Abstract
Aims
Our objective was to conduct a systematic review and meta-analysis, to establish whether differences arise in clinical outcomes between autologous and synthetic bone grafts in the operative management of tibial plateau fractures.
Methods
A structured search of MEDLINE, EMBASE, the online archives of Bone & Joint Publishing, and CENTRAL databases from inception until 28 July 2021 was performed. Randomized, controlled, clinical trials that compared autologous and synthetic bone grafts in tibial plateau fractures were included. Preclinical studies, clinical studies in paediatric patients, pathological fractures, fracture nonunion, or chondral defects were excluded. Outcome data were assessed using the Risk of Bias 2 (ROB2) framework and synthesized in random-effect meta-analysis. The Preferred Reported Items for Systematic Review and Meta-Analyses guidance was followed throughout.
Results
Six studies involving 353 fractures were identified from 3,078 records. Following ROB2 assessment, five studies (representing 338 fractures) were appropriate for meta-analysis. Primary outcomes showed non-significant reductions in articular depression at immediate postoperative (mean difference -0.45 mm, p = 0.25, 95%confidence interval (CI) -1.21 to 0.31, I2 = 0%) and long-term (> six months, standard mean difference -0.56, p = 0.09, 95% CI -1.20 to 0.08, I2 = 73%) follow-up in synthetic bone grafts. Secondary outcomes included mechanical alignment, limb functionality, and defect site pain at long-term follow-up, perioperative blood loss, duration of surgery, occurrence of surgical site infections, and secondary surgery. Mean blood loss was lower (90.08 ml, p < 0.001, 95% CI 41.49 to 138.67) and surgery was shorter (16.17 minutes, p = 0.04, 95% CI 0.39 to 31.94) in synthetic treatment groups. All other secondary measures were statistically comparable.
Conclusion
All studies reported similar methodologies and patient populations; however, imprecision may have arisen through performance variation. These findings supersede previous literature and indicate that, despite perceived biological advantages, autologous bone grafting does not demonstrate superiority to synthetic grafts. When selecting a void filler, surgeons should consider patient comorbidity, environmental and societal factors in provision, and perioperative and postoperative care provision.
Cite this article: Bone Jt Open 2022;3(3):218–228.
Introduction
Fractures of the tibial plateau, although relatively uncommon with a global yearly incidence of 10.3 per 100,000 people, have substantial and deleterious impacts on patients’ quality of life.1-3 Two distinct mechanisms of injury are observed: high-energy trauma in younger patients and low-energy trauma in osteopenic patients.1-3 In both settings, there is significant risk of malunion, early osteoarthritis, and deep infection, which is severely debilitating in younger patients.4-6
Classification of tibial plateau fracture patterns, most commonly with Schatzker or AO/OTA nomenclature, indicate the degree of anatomical stability, and thus inform management strategies.6-10 The span of injury patterns, both bony and soft-tissue, results in a wide variety of treatment methods and outcomes. Operative managements of complex fractures (AO/OTA 41 C1,2,3; Schatzker IV-VI) include uni-/bicondylar fixation, arthroplasty, or external fixation in soft-tissue injury.6,9,11-13 Treatment seeks to achieve a stable, mechanically aligned leg with restoration of the joint surface.2,3 Subsequently, in these complex fractures, bone grafting may support this reduction.3
Bone grafting is indicated to augment the open reduction and internal fixation of intra-articular tibial plateau fractures, improving the mechanical environment and promoting bone growth within the fracture defect void.14-16 Historically, autologous bone grafting (ABG), typically from the anterior iliac spine, has been preferred given its proposed structural and osteogenic properties.14
However, this intervention is not without significant complication profiles, both at the recipient fracture site and the site of bone graft harvest.17,18 Additional donor site morbidity is associated with an 8.6% risk of major complications, including infection and reoperation.18 Furthermore, following anterior iliac spine graft harvesting, approximately 40% of patients will still suffer from pain six-months postoperatively.19
Subsequently, interest in synthetic bone graft substitutes has increased over recent decades, underpinned by translational research into advanced, bioengineered, biomaterials.20 Animal studies have demonstrated the biomechanical superiority of calcium phosphate cements relative to cancellous ABG in maintaining the reduction of depression following intra-articular tibial plateau defects.21 Cadaveric studies corroborate this biomechanical advantage, demonstrating improved stiffness and decreased displacement in synthetic bone grafts.22 Despite the perceived advantages of an improved mechanobiological environment and no donor site morbidity, to date there is limited high-quality clinical evidence to warrant the perceived additional costs of synthetic grafts.
This meta-analysis aims to assimilate the relevant high-quality randomized controlled trials (RCTs) to ascertain whether ABGs demonstrate clinical superiority to synthetic bone grafts in the management of tibial plateau fractures.
Methods
Protocol and registration
This systematic review and meta-analysis was undertaken in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA)23 guidance and the Cochrane Handbook for Systematic Reviews of Interventions.24 The review protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) database on 7 September 2021 (accessible under CRD42021270073). Ethical approval and informed consent were not required for this research.
Eligibility criteria
Eligibility criteria were considered with respect to the population, intervention, comparator, outcome and study design (PICOS) framework.24 We interrogated reported populations of tibia plateau fractures patients, to compare the use of synthetic bone substitutes with the standard of care ABG in RCTs.16,17 Our primary outcome was postoperative articular depression. Secondary outcomes included mechanical alignment, satisfaction with reduction, return to functionality, perioperative blood loss, duration of surgery, defect site pain at long-term follow-up and frequencies of defect site infections and secondary surgical interventions.
In vitro or cadaveric experiments, and observational and non-randomized clinical studies were ineligible, ensuring the highest applicability to clinical practice. Studies investigating tibial plateau fractures in patients under 16 years old, benign or malignant bone tumours, or fracture nonunion were also excluded, as were studies exploring chondral defect repair.
We searched databases from their inception to 28 July 2021. When indicated, we requested manuscripts from non-English language publications and unpublished literature. These were only excluded if the corresponding authors did not respond.
