Abstract
Objectives
Surgical marking during tendon surgery is often used for technical and teaching purposes. This study investigates the effect of a gentian violet ink marker pen, a common surgical marker, on the viability of the tissue and cells of tendon.
Methods
In vitro cell and tissue methods were used to test the viability of human hamstring explants and the migrating tenocytes in the presence of the gentian violet ink.
Results
The outcome of this study was that a constituent of the surgical marker pen causes cell and tissue death in culture, implying the same would occur in vivo.
Conclusions
This is a cause for concern when marking tendon during surgical procedures, as it may compromise healing and repair and potentially contribute to a poor outcome. The authors suggest that an alternative surgical marking procedure should be found, or that all marker pens should undergo testing on human tendon tissue in vitro prior to use.
Article focus
Do surgical marker pens have detrimental effects on tendon tissue and cells?
Key messages
A surgical marker pen containing gentian violet causes cell and tissue death
The surgical marker pen potentially impairs cell migration
This is a theoretical concern for marking during tendon procedures
Strengths and limitations
Data provide evidence of cell death in 100% of marked tissue explants
Death of cells may not affect surgical -procedures that succeed with a decellular-ised tissue scaffold
Further investigation into the mechanism of toxicity is required
Introduction
Ink surgical marker pens are used universally in most surgical specialties. Pre-operative skin marking is strongly recommended to avoid wrong-site surgery and is well documented by the guidelines of the American Academy of Orthopaedic Surgeons1 and the United Kingdom National Patient Safety Agency.2 Sterile, single-use marker pens are also used extensively to design and plan skin- and soft-tissue incisions and osteotomies, to assist in the orientation of tissues during procedures and to facilitate the training of junior surgeons.3-5 Gentian violet is the most common dye found in surgical marking pens. It is a triaryl-methane dye with a maximum absorbance at 590 nm, giving it a blue-violet colour.6 Gentian violet has antiseptic properties and has been used in medicine for over 100 years.7 The dye also forms the basis of Gram’s staining method for the classification of bacteria.
The intra-operative use of marker pens for technical and training purposes is ingrained into surgical practice with very little in the literature documenting the potential complications or risks. Rarely, skin marking with gentian violet at the site of incision can lead to permanent tattooing.4 There are reports that the intra-operative use of gentian violet to determine the nature and extent of a small tear of the rotator cuff can lead to chondroylsis of the glenohumeral joint, with severe degenerative changes seen up to seven years post--operatively.8 There is in vitro evidence for endothelial damage in Descemet’s stripping automated endothelial keratoplasty (DSAEK) partial thickness corneal transplant donor tissue with the use of a gentian violet surgical marker.9
Tendon procedures including repair, transfer, shortening-/lengthening and reconstruction are common examples of operations in which marker pens are used in orthopaedic surgery, but there are no studies investigating any potential detrimental effects. This study investigated the effect of using a gentian violet surgical marker on the viability and outgrowth vigour of tendon cells (tenocytes) derived from hamstring tendon harvested during anterior cruciate ligament (ACL) reconstruction.
Materials and Methods
Patient selection
Tendon tissue was obtained from the Oxford Musculoskeletal BioBank, Oxford, United Kingdom, and was collected with informed donor consent in full compliance with national and institutional ethical requirements, the United Kingdom Human Tissue Act, and the Declaration of Helsinki. Five patients undergoing ACL reconstruction with hamstring tendon autograft were selected at random. They were all male with a mean age of 36.4 years (25 to 48) (Table I).
Table I
Values of the in vitro tendon cell migration from healthy human hamstring explants for both control and inked tissue. Data shows number of wells containing explants with those showing cell migration in brackets
| Patient | Age (yrs) | Un-inked (cell migration) | Inked (cell migration) |
|---|---|---|---|
| 1 | 36 | 7 (7) | 5 (0) |
| 2 | 26 | 2 (2) | 2 (0) |
| 3 | 48 | 6 (6) | 6 (0) |
| 4 | 25 | 8 (8) | 3 (0) |
| 5 | 47 | 3 (3) | 3 (0) |
Explanting of human hamstring tissue
Unmarked excess hamstring tendon was excised at the tibial tunnel following graft placement during ACL reconstruction surgery. The tendon was placed in Dulbecco’s Modified Eagle Medium (DMEM: F-12 1:1 w/15 mM Hepes, L-Gln) (Lonza, Basel, Switzerland) immediately following the surgical procedure.
