Abstract
This review briefly summarises some of the definitive studies of articular cartilage by microscopic MRI (µMRI) that were conducted with the highest spatial resolutions. The article has four major sections. The first section introduces the cartilage tissue, MRI and µMRI, and the concept of image contrast in MRI. The second section describes the characteristic profiles of three relaxation times (T1, T2 and T1ρ) and self-diffusion in healthy articular cartilage. The third section discusses several factors that can influence the visualisation of articular cartilage and the detection of cartilage lesion by MRI and µMRI. These factors include image resolution, image analysis strategies, visualisation of the total tissue, topographical variations of the tissue properties, surface fibril ambiguity, deformation of the articular cartilage, and cartilage lesion. The final section justifies the values of multidisciplinary imaging that correlates MRI with other technical modalities, such as optical imaging. Rather than an exhaustive review to capture all activities in the literature, the studies cited in this review are merely illustrative.
Introduction
The gradual degradation of articular cartilage is a hallmark of osteoarthritis (OA), a major musculoskeletal disease that contributes to the number one cause of disability in adults.1-3 Anatomically, articular cartilage is a thin layer of connective tissue covering the load bearing ends of bones in joints to absorb shocks and distribute loads. While the structure and properties of healthy and diseased cartilage have been studied extensively, an accurate diagnosis of early OA in humans, at a stage when a clinical intervention might potentially be useful, remains elusive in practice.4 The early diagnosis has two main obstacles: 1) the early changes in the tissue’s fine structure and delicate functions markedly precede the development of OA as a clinical disease; and 2) current diagnostic imaging has insufficient resolution and unsatisfactory sensitivity to detect early (i.e., small) lesions in cartilage.
In order to overcome the first obstacle, animal models of the human disease have been established. Since OA is a slow-progression disease spanning many years, the disease remains silent at the early stages. When a patient presents to a clinic, it is usually due to complaining of pain in the joints – unfortunately, pain correlates poorly with what the cartilage looks like, whether histologically, arthroscopically, radiologically or on MRI.5-9 As one cannot initiate a disease in humans, and is it not possible to test some potential disease-modification drugs on humans before the drugs’ effectiveness is demonstrated, animal models serve an essential purpose in human biomedical research. Once we understand the degradation of tissue from the very beginning (the most vital period) to the end, step-by-step, in animal models, an intervention at the early stages before the point-of-no-return could be designed to eventually save human joints.
In order to overcome the second obstacle, one must have high-resolutions in any diagnostic imaging.10 This is because articular cartilage contains complex, depth-dependent structures and localised progression of disease across its thin thickness. Since MRI is the only totally non-invasive imaging tool with excellent soft-tissue contrast, microscopic MRI (µMRI) is the logical bridge that would be able to translate the findings between invasive procedures (e.g., light microscopy or biochemical assay) and clinical MRI diagnostics. As there is no difference in physics principles and engineering architectures between µMRI and clinical MRI, there exists no fundamental obstacle that will prevent the realisation of higher resolutions in clinical MRI scanners.10,11 Since the tissue or organ in common animal models are smaller than human tissues and organs, and since the goal of any biomedical research is to identify the early lesion, the use of high-resolution µMRI in animal studies is an effective and logical strategy to identify the parameter or procedure that might be clinically useful.
This review briefly summarises some of the definitive studies of articular cartilage by µMRI that have the highest spatial resolutions. Rather than an exhaustive review to capture all activities in the literature, the studies cited are merely illustrative. The interested readers can easily find additional readings and many equally important studies with a key-word search.
Articular cartilage
Articular cartilage in large animals and humans contains a scattered population of living cells (chondrocytes), which accounts for 1% to 2% of the total tissue.12 Most of the cartilage is extracellular and composed primarily of three molecules: water (approximately 70%), collagen (approximately 20%) and proteoglycan (approximately 5%).13,14 The collagen in the tissue is primarily type II triple-helical fibrils that form a 3D matrix.15 The proteoglycan (PG) has a bottle-brush-like structure with numerous side-chains of sulfated glycosaminoglycans (GAGs).16 Structurally, articular cartilage is highly ordered and is commonly considered to comprise three sub-tissue zones based on the local orientation of the collagen fibrils (Fig. 1a).17,18 These three zones are the superficial zone (SZ) where the collagen is orientated parallel with the articular surface, the transitional zone (TZ) where the collagen is orientated rather randomly, and the radial zone (RZ) where the collagen is orientated perpendicularly to the articular surface. In addition, many of the biological and physical properties of articular cartilage are also known to be depth-dependent (Fig. 1b).13,14,16,19
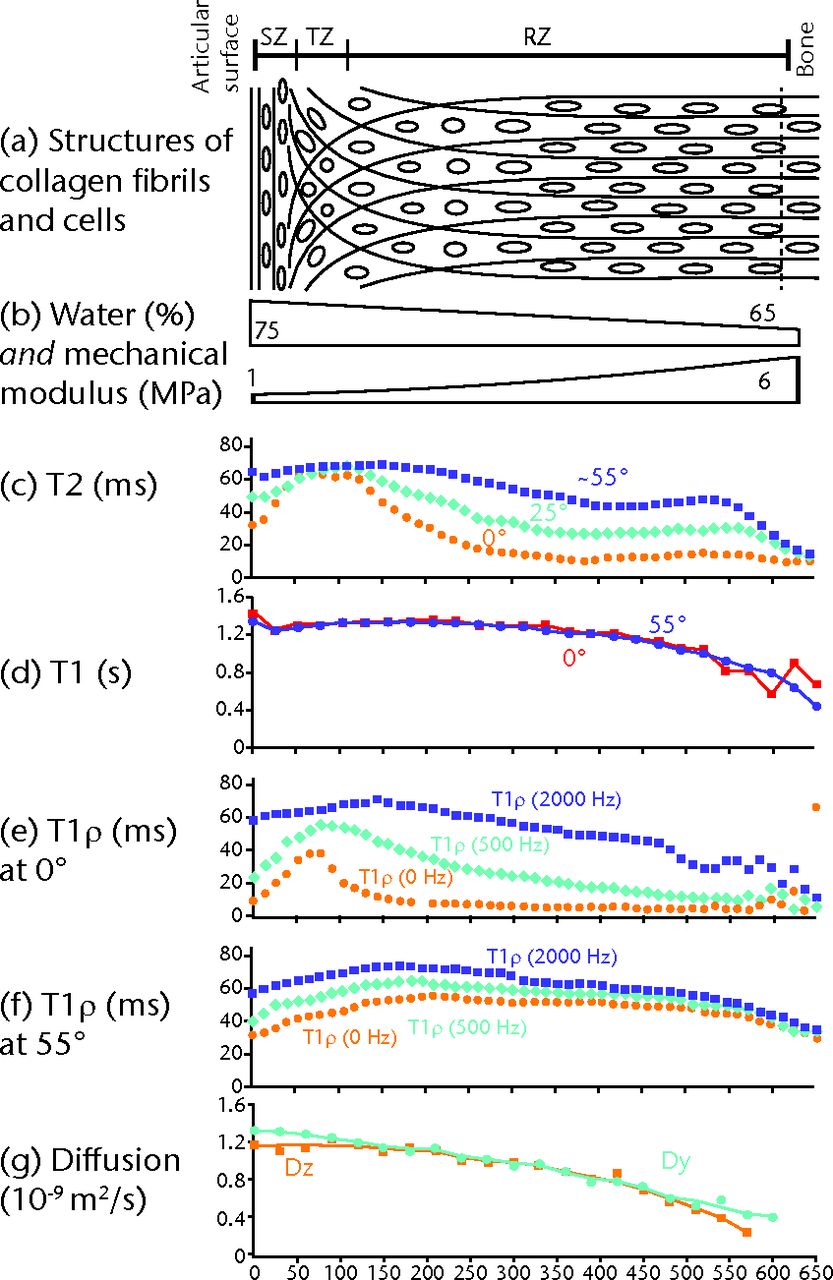
Fig. 1
The depth-dependent structures and properties of canine humeral articular cartilage by µMRI. Figure 1a – schematic diagram of cartilage showing the orientation of the collagen fibrils (the short lines) in different histological zones and the orientation of the chondrocytes (the circles and ovals) (not to scale). Figure 1b – approximate water concentration and depth-dependent compressive modulus in cartilage. Figure 1c – T2 anisotropy profiles of articular cartilage at different specimen orientations (0° is when the articular surface is perpendicular to the magnetic field direction). Figure 1d – T1 profiles of articular cartilage at two specimen orientations. Figures 1e and 1f – T1ρ profiles of articular cartilage under three different spin-lock powers e) at 0° and f) at 55°. Figure 1g – self-diffusion profiles of articular cartilage when the diffusion gradient is applied in the two different directions.