Information sources and search strategy
Our search strategy (Supplementary Table i) was executed on the “MEDLINE(R) and In-Process, In-Data-Review & Other Non-Indexed Citations 1946 to July 28th, 2021”, “EMBASE 1980 to 2021 Week 30”, and “Cochrane Central Register of Controlled Trials” databases. This was augmented by a further Boolean search: “Tibia Plateau Fracture AND (Bone Substitute OR Bone Graft)” on the National Institute of Health Clinical Trials Registry and the Bone and Joint Database. Manual reference list screening was performed on all relevant reviews and included articles.
Study selection and risk of bias assessment
After de-duplication, 3,078 records were screened by at least two reviewers (GC and MK). Disagreements between reviewers were resolved by the senior author (DS). Cohen’s kappa was calculated to assess inter-rater reliability between reviewers.25
Risk of bias was assessed using the contemporaneous Cochrane Risk of Bias 2 (ROB2) tool.26 The overall risk of bias was assessed for each set of outcome data included in our synthesis and was ascribed, according to its worst domain, as either low-risk, some concerns, or high-risk. High-risk outcome data were excluded from our synthesis. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework was used to assess the certainty of each assimilated outcome.27
Data collection process, data items, and effect measures
Data relating to the primary and secondary outcomes was extracted from each article under the observation of at least one other author. Where necessary, any further or missing outcome data was requested from corresponding authors. We sought further descriptive population-level values for study treatment groups (e.g. sample size, mean patient age, and proportion of female sex) and to capture possible ascertainment biases and indicate sources of heterogeneity.
We defined the immediate postoperative period as any time within the contiguous two weeks and long-term follow-up, as the last reported measurements, taken at least six months postoperatively.
Continuous outcomes were directly compared by mean difference and 95% confidence intervals (CIs). Similarly, discontinuous outcomes were compared by calculating odds ratio (OR) and 95%CIs.
Where outcomes were reported as continuous or discontinuous measurements between studies, we contacted the authors for continuous outcome data. If necessary, standard mean differences (SMD) and corresponding standard error (SE) of discontinuous outcome data were calculated from ORs and 95% CIs using Chinn’s method.28 This approach is recommended by the Cochrane Handbook for Systematic Review of Intervention and summarized in Supplementary Methods 1.28 Where necessary, standard deviation (SD) was imputed from sample range value using Wan et al’s29 adaptive method, outlined in Supplementary Methods 2.
Statistical analysis
Pairwise meta-analyses were performed using an inverse-variance, random effects model. The corresponding forest plots were generated using Review Manager v. 5.4 (The Cochrane Collaboration, UK).30
Heterogeneity was assessed according to the I2 statistic. Post-hoc sensitivity analysis was indicated in assimilations of three or more studies, where heterogeneity was, at least, moderate (I2 > 30%).24
A prespecified subgroup was assembled from synthetic, calcium phosphate cement (CPC), substitutes. Post-hoc, we identified bioactive glass granule and porous titanium granule subgroups. Subgroup analyses were also conducted in Review Manager 5.4 using the Χ2 function.30
Results
Following de-duplication, 3,078 records were identified. After screening and manuscript assessment, six studies were initially identified in our review (Figure 1).31-36 Inter-rater reliability indicated substantial agreement (Cohen’s k = 0.72).26 Additional information on almost-eligible studies is presented in Supplementary Results 1.
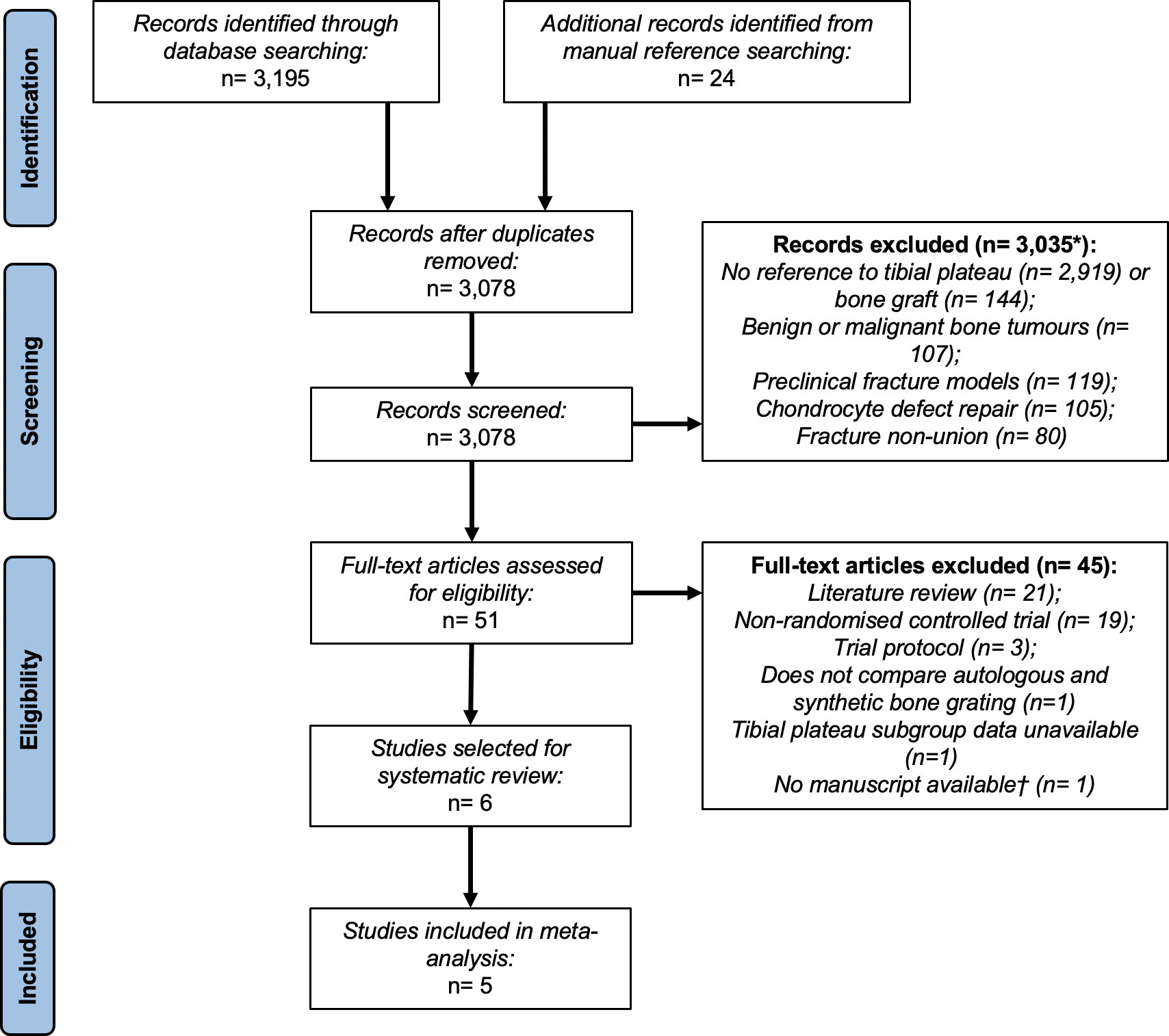
Fig. 1
A Preferred Reported Items for Systematic Review and Meta-Analyses flow diagram summarizing the selection of studies for systematic review and meta-analysis. Five studies were suitable for meta-analysis from 3,078 identified records. *Studies could be excluded for multiple reasons. †This conference abstract was excluded due to a lack of available data after contacting the corresponding author(s).
