric loading programme. The setting was a sports medicine outpatient clinic and patients were recruited through web-based advertisements and posters. The authors did not demonstrate any differences between the two groups using the Victorian Institute of Sports Assessment-Achilles (VISA-A) score.14 However, the study was underpowered, with only 27 patients in each group, and it did not address the chronic subgroup of Achilles tendinopathy patients that present for a surgical opinion described above.13
In order to address the need to study this specific subgroup of patients, the aim of this current research was to perform a pilot RCT comparing PRP injections with the gold-standard eccentric loading programme in the chronic subgroup of patients who have failed previous non-operative management. This pilot trial is key to determining the feasibility of evaluating alternative non-operative interventions, such as PRP, in this chronic subgroup who have already undergone conservative procedures and are seeking a surgical opinion. Data will be collected regarding the feasibility of recruitment procedures, the conversion of the number of approaches to those randomised and provide important information required for later determining of sample size calculations for a full trial in this group of patients.
This information will subsequently enable conclusions to be drawn regarding the feasibility of completing a full study which would aim to answer: ‘In patients who have failed previous non-operative management, is there a difference in VISA-A scores at six months between those managed with a PRP injection and those managed with an eccentric loading programme’.
Patients and Methods
Design and research governance
This was a pilot RCT to evaluate the feasibility of completing a subsequent full trial. It was approved by NRES Committee (Coventry Research Ethics Committee and University Hospitals Coventry and Warwickshire Research and Development Department). It was also registered on the current controlled trials database ISRCTN95369715.
Setting and participants
This study was conducted between May 2009 and March 2012 in an outpatient department of a large United Kingdom teaching hospital. All patients were referred to this clinic for a surgical opinion regarding their mid-substance Achilles tendinopathy, having already failed previous non-operative management. On first presentation all patients were first seen by the orthopaedic lead for the clinic who screened all patients for eligibility.
Inclusion criteria were all patients with a clinical diagnosis of mid-substance Achilles tendinopathy. This comprised a subjective history of increasing pain on loading activities for a minimum duration of three months, objective findings of pain on palpation at a level 2 cm to 6 cm above the tendon insertion, and confirmation on ultrasonography of local tendon thickening with hypoechoic areas and irregular fibre orientation.
Patients were excluded if the tendinopathy was secondary to a systemic condition such as rheumatoid arthritis or diabetes. Achilles tendinopathies presenting at the insertion were also excluded. It is recognised that both of these subgroups of patients represent a separate population with separate underlying causes.13 Additionally, patients who had sustained a previous rupture or had undergone previous surgery on the Achilles tendon were excluded, as were patients who had had previous lower limb injuries in the previous twelve months.
All eligible patients that were screened by the lead orthopaedic consultant (MLC) were then referred to the research physiotherapist, also present within the clinic. The research physiotherapist (RSK) was responsible for providing all eligible patients with an information pack, approved by the ethics committee and the opportunity to ask questions about study participation.
Patients who agreed to participate in the study signed a consent form before being randomly allocated to one of the two treatment groups in a 1:1 ratio. Treatment allocation was determined using a computer-generated random number sequence and administered by an independent trial co-ordinator. All patients then received one of two standardised interventions.
Sample size
Given the pilot nature of this study, no formal sample size calculation was performed, but a sample of 20 in total was considered to be sufficient to assess variability between patients and crudely estimate the nature of any treatment effect. This was a small study, so the size of the treatment effect was unlikely to be estimated with great precision.
Control intervention
All patients allocated to receive the eccentric loading programme were provided with a written instructional manual and shown how to perform the exercises by the research physiotherapist (RSK). This protocol had two exercises; the first involved the patient being in a standing position with the heel over the edge of a step with the legs straight. The patient then lowered their heels beyond the level of the step. The second exercise followed the same sequence but with the knee slightly bent, to maximise activation of the soleus muscle. These exercises were performed twice a day, seven days a week for 12 weeks and then progressed as pain allowed by advancing from double-leg exercises to single-leg exercises and finally single-leg exercises with added weight via a backpack. At each session the patient completed three sets of 15 repetitions of the two exercises (i.e., 180 repetitions/day). This programme followed the original programme published by Alfredson et al.15
Study intervention
All patients allocated to receive an injection of PRP had 52 ml of whole blood withdrawn from the antecubital fossa, which was combined with 5 ml of citrate anticoagulant. This was immediately centrifuged for 12 minutes at 2400 rpm (GenesisCS Component Concentrating System, Fort Myers, Florida). After centrifugation the platelet layer (approximately 3 ml to 5 ml) was extracted using a syringe and then injected into the Achilles tendon using a peppering technique.12 This technique involved a single-skin portal followed by five penetrations of the tendon. All patients then received standardised post-treatment advice. This included advising the patient to gradually return to activities of daily living and sports as pain allowed, in addition to being advised of possible adverse events.