It should be noted, however, that the common definition of these ‘sub-tissue zones’ is only a concept, not a clearly defined reality.19 Anyone who uses a high-resolution imaging tool to examine a piece of articular cartilage would not find any sharp ‘line’ or ‘border’ across which the property of tissue differs distinctively. Instead, the tissue changes gradually over a finite distance, which yields a continuous function of tissue properties. Consequently, any criterion to truncate the continuous function into several discrete zones is intrinsically arbitrary and model-dependent.19 The concept of these ‘discrete zones’, however, is a useful one in terms of focusing attention to a particular portion of tissue.
MRI and µMRI
The principles of nuclear magnetic resonance imaging (NMR Imaging or MRI) are well understood. At the heart of the technique is the linear proportionality between the precessional frequency of the nuclei (e.g., protons in water) and the magnitude of the external magnetic field in which the nuclei are immersed. By making the magnetic field deliberately non-uniform (commonly a linear gradient), the frequency of these nuclei will differ from one location to another across the sample. The location of the nuclei is, therefore, encoded by a shift of their precessional frequencies. By setting up three gradient fields in three orthogonal directions, the nuclei (or water molecules) can be spatially encoded in any 3D or 2D space.
With the scaling down of the receiver coil and fine-tuning of the instrument, the resolution of MRI could be as fine as 10 µm. When the size of one volume element (voxel) of the image is < 100 µm, MRI is termed NMR microscopy (microscopic MRI, or µMRI).11,20-22 µMRI is exceptionally well suited for thestudy of spatially heterogeneous biological materials in animal models because of its high sensitivity to the molecular environments. The ability to produce microscopic-resolution images of molecular-level environments and activities distinguishes µMRI from other microscopic imaging methods such as ultrasound or CT. The fact that µMRI shares identical physics principles and engineering architectures with clinical MRI ensures the direct relevance of the µMRI results to clinical MRI research. Since the proton (1H) is the most common nucleus in biological systems, and also the most sensitive nucleus among all nuclear species, the majority of MRI experiments are carried out to measure the protons in water molecules. In articular cartilage, MRI essentially measures extracellular matrices (the cells are invisible in MRI), where the depth-dependent characteristics of the collagen matrix play the foremost role.
Concept of MRI contrast
The exquisite sensitivity of MRI signals to molecular motion and molecular dynamics is the true value of this non-destructive and non-invasive imaging technique, a technique that can best be understood in terms of image contrasts.11 There are several fundamental mechanisms in MRI contrasts. For example, if the molecule moves physically from one location to another during an imaging experiment (such as the blood flow in animal or human, or vascular flow in plants), the MRI signal will carry an extra phase shift associated with the flowing molecule. The molecule’s velocity can be quantified in the subsequent image analysis.23,24 Another example relates to the time delay for the molecules when they are excited and sampled, which will manipulate the MRI signal with a complex attenuation that can also be quantified. In principle, it is difficult to acquire a map of the true water density in MRI, as any image in MRI is always weighted by several intrinsic image contrasts regardless of whether the experimentalist intends to image the contrast or not. A perfect example of this type of unwanted image contrast is the ‘magic angle effect’ in MRI of cartilage25; this effect has an orientation-dependent inconsistency between the known water content and the image intensity of the specimen.
Three relaxation times are the most important in MRI of cartilage. They are T1 (the spin-lattice relaxation time), T2 (the spin-spin relaxation time), and T1ρ (the spin-lattice relaxation time in the rotating frame). The fundamental principles of spin relaxation in MRI reside deep in NMR physics and are beyond the scope of this review. In essence, these relaxation times are closely related to different frequencies of the molecular dynamics. It can be shown that T1 is sensitive to the highest frequencies of molecular motions, or frequencies close to the precessional frequencies in the MHz range; T1ρ is sensitive to the intermediate frequencies of molecular motions, probably in several or tens of kHz; and T2 is sensitive to the lowest frequencies of molecular motions involving static or slowly fluctuating magnetic fields.26
A subtle feature of the relaxation time measurement in biological systems is the possible dependency of their values on the specimen orientation in the external magnetic field, where the relaxation times are said to have anisotropy. The main cause of the relaxation anisotropy is the dipolar interaction between the water molecules and the (less mobile) macromolecules in the tissue. In addition, the exchange between different molecular environments and cross relaxation between protein macromolecules and water are important aspects of the relaxation mechanism in biological materials. For articular cartilage, T1 relaxation was found to be isotropic while T2 relaxation was found to have a strong orientational dependence that varied as (3cos2θ – 1),27 the geometrical factor that dominates the non-zero dipolar Hamiltonian. These characteristics are the indications of slow macromolecular motion in cartilage, likely related to the highly constrained motion of swollen proteoglycans in the collagen matrix.28
In addition to three relaxation times, several other MRI parameters can also cause the image contrast of cartilage in MRI, including self-diffusion (D) and magnetisation transfer (MT). Self-diffusion measures the random Brownian motion, a motion that results in the loss of the MRI signal amplitude and can be quantified with the use of specific imaging sequences. The diffusion images from articular cartilage also have some anisotropy,29 which is weaker than the T2 anisotropy in cartilage. Since D and MT are smaller effects than the relaxation times are, measurements of self-diffusion and magnetisation transfer are less common in the MRI of cartilage.