The selected RCTs represent 353 fractures across 352 patients from 1989 to 2019.31-36 One study was set in the USA, one in both Canada and the USA, and four across Northern or Central Europe (Table I). Study populations and treatment arms were directly comparable.31-36
Table I.
Summary characteristics of randomized controlled trials included in meta-analysis and systematic review.
| Study name | Study setting | Patient population (treatment group, control group) | Treatment groups | Outcomes included in review | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | Country | Study centres, n | Patients, n | Tibial plateau fractures, n | Mean patient age, yrs (SD) | Patient sex, n (% female) |
Mean follow-up, mths (SD) | Treatment void filler | Control void filler | ||
| Bucholz et al (1989)31 | 1981 to 1985 | USA | 1 | 20, 20 | 20, 20 | 37.5 (N/A), 36.7 (N/A) | 9 (45), 7 (35) | 34.5 (N/A), 15.4 (N/A) | CPC | ABG (Cancellous) | A, D, E |
| Russell et al (2008)32 | 1999 to 2002 | USA, Canada | 12 | 119 | 82, 38 | 43.0 (N/A), 43.0 (N/A) | 46 (39) | 12* (N/A) | CPC | ABG (Anterior Iliac Crest) | B, C, D, E |
| Heikkilä et al (2010)33 | 1995 to 1999 | Finland | 1 | 14, 11 | 14, 11 | 57.0 (N/A), 50.0 (N/A) | 7 (50), 6 (55) | 12* (N/A) | BG | ABG (Anterior Iliac Crest) | A, B, C, G |
| Pernaa et al (2011)34 | 1995 to 2010 | Finland | 1 | 5, 10 | 5, 10 | 52.0 (N/A), 58.0 (N/A) | 8 (53) | 132 (N/A) | BG | ABG (Anterior Iliac Crest) | A, B, C, D, F |
| Jónsson and Mjöberg (2015)35 | 2008 to 2012 | Sweden | 1 | 11, 9 | 11, 9 | 48.7 (19.3), 49.4 (15.5) | 6 (55), 5 (56) | 12* | PTG | ABG (Unspecified) | A, B, D, E, F, H |
| Hofmann et al (2019)36 | 2013 to 2017 | Germany | 20 | 65, 68 | 65, 68 | 47.0 (12.4), 46.3 (11.2) | 36 (55%), 39 (57%) | 6* | Biphasic CPC and CSC | ABG (Anterior Iliac Crest) | B, C, D, G, H |
-
Bucholz et al’s31 patient population was younger and, along with Russell et al,32 proportionally less female, although these differences were not substantial.
-
*
Where mean duration of follow-up was not reported, the maximum per-protocol follow-up was reported instead.
-
A, postoperative articular depression; ABG, autologous bone graft; B, articular depression at long-term follow-up; BG, bioactive glass granules; C, mechanical alignment at long-term follow-up; CPC, calcium phosphate cement; CSC, calcium sulphate cement; D, frequency of surgical site infection at tibial defect site; E, frequency of secondary surgical interventions; F, defect site pain at long-term follow-up; G, perioperative blood loss; H, duration of surgery; HA, hydroxyapatite; N/A, not available; PTG, porous titanium granules.
Study-specific sources of bias and reporting biases
Following risk of bias assessments (Supplementary Table ii), we excluded Pernaa et al34 from our meta-analysis. This paper presented mean 11-year follow-up data of Heikkilä et al.33 However, it included a substantive loss of subjects to follow-up (approximately 50%), which exacerbated attrition bias.33,34 Thus, our study selection process identified five studies (comprising 338 fractures) suitable for meta-analysis (Figure 1).
Following patient randomization according to the date of presentation, the unblinded nature of Bucholz et al31 represents clear loss of allocation concealment, introducing concerns around ascertainment biases.26 Despite this, the baseline characteristics of both groups were highly comparable, indicating researchers’ equipoise and limiting the magnitude of these concerns.