Data collection and outcome measures
All patients were assessed at standard clinical follow-up at six weeks, three months and six months. At these timepoints the VISA-A questionnaire was administered, which was the primary outcome measure.14 This questionnaire comprises eight questions covering the three domains of pain, function and activity, and the final score ranges from 100 (asymptomatic) to 0 (greatest disability). A change in the VISA-A of 12 points reflects a change in performing activities such as walking, jumping and sports with mild pain versus no pain.13 Such an improvement is important to patients on individual and population levels, and will lead to a change in clinical practice both locally and nationally.13 The EuroQol 5-Dimension questionnaire (EQ-5D)16 was also administered as a secondary outcome measure. This is a validated quality-of-life questionnaire comprising five domains related to daily activities, with a three-level answer possibility and a VAS ‘thermometer’, which is the participants self-rating of their health status. It has end points of 100 (best imaginable state) and 0 (worst imaginable health state).16 Any complications were recorded.
It was not possible to blind the clinician administering the intervention or the patient receiving the intervention. However, the primary data was patient-reported and the trial statistician was blinded to the intervention groups throughout.
Statistical analysis
The main analysis investigated differences in the primary outcome measure (VISA-A) between the two treatment groups on an intention to treat basis at six months after treatment. Six months was chosen from previous studies in the area13 as the point at which a patient would have improved clinically and subsequently discharged, or alternatively deemed to have failed treatment and offered further alternative management.
As this was a pilot study the analysis was exploratory in nature, with the primary aim to assess the size and direction of observed differences between the treatment groups. Formal significance testing was undertaken, assuming an approximate normal distribution for the VISA-A scores at six months and using a Student’s t-test and linear regression analysis to adjust for any imbalance in the baseline characteristics between treatment groups. Diagnostic quantile–quantile plots of the residuals were used to assess the normality assumptions. Mann–Whitney tests were used to compare EQ-5D and EQ VAS scores at six months. Statistical significance was set at the 5% level for all tests. Analyses and graphical summaries were produced using the statistical package R (R Foundation for Statistical Computing, Vienna, Austria).
