Abstract
Clinical studies evaluating the effects of vitamin D alone or in combination with calcium on physical function, falls and fractures have been inconsistent. Vitamin D has, however, been the focus of much orthopaedic, trauma and endocrine research. Playing a central role in muscle and bone metabolism, some studies on Vitamin D therapies offer the tantalising suggestion of a reduction in falls and fractures simply with vitamin D supplementation. We review the background and evidence behind vitamin D.
The production of vitamin D
Vitamin D deficiency is a well-recognised global health problem caused mainly by insufficient exposure to sunlight. It is estimated that one billion people have vitamin D deficiency or insufficiency worldwide,1 and it is particularly prevalent among the elderly.2 Vitamin D exists in two forms – cholecalciferol (D3) and ergocalciferol (D2). Ergocalciferol is obtained from yeast and plants. Cholecalciferol is obtained from the diet through the ingestion of vitamin D-containing products (fatty fish and eggs), vitamin D-fortified milk or margarine, and /or the use of multivitamins. However, the primary source of cholecalciferol (80% to 90% of the body stores) is via ultraviolet irradiation of the precursor molecule 7-dehydrocholesterol in the skin. Vitamin D (D2 and D3) are then subsequently hydroxylated in the liver by 25-hydroxylase to produce 25-hydroxyvitamin D (25OHD). 25OHD is then further hydroxylated in the kidney by the 1-alpha hydroxylase to form 1,25-dihydroxyvitamin D ((1,25(OH)2D) or calcitriol), which is the biologically- active form of vitamin D (Fig. 1).
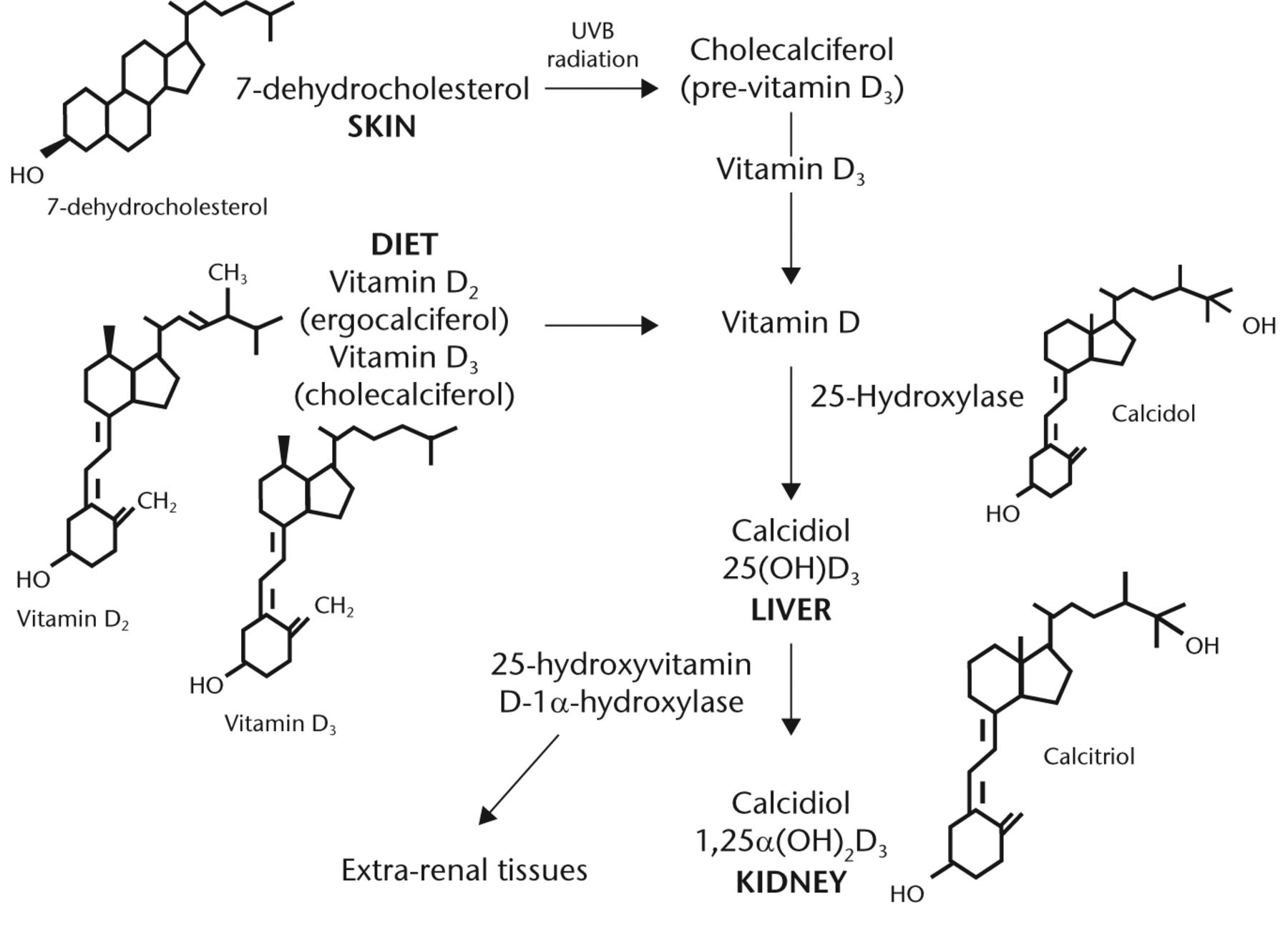
Fig. 1
FigCapVitamin D metabolism (adopted from Dirks-Naylor 2011).3 Metabolism of vitamin D from dietary intake and the skin precursor, 7-dehydrocholesterol by UV radiation to pre-vitamin D, and its subsequent hydroxylation in the liver and kidney to its active form.
The 1-alpha hydroxylation can also occur in a multitude of other tissues (extra renal tissue, Fig. 1), generating locally active vitamin D, which leads to auto and /or paracrine effects. The principal measured index of vitamin D status is the serum 25OHD concentration, with a half-life of about three weeks, as compared with the biologically active form 1,25(OH)2D which has a half-life of only four to six hours.4
Diagnostic definition