Experimentally, since the images from MRI are intrinsically weighted by some of these image contrast factors, one can use specific pulse sequences to purposely acquire a weighted image (e.g., T1- or T2-weighted). Alternatively, one can acquire a series of images, each weighted by the same contrast factor but by a different amount. A subsequent pixel-by-pixel calculation can construct a parameter image, such as a T1 or T2 image. The practice of calculating the parameter images has become common clinically.
Characteristics of articular cartilage by MRI at microscopic resolution
It has been known for two decades that normal (i.e., healthy) articular cartilage can appear laminated in MRI when the tissue is placed at certain orientations with respect to the external magnetic field.25,30-33 In other words, instead of having a uniform intensity, several thin sub-layers that are parallel with the articular surface (the surface of the tissue) can be seen inside the cartilage. Each of the sub-layers has a different intensity and thickness.31,33,34 When the tissue surface is placed at about 55° with respect to the main magnetic field, the tissue intensity can become homogeneous and higher than at other angles. Since 54.57° is known as the magic angle in MRI physics, this laminar appearance of articular cartilage is also known as the magic angle effect of cartilage in MRI literature.35-37
T2 relaxation
The origin of the magic angle effect in MRI of cartilage soon became clear with the discovery of T2 anisotropy in articular cartilage in the mid-1990s. In essence, T2 in articular cartilage is both depth-dependent and orientation-dependent, as shown in Figure 1c.27,38 These features of T2 anisotropy were immediately interpreted as being caused mainly by the depth-dependent structure of the collagen matrix in cartilage,25 which imposes a depth-dependent motional anisotropy for the protons in the tissue due to their close interaction with the collagen matrix. A set of quantitative criteria was soon developed in a combined µMRI and polarised light microscopy study that successfully divided the tissue into three MRI zones, which were statistically equivalent to the three histological zones in cartilage.39 This equivalency between the µMRI zones and histological zones is important because it demonstrates that just as noncalcified cartilage can be conceptually subdivided based on the orientation of the collagen fibers into three distinct structural zones in histology, in µMRI, a piece of articular cartilage can also be subdivided based on the regional characteristics of T2 relaxation into three structural zones. This enables multidisciplinary research of cartilage.
T1 relaxation
In contrast to the strong T2 anisotropy in articular cartilage, T1 in articular cartilage is known to be isotropic to the magnetic field direction and has only a small dependency on its tissue depth, as shown in Figure 1d.25,40 This isotropic T1 and anisotropic T2 in healthy cartilage tissue indicate precisely the very slow motion of the water molecules, which came from the highly constrained proteoglycans in cartilage (hence the lack of high frequency motions). In most imaging experiments, therefore, T1 itself would cause few complications in MRI of articular cartilage. Recently, a laminar appearance that had the opposite intensity pattern was observed in MRI of cartilage by using some fast imaging sequences.41 This reversed laminae was attributed to the small reduction of T1 in the deep tissue (Fig. 1d), which causes the intensity elevation of the deep tissue in MRI, a phenomenon noted earlier as “uncertain etiology” by McCauley and Disler.42 This reversed laminae in cartilage reminded us that the underlying molecular structure in cartilage and the imaging methodology should both be considered when one examines the appearance of cartilage in MRI.
The usefulness of T1 relaxation in MRI of cartilage came from the development of the dGEMRIC (delayed Gadolinium Enhanced Magnetic Resonance Imaging of Cartilage) protocol in MRI. This protocol doped the tissue with the Gd(DTPA)2- contrast agent. Since these charged ions distribute in the cartilage in an inverse relation compared to the concentration of the negatively charged GAG molecules, and since gadolinium is a paramagnetic ion that shortens the T1 relaxation, a GAG image can be constructed based on two T1 images acquired before and after the patient is injected with the Gd contrast agent.43-48 This dGEMRIC procedure has become important in the clinical detection and management of joint diseases, since the reduction of GAG in tissue will result in a biochemically and biomechanically weakened cartilage, an early sign of the tissue degradation. A set of high-resolution GAG profiles was obtained in µMRI,49 which shows that the GAG concentration in canine cartilage is approximately a linear function, increasing from the superficial zone to the radial zone. One peculiar feature in the original dGEMRIC protocol was an arbitrary scaling constant of two,43 which was needed to make the experimental values between MRI and histochemistry agree with each other. This arbitrary scaling factor was recently found to be unnecessary.50
T1ρ relaxation
The sensitivity of T1ρ relaxation to molecular motion is in a frequency range between T1 and T2; the slow (but not static) motional interactions between the confined water molecules and the macromolecules makes T1ρ less sensitive to the local fibril orientation. A unique feature (or complication) of T1ρ relaxation is its dependency on the strength of the spin-lock field (the radiofrequency field that locks the magnetisation in the transverse plane), a phenomenon termed as T1ρ dispersion (Figs 1e and 1f).51 When the power of the spin-lock field is zero, T1ρ relaxation is identical to T2 relaxation, which has all the features of T2 anisotropy (note the similarity between T1ρ at 0 Hz in Fig. 1e and T2 at 0° in Fig. 1c). With an increase of the spin-lock strength, T1ρ becomes less anisotropic, less depth-dependent, and has higher values. With a sufficiently high spin lock field (e.g., > 2000 Hz), the influence of the dipolar interaction to spin relaxation is sufficiently minimised to yield the measurement of a ‘true’ T1ρ (note the similarity of the T1ρ profiles at 2000 Hz in Figures 1e and 1f).
In most clinical MRI scanners, this ‘sufficient spin-lock power’ condition is never met, since most clinical MRI can only have the spin-lock field < 1000 Hz. The T1ρ value and profiles from the clinical MRI experiments are hence not ‘pure’ and still subject to the influence of the dipolar interaction (hence a small amount of the magic angle effect). Nevertheless, a reduced sensitivity to the dipolar interaction (and hence to the specimen orientation in the magnetic field) is a welcome feature in human imaging where the subject orientation in the magnet cannot be adjusted easily.