Results
A CONSORT diagram of participant flow through the trial is shown in Figure 1. Following exclusions, a total of 20 patients were randomised to one of the two treatment interventions. All randomised patients received and completed their allocated intervention. The baseline demographics for the two groups can be found in Table I. One patient in the PRP group was lost to follow-up before the three-month assessment because they failed to respond to follow-up procedures.
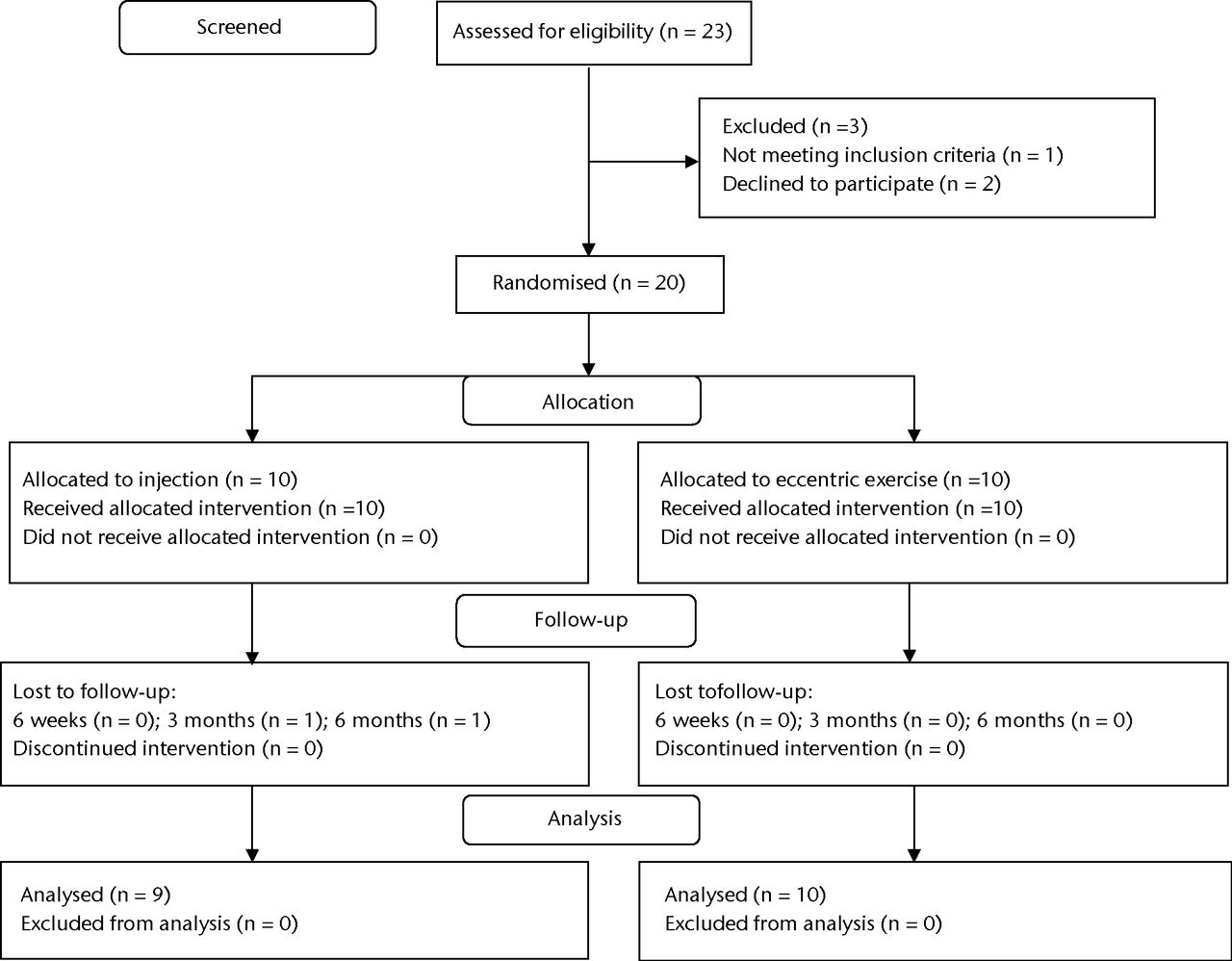
Fig. 1
CONSORT flow diagram.
Table I
Baseline demographics (PRP, platelet-rich plasma)
| Treatment group | |||
|---|---|---|---|
| Characteristic* | Eccentric exercise (n = 10) | PRP injection (n = 10) | |
| Male (n, %) | 3 (30) | 4 (40) | |
| Mean age (yrs) (range) | 49.9 (36 to 66) | 47.8 (35 to 59) | |
| Mean height (cm) (range) | 169.8 (162 to 187) | 170.7 (163 to 182) | |
| Mean weight (kg) (range) | 78.6 (57 to 112) | 82.4 (64 to 103) | |
| Smoker (n, %) | 2 (20) | 0 (0) | |
| Mean symptom duration (mths) (range) | 28.1 (8 to 144) | 30.8 (9 to 156) | |
| Mean VISA-A score (range) | 36 (5 to 71) | 41 (23 to 73) | |
| Mean EQ-5D (range) | 0.56 (0.09 to 1.00) | 0.75 (0.62 to 1.00) | |
-
* VISA-A, Victorian Institute of Sports Assessment-Achilles scores; EQ-5D, EuroQol 5-Dimension questionnaire
Figure 2 and Table II show the temporal trends for the VISA-A and EQ-5D scores for both groups. The mean VISA-A score for the PRP injection group at the primary endpoint of six months was 76.0 (95% confidence interval (CI) 58.3 to 93.7) compared with 57.4 (95% CI 38.1 to 76.7) for the exercise group; giving a crude difference of 18.6. After adjusting for the baseline VISA-A scores in a linear regression, a more realistic estimate of the treatment effect was generated of 13.3 (95% CI -6.3 to 32.9), which was not a statistically significant difference (p = 0.171). Using the same described linear regression model, patient age, gender and symptom duration were not significant modifiers of the treatment effect (p > 0.05).