Both Hofmann et al36 and Russell et al32 reported partial or complete missing data in approximately 15% of patients randomized. These were primarily attributed to loss to follow-up. Given the challenges associated with maintaining patient engagement in large surgical trials, we did not feel these indicated a particular risk of bias.26,32,36 The small number of studies precluded assessment of publication bias with Egger’s test.24 The certainty of each synthesized outcome, as determined by the GRADE framework, is presented in Supplementary Table iii.27
Articular depression and mechanical alignment
All RCTs included in our synthesis reported on articular reduction, which was measured with anteroposterior radiographs.31-33,35,36 An absence of statistically improved reduction was observed by three studies at immediate postoperative follow-up, with pooled analysis observing a non-significantly smaller malreduction with the use of synthetic grafts (mean difference -0.45 mm, p = 0.25, 95% CI -1.21 to 0.31, I2 = 0%, Figure 2a).31,33,35 Bucholz et al31 was the only study included in this outcome analysis without a low overall risk of bias. Here, some concerns were attributed to the ascertainment of treatment groups and uncertainty as to whether radiological outcome data collection was blinded.26
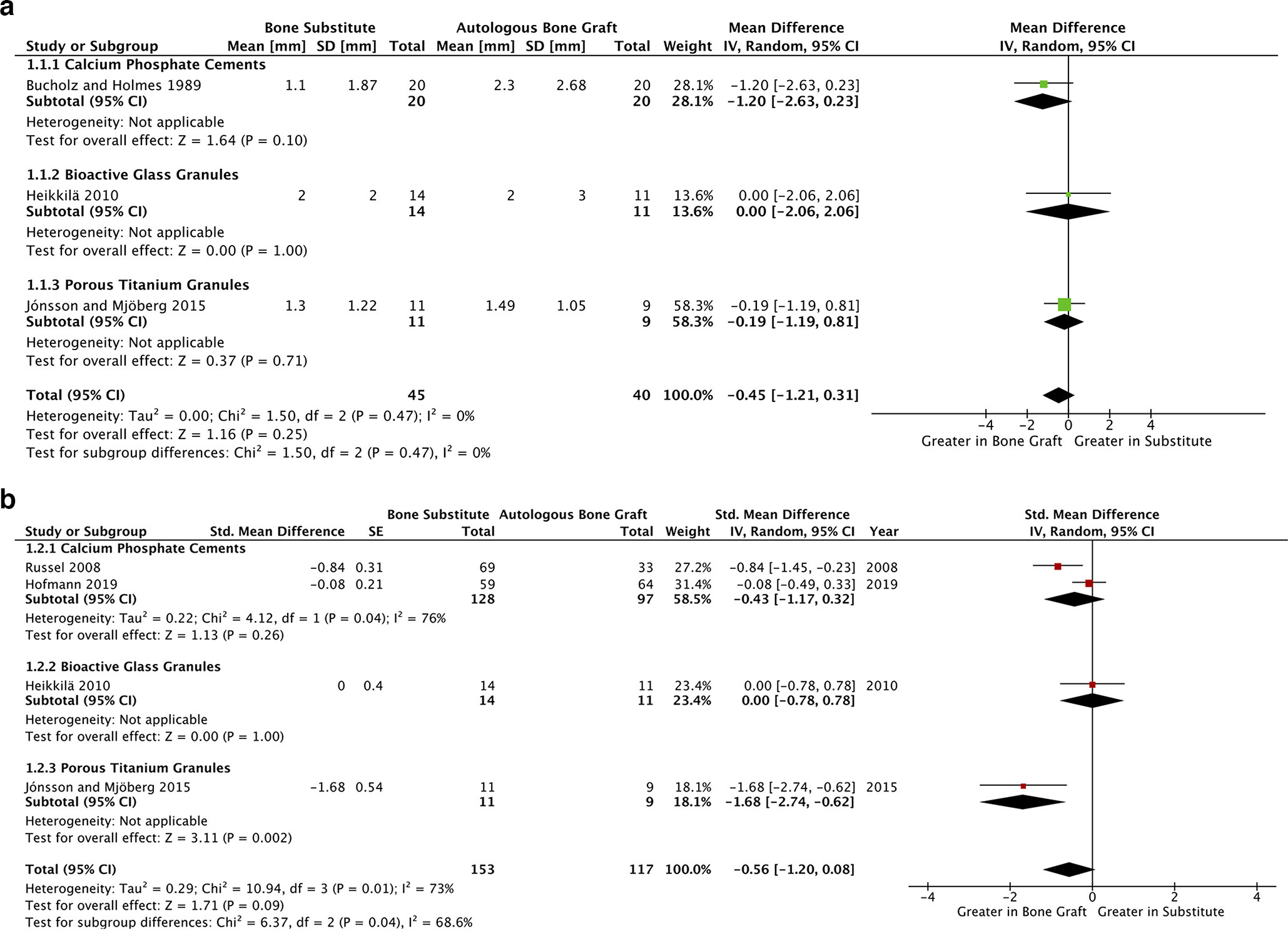
Fig. 2
a) Forest plot of postoperative articular depression outcome data. This figure presents a forest plot of articular reduction at postoperative follow-up, using data from three studies.31,33,35 b) Forest plot of long-term articular depression outcome data. This figure presents a forest plot of articular reduction at long-term follow-up (≥ six months postoperatively). This panel includes data from four studies.32,33,35,36 CI, confidence interval; IV, inverse variance; SD, standard deviation; SE, standard error.
When comparing synthetic grafting to ABG, a statistically non-significant improvement in articular reduction was observed in long-term (> six months) follow-up analysis of four studies, each with low overall risks of bias (SMD -0.56, p = 0.09, 95% CI -1.20 to 0.08, I2 = 73%, Figure 2b).32,34-36 Sensitivity analyses (Figures 3a and 3b) implicate both Russell et al32 and Jónsson and Mjöberg’s35 reported effect-sizes in the heterogeneity of this outcome.