Diffusion
Self-diffusion quantifies random Brownian motion, which can be measured in µMRI by the incorporation of a pair of specific gradient pulses in the imaging sequence.24 In early MRI literature, the value of self-diffusion was shown to be relatively uniform for most of the upper tissue and to decrease in the deep tissue. In addition, a small diffusion anisotropy (i.e., diffusion tensor52,53) was found in articular cartilage (Fig. 1g)29,54 and found to occur at surface and deep portions of the tissue when the diffusion gradients were pointed to different directions (Fig. 1g). Several groups reported the increase of self-diffusion by about 20% to 30% upon trypsin-induced cartilage degradation.55,56 A more comprehensive study of the role of the diffusion measurement in cartilage degradation involved the use of several biochemical assays - each manipulated the GAG and collagen contents in cartilage distinctly differently.57 The measurement suggested that the diffusion wasnot sensitive solely to the proteoglycan content of cartilage. Furthermore, analysis for collagen degradation established that diffusion does not depend solely on the collagen content of the tissue either. A likely mechanism to account for the increase in MRI diffusion values in both naturally occurring lesioned and biochemically-degraded cartilage is one in which microscopic holes are created that take the place of the macromolecules that have been degraded. Water fills these holes, and it is the enhanced freedom of water to diffuse within these holes that produces an increase in diffusion. This suggestion is supported by the observation that degenerated cartilage contains more water than normal tissue.58 Compared with the widespread usage of relaxation measurement in MRI of cartilage, the potential for diffusion in MRI of cartilage may require further work.59
Multi-component relaxation
Since the dynamics of water molecules closely reflect the macromolecules they are associated (interacted) with, there must be more than one population of water in articular cartilage or any biological tissue. For example, one can speculate about the existence of the water associated with the collagen (the crystalline water), the water associated with the proteoglycan (the hydration water), and the relatively free water60 (ignoring for the moment the exchanges among the different populations and the distribution of each population). Therefore, each relaxation time could have at least three components, each corresponding to one particular type of water population. In other words, the general practice in MRI that assigns only one value to T1, T2, or T1ρ at any pixel location is an approximation. While this is a correct statement in principle, whether one can measure all the components experimentally depends upon many practical factors. First, the individual components must be sufficiently different from each other for them to be resolved. Secondly, the exchange process among the spin populations must be sufficiently small to allow the differentiation of the individual components. Thirdly, the MRI instrument and experimental protocols must be capable of resolving the individual components. Finally, one must have an adequately small imaging voxel (i.e., high resolution) to avoid simple volume averaging of different morphological structures. What contributes to each of the four factors is beyond the scope of this brief review. Some succinct statements on multi-component relaxation can be made here.
1. Just as the fact that the notion of the discrete sub-tissue zones in articular cartilage is only a concept, the notion of three discrete water populations in cartilage is also only a concept. In reality, there are layers and layers of water molecules surrounding any macromolecule. Each outside layer would have weaker interactions with the macromolecule. In addition, the water molecules in each layer are also constantly moving to different layers. Hence, the distribution of any water population could never be a delta function, but would be a broad distribution peak. By the theory of relaxation physics, exchanges among these different populations do exist, which would further blur the distinction of the individual relaxation peaks.
2. Common protocols in clinical MRI and µMRI all have a minimum echo time of several milliseconds, during which the molecular information is not accessible. Most imaging experiments, hence, cannot resolve the shortest relaxation components (the water molecules that tightly interact or bind with the macromolecules). In addition, most relaxation protocols do not have sufficient resolutions to differentiate between different relaxation components. Consequently, almost all quantitative relaxation experiments by clinical MRI and µMRI in literature resolve only one relaxation component, which is influenced jointly by different amounts of all water populations. The only way to resolve multi-component relaxation in any imaging experiment is to reduce the min echo time61,62 and to increase the relaxation resolution.63-67
3. The result of a multi-component relaxation experiment depends greatly upon the size and orientation of the imaging voxel. This is because any bulk (i.e., by spectroscopy) or low-resolution imaging must have its signal from a tissue volume that is large enough to contain many different structures, and hence, exhibiting several relaxation components. Only by µMRI can one measure multi-component relaxation coexisting intrinsically in the tissue. Recently, the role of dipolar interactions toward the measurements of multi-component relaxation in MRI of cartilage was noticed in several studies. Briefly, bovine nasal cartilage was found to have two T2 components in some studies67-69 and one T2 component in some other studies.63,65 Based on a ‘re-discovery’ that nasal cartilage has a residual fibril anisotropy,70 we recently provided the explanation that can unite the inconsistency in the literature66: there is a transition between multi-components and the mono-component in nasal cartilage, due to the influence of the residual collagen anisotropy and the strength of the spin-lock field.66 These recent results demonstrate that the specimen orientation and experimental parameters must be considered for any multi-component analysis, even for nasal cartilage that is commonly considered homogenously structured.71,72
Factors that influence the cartilage appearance in MRI
In addition to the contrast factors that are important in MRI of cartilage, many experimental and tissue factors can also influence the appearance of articular cartilage in MRI and the detection of cartilage lesion by MRI. Detailed features of these experimental and tissue factors are beyond the scope of this review. Several factors are briefly discussed in this section.
The resolution scaling law in MRI of articular cartilage
The image resolution in MRI has non-trivial effects on the outcomes of the experiment. The dimension of an image voxel includes not only the common pixel resolution and slice thickness, but also the orientation of the universally pencil-shaped voxel in MRI.10 The goal in any biomedical MRI is to tailor the dimensions and orientation of the image voxel to maximise the homogeneity of the molecular environment in each voxel, consequently reducing any artifacts due to partial volume averaging among different molecular populations (e.g., small lesion and large background tissue) within the same voxel. A resolution scaling law in MRI of cartilage10 was formulated several years ago, which concluded that the transverse resolution in MRI for cartilage should ideally be 2% of the relative tissue depth per image pixel, which translates to about 40 µm for a 2 mm thick tissue (such as human knee or hip). This ideal resolution, at the present time, still poses challenges to whole-body MRI scanners. However, the societal importance of managing joint diseases, which is the number one cause of disabilities in the population,3 is a sufficient motivation for all of us to work together to design higher-resolution MRI systems (e.g., extremity MRI) around this problem, and to develop novel MRI protocols (e.g., short echo-time protocols) that are exquisitely sensitive to the unique events in the early degradation of cartilage.
Strategies in image analysis for MRI of articular cartilage
Whether or not we have high resolution to resolve the details of articular cartilage, the layered-fan strategies in image analysis can improve the sensitivity and specificity of our MRI results. First, since T2 anisotropy is always present in articular cartilage, we should always sub-divide the tissue in post-acquisition image analysis into several parallel layers, i.e., surface layer, middle layer, and deep layer. This layered analysis would always produce better results than obtaining a value that averages across the tissue depth. Secondly, instead of dividing the tissue into three equal-thickness layers, if the data allows, we should give the layers different thicknesses, with the first and second layers being the thinnest possible, and the last layer representing the thickest deep tissue. This way, one tries to separate and emphasise the differences among the three zones. Finally, instead of grouping all the values in each layer together, we should divide the tissue into several fan-segments (each at a different orientation with the magnetic field) and group all the values in any layer that have approximately the same orientation with respect to the magnetic field, in order to account for the consequences of the magic angle effect.
Imaging visualisation of the total tissues
Not all cartilage can be visualized in an MRI experiment. Since the echo time (TE) in common MRI pulse sequence is finite (typically 10 ms), it is difficult to image the portion of the tissue/water that has a T2 relaxation time much shorter than the experimental TE. In cartilage MRI, the finite-width dark band between the soft tissue and bone might contain the invisible tissue. This is because the effects of TE and the relaxation time (e.g., T2) are accounted for in MRI as a ratio: exp(-TE/T2). For example, if the TE equals the T2, the value of exp(-1) is 0.37, which means that this particular tissue/molecule component that has a T2 relaxation time of 10 ms makes only a small contribution towards the total signal. If the ratio of TE over T2 becomes -5, this portion of the tissue would contribute less than 1% towards the total signal, i.e., this portion of the tissue with a T2 of 2 ms, when imaged under a TE of 10 ms, will be practically invisible on MRI. In order to image the total tissue, therefore, one needs to minimise the echo time of an MRI experiment.