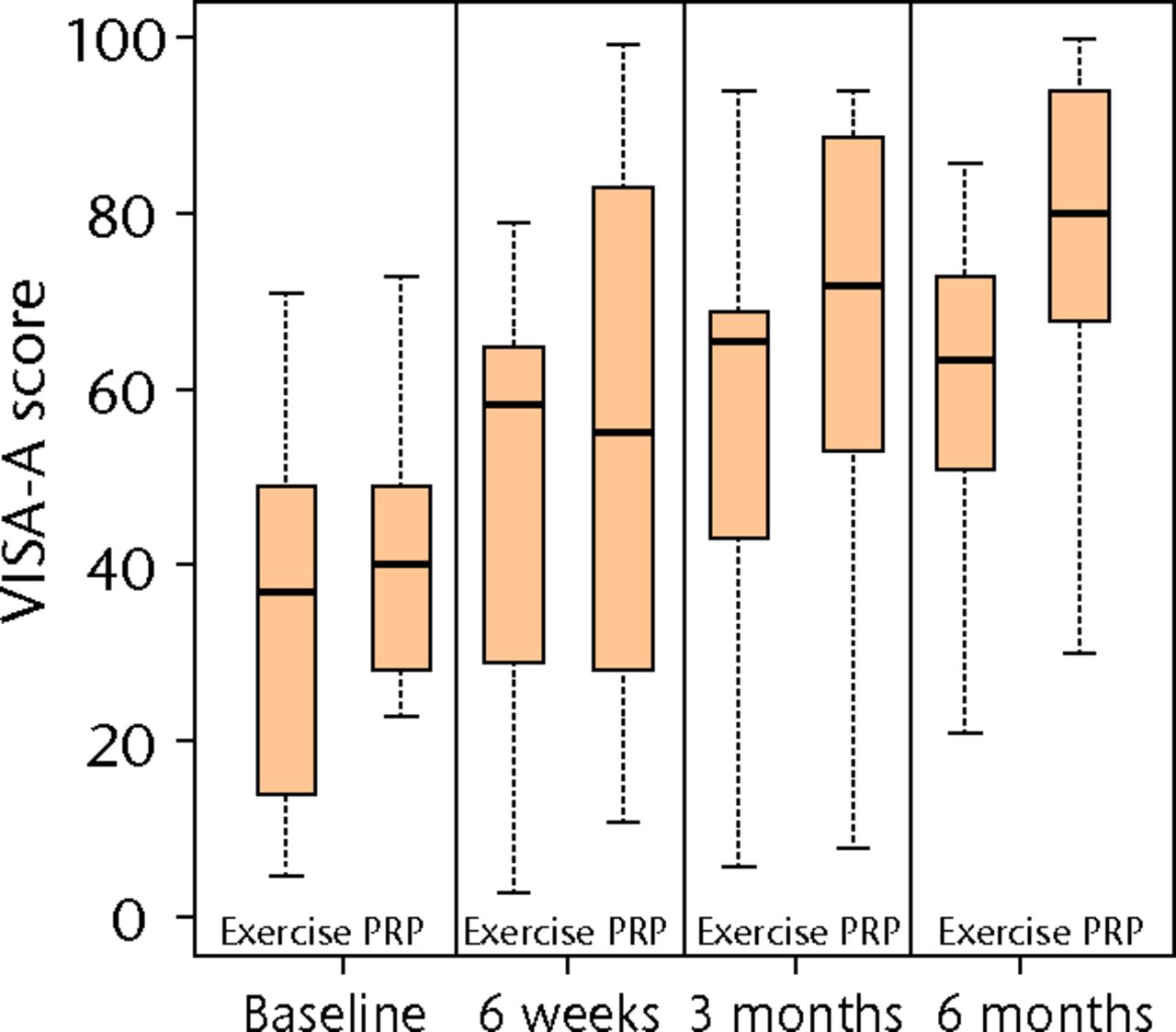
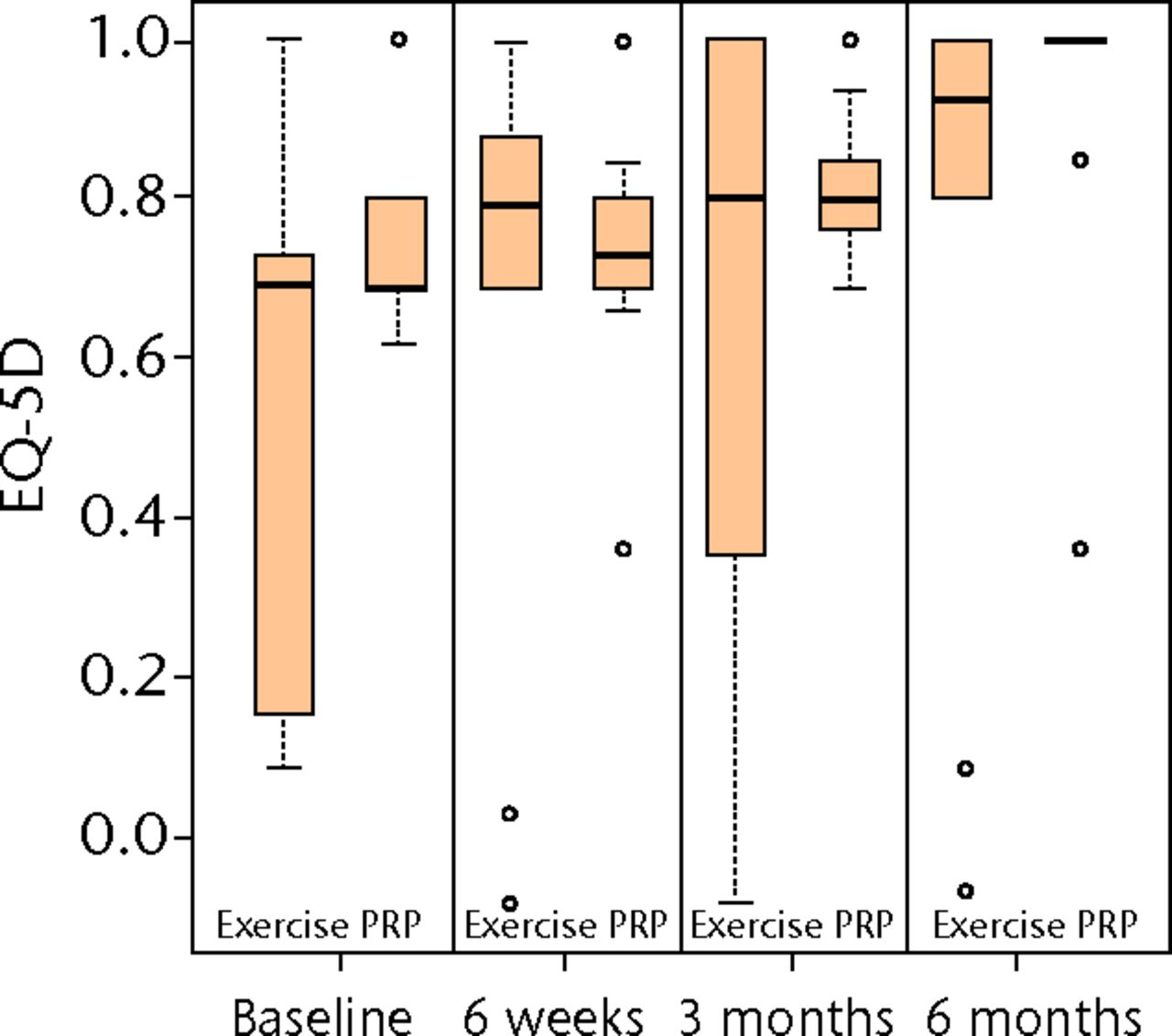
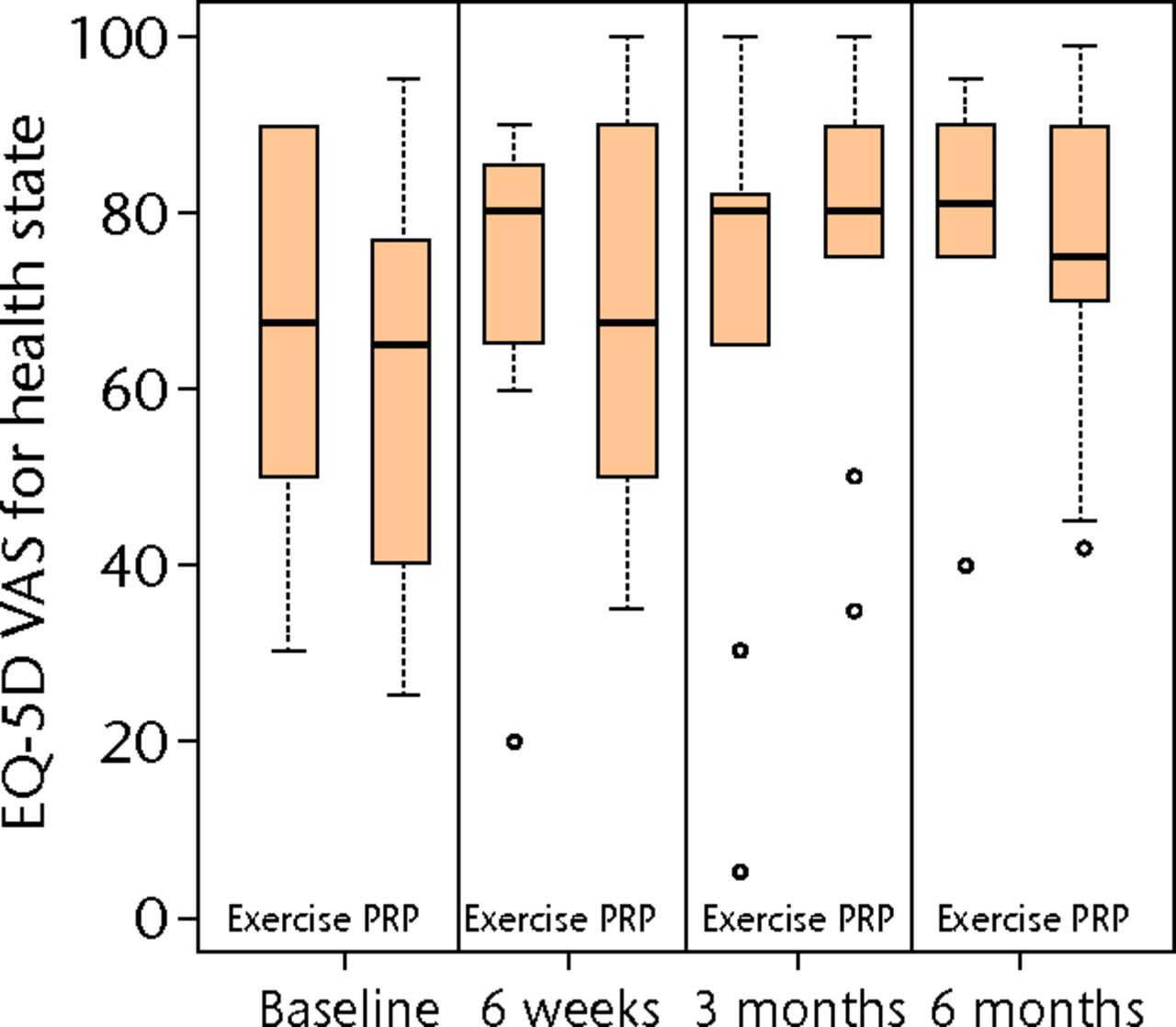
Figs. 2a - 2c
Boxplots showing a) the Victorian Institute of Sports Assessment-Achilles (VISA-A) score, b) the EuroQol 5-Dimension (EQ-5D) score and c) the EQ-5D visual analogue scale for health state at baseline, six weeks, three months and six months. The boxes denote the median and interquartile range, the whiskers denote the range of data and ° denotes outlying data.
Table II
Outcomes for both groups (PRP, platelet-rich plasma)
| Mean score (sd) | ||