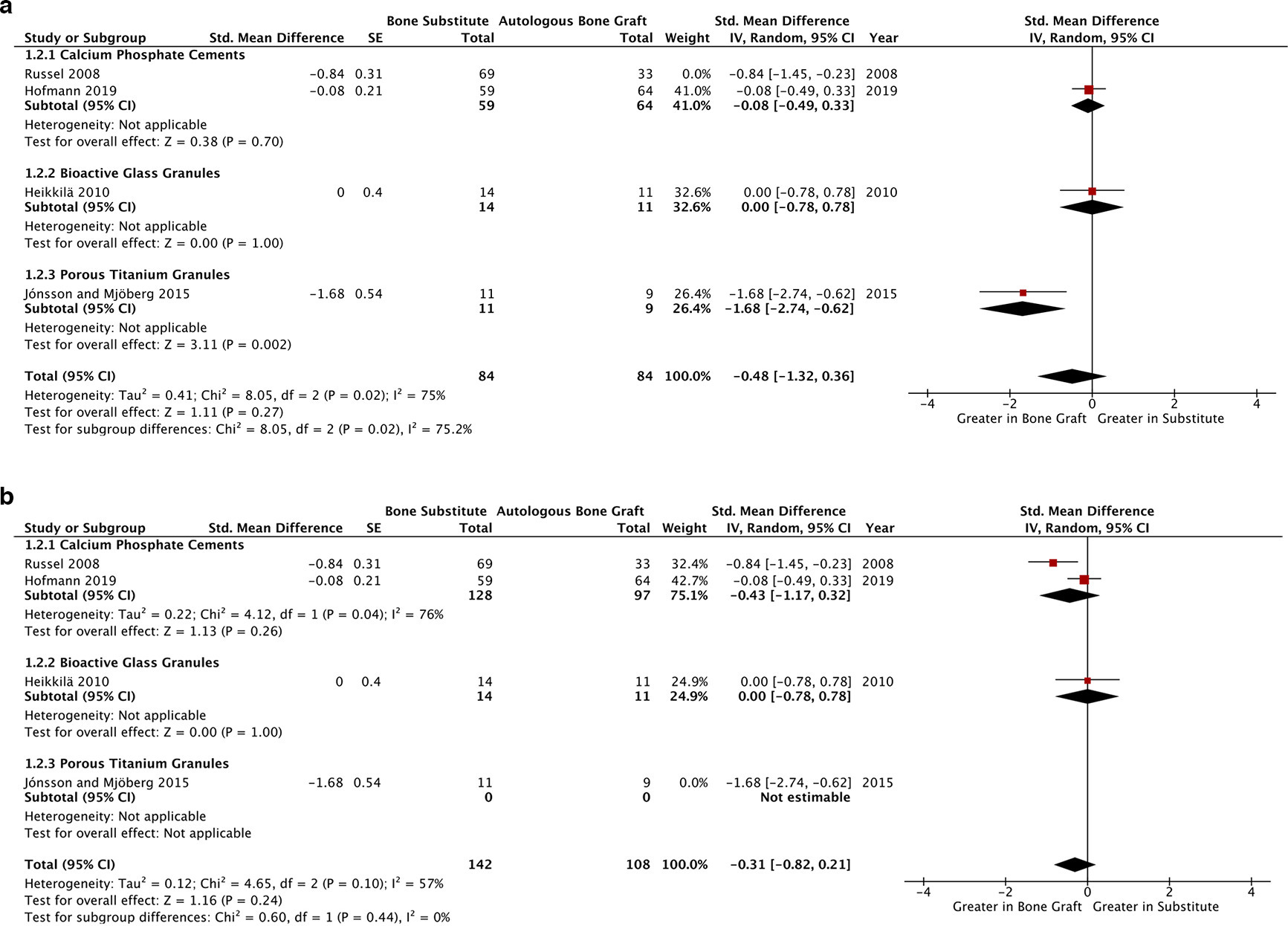
Fig. 3
a) Sensitivity analysis of Figure 2b exploring the impact of Russell et al’s32,33,35,36 reported effect sizes in contributing heterogeneity within the long-term articular reduction outcome. b) Sensitivity analysis of Figure 2b exploring the impact of Jónsson and Mjöberg’s32,33,35,36 reported effect sizes in contributing heterogeneity within the long-term articular reduction outcome.CI, confidence interval; IV, inverse variance; SE, standard error.
Pernaa et al34 reported mean postoperative articular depression of 1.4 mm (0 to 2) in the bioactive glass and 1.6 mm (0 to 5) in the ABG groups, respectively. Similarly, at final follow-up (mean 11 years; 10 to 14), articular depression was 1.4 mm (0 to 2) and 1.4 mm (0 to 4) in the bioactive glass and ABG groups; indicating, despite its high risk of bias, extended long-term efficacy of bioactive glass void filler.26,33,34
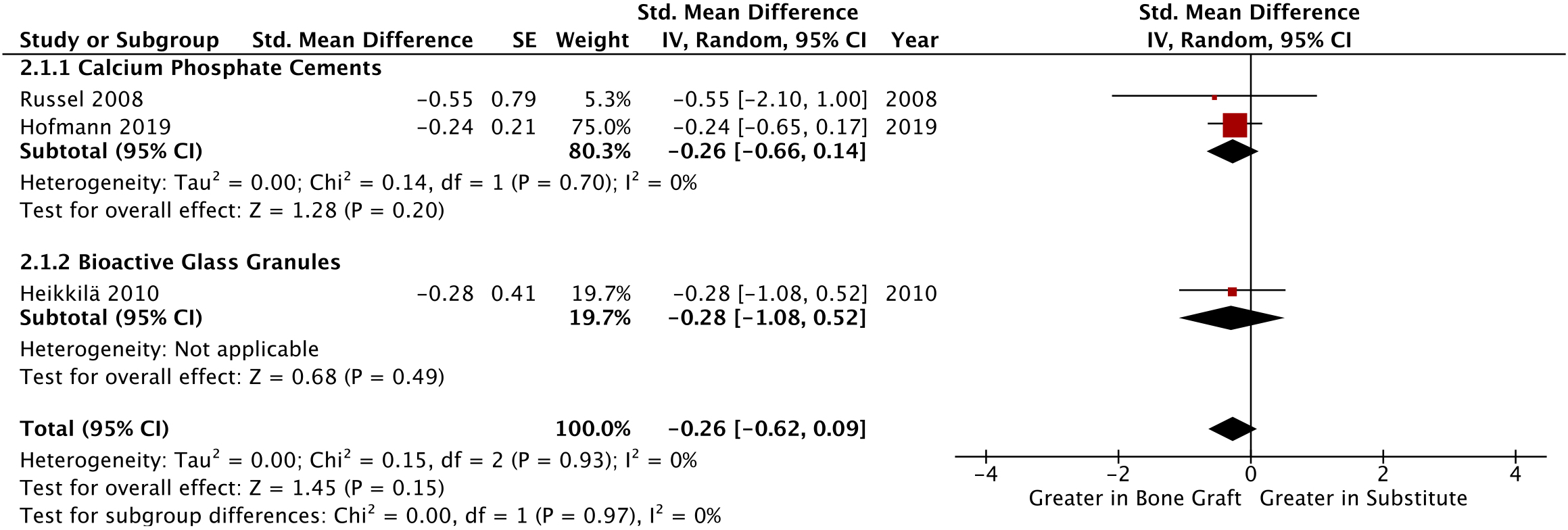
Mechanical alignment at long-term follow-up was compared radiologically in three studies, demonstrating insignificant differences in alignment when compared to the other leg using weightbearing, anteroposterior radiographs (SMD -0.26, p = 0.15, 95% CI -0.62 to 0.09, I2 = 0%, Figure 4).32,33,36 This finding correlates with Pernaa et al,34 which reported no significant mean contralateral (affected/unaffected knee) differences in the tibiofemoral angle in the bioactive glass and ABG groups. All studies assimilated in this outcome had a low overall risk of bias. Overall certainty in the quality of these three outcomes was high, according to our GRADE analysis (Supplementary Table iii).27
Anticipated adverse events
Defect site pain at long-term follow-up was extracted from three studies.31,33,35 Two of these studies had had high overall risks of bias, so were excluded from the synthesis. Pernaa et al34 (bioactive glass: mean 0.4; ABG: mean 1.0, using a ten-point visual analogue scale) reported little difference. Bucholz et al31 was also excluded from this outcome synthesis as there was no reported methodology for measuring defect site pain at long-term follow-up, introducing high risk of information biases. However, this study too reported little difference when comparing synthetic and ABG interventions (OR 0.78, p = 0.72, 95% CI 0.19 to 3.13).