Vitamin D status is assessed by measuring blood levels of 25OHD. The Institute of Medicine (IOM) considers that the recommended daily allowance for vitamin D should lead to a serum 25OHD level of at least 50 nmol/L and that individuals below that level should receive vitamin D supplementation.5,6 In parallel, both the US Endocrine Society7 and the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO)8 recommend higher treatment targets (≥ 70 nmol/L to 75 nmol/L) for health benefits. The recently published National Osteoporosis Society UK guidance recommends 25OHD > 50 nmol/L for those at high risk of fragility fractures.9
The function of vitamin D
The vitamin D endocrine system plays a primary role in the maintenance of extracellular fluid calcium concentration. The association between vitamin D deficiency and bone disease, such as rickets, osteomalacia and osteoporosis, is well recognised, however, increasingly the relationship between vitamin D deficiency and other conditions has been identified (Table I).10
Table I. Vitamin D deficiency and associated conditions
| Vitamin D deficiency | Associated conditions |
|---|---|
| Cardiovascular | Cardiovascular disease, aortic dilatation, orthostatic hypotension |
| Respiratory | Bronchiectasis, asthma, cystic fibrosis, bronchiolitis, obstructive sleep apnoea |
| Gastrointestinal | Inflammatory bowel disease, chronic hepatitis, liver cirrhosis, pancreatitis |
| Neurological | Multiple sclerosis, myasthenia gravis, meningomyelocele, depression |
| Musculoskeletal | Muscle weakness, osteoarthritis, rheumatoid arthritis, juvenile arthritis |
| Metabolic | Metabolic syndrome, diabetes mellitus, diabetic nephropathy, infertility (male), chronic kidney disease |
| Cancer | Breast, colorectal, ovarian, lung, prostate |
| Skin | Psoriasis, systemic lupus erythematosus, eczema |
Another important relationship to the orthopaedic surgeon is that between vitamin D and muscle function. Vitamin D deficiency leads to weakness of the proximal muscle groups, affecting weight-bearing antigravity muscles of the lower limb necessary for postural balance and walking. Patients usually complain of symptoms such as 'heaviness in the legs', tiring easily, and difficulty in climbing stairs or rising from a chair.11,12 Functionally, the result is slower walking speed, prolonged sit-to-stand time, lower quadriceps strength13 and an increased risk of falls and fall-related fractures.14,15 In addition to muscle weakness, falls involve neural responses, which are also influenced by vitamin D status.16 Furthermore, vitamin D deficiency is associated with secondary hyperparathyroidism17 as well as hypophosphataemia,18 which also leads to muscle weakness.