|---|---|---|
| Outcome measure* | Eccentric loading | PRP injection |
| VISA-A | ||
| Baseline | 36 (21) | 41 (16) |
| Six weeks | 49 (26) | 56 (30) |
| Three months | 56 (27) | 63 (29) |
| Six months | 57 (27) | 76 (23) |
| EQ-5D | ||
| Baseline | 0.56 (0.32) | 0.75 (0.14) |
| Six weeks | 0.67 (0.38) | 0.73 (0.16) |
| Three months | 0.66 (0.41) | 0.74 (0.28) |
| Six months | 0.74 (0.39) | 0.82 (0.35) |
| EQ-5D VAS | ||
| Baseline | 67 (21) | 61 (23) |
| Six weeks | 71 (20) | 68 (23) |
| Three months | 68 (29) | 69 (32) |
| Six months | 76 (20) | 68 (30) |
-
* VISA-A, Victorian Institute of Sports Assessment-Achilles; EQ-5D, EuroQol 5-Dimension; VAS, visual analogue scale for general health state
Mann–Whitney tests at six months for secondary outcomes, EQ-5D and EQ-VAS, provided no evidence for significant treatment effects (p = 0.202 and p = 0.999, respectively). These scores represent an unadjusted analysis and the data are represented in Figure 2 and Table II. Quantile–quantile plots, and the symmetry of the six-month boxplots shown in Figure 2a, indicate that the assumption of approximate normality for VISA-A at six months was acceptable.