A piece of tissue sized 2 cm2 × 1 cm2 was removed from one surgically cut end of the tendon. This section was sliced longitudinally through the centre of the tissue, giving two pieces of similar size from an identical anatomical location. One piece was marked with three stripes using an NHS-approved surgical marker pen (Aspen Surgical Products Inc., Caledonia, Michigan) that contained up to 10% gentian violet and 50% isopropanol (Fig. 1). The other piece was not marked with the pen and served as a control.

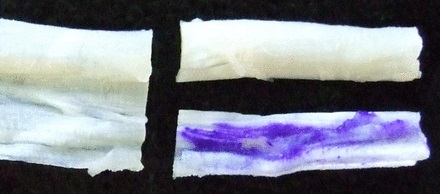
Figs. 1a - 1b
Photographs showing the preparation of the tendon, a) a piece of healthy human hamstring tissue, such as that prepared for an anterior cruciate -ligament graft, and b) showing the tendon divided lengthways at one end, one half marked with gentian violet pen and the other remaining un-inked as a control.
The tendon tissue was then carefully cut into pieces about 2 mm3 and placed into wells of 6-well dishes. Each tendon piece was submerged in a drop of 50% heat in-activated foetal bovine serum (FBS) (Biosera, Ringmer, United Kingdom) /DMEM-F12 containing 1% penicillin/streptomycin (Sigma, Poole, United Kingdom) in order to encourage cell migration while reducing infection. The dishes were subsequently placed in a humidifying chamber at 37°C, 5% CO2 and atmospheric oxygen, and left for 48 hours. After this time the media were replaced every three days until the first sign of cell migration occurred, at which point the media were changed to 10% FBS/DMEM-F12. Explanted tissue was kept for up to three weeks, with tenocytes typically migrating out of the tissue within seven to ten days.
Explant viability and imaging
In order to assess the viability of the explants, a Live & Dead stain (Invitrogen, -Paisley, United Kingdom) was used according to the manufacturer’s protocol. Calcein AM fluoresces green when cleaved by intracellular esterases in live cells, whereas dead cells fluoresce red since increased membrane permeability permits the take up of the ethidium homodimer dye. The Live & Dead stain was carried out on randomly selected tendon pieces taken two weeks after explanting. Explants were imaged using a Nikon TE300 microscope (Nikon Instruments, Tokyo, Japan) with Retiga CCD camera (QImaging, British Columbia, Canada) and ImagePro computer software (MediaCybernetics, Bethesda, -Maryland). The number of live and dead cells was quantified in a subset of explants (n = 3 for each condition).
At ten days after explanting, all wells were visualised using the Nikon microscope and camera and light microscopic images were taken of migrating cells from the explants. The number of cells which had migrated out from the explants was quantified (n = 6 for each -condition).
Scoring of migrated tendon cells
Tendon explants were microscopically checked daily for three weeks to identify migrated tenocytes from the explanted tissue. Over the three-week period it was noted how many of the explants showed cell migration.
Statistical analysis
Results are expressed as mean with standard error of the mean (sem). Statistical analysis was performed using GraphPad Prism 4.5.03 software (GraphPad Software Inc., LaJolla, California). A two-tailed paired t-test was used to compare cell migration data. Repeated measures analysis of variance (ANOVA) and post-hoc Tukey’s multiple comparison test were used to compare cell viability data. A p-value ≤ 0.05 was considered to indicate statistical significance.
Results
Lack of migrating cells from inked hamstring tendon tissue
Every piece of explanted tissue which had been marked with the gentian violet ink surgical pen showed complete absence of local migratory cells (Table I). Wells containing these inked explants contained no tenocytes whatsoever. Migrating tenocytes were already seen emerging from some pieces of un-inked tissue from seven days post explanting. This pattern was noted throughout the three weeks, at which time cell migration was seen from all control explants.
Figure 2 depicts the findings at ten days after explanting. In the control explants it is clear that specific areas of the tissue demonstrate migration of tenocytes (Fig. 2a). Even though there would be a degree of proliferation occurring at this point, it is certain that a high proportion of cell migration occurred after a short period of time in culture. In those images taken of inked tissue it was clear that no cell migration had occurred at the same time point as controls (Fig. 2b). Statistical analysis confirmed that there was a significant reduction in cells migrating from tissue explants which had been exposed to the gentian violet ink (p = 0.0182) (Fig. 2c).