Cellular effects of vitamin D
Histopathologically, vitamin D deficiency leads to atrophy of the type II muscle fibres.19 Birge and Haddad20 were the first to show that 25OHD directly influences muscle phosphate metabolism in the diaphragms of vitamin D-deficient rats. The vitamin D receptor (VDR) is expressed in the cell nuclei of human muscle cells21,22 and vitamin D has been shown to affect muscle cell contractility.23 The number of VDRs decreases with age, which may contribute to the observed reduction in muscle strength with ageing. The effects of vitamin D on muscle cells are further supported by muscle biopsy and electrophysiological studies. Low vitamin D levels have been shown to cause an abnormal pattern with reduced motor unit potential duration, a decrease in amplitude and an increase in polyphasicity, without concomitant signs of denervation.24 Treatment with vitamin D leads to reversal of muscle atrophy, including an increase in both the number and cross-sectional area of the fibres.25,26
Vitamin D acts directly through genomic27-29 and non-genomic mechanisms30,31 (Fig. 2).
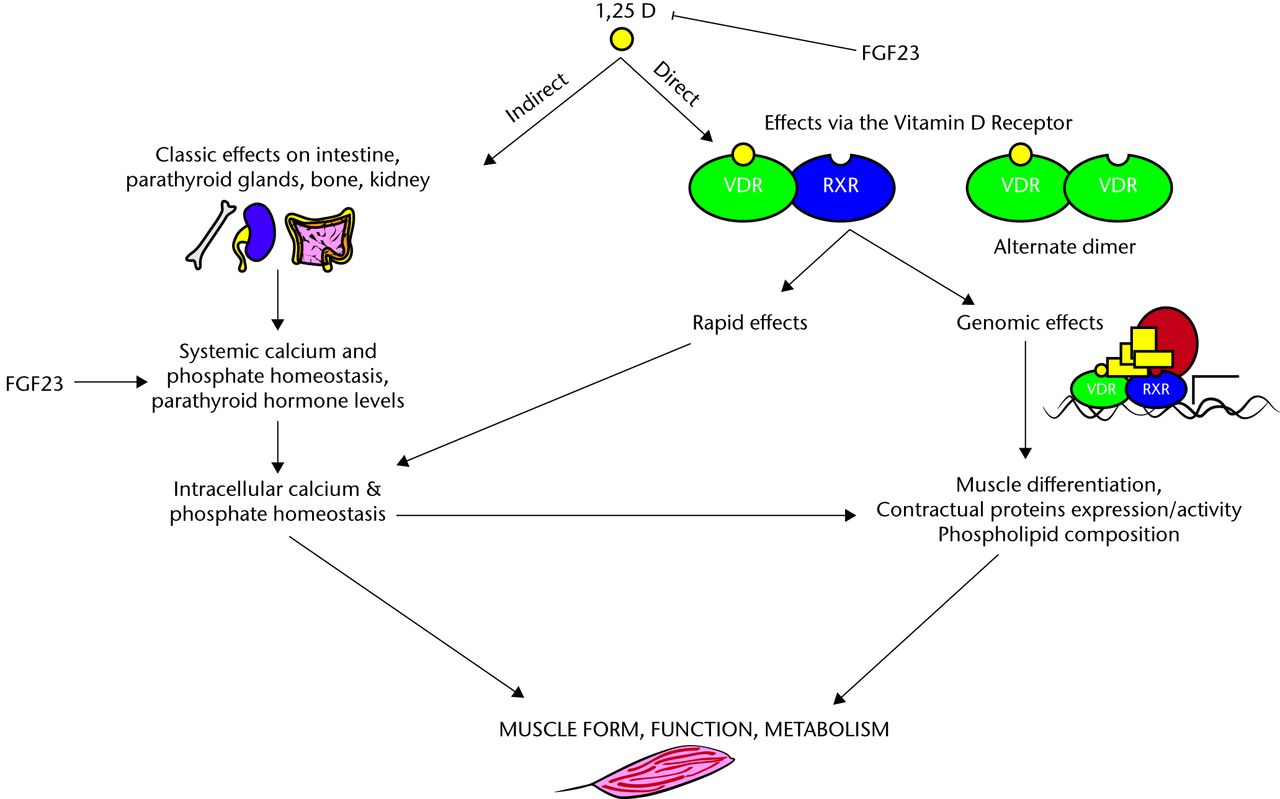
Fig. 2
FigCapGenomic and non-genomic effects of vitamin D on muscle (adapted from Girgis 2013).32
Genomic effects are initiated by the binding of vitamin D to the vitamin D receptor (VDR), which results in changes in gene transcription. Non-genomic effects of vitamin D are rapid and mediated by the membrane-bound VDR, and it is these effects which have been shown to be primarily related to muscle. The evidence for this is strengthened further by the generation of the VDR knockout mice model. In the study by Endo et al33 which was designed to investigate the physiological roles of vitamin D in skeletal muscle development, they examined skeletal muscle in VDR gene-deleted mice. They found that each muscle fibre was small and variable in size although overall myocyte differentiation occurred normally and the effects were independent of secondary metabolic changes such as hypocalcaemia and hypophosphataemia.
Effects on physical function, falls and fractures
Clinical studies evaluating the effects of vitamin D alone, or in combination with calcium, on physical function, falls and fractures have given inconsistent results. In a recent systematic review,34 16 randomised controlled trials (RCTs) evaluating the effects of vitamin D on muscle function were reviewed. Half of the studies showed a beneficial effect in terms of improved muscle strength,35,36 reduced body sway,37 an improved timed up-and-go test,36,37 increased 12-minute gait speed37 and improved aggregated measure