It is not often that a new drug becomes available that is likely to affect the workload of orthopaedic surgeons, but denosumab has that potential. This edition of 360 carries a summary of a potentially landmark RCT examining the use of denosumab in giant cell tumour of bone.
Denosumab is a fully human monoclonal antibody that inhibits RANK-L (Receptor activator of nuclear factor kappa-B ligand). RANK-L drives osteoclast formation, function and survival and an excess of RANK-L causes an increase in bone destruction, thus inhibiting osteoclast mediated bone destruction. To date, denosumab has found a role in three areas of interest in orthopaedics. These include osteoporosis, metastatic bone disease and giant cell tumour of bone.
The evidence for its use in osteoporosis has been confirmed by several randomised controlled trials, the largest of which, the FREEDOM study, showed a significant risk reduction of new vertebral fractures in patients taking denosumab, compared with placebo.1 It also demonstrated improved bone mineral density the longer the treatment was continued.2 In October 2010, NICE approved denosumab as an option for post-menopausal women to prevent them suffering either a first osteoporotic fracture or further osteoporotic fracture if they were unable to take bisphosphonates (the normal first line therapy).3 The guidance gives quite clear indications as to who is suitable for this treatment which is given as a subcutaneous injection of 60 mg once every six months.
Denosumab has been shown to be clinically effective in reducing skeletal-related events (SRE) in patients with bone metastases from solid tumours, and has been sanctioned by NICE for use in metastatic bone disease (apart from prostate metastases).4 A systematic review of the clinical and cost effectiveness of denosumab for the treatment of bone metastases from solid tumours has been reported recently.5 This has shown that in a series of RCTs, denosumab, given monthly at a dose of 120 mg s/c, was effective in delaying the time to first SRE and reducing the risk of multiple SREs compared with zoledronic acid. It had a similar incidence of side effects to zoledronic acid and was cost effective if a reduced-price patient-access scheme was available. Appropriate treatment will undoubtedly reduce the incidence of SREs which will benefit patients and in turn will reduce the orthopaedic workload.
More recently there have been several reports on the use of denosumab in giant cell tumour (GCT) of bone.6 The latest study reports on a phase 2 study of patients who either had inoperable GCT or a GCT in a location where surgery (the conventional treatment) would have resulted in major morbidity.7 The primary endpoint of the study was the safety profile of denosumab, while the secondary endpoint was time to disease progression. In this study it was given monthly at a dose of 120 mg s/c, after three weekly loading doses. The results have been dramatic: there has been a 96% clinical response rate in that progression of disease has stopped and there have been improvements in pain, mobility and function. In many cases where morbid surgery was planned it was avoided or a lesser procedure was carried out. Objective radiological responses were observed in 72% of cases, with most patients demonstrating a decrease in the size of the soft-tissue extent of the tumour, and about one third a dramatic calcification of the tumour (Fig. 1). The FDA in the USA was clearly impressed with these results, licensing denosumab on 13th June 2013 for unresectable GCT or those where surgical resection was likely to result in severe morbidity.8 It is still, however, not licenced in Europe for the treatment of GCTs.
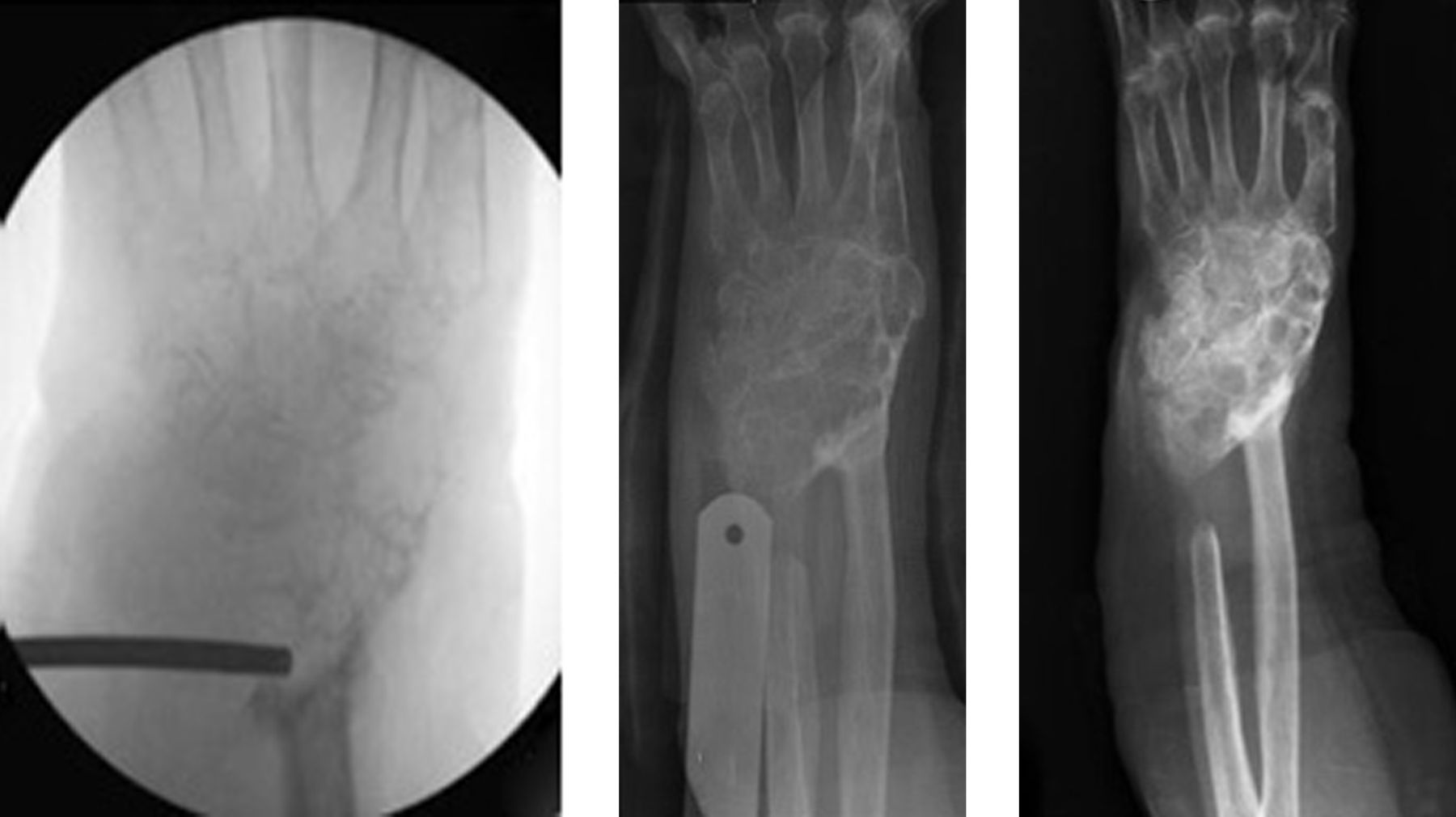
Fig. 1
A 79-year-old woman presented with extensive recurrence of a GCT involving the distal radius and carpus. The biopsy radiograph shows no discernible bone structure remaining. Within six weeks of starting denosumab, ossification was apparent and by three months she had dense calcification and was pain free.