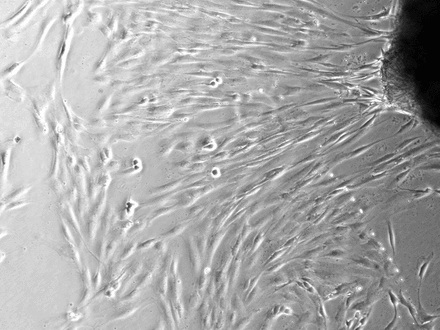

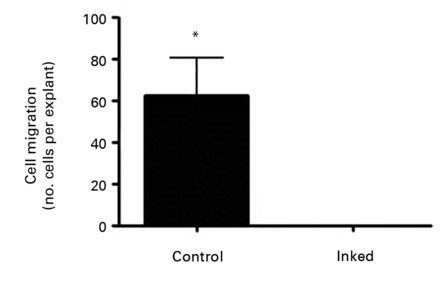
Figs. 2a - 2c
Figures 2a and 2b – photomicrographs showing hamstring tendon explants in the culture environment in a) control tissue, showing tenocytes migrating from various points of the explants, which then continued to migrate and proliferate, and b) in tissue exposed to the gentian violet ink marker, showing no cell migration. Figure 2c – histogram comparing mean cell migration between the control and inked tissues, showing a statistically significant difference (* p = 0.0182). The error bars show the standard error of the mean.
Explant viability
Staining of the explants using a Live & Dead viability assay illustrated healthy live ‘green’ cells within the tissue of control explants after three weeks in culture (Fig. 3a). However, in those explants which had been exposed to the gentian violet marker pen, all cells were ‘red’ – therefore dead (Fig. 3b). This suggests an explanation for the lack of migrating cells from inked explants. Statistical analysis confirmed that the number of live cells was dramatically reduced in explants exposed to the gentian violet marker pen compared with the numbers seen in control explants (p = 0.0055) (Fig. 3c).
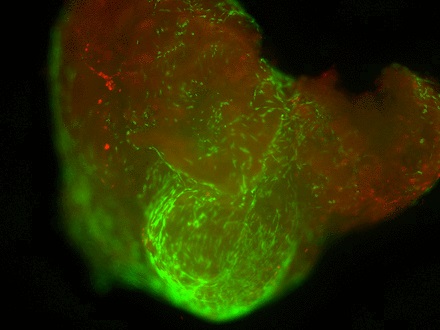
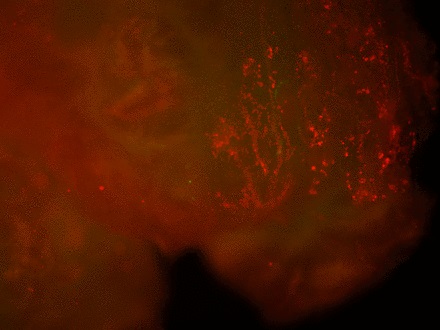
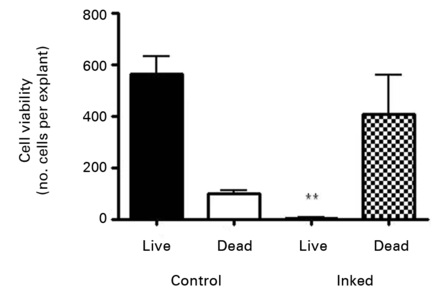
Figs. 3a - 3c
Figures 3a and 3b – fluorescent photomicrographs of tendon explants using Live/Dead staining, in a) control tissue, showing a high proportion of live tenocytes (in green) with few dead cells (in red) present, and b) in tissue exposed to the gentian violet ink marker, showing the majority of present cells as being dead (in red). Figure 3c – histogram comparing mean cell viability between control and inked implants, showing significantly less live cells in inked implants compared with the controls (** p = 0.0055). The error bars show the standard error of the mean.
Discussion
The use of intra-operative marking to aid surgical technique and training is regarded as non-detrimental, and is common across surgical specialties. Despite the common use of gentian violet marker pens during many surgical procedures on tendon, the authors are not aware of any studies into the biological effects on the tissue. This in vitro study shows that explanted human hamstring -tendon exposed to surgical-style marking produced a total failure of explant vigour, with zero tenocyte emigration and evidence of drastically reduced tissue viability in longer term culture.