Jónsson and Mjöberg35 identified a non-significant signal for reduced long-term defect site pain between synthetic substitutes and ABG (OR 0.34, p = 0.27, 95% CI 0.05 to 2.26). Overall certainty in this outcome was moderate (Supplementary Table iii), following the relative imprecision in the context of the demonstrable patient impact, and additional concerns of information biases arose following unblinded data collection; however, these concerns were limited by an acceptable, standardized methodology.26,27,35
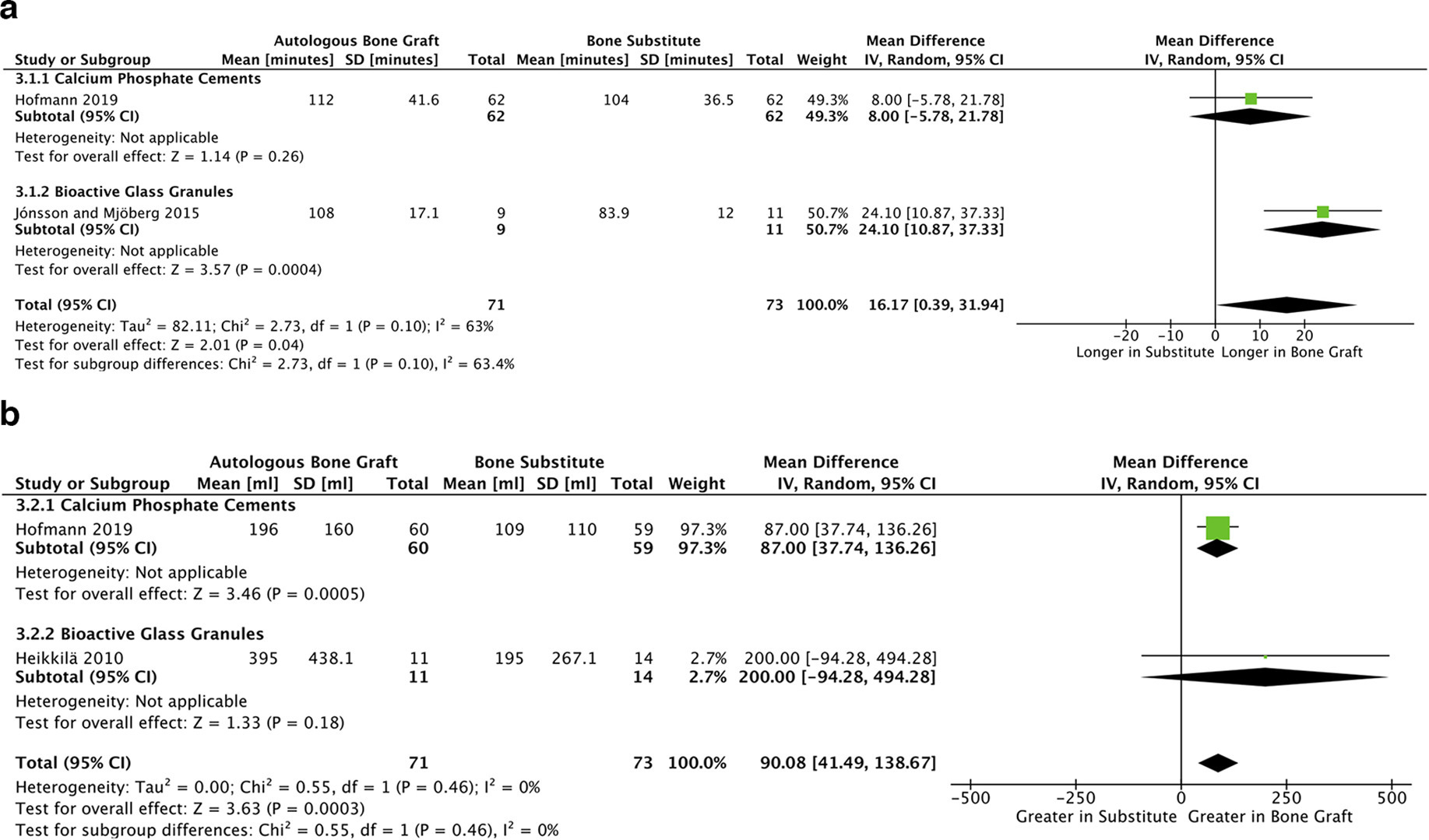
Small but statistically significant reductions in duration of surgery and blood loss were observed. Assimilation of two studies identified this reduction in surgery duration (mean difference 16.17 minutes, p = 0.04, 95% CI 0.39 to 31.94, I2 = 63%, Figure 5a).35,36 Similarly two different studies identified the reduction in blood loss (mean difference 90.08 ml, p < 0.001, 95% CI 41.49 to 138.67, I2 = 0%, Figure 5b).33,36 All studies synthesized for these two outcomes showed low risk of bias in their respective outcomes.26 Overall certainty in the quality of both these outcomes was high, according to our GRADE analysis (Supplementary Table iii).27
Unanticipated adverse events
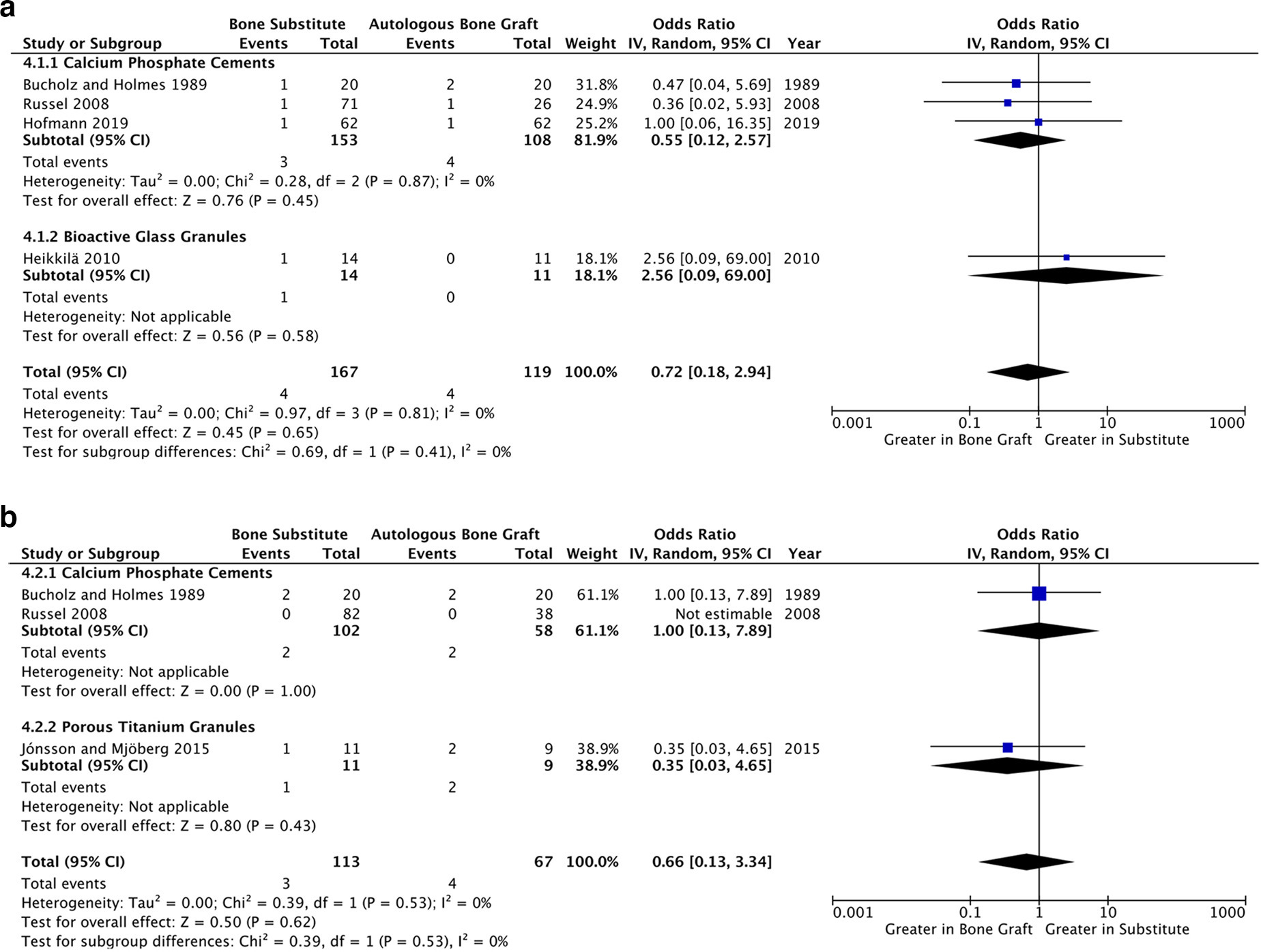
A synthesis of four studies accumulated data on 294 fractures, giving an overall surgical site infection rate of 2.7% (8/294) (Figure 6a).31-33,36 Pooled analysis revealed no difference between substitutes and ABG (OR 0.72, p = 0.58, 95% CI 0.18 to 2.94, I2 = 0%). In Pernaa et al,34 one patient in the ABG group developed a mild wound infection but none did from the bioactive glass group (OR 0.63, p = 0.79, 95% CI 0.02 to 18.37), showing no significant difference from the consensus of analyzed literature.