of physical abilities.38,39 Hand grip strength, which is commonly used to measure muscle function in clinical research, has also been shown to respond to vitamin D supplementation in observational studies,40,41 but no effect has been seen in RCTs. This observation supports the hypothesis that vitamin D primarily affects the proximal muscles, causing proximal myopathy in the state of severe deficiency. The overall conclusion of the IOM was that observational data provide some support for a link between vitamin D status and physical performance and that RCTs suggest that vitamin D supplementation of at least 800 IU/day, with or without calcium, may be beneficial for physical performance.
With respect to Vitamin D's effect on falls, the recent meta-analysis commissioned by the Endocrine Society found that the odds ratio for falling in those randomised to vitamin D supplementation with or without calcium was 0.86 (95 % confidence interval (CI) 0.77 to 0.96), however, the quality of analysis was moderate given significant heterogeneity within the included studies.42 A more recent review by Bolland et al43 which identified 20 RCTs involving 29 535 participants repeated the analysis but with stricter inclusion criteria and utilising all available data from factorial and multi-arm studies. In this review, they found no effect of vitamin D on falls, whether used alone or in combination with calcium. Other subgroup analyses showed no influence on outcome of baseline 25OHD, 25OHD achieved, study duration, residential status, or whether falls were primary or secondary endpoints. The Cochrane analysis of trials in community-dwelling individuals similarly found no benefit unless trial subjects were preselected for vitamin D deficiency, where the risk ratio was 0.70 (95 % CI 0.56 to 0.87).44 In care facilities, on the other hand, the Cochrane review by Cameron et al45 found that vitamin D supplementation reduced the rate of falls (rate ratio, 0.63 (95 % CI 0.46 to 0.86); five trials, 4603 participants). Similar conclusions were drawn from the meta-analysis by Bischoff-Ferrari et al,39 who showed that vitamin D in doses of > 700 IU per day reduced falls risk (RR, 0.81 (95 % CI 0.71 to 0.92); n = 1921 from seven trials), whereas lower doses did not. It seems likely, given these conflicting outcomes from well-conducted trials, that vitamin D supplementation does have an effect on likelihood of falls, but only in patients with initial deficiency. There would be a reasonable argument within the general orthopaedic setting for measuring and correcting vitamin D deficiency on patients admitted with falls.