Poor surgical technique with heavy tissue handling can cause iatrogenic mechanical injury to tendons with associated loss of tenocyte viability.10 Mechanical damage during hamstring harvest (i.e. tendon stripping), incision, and explant preparation is unlikely to be the major cause of the observed loss of tissue viability in this study, since unmarked control explants retained large numbers of viable cells. For marked explants it is possible that contact pressure during ink application may have caused some local cell death, but cannot explain the total loss of viable cells throughout all 2 mm2 explants from the marked -tendon pieces.
Gentian violet dye or isopropanol, the two known ingredients of the marker pen, are the most likely cause of tenocyte death in the explanted hamstring tendon. Gentian violet ink has in the past been linked to chondrolysis and endothelial cell damage,8 but this is the first known report of a detrimental effect on tendon. Gentian violet has been shown to be cytotoxic to mammalian cells by inducing chromosomal damage11,12 and this may be a potential mechanism of the loss of tenocyte viability. It has not been reported that isopropanol, in the concentration found in the pen, is detrimental to cell and tissue viability. However, this has not been formally tested in this explant study.
The authors would like to highlight that the findings of this study raise a theoretical concern regarding surgical marking during tendon operations in general, and also in particular for end-to-end repair, transfer procedures and interposition grafting, as these require the direct healing of tendon to tendon. Much literature exists on healing of tendon grafts in ACL reconstruction.13-15 In the scenario of hamstring tendon ACL reconstruction, one could argue that marker pen ink may have potential adverse effects on “ligamentisation” of the autograft and tendon-bone--integration within the bone-tunnels.
In spite of the very common use of surgical marking pens in tendon operations, there does not seem to be the poor clinical outcome that would be expected by the loss of tenocyte viability and migration demonstrated in this in vitro explant study. There are a number of explanations for this. The surgical site has local bleeding and vascular inflow. For example, after ACL reconstruction there is an acute post-surgical haemarthrosis with bleeding from the bone tunnels. This in itself may dilute and remove the ink, thus reducing exposure to its toxic effects. The ensuing inflammatory and tissue healing pathways are associated with an influx of growth factors i.e. transforming growth factor beta (TGF-β1), platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and fibroblast growth factors-2 (FGF-2) and the recruitment of inflammatory cells, pleuripotent mesenchymal stem cells and fibroblasts.14 This physiological environment may negate and overcome any cytotoxic and anti-migratory effects of gentian violet based ink on tenocytes. This would be more likely if the amount of marking in relation to the tendon size or cross-sectional area was small. The FBS in the culture medium is an inferior source of anabolic factors compared with the inflammatory haematoma and there is no cellular component. In addition, the amount of marking in relation to the size of the tendon tissue (2 mm2 explants) is likely to be much greater than during a surgical procedure.
Tendon procedures are not universally successful and failure can be defined in many ways including re--operation, rupture, biomechanical laxity or persistent loss of function. Whatever the definition, failure is due to a combination of surgical, biomechanical and biological factors. Surgical factors such as timing, technique, implants (i.e. sutures) and post-operative management have, arguably, the greatest impact. It is not possible to retrospectively investigate tendon procedures with regard to surgical marking because the use and location of surgical marking during surgery is not usually documented. For any given tendon procedure, we cannot compare clinical or biomechanical endpoints for marked versus unmarked tendon, or assess for an association with poor outcome. Therefore, it remains possible that excessive use of gentian violet based marker at sites of tendon repair or integration may cause delayed healing, prolonged recovery or subclinical laxity that are not considered as failure per se. These settings could reduce the threshold for subsequent overt failure by other more significant mechanisms.
We are not aware of any previous studies demonstrating the deleterious effects of gentian violet based ink on explanted human tendon tissue. Although the number of patients was small (n = 5), the comparison of paired marked and control (unmarked) tissue from the same patient and the highly statistically significant differences observed does add weight to the findings of this preliminary study. However, there are a number of limitations to this study that should be addressed in future work. Only a single make and brand of surgical marking pen was investigated. Further studies are required to see if the detrimental effects are reproduced with other gentian violet based marking pens. The cytotoxic effects seen in this study may be due to the combined action of gentian violet, isopropanol and minor constituents of the ink, which may vary between brands. We did not assess the possibility of a dose-dependent nature to the effects of tendon marking, for example by varying the surface area of marking and size of the explant. This would be particularly pertinent to the clinical relevance of the present findings as discussed previously. Similarly, the biomechanical consequences of reduced tissue viability on tendon integrity and strength needs further investigation both in vitro and in vivo, using animal models. The cytotoxic effects of marker pen ink on other commonly marked tissues such as muscle and bone also needs assessment.