Three studies (representing 174 fractures) comprehensively reported secondary surgical interventions throughout their follow-up period (Table I), which are summarized in Supplementary Table iv.31,32,36 These showed a combined occurrence rate of 4.3% (7/164) and a statistically non-significant signal towards increased frequency in patients receiving ABG, relative to the synthetic bone substitute group (OR 0.66, p = 0.53, 95% CI 0.13 to 3.34, I2 = 0%, Figure 6b). Noticeably, Russell et al32 reported no secondary surgical interventions in both treatment groups, and thus this studies effect size could not be estimated or assimilated.
In both outcomes, the concerns around population ascertainment in Bucholz et al31 were the only potential risks of bias identified.32,33,35,36 Following outcome imprecision, in the context of demonstrable patient impact, overall certainty in the quality of both frequency of surgical site infection and secondary surgical interventions outcomes was moderate, according to our GRADE analysis (Supplementary Table iii).27
Limb functionality
Heterogeneity arising from several measures of limb functionality precluded comparisons of this domain. Bucholz et al31 reported total return to employment in both ABG and synthetic study arms (20/20, n = 20). Russell et al32 identified no statistical differences in either knee flexion and extension at both six- and 12-month follow-up. Heikkilä et al33 and Pernaa et al34 present patient reported satisfactions at long-term follow-up. We elected not to synthesize these measures, given the overlapping patient groups and high attrition in Pernaa et al.34 Furthermore, both patient populations were unblinded, introducing possible information biases. Both studies reported no significant differences in “Excellent” results (Heikkilä et al33 OR 0.90, p = 0.90, 95% CI 0.18 to 4.41; Pernaa et al34 OR 7.00 p = 0.26, 95% CI 0.24 to 206.80) between ABG and synthetic grafts.