The data on anti-fracture efficacy is perhaps the most conflicting. The Cochrane analysis of vitamin D and related vitamin D compounds for preventing fractures resulting from osteoporosis in older people by Avenell et al46 concluded that vitamin D alone is unlikely to prevent fractures in the doses and formulations tested so far in older people, but supplements of vitamin D and calcium may prevent hip or any type of fracture. This is similar to the conclusions drawn from the DIPART-patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials conducted in the US and Europe.47 By contrast, the meta-analysis by Bischoff-Ferrari et al48 concluded that high-dose vitamin D supplementation (≥ 800 IU daily) was effective in the prevention of hip fracture and any non-vertebral fracture in persons 65 years of age or older. A more recent review by Lips et al,49 evaluating 19 RCTs and 13 meta-analyses with vitamin D, either alone or in combination with vitamin D, showed a decrease in fracture incidence in seven RCTs, a neutral outcome in ten RCTs, and in two trials with yearly high-dose vitamin D, an increase in fracture incidence.50,51 In three out of four well-powered trials that used the recommended doses of daily vitamin D of between 700 IU and 1000 IU, vitamin D supplementation did not significantly influence fracture risk. However, in one of these trials, a statistically significant fracture reduction was observed in care home residents with severe vitamin D deficiency, low calcium intake and good compliance.52 Of the 13 meta-analyses, 11 showed a significant decrease in fracture incidence in the supplemented groups (analyses for vertebral fractures were negative in all cases). The review concluded that a vitamin D supplement of 800 IU per day in combination with calcium may decrease the incidence of non-vertebral fractures, especially in older people with a low-baseline vitamin D status, low calcium intake and showing good compliance.
Key points:
-
The prevalence of vitamin D deficiency is high worldwide, particularly in the elderly
-
25 hydroxyvitamin D (25OHD) is the best marker of vitamin D status and is defined as a 25OHD < 30 nmol/L
-
The primary role of vitamin D is the maintenance of extracellular fluid calcium concentrations, but more recently it has been associated with many other conditions
-
Vitamin D deficiency is associated with muscle weakness, predominantly of the proximal muscle groups through both genomic and non-genomic pathways
-
Muscle weakness due to vitamin deficiency is reversible with vitamin D supplementation
-
Vitamin D supplement of 800 IU per day in combination with calcium may decrease the incidence of non-vertebral fractures, especially in older people with a low-baseline vitamin D status and low calcium intake
Conflict of Interest
O. Sahota received honoraria from Eli Lilly, Takeda and Consilent Healthcare in the last 12 months.
1 Holick MF . Vitamin D deficiency. N Engl J Med2007;357:266–281.CrossrefPubMed Google Scholar
2 Kuchuk NO , PluijmSM, van SchoorNM, et al.Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab2009;94:1244–1250.CrossrefPubMed Google Scholar
3 Dirks-Naylor AJ , Lennon-EdwardsS. The effects of vitamin D on skeletal muscle function and cellular signaling. J Steroid Biochem Mol Biol2011;125:159–168.CrossrefPubMed Google Scholar
4 Zerwekh JE . Blood biomarkers of vitamin D status. Am J Clin Nutr2008;87:1087S–1091S.CrossrefPubMed Google Scholar
5 Aloia JF . Clinical Review: The 2011 report on dietary reference intake for vitamin D: where do we go from here?J Clin Endocrinol Metab2011;96:2987–2996.CrossrefPubMed Google Scholar
6 No authors listed. Dietary reference intakes for calcium and vitamin D. Institute of Medicine 2010. http://www.iom.edu/reports/2010/dietary-reference-intakes-for-calcium-and-vitamin-d.aspx (Date last accessed 24 April 2015). Google Scholar