There were no complications recorded for either group during the six-month follow-up. At this time point, 15 patients were discharged, one was lost to follow-up and two patients from each group opted to receive a further six-month period of the non-operative treatment that they were not initially randomised to. Of these four patients, two were discharged after the further six months of non-operative management and two opted for a surgical procedure. In both cases this consisted of an initial percutaneous tenotomy followed by a later tendon debridement. No results were collected beyond the six-month trial period.
Discussion
The aim of this current research was to perform a pilot RCT in patients with failed previous non-operative management for mid-substance Achilles tendinopathy, assessing the clinical effectiveness of PRP injections in comparison with a gold-standard eccentric loading programme. The results of this pilot trial would enable conclusions to be drawn regarding the feasibility of completing a full study to evaluate the clinical effectiveness of PRP injections.
The study demonstrated that patients are prepared to forego surgery in favour of a final programme of non-operative management, with only two patients refusing to take part from a total of 22 approached. It has also provided valuable information on the possible size and direction of treatment effects for a larger study. The estimate of the standard deviation of the VISA-A score at six months was approximately 20 points. Assuming a conservative treatment effect of 12 points, an overall minimum sample size for a full trial of the PRP intervention would be 90 patients (45 in each group), for assumed normality of the outcome measure, using a two-armed parallel group trial based on 80% power and 5% significance. A study of this sample size would be feasible across a small number of additional centres.
At the time of starting this study (2009), NICE had recently performed a systematic review of the literature relating to tendinopathy and autologous blood injections. They found no RCTs in this area. Further systematic reviews on this topic were published throughout 201017-19; these highlighted one RCT13 published since the NICE review. This study investigated the superiority of an eccentric loading programme plus PRP injection versus an eccentric loading programme plus a saline injection for mid-substance Achilles tendinopathy. No statistically significant difference between the two treatments was found. However, this may be secondary to a type II error, secondary to the small sample of 27 in each group for this study. Despite there being no statistically significant findings, the direction of the treatment effect was in favour of the PRP injection group.13
Although it could be argued that what is needed now is an appropriately powered RCT to assess the clinical effectiveness of PRP, this further pilot stage was essential. The trial described above studied a subgroup of patients with Achilles tendinopathy who had not previously undergone an eccentric loading programme,13 whereas our population comprised only those who had undergone previous non-operative management. Furthermore the recruitment strategy for the previous study was through advertisement, meaning that their patients were inherently motivated and subsequently compliant, as opposed to the challenges faced by the patient presenting in a NHS tertiary referral clinic. These differences therefore warranted further piloting of trial process prior to undertaking a larger study.
No further RCTs in relation to mid-substance Achilles tendinopathy have been published comparing PRP injections with traditional eccentric loading exercises, but additional case series have. These have demonstrated clinically relevant improvements in patient-reported outcome measures and, in keeping with this study, no reported complications.20,21
It is clear that there is no definitive answer regarding the superiority of PRP injections for patients with mid-substance Achilles tendinopathy. This pilot study has demonstrated the feasibility of conducting such a study in a United Kingdom NHS setting. Before a full trial could be attempted, the limitations of this pilot study would need to be addressed. The main considerations include the blinding of assessors and recording of exercise adherence through possible inclusion of patient diaries.
Additionally, given the relatively small number of patients required for a full trial, further consideration will be required regarding the randomisation procedures, such as stratifying for known confounding factors. Finally there are also relative advantages and disadvantages to the inclusion or exclusion of a placebo arm that should be carefully considered. A placebo arm was included in the previous RCT,13 but not in our pilot study. Our main reason for this omission was because a true placebo injection for Achilles tendinopathy does not exist: both saline injections and dry needling have both been advocated by NICE as having a possible treatment effect.12 However, if a placebo arm could be implemented not eliciting a treatment effect, then this could provide an advantageous alternative to an active treatment arm to determine treatment efficacy. Additionally if an active treatment (such as the eccentric loading programme) is added to both treatment arms, as it was in the previous RCT,13 there is an unknown interaction effect of adding an eccentric loading treatment to demonstrate any differences. Therefore these considerations must be weighed appropriately against the potential importance of a placebo controlled trial for any future studies.