Topographical variations of tissue properties
Cartilage is not a homogenous material.73-82 This is certainly true for cartilage from different joints or from different species. Even for cartilage from different sampling sites of the same joint, a number of topographical variations in the chemical, physical, optical, and mechanical properties have been observed83; all of these topographical variations are also age-dependent,84 which is probably a consequence of the load-bearing and motional patterns for particular joints and animals. Because of these topographical variations, one must keep in mind that a different sampling site within a relatively small area of the same joint surface may significantly alter the outcomes of the study, regardless of whether the study is morphological or mechanical, physical or chemical, in vivo or in vitro, clinical or in laboratory, or spectroscopic or imaging.
Surface fibril ambiguity
The orientational change of 90° for collagen fibrils between the SZ and the RZ is well accepted. This 90° orientational change, however, still leaves the freedom for the surface fibres to distribute themselves in any orientation in a 2D plane that is parallel with the articular surface: hence the observation of a “surface fibril ambiguity” in MRI literature.38 Consequently, although the deep portion of the profiles of T2 or T1ρ at small spin-lock powers is consistent among a group of cartilage specimens, the surface portion of the profiles of T2 or T1ρ at small spin-lock frequencies can still fluctuate. Several years ago, a µMRI experiment was conducted purposefully to examine the surface fibril anisotropy.85 A clear periodicity was found in the T2 anisotropy at the superficial zone, which demonstrates that the distribution of the collagen fibrils in the superficial zone is not random. A collagen architecture model was formulated to interpret the experimental data, which suggests a potential mechanism that might be used to detect the fibrillation on the surface of articular cartilage in vivo and would provide an early indication of tissue degradation and joint disease.
Deformation of articular cartilage due to external loading
Since collagen orientation can significantly alter the intensity pattern of cartilage in MRI, a deformed collagen matrix in cartilage (which is natural to cartilage as a load-bearing tissue) must have some consequences in MRI of cartilage. Several µMRI studies during the last ten years have documented the functional changes of the tissue due to the external loading. In essence, the value of the relaxation will decrease in loaded cartilage. In addition, contrary to what has been observed in normal (unloaded) cartilage at the magic angle (Fig. 1c), the loaded tissue at the magic angle no longer appears homogenous. Instead, there is a distinctly laminar appearance at the magic angle, which contains a strain-dependent dark band.86,87 This work implies that compression can become a controllable mechanism in MRI to induce new image contrast and can be, in every sense, a functional study of the tissue’s structures and properties – compression may be used to study the damage to the fine structure of the collagen matrix, and the reduction of the proteoglycan contents in cartilage. This topic is being studied actively in our lab in recent years.88-90 MRI of loaded cartilage has also been realised in recent years in clinical MRI.91,92
Cartilage lesions
The theory of MRI physics implies that the values of the relaxation and diffusion would increase if the water molecules were in a less viscous and freer environment. The mechanisms of cartilage degradation leads to the knowledge that cartilage would have less proteoglycan, more water, and bigger spaces between the fibrils during the early stages of tissue degradation. Consequently, one can conclude that the values of the relaxations and diffusion would increase when the tissue is degraded (provided that the fibril matrix is still intact), which has indeed been observed in T2 relaxation,63,93 T1ρ relaxation44-48,51 and self-diffusion.55-62
The accuracy and sensitivity of these molecular-level imaging biomarkers, however, are less than ideal at the present time4 due to some of the factors discussed previously in this review. By reducing the size of the imaging voxel, one can improve the homogeneity of the molecular environment, consequently reducing any artifacts due to partial volume averaging, competing mechanisms, and topographical variations. By reducing the echo time and improving the MRI system, one can reduce the dipolar interaction, consequently reducing orientational influences to T2 and T1ρ. Finally, by using multidisciplinary techniques, one can discriminate among the various factors and changes and their influence on the functional integrity of cartilage as a load-bearing biological tissue. This would provide critical information towards the development of novel methods for early detection and effective monitoring of the aetiology of cartilage diseases at both clinical and molecular levels.
Conclusions
In contrast to most clinical MRI studies of cartilage for which the imaging resolution is about several hundreds of microns or poorer, µMRI can have a transverse resolution as fine as tens of microns across the depth of the cartilage tissue. At the present time, this high resolution is not possible in clinical MRI. Hence, µMRI at the present stage is a basic research tool for the benches in the labs. Given the fact that µMRI shares the same physics and engineering principles with clinical MRI, however, µMRI study of animal models of human diseases presents itself as a logical and ideal combination. µMRI has direct and immediate clinical relevance in that one can identify the parameter or procedure that might be useful clinically. In addition, since µMRI resolutions sit between those of ultrastructural optical/electronic imaging and clinical MRI, the µMRI projects provide an ideal intermediate-resolution platform to coarse-grain the ultrastructural results for an eventual clinical application.
We have been studying articular cartilage for nearly 20 years in our lab, initially using µMRI because it can map the physical properties of viable cartilage in its near-native environment. In the later 1990s, we started to incorporate PLM (polarised light microscopy) in our work, because PLM (the gold standard in histology) can image the collagen organisation,39,90,93 which modulates the µMRI signal. As our projects progressed, we needed to image the molecular concentrations in the early lesion cartilage as directly as possible, at high resolutions. For this reason, since 2005, we started to use FTIRI (Fourier-transform infrared imaging) in our work. FTIRI is sensitive to the vibration of dipole moments of chemical bonds in tissue.94-97 In addition to these high-resolution imaging tools, we also employ several biomechanical and biochemical methods – each measures a unique aspect of the tissue’s bulk properties and can be correlated with the spatially resolved changes in imaging. This multidisciplinary research approach is a recognition that many of today’s biomedical problems are best addressed using multi-disciplinary techniques; each method has its own scientific merit.19 In view of the molecular and ultrastructural changes due to early diseases and the interdependent structure-function-property relationships in tissue, applying multi-disciplinary techniques can discriminate among the various factors and changes and their influence on the functional integrity of cartilage as a load-bearing material. Even though neither µMRI nor PLM nor FTIRI has the resolution to identify individual collagen fibrils or other molecules, a multidisciplinary micro-imaging approach can identify subtle changes in the morphological structure and molecular concentration in cartilage due to natural lesions and mechanical loading, enabling the monitoring and prediction of early changes in tissue that lead to cartilage degradation as a clinical disease.