Densosumab is simple to administer but does have contraindications and important side effects. Any woman of childbearing age on the drug must not get pregnant and it is contraindicated in anyone with existing hypocalcaemia, or with dental infections, due to its risk of producing osteonecrosis of the jaw (1-2%). Patients must take daily supplements of calcium and Vitamin D. If these precautions are observed, side effects are few and the treatment is well tolerated.
The paper by Chawla et al7 confirms that denosumab works for inoperable GCTs in the short term but does not indicate what role this powerful agent may have for the more common 'operable' GCT or for the longer-term use in these inoperable cases. At the moment the trial simply shows that GCTs can be controlled for up to five years by monthly injections. Does this mean they will need to continue on this regime for life? Evidence suggests that if denosumab is stopped, the giant cells will return over a period of around nine months in most cases. What are the possible side effects of being on denosumab, potentially for life, particularly in younger patients? Already there have been reports of atypical femoral fractures in patients on prolonged treatment with denosumab.9 Can the dose or the frequency of the injections be safely reduced without risking recurrence? Of even more importance is the query as to what is the potential role in conventional GCT. Could it be used as a neoadjuvant to 'shrink' a GCT prior to surgery (much like neoadjuvant chemotherapy for bone sarcomas) and would that help improve local recurrence rates? Should it be used as an adjuvant after curettage and, if so, for how long?
Finally, could denosumab have a role in other areas of orthopaedics where osteoclast mediated bone resorption takes place? One of the more obvious is Paget's disease but a more interesting consideration could be in osteoclast-mediated aseptic loosening around joint replacements. It is already being trialled as a potential agent to reduce bone resorption in patients with rheumatoid arthritis.10 This is certainly a drug to keep your eye on.
1 Keaveny T, McClung M, Genant H, et al. denosumab improves both femoral and vertebral strength in women with osteoporosis: results from the FREEDOM trial [abstract]. Annual meeting of the American Society for Bone and Mineral Research; Canada: 2010. Google Scholar
2 Austin M , YangYC, VittinghoffE, et al.Relationship between bone mineral density changes with denosumab treatment and risk reduction for vertebral and nonvertebral fractures. J Bone Miner Res2012;27:687–693.CrossrefPubMed Google Scholar
3 No authors listed. National Institute for Health and Clinical Excellence http://guidance.nice.org.uk/TA204/Guidance/pdf/English (date last accessed 4 September 2013). Google Scholar
4 No authors listed. National Institute for Health and Clinical Excellence http://www.nice.org.uk/nicemedia/live/13939/61129/61129.pdf (date last accessed 4 September 2013). Google Scholar
5 Ford J , CumminsE, SharmaP, et al.Systematic review of the clinical effectiveness and cost-effectiveness and economic evaluation, of denosumab for the treatment of bone metastases from solid tumours. Health Technol Assess2013;17:1–386. Google Scholar
6 Thomas D , HenshawR, SkubitzK, et al.denosumab in patients with giant cell tumour of bone: an open-label phase 2 study. Lancet Oncol2010;11:275–280. Google Scholar
7 Chawla S , HenshawR, SeegerL, et al.Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel group, phase 2 study. Lancet Oncol2013;14:901–908. Google Scholar
8 No authors listed. Food and Drug Administration http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm356528.htm (date last accessed 4 September 2013). Google Scholar
9 No authors listed. Medicines and Healthcare Products Redulatory Agency. http://www.mhra.gov.uk/Safetyinformation/DrugSafetyUpdate/CON239411 (date last accessed 4 September 2013). Google Scholar
10 Cohen SB , DoreRK, LaneNE, et al.denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum2008;58:1299–1309.CrossrefPubMed Google Scholar