7 Holick MF , BinkleyNC, Bischoff-FerrariHA, et al.Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab2011;96:1911–1930.CrossrefPubMed Google Scholar
8 Rizzoli R , BoonenS, BrandiML, et al.Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr Med Res Opin2013;29:305–313. Google Scholar
9 No authors listed. Vitamin D and bone health: a practical clinical guideline for patient management. http://www.nos.org.uk/document.doc?id=1352 (date last accessed 24 April 2015). Google Scholar
10 Reid IR , BollandMJ. Skeletal and nonskeletal effects of vitamin D: is vitamin D a tonic for bone and other tissues?Osteoporos Int2014;25:2347–2357.CrossrefPubMed Google Scholar
11 Prabhala A , GargR, DandonaP. Severe myopathy associated with vitamin D deficiency in Western New York. Arch Intern Med2000;160:1199–1203.CrossrefPubMed Google Scholar
12 Kojima G , TamaiA, MasakiK, et al.Prevalence of vitamin D deficiency and association with functional status in newly admitted male veteran nursing home residents. J Am Geriatr Soc2013;61:1953–1957.CrossrefPubMed Google Scholar
13 Dretakis OE , TsatsanisC, FyrgadisA, et al.Correlation between serum 25-hydroxyvitamin D levels and quadriceps muscle strength in elderly cretans. J Int Med Res2010;38:1824–1834.CrossrefPubMed Google Scholar
14 Bischoff-Ferrari HA , Dawson-HughesB, WillettWC, et al.Effect of Vitamin D on falls: a meta-analysis. JAMA2004;291:1999–2006.CrossrefPubMed Google Scholar
15 Annweiler C , BeauchetO. Questioning vitamin D status of elderly fallers and nonfallers: a meta-analysis to address a 'forgotten step'. J Intern Med2015;277:16–44.CrossrefPubMed Google Scholar
16 Annweiler C , Montero-OdassoM, SchottAM, et al.Fall prevention and vitamin D in the elderly: an overview of the key role of the non-bone effects. J Neuroeng Rehabil2010;7:50–56.CrossrefPubMed Google Scholar
17 Baczynski R , MassrySG, MagottM, et al.Effect of parathyroid hormone on energy metabolism of skeletal muscle. Kidney Int1985;28:722–727.CrossrefPubMed Google Scholar
18 Schubert L , DeLucaHF. Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch Biochem Biophys2010;500:157–161.CrossrefPubMed Google Scholar
19 Russell JA . Osteomalacic myopathy. Muscle Nerve1994;17:578–580.CrossrefPubMed Google Scholar
20 Birge SJ , HaddadJG. 25-hydroxycholecalciferol stimulation of muscle metabolism. J Clin Invest1975;56:1100–1107.CrossrefPubMed Google Scholar
21 Bischoff-Ferrari HA , BorchersM, GudatF, et al.Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res2004;19:265–269.CrossrefPubMed Google Scholar
22 Simpson RU , ThomasGA, ArnoldAJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem1985;260:8882–8891.PubMed Google Scholar
23 Marcinkowska E . A run for a membrane vitamin D receptor. Biol Signals Recept2001;10:341–349.CrossrefPubMed Google Scholar
24 Boland R . Role of vitamin D in skeletal muscle function. Endocr Rev1986;7:434–448.CrossrefPubMed Google Scholar
25 Sato Y , IwamotoJ, KanokoT, SatohK. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis2005;20:187–192.CrossrefPubMed Google Scholar
26 Sørensen OH , LundB, SaltinB, et al.Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci (Lond)1979;56:157–161.CrossrefPubMed Google Scholar
27 Boland R , NormanA, RitzE, HasselbachW. Presence of a 1,25-dihydroxy-vitamin D3 receptor in chick skeletal muscle myoblasts. Biochem Biophys Res Commun1985;128:305–311.CrossrefPubMed Google Scholar
28 Bischoff HA , BorchersM, GudatF, et al.In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J2001;33:19–24.CrossrefPubMed Google Scholar
29 Costa EM , BlauHM, FeldmanD. 1,25-dihydroxyvitamin D3 receptors and hormonal responses in cloned human skeletal muscle cells. Endocrinology1986;119:2214–2220.CrossrefPubMed Google Scholar
30 Nemere I , DormanenMC, HammondMW, OkamuraWH, NormanAW. Identification of a specific binding protein for 1 alpha,25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J Biol Chem1994;269:23750–23756.PubMed Google Scholar