The author is grateful to the R01 grant from the National Institutes of Health (NIH AR 052353), to the students and staff in the laboratory who undertook the original cartilage imaging work, to Dr C. Les (Henry Ford Hospital, Detroit) for stimulating discussions, to Miss A. Xia (College of Engineering, University of Michigan) and Ms C. Searight (Department of Physics, Oakland University) for editorial comments on the manuscript.
1 Helmick CG , FelsonDT, LawrenceRC, et al.Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum2008;58:15–25. Google Scholar
2 Lawrence RC , FelsonDT, HelmickCG, et al.Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part II. Arthritis Rheum2008;58:26–35. Google Scholar
3 Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults (United States, 2005). Morbidity and Mortality Weekly Report May 1, 2009;58:421–426. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5816a2.htm (date last accessed 26 September 2012). Google Scholar
4 Burstein D , GrayML. Is MRI fulfilling its promise for molecular imaging of cartilage in arthritis?Osteoarthritis Cartilage2006;14:1087–1090.CrossrefPubMed Google Scholar
5 Schaible HG . Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep2012;14:549–556.CrossrefPubMed Google Scholar
6 Parks EL , GehaPY, BalikiMN, et al.Brain activity for chronic knee osteoarthritis: dissociating evoked pain from spontaneous pain. Eur J Pain2011;15:843–841.CrossrefPubMed Google Scholar
7 Sofat N , EjinduV, KielyP. What makes osteoarthritis painful?: the evidence for local and central pain processing. Rheumatology (Oxford)2011;50:2157–2165. Google Scholar
8 Inoue R , IshibashiY, TsudaE, et al.Knee osteoarthritis, knee joint pain and aging in relation to increasing serum hyaluronan level in the Japanese population. Osteoarthritis Cartilage2011;19:51–57.CrossrefPubMed Google Scholar
9 Goddard NJ , GoslingPT. Intra-articular fluid pressure and pain in osteoarthritis of the hip. J Bone Joint Surg [Br]1988;70-B:52–55.CrossrefPubMed Google Scholar
10 Xia Y . Resolution ‘scaling law’ in MRI of articular cartilage. Osteoarthritis Cartilage2007;15:363–365. Google Scholar
11 Xia Y . Contrast in NMR imaging and microscopy. Concepts Magn Reson1996;8:205–225. Google Scholar
12 Hunziker EB , QuinnTM, HäuselmannHJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage2002;10:564–572.CrossrefPubMed Google Scholar
13 Venn M , MaroudasA. Chemical composition and swelling of normal and osteoarthritic femoral head cartilage: I: Chemical composition. Ann Rheum Dis1977;36:121–129. Google Scholar
14 Maroudas A , BaylissMT, VennM. Further studies on the composition of human femoral head cartilage. Ann Rheum Dis1980;39:514–534.CrossrefPubMed Google Scholar
15 Clarke IC . Articular cartilage: a review and scanning electron microscope study: 1: The interterritorial fibrillar architecture. J Bone Joint Surg [Br]1971;53-B:732–750. Google Scholar
16 Bayliss MT , VennM, MaroudasA, AliSY. Structure of proteoglycans from different layers of human articular cartilage. Biochem J1983;209:387–400.CrossrefPubMed Google Scholar
17 Maroudas A , WachtelEJ, GrushkoG, KatzEP, WeinbergP. The effect of osmotic and mechanical pressures on water partitioning in articular cartialge. Biochim Biophys Acta1991;1073:285–294. Google Scholar
18 Mow VC , GuoXE. Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies. Ann Rev Biomed Eng2002;4:175–209.CrossrefPubMed Google Scholar
19 Xia Y . Averaged and depth-dependent anisotropy of articular cartilage by microscopic imaging. Semin Arthritis Rheum2008;37:317–327.CrossrefPubMed Google Scholar
20 Callaghan PT. Principles of nuclear magnetic resonance microscopy. Oxford: Oxford University Press, 1991. Google Scholar
21 Blümich B, Kuhn W. Magnetic resonance microscopy: methods and application in materials science, agriculture and biomedicine. Weinheim: VCH, 1992. Google Scholar
22 Blümler P, Blümich B, Botto R, Fukushima E, eds. Spatially resolved magnetic resonance: methods, materials, medicine, biology, rheology, geology, ecology, hardware. Weinheim: Wiley-VCH, 1998. Google Scholar
23 Jenner CF , XiaY, EcclesCD, CallaghanPT. Circulation of water within wheat grain revealed by nuclear magnetic resonance micro-imaging. Nature1988;336:399–402. Google Scholar
24 Callaghan PT , XiaY. Velocity and diffusion imaging in dynamic NMR microscopy. J Magn Reson1991;91:326–352. Google Scholar
25 Xia Y , FarquharT, Burton-WursterN, LustG. Origin of cartilage laminae in MRIJ. Magn Reson Imaging1997;7:887–894.CrossrefPubMed Google Scholar
26 Slichter CP. Principles of magnetic resonance. Berlin: Springer-Verlag, 1992. Google Scholar
27 Xia Y . Relaxation anisotropy in cartilage by NMR microscopy (muMRI) at 14-micron resolution. Magn Reson Med1998;39:941–949. Google Scholar
28 Muir H . Proteoglycans as organizers of the intercellular matrix. Biochem Soc Trans1983;11:613–622.CrossrefPubMed Google Scholar
29 Xia Y , FarquharT, Burton-WursterN, JelinskiLW. Microscopic MRI studies of cartilage. Orthop Trans1994;18:403–404. Google Scholar
30 Tyrrell RL , GluckertK, PathriaM, ModicMT. Fast three-dimensional MR imaging of the knee: comparison with arthroscopy. Radiology1988;166:865–872.CrossrefPubMed Google Scholar
31 Lehner KB , RechlHP, GmeinwieserJK, et al.Structure, function, and degeneration of bovine hyaline cartilage: assessment with MR imaging in vitro. Radiology1989;170:495–499.CrossrefPubMed Google Scholar
32 Hayes CW , SawyerRW, ConwayWF. Patellar cartilage lesions: in vitro detection and staging with MR imaging and pathologic correlation. Radiology1990;176:479–483.CrossrefPubMed Google Scholar
33 Modl JM , SetherLA, HaughtonVM, KneelandJB. Articular cartilage correlation of histologic zones with signal intensity at MR Imaging. Radiology1991;181:853–855. Google Scholar
34 Hayes CW , ParelladaJA. The magic angle effect in musculoskeletal MR imaging. Top Magn Reson Imaging1996;8:51–56.PubMed Google Scholar
35 Rubenstein JD , KimJK, Morava-ProtznerI, StanchevPL, HenkelmanRM. Effects of collagen orientation on MR imaging characteristics of bovine articular cartilage. Radiology1993;188:219–226.CrossrefPubMed Google Scholar