The authors would like to acknowledge H. Richmond for her initial contribution to the conduct of the study and the support of Warwick Medical School and University Hospitals of Coventry and Warwickshire for their support throughout the completion of the study.
1 Riley G . Tendinopathy: from basic science to treatment. Nat Clin Pract Rheumatol2008;4:82–89. Google Scholar
2 Maffulli N , KaderD. Tendinopathy of tendo achillis. J Bone Joint Surg [Br]2002;84-B:1–8.PubMed Google Scholar
3 de Jonge S , van den BergC, de VosRJ, et al.Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med2011;45:1026–1028.CrossrefPubMed Google Scholar
4 No authors listed. Management of chronic Achilles tendinopathy. Drug Ther Bull2012;50:93–96CrossrefPubMed Google Scholar
5 Sussmilch-Leitch SP , CollinsNJ, BialocerkowskiAE, WardenSJ, CrossleyKM. Physical therapies for Achilles tendinopathy: systematic review and meta-analysis. J Foot Ankle Res2012;5:15.CrossrefPubMed Google Scholar
6 McLauchlan GJ , HandollHH. Interventions for treating acute and chronic Achilles tendinitis. Cochrane Database Syst Rev2001;2:CD000232.CrossrefPubMed Google Scholar
7 Magnussen RA , DunnWR, ThomsonAB. Nonoperative treatment of midportion Achilles tendinopathy: a systematic review. Clin J Sport Med2009;19:54–64.CrossrefPubMed Google Scholar
8 Maffulli N , LongoUG, DenaroV. Novel approaches for the management of tendinopathy. J Bone Joint Surg [Am]2010;92-A:2604–2613.CrossrefPubMed Google Scholar
9 Bohu Y , LefèvreN, BauerT, et al.Surgical treatment of Achilles tendinopathies in athletes; multicenter retrospective series of open surgery and endoscopic techniques. Orthop Traumatol Surg Res2009;95(Suppl 1):S72–S77. Google Scholar
10 Andres BM , MurrellGA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res2008;466:1539–1554. Google Scholar
11 Sayana MK , MaffulliN. Eccentric calf muscle training in non-athletic patients with Achilles tendinopathy. J Sci Med Sport2007;10:52–58.CrossrefPubMed Google Scholar
12 National Institute for Health and Clinical Excellence (NICE). NICE guidance IPG279: autologous blood injection for tendinopathy, 2009. Google Scholar
13 de Vos RJ , WeirA, van SchieHT, Bierma-ZeinstraSM, et al.Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA2010;303:144–149.CrossrefPubMed Google Scholar
14 Robinson JM , CookJL, PurdamC, et al.The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med2001;35:335–341.CrossrefPubMed Google Scholar
15 Alfredson H , PietiläT, JonssonP, LorentzonR. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med1998;26:360–366.CrossrefPubMed Google Scholar
16 Brooks R . EuroQol: the current state of play. Health Policy1996;37:53–72.CrossrefPubMed Google Scholar
17 Coombes BK , BissetL, VicenzinoB. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet2010;376:1751–1767.CrossrefPubMed Google Scholar
18 Kampa RJ , ConnellDA. Treatment of tendinopathy: is there a role for autologous whole blood and platelet rich plasma injection?Int J Clin Pract2010;64:1813–1823.CrossrefPubMed Google Scholar
19 Engebretsen L , SteffenK, AlsousouJ, et al.IOC consensus paper on the use of platelet-rich plasma in sports medicine. Br J Sports Med2010;44:1072–1081.CrossrefPubMed Google Scholar
20 Monto RR . Platelet rich plasma treatment for chronic Achilles tendinosis. Foot Ankle Int2012;33:379–385.CrossrefPubMed Google Scholar
21 Gaweda K , TarczynskaM, KrzyzanowskiW. Treatment of Achilles tendinopathy with platelet-rich plasma. Int J Sports Med2010;31:577–583.CrossrefPubMed Google Scholar
Funding statement:
Chartered Society Research Foundation provided funding for this pilot study. They did not have a role in study design, collection, analysis/interpretation of data, writing of the manuscript or in the decision to submit the manuscript for publication.
Author contributions:
R. S. Kearney: Study design, Data collection and analysis, Final write-up
N. Parsons: Study design, Data analysis, Final write-up
M. L. Costa: Study design, Data analysis, Final write-up
ICMJE Conflict of Interest:
None declared
©2013 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