31 Nemere I , SchwartzZ, PedrozoH, et al.Identification of a membrane receptor for 1,25-dihydroxyvitamin D3 which mediates rapid activation of protein kinase C. J Bone Miner Res1998;13:1353–1359.CrossrefPubMed Google Scholar
32 Girgis CM , Clifton-BlighRJ, HamrickMW, HolickMF, GuntonJE. The roles of vitamin D in skeletal muscle: form, function, and metabolism. Endocr Rev2013;34:33–83.CrossrefPubMed Google Scholar
33 Endo I , InoueD, MitsuiT, et al.Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology2003;144:5138–5144.CrossrefPubMed Google Scholar
34 Rejnmark L . Effects of vitamin D on muscle function and performance: a review of evidence from randomized controlled trials. Ther Adv Chronic Dis2011;2:25–37.CrossrefPubMed Google Scholar
35 Moreira-Pfrimer LDF , PedrosaMAC, TeixeiraL, Lazaretti-CastroM. Treatment of vitamin D deficiency increases lower limb muscle strength in institutionalized older people independently of regular physical activity: a randomized double-blind controlled trial. Ann Nutr Metab2009;54:291–300.CrossrefPubMed Google Scholar
36 Pfeifer M , BegerowB, MinneHW, et al.Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int2009;20:315–322.CrossrefPubMed Google Scholar
37 Bunout D , BarreraG, LeivaL, et al.Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol2006;41:746–752.CrossrefPubMed Google Scholar
38 Dhesi JK , JacksonSHD, BearneLM, et al.Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing2004;33:589–595.CrossrefPubMed Google Scholar
39 Bischoff HA , StähelinHB, DickW, et al.Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J Bone Miner Res2003;18:343–351.CrossrefPubMed Google Scholar
40 Foo LH , ZhangQ, ZhuK, et al.Low vitamin D status has an adverse influence on bone mass, bone turnover, and muscle strength in Chinese adolescent girls. J Nutr2009;139:1002–1007.CrossrefPubMed Google Scholar
41 Stewart JW , AlekelDL, RitlandLM, et al.Serum 25-hydroxyvitamin D is related to indicators of overall physical fitness in healthy postmenopausal women. Menopause2009;16:1093–1101.CrossrefPubMed Google Scholar
42 Murad MH , ElaminKB, Abu ElnourNO, et al.Clinical review: The effect of vitamin D on falls: a systematic review and meta-analysis. J Clin Endocrinol Metab2011;96:2997–3006.CrossrefPubMed Google Scholar
43 Bolland MJ , GreyA, GambleGD, ReidIR. Vitamin D supplementation and falls: a trial sequential meta-analysis. Lancet Diabetes Endocrinol2014;2:573–580.CrossrefPubMed Google Scholar
44 Gillespie LD , RobertsonMC, GillespieWJ, et al.Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev2012;9:CD007146.CrossrefPubMed Google Scholar
45 Cameron ID , MurrayGR, GillespieLD, et al.Interventions for preventing falls in older people in nursing care facilities and hospitals. Cochrane Database Syst Rev2010;1:CD005465.CrossrefPubMed Google Scholar
46 Avenell A , MakJC, O'ConnellD. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev2014;4:CD000227.CrossrefPubMed Google Scholar
47 Abrahamsen B , MasudT, AvenellA, et al.Patient level pooled analysis of 68 500 patients from seven major vitamin D fracture trials in US and Europe. BMJ2010;340:5463.CrossrefPubMed Google Scholar
48 Bischoff-Ferrari HA , WillettWC, OravEJ, et al.A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med2012;367:40–49.CrossrefPubMed Google Scholar
49 Lips P , GielenE, van SchoorNM. Vitamin D supplements with or without calcium to prevent fractures. Bonekey Rep2014;3:512. Google Scholar
50 Smith H , AndersonF, RaphaelH, et al.Effect of annual intramuscular vitamin D on fracture risk in elderly men and women--a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford)2007;46:1852–1857.CrossrefPubMed Google Scholar
51 Sanders KM , StuartAL, WilliamsonEJ, et al.Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA2010;303:1815–1822.CrossrefPubMed Google Scholar
52 Chapuy MC , ArlotME, DuboeufF, et al.Vitamin D3 and calcium to prevent hip fractures in the elderly women. N Engl J Med1992;327:1637–1642. Google Scholar