36 Xia Y . Magic angle effect in MRI of articular cartilage: a review. Invest Radiol2000;35:602–621. Google Scholar
37 Bydder M , RahalA, FullertonGD, BydderGM. The magic angle effect: a source of artifact, determinant of image contrast, and technique for imaging. J Magn Reson Imaging2007;25:290–300.CrossrefPubMed Google Scholar
38 Xia Y , MoodyJ, AlhadlaqH. Orientational dependence of T2 relaxation in articular cartilage: a microscopic MRI (microMRI) study. Magn Reson Med2002;48:460–469.CrossrefPubMed Google Scholar
39 Xia Y , MoodyJ, Burton-WursterN, LustG. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage2001;9:393–406.CrossrefPubMed Google Scholar
40 Zheng S , XiaY, BidthanapallyA, et al.Damages to the extracellular matrix in articular cartilage due to cryopreservation by microscopic magnetic resonance imaging and biochemistry. Magn Reson Imaging2009;27:648–655.CrossrefPubMed Google Scholar
41 Xia Y , ZhengS. Reversed laminar appearance of articular cartilage by T1-weighting in 3D fat-suppressed spoiled gradient recalled echo (SPGR) imaging. J Magn Reson Imaging2010;32:733–737.CrossrefPubMed Google Scholar
42 McCauley TR , DislerDG. MR imaging of articular cartilage. Radiology1998;209:629–640.CrossrefPubMed Google Scholar
43 Bashir A , GrayML, BursteinD. Gd-DTPA2- as a measure of cartilage degradation. Magn Reson Med 1996;36:665–673.CrossrefPubMed Google Scholar
44 Trattnig S , MlynárikV, BreitenseherM, et al.MRI visualization of proteoglycan depletion in articular cartilage via intravenous administration of Gd-DTPA. Magn Reson Imaging1999;17:577–583.CrossrefPubMed Google Scholar
45 Nieminen MT , RieppoJ, SilvennoinenJ, et al.Spatial assessment of articular cartilage proteoglycans with Gd-DTPA-enhanced T1 imaging. Magn Reson Med2002;48:640–648.CrossrefPubMed Google Scholar
46 Nieminen MT , TöyräsJ, LaasanenMS, et al.Prediction of biomechanical properties of articular cartilage with quantitative magnetic resonance imaging. J Biomech2004;37:321–328.CrossrefPubMed Google Scholar
47 Samosky JT , BursteinD, Eric GrimsonW, et al.Spatially-localized correlation of dGEMRIC-measured GAG distribution and mechanical stiffness in the human tibial plateau. J Orthop Res2005;23:93–101.CrossrefPubMed Google Scholar
48 Wedig M, Bae W, Temple M, Sah R, Gray M. [GAG] profiles in “normal” human articular cartilage. Procs Orthopedic Research Society Meeting, Washington DC, 2005. Google Scholar
49 Xia Y , ZhengS, BidthanapallyA. Depth-dependent profiles of glycosaminoglycans in articular cartilage by microMRI and histochemistry. J Magn Reson Imaging2008;28:151–157.CrossrefPubMed Google Scholar
50 Zheng S , XiaY. The impact of the relaxivity definition on the quantitative measurement of glycosaminoglycans in cartilage by the MRI dGEMRIC method. Magn Reson Med2010;63:25–32.CrossrefPubMed Google Scholar
51 Wang N , XiaY. Depth and orientational dependencies of MRI T(2) and T(1rho) sensitivities towards trypsin degradation and Gd-DTPA(2-) presence in articular cartilage at microscopic resolution. Magn Reson Imaging2012;30:361–370. Google Scholar
52 Basser PJ , JonesDK. Diffusion-tensor MRI: theory, experimental design and data analysis: a technical review. NMR Biomed2002;15:456–467. Google Scholar
53 Momot KI . Diffusion tensor of water in model articular cartilage. Eur Biophys J2011;40:81–91.CrossrefPubMed Google Scholar
54 Xia Y , FarquharT, Burton-WursterN, RayE, JelinskiLW. Diffusion and relaxation mapping of cartilage-bone plugs and excised disks using microscopic magnetic resonance imaging. Magn Reson Med1994;31:273–282.CrossrefPubMed Google Scholar
55 Burstein D , GrayML, HartmanAL, GipeR, FoyBD. Diffusion of small solutes in cartilage as measured by nuclear magnetic resonance (NMR) spectroscopy and imaging. J Orthop Res1993;11:465–478.CrossrefPubMed Google Scholar
56 Xia Y, Tso D, Farquhar T, Burton-Wurster N, Jelinski LW. Microscopic MRI studies of cartilage. Procs Orthopedic Research Society Meeting, New Orleans, 1994. Google Scholar
57 Xia Y , FarquharT, Burton-WursterN, et al.Self-diffusion monitors degraded cartilage. Arch Biochem Biophys1995;323:323–328.CrossrefPubMed Google Scholar
58 Burton-Wurster N , Hui-ChouCS, GreisenHA, LustG. Reduced deposition of collagen in the degenerated articular cartilage of dogs with degenerative joint disease. Biochem Biophys Acta1982;718:74–84.CrossrefPubMed Google Scholar
59 Deng X , FarleyM, NieminenMT, GrayM, BursteinD. Diffusion tensor imaging of native and degenerated human articular cartilage. Magn Reson Imaging2007;25:168–171.CrossrefPubMed Google Scholar
60 Fullerton GD , PotterJL, DornbluthNC. NMR relaxation of protons in tissues and other macromolecular water solutions. Magn Reson Imaging1982;1:209–226.CrossrefPubMed Google Scholar
61 Du J , DiazE, CarlM, et al.Ultrashort echo time imaging with bicomponent analysis. Magn Reson Med2012;67:645–649.CrossrefPubMed Google Scholar
62 Qian Y , WilliamsAA, ChuCR, BoadaFE. Multicomponent T2* mapping of knee cartilage: technical feasibility ex vivo. Magn Reson Med2010;64:1426–1431.CrossrefPubMed Google Scholar
63 Zheng S , XiaY. Multi-components of T2 relaxation in ex vivo cartilage and tendon. J Magn Reson 2009;198:188–196.CrossrefPubMed Google Scholar
64 Reiter DA , LinPC, FishbeinKW, SpencerRG. Multicomponent T2 relaxation analysis in cartilage. Magn Reson Med2009;61:803–809.CrossrefPubMed Google Scholar
65 Zheng S , XiaY. On the measurement of multi-component T2 relaxation in cartilage by MR spectroscopy and imaging. Magn Reson Imaging2010;28:537–555.CrossrefPubMed Google Scholar
66 Wang N , XiaY. Dependencies of multi-component T2 and T1rho relaxation on the anisotropy of collagen fibrils in bovine nasal cartilage. J Magn Reson2011;212:124–132. Google Scholar
67 Reiter DA , RoqueRA, LinPC, et al.Mapping proteoglycan-bound water in cartilage: Improved specificity of matrix assessment using multiexponential transverse relaxation analysis. Magn Reson Med2011;65:377–384.CrossrefPubMed Google Scholar
68 Keinan-Adamsky K , ShinarH, NavonG. The effect of detachment of the articular cartilage from its calcified zone on the cartilage microstructure, assessed by 2H-spectroscopic double quantum filtered MRI. J Orthop Res2005;23:109–117.CrossrefPubMed Google Scholar