The authors suggest that intra-operative tendon marking with gentian violet/isopropanol pens should be done sparingly and with caution. We speculate that excessive surgical marking is a potential biological factor that may predispose to poor outcome. Alternative marking techniques should be considered when possible.
1 American Academy of Orthopaedic Surgeons. Information statement: wrong-site surgery, 2008. http://www.aaos.org/about/papers/advistmt/1015.asp (date last accessed 14 March 2012). Google Scholar
2 NHS National Patient Safety Agency. Patient safety alert 06: correct site surgery, 2005. http://www.rcseng.ac.uk/media/medianews/Nationalpatientsafetyagency/ (date last accessed 22 June 2011). Google Scholar
3 Tatla T Lafferty K . Making your mark again in surgery. Ann R Coll Surg Engl2002;84:129–130.PubMed Google Scholar
4 Granick MS Heckler FR Jones EW . Surgical skin-marking techniques. Plast Reconstr Surg1987;79:573–580.CrossrefPubMed Google Scholar
5 Ayhan M Silistreli O Aytug Z Gorgu M Yakut M . Skin marking in plastic surgery. Plast Reconstr Surg2005;115:1450–1451.CrossrefPubMed Google Scholar
6 Adams EQ Rosenstein L . The color and ionization of crystal-violet. J American Chem Soc1914;36:1452–1473. Google Scholar
7 Docampo R Moreno SN . The metabolism and mode of action of gentian violet. Drug Metab Rev1990;22:161–178.CrossrefPubMed Google Scholar
8 Shibata Y Midorikawa K Koga T Honjo N Naito M . Chondrolysis of the glenohumeral joint following a color test using gentian violet. Int Orthop2001;25:401–403.CrossrefPubMed Google Scholar
9 Ide T Yoo SH Kymionis GD , et al.. Descemet-stripping automated endothelial keratoplasty (DSAEK): effect of nontoxic gentian violet marking pen on DSAEK donor tissue viability by using vital dye assay. Cornea2008;27:562–564.CrossrefPubMed Google Scholar
10 Scott A Khan KM Heer J , et al.. High strain mechanical loading rapidly induces tendon apoptosis: an ex vivo rat tibialis anterior model. Br J Sports Med2005;39:25.CrossrefPubMed Google Scholar
11 Au W Pathak S Collie CJ Hsu TC . Cytogenetic toxicity of gentian violet and crystal violet on mammalian cells in vitro. Mutat Res1978;58:269–276.CrossrefPubMed Google Scholar
12 Thomas SM MacPhee DG . Crystal violet: a direct-acting frameshift mutagen whose mutagenicity is enhanced by mammalian metabolism. Mutat Res1984;140:165–167.CrossrefPubMed Google Scholar
13 Claes S Verdonk P Forsyth R Bellemans J . The “ligamentization” process in anterior cruciate ligament reconstruction: what happens to the human graft?: a systematic review of the literature. Am J Sports Med2011;39:2476–2483. Google Scholar
14 Deehan DJ Cawston TE . The biology of integration of the anterior cruciate ligament. J Bone Joint Surg [Br]2005;87-B:889–895.CrossrefPubMed Google Scholar
15 Lui P Zhang P Chan K Qin L . Biology and augmentation of tendon-bone insertion repair. J Orthop Surg Res2010;5:59.CrossrefPubMed Google Scholar
None declared
S. L. Franklin: Writing the paper, Data collection, Data analysis
C. Jayadev: Writing the paper, Specimen collection
R. Poulsen: Data collection
P. Hulley: Data collection, Advising data analysis, Writing the paper
A. Price: Idea development, Specimen collection, Writing the paper
None declared
©2012 British Editorial Society of Bone and Joint Surgery. This is an open-access article distributed under the terms of the Creative Commons Attributions licence, which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