69 Reiter DA , PeacockA, SpencerRG. Effects of frozen storage and sample temperature on water compartmentation and multiexponential transverse relaxation in cartilage. Magn Reson Imaging2011;29:561–567.CrossrefPubMed Google Scholar
70 Xia Y , ZhengS, SzarkoM, LeeJ. Anisotropic properties of bovine nasal cartilage. Microsc Res Tech2012;75:300–306.CrossrefPubMed Google Scholar
71 Glasgold MJ , KatoYP, ChristiansenD, et al.Mechanical properties of septal cartilage homografts. Otolaryngol Head Neck Surg1988;99:374–379.CrossrefPubMed Google Scholar
72 Grellmann W , BerghausA, HaberlandEJ, et al.Determination of strength and deformation behavior of human cartilage for the definition of significant parameters. J Biomed Mater Res A2006;78:168–174.CrossrefPubMed Google Scholar
73 Weiss C , RosenbergL, HelfetAJ. An ultrastructural study of normal young adult human articular cartilage. J Bone Joint Surg [Am]1968;50-A:663–674.CrossrefPubMed Google Scholar
74 Meachim G . Effect of age on the thickness of adult articular cartilage at the shoulder joint. Ann Rheum Dis1971;30:43–46. Google Scholar
75 Zimny ML , RedlerI. Morphological variations within a given area of articular surface of cartilage. Z Zellforsch Mikrosk Anat1974;147:163–167.CrossrefPubMed Google Scholar
76 Kincaid SA , Van SickleDC. Regional histochemical and thickness variations of adult canine articular cartilage. Am J Vet Res1981;42:428–432.PubMed Google Scholar
77 Slowman SD , BrandtKD. Composition and glycosaminoglycan metabolism of articular cartilage from habitually loaded and habitually unloaded sites. Arthritis Rheum1986;29:88–94.CrossrefPubMed Google Scholar
78 Kiviranta I , TammiM, JurvelinJ, HelminenHJ. Topographical variation of glycosaminoglycan content and cartilage thickness in canine knee (stifle) joint cartilage: application of the microspectrophotometric method. J Anat1987;150:265–276. Google Scholar
79 Korvick D , AthanasiouK. Variations in the mechanical properties of cartilage from the canine scapulohumeral joint. Am J Vet Res1997;58:949–953.PubMed Google Scholar
80 Farquhar T , BertramJ, TodhunterRJ, Burton-WursterN, LustG. Variations in composition of cartilage from the shoulder joints of young adult dogs at risk for developing canine hip dysplasia. J Am Vet Med Assoc1997;210:1483–1485.PubMed Google Scholar
81 Fragonas E , MlynárikV, JellúsV, et al.Correlation between biochemical composition and magnetic resonance appearance of articular cartilage. Osteoarthritis Cartilage1998;6:24–32.CrossrefPubMed Google Scholar
82 Brama PA , TeKoppeleJM, BankRA, van WeerenPR, BarneveldA. Influence of site and age on biochemical characteristics of the collagen network of equine articular cartilage. Am J Vet Res1999;60:341–345.PubMed Google Scholar
83 Xia Y , MoodyJ, AlhadlaqH, Burton-WursterN, LustG. Characteristics of topographical heterogeneity of articular cartilage over the joint surface of a humeral head. Osteoarthritis Cartilage2002;10:370–380.CrossrefPubMed Google Scholar
84 Xia Y , MoodyJ, AlhadlaqH, HuJN. Imaging the physical and morphological properties of a multi-zone young articular cartilage at microscopic resolution. J Magn Reson Imaging2003;17:365–374.CrossrefPubMed Google Scholar
85 Zheng S , XiaY. The collagen fibril structure in the superficial zone of articular cartilage by µMRI. Osteoarthritis Cartilage2009;17:1519–1528. Google Scholar
86 Alhadlaq H , XiaY. The structural adaptations in compressed articular cartilage by microscopic MRI (microMRI) T(2) anisotropy. Osteoarthritis Cartilage2004;12:887–894. Google Scholar
87 Alhadlaq HA , XiaY. Modifications of orientational dependence of microscopic magnetic resonance imaging T(2) anisotropy in compressed articular cartilage. J Magn Reson Imaging2005;22:665–673.CrossrefPubMed Google Scholar
88 Alhadlaq HA , XiaY, HansenFM, LesCM, LustG. Morphological changes in articular cartilage due to static compression: polarized light microscopy study. Connect Tissue Res2007;48:76–84.CrossrefPubMed Google Scholar
89 Xia Y , AlhadlaqH, RamakrishnanN, et al.Molecular and morphological adaptations in compressed articular cartilage by polarized light microscopy and Fourier-transform infrared imaging. J Struct Biol2008;164:88–95.CrossrefPubMed Google Scholar
90 Xia Y , WangN, LeeJ, BadarF. Strain-dependent T1 relaxation profiles in articular cartilage by MRI at microscopic resolutions. Magn Reson Med2011;65:1733–1737.CrossrefPubMed Google Scholar
91 Gold GE , BesierTF, DraperCE, et al.Weight-bearing MRI of patellofemoral joint cartilage contact area. J Magn Reson Imaging2004;20:526–530.CrossrefPubMed Google Scholar
92 Stehling C , SouzaRB, GraverandMP, et al.Loading of the knee during 3.0 T MRI is associated with significantly increased medial meniscus extrusion in mild and moderate osteoarthritis. Eur J Radiol2012;81:1839–1845. Google Scholar
93 Alhadlaq H , XiaY, MoodyJB, MatyasJ. Detecting structural changes in early experimental osteoarthritis of tibial cartilage by microscopic magnetic resonance imaging and polarized light microscopy. Ann Rheum Dis2004;63:709–717. Google Scholar
94 Xia Y , RamakrishnanN, BidthanapallyA. The depth-dependent anisotropy of articular cartilage by Fourier-transform infrared imaging (FTIRI). Osteoarthritis Cartilage2007;15:780–788.CrossrefPubMed Google Scholar
95 Ramakrishnan N , XiaY, BidthanapallyA. Polarized IR microscopic imaging of articular cartilage. Phys Med Biol2007;52:4601–4614.CrossrefPubMed Google Scholar
96 Yin JH , XiaY. Macromolecular concentrations in bovine nasal cartilage by fourier transform infrared imaging and principal component regression. Appl Spectrosc2010;64:1199–1208.CrossrefPubMed Google Scholar
97 Yin JH , XiaY, RamakrishnanN. Depth-dependent anisotropy of proteoglycan in articular cartilage by Fourier transform infrared imaging. Vib Spectrosc2011;57:338–341.CrossrefPubMed Google Scholar
Funding statement:
This work was supported by a NIH R01 Grant (AR052353).
Author contributions:
Y. Xia: Researched and wrote the paper
ICMJE Conflict of Interest:
None declared
©2013 The British Editorial Society of Bone & Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