Jónsson and Mjöberg35 found no significant difference in Lysholm knee score at 12 months between ABG and synthetic grafts (mean difference 2.920, p = 0.65, 95% CI -10.2465 to 16.0874). Finally, Hofmann et al36 graphically presented quality of life and functionality using the 12-item short form survey (SF-12) mental and physical component summaries, respectively. Although these data could not be extracted, no clinically or statistically significant difference between synthetic and ABG treatments were observed.36
Discussion
While it is widely accepted that open reduction and internal fixation of complex tibial plateau fractures is the gold standard for management, it is often assumed that the presence of a metaphyseal void mandates immediate surgical intervention with void filler.6,11,15-18,32,37,38 However, biomechanical studies do not replicate the evolving scenario seen with fracture healing, and there remain no quality in vivo experiments that replicate the biomechanics of a bipedal plateau void.39
Historically, ABG has been considered the preferred defect void filler, given its proposed satisfaction of ideal biological, mechanical, and economic criteria for bone grafts.14-16,31–40-43 However, harvesting ABGs imparts additional morbidity.17-19,44 Structural concerns surround the relatively low density of cancellous iliac bone, exacerbating resorption in acute injury and impairing the maintenance of reduction.45 Furthermore, a recent systematic review concluded that, despite ongoing research, there is currently insufficient evidence to elucidate the utility of biologically active bone grafts in fracture healing.46 Indeed, consensus indicates that synthetic bone grafts may provide a viable alternative void filler in the setting of tibial plateau fractures.31-37,40,47-49
This meta-analysis represents the most contemporary synthesis of high-quality (Oxford Centre for Evidence-Based Medicine, OCEBM, Level 1), randomized-controlled literature comparing synthetic and autologous bone grafts for tibial plateau fractures, and supersedes a previous systematic review containing lower-level literature.40,50 The primary outcomes of this analysis indicate non-significant signals towards increased accuracy of initial surgical reduction and the preservation of articular reduction at the long-term (> six months) follow-up period, when comparing synthetic and autologous grafts. This is consistent with the limited mechanical characteristics of ABGs, relative to synthetic grafts in preclinical literature.51,52
The substantial heterogeneity (I2 = 73%) in long-term articular reduction was driven by two factors. Firstly, a large magnitude of effect attributed to titanium granules perhaps indicates resilience towards early resorption.35 Secondly, despite both being pragmatic, comparable RCTs, Russell et al32 and Hofmann et al36 reported inconsistent estimates of the effect of CPC compared to ABG on long-term articular reduction. However, Russell et al32 followed patients 12 months postoperatively, while Hofmann et al’s36 final follow-up was six months postoperatively. Consequently, this potentially masks the true effect size in our observed findings.
Small but significant improvements in duration of surgery and blood loss were observed in synthetic bone grafts, relative to ABGs, when measured. The magnitude of these (16.17 minutes and 90.08 ml) are consistent with graft harvesting in previous literature.17-19,45,53 The heterogeneity identified in duration of surgery may arise from differences in study setting, including procedural familiarity and local incidences between the single-centre in Jónsson and Mjöberg,35 and the multicentre Hofmann et al.36 Alternatively, it may indicate greater simplicity in the application of titanium granules relative to biphasic calcium phosphate and sulphate cements.35,36 Regardless, these findings have implications for contemporary practice, representing marginal but statistically significant gains in perioperative morbidity and, in this context, demonstrating the non-inferiority of synthetic grafting. Blood loss is a key surgical morbidity, and reduced duration of surgery impacts provision of both orthopaedic and anaesthetic services, especially given widespread increased systemic pressures around the COVID-19 pandemic, the additional morbidity associated with prolonged anaesthesia, and environmental concerns surrounding inhaled anaesthetics.54-57
Three studies reported on mechanical alignment, which may be predictive of impaired long-term functionality and subsequent development of osteoarthritis, at follow-up, and concluded there was no difference in alignment between synthetic and autologous bone grafts.32,33,35,47,58 Functional outcome data in our evidence base was limited and showed no preference for synthetic or autologous bone grafts. There were non-significant differences in measured adverse event outcomes – specifically pain – frequency of surgical site infection, or in adverse events that required secondary surgery, which favoured synthetic rather than autologous grafts. Subsequently, we could not observe statistical divergence in the adverse event profiles of the graft types we explored; however, delayed synthetic graft resorption might be reasonably expected in synthetic bone graft subtypes.20,59
Despite its importance to patients and prominence in epidemiological literature, there was limited high-quality reporting of long-term defect site pain.1-5,31,34,35 Measures of patient-reported functional outcomes and quality of life, which are at the core of contemporary orthopaedic research, were highly heterogenous in our evidence base and thus limited assimilation.60 Furthermore, there is a noticeable lack of outcome data beyond 12 months and cost-benefit analyses, limiting comparison of these treatments in these contexts. These shortcomings indicate the need for a further, large-scale, pragmatic RCT in this setting to consolidate these limitations in the literature. In the setting of advancing synthetic graft materials and the adverse effects of autologous grafting, researchers may also seek to compare osteosynthesis using synthetic grafts versus fixation alone.17-20,59,61,62 In this case, it will be important for the pragmatic design of this trial to optimize the selection of .
It should not be concluded that synthetic void fillers are superior to ABGs. Instead, we suggest that the biological attributes of ABG provide little measurable benefit to tibial plateau fracture patients. There are several key limitations to our study. As with many RCTs, there is often an unseen selection bias which can be compounded by meta-analysis.61 In this case, patients are more likely to be included in trials who have larger defect voids and more complex fracture patterns. Consequently, while these results are not likely generalizable to the wider setting of tibial plateau fractures (where bone grafting is less frequently indicated), this self-selection may act to limit variation in fracture patterns within study populations, thus maintaining the internal validity of this review.3
Furthermore, surgeons with a certain preference or familiarity with a particular graft may introduce performance biases.63 Additionally, postoperative variation arises between geographical and temporal variance in rehabilitation pathways, and the psychosocial and economic factors determining patient engagement within these pathways introduce heterogeneity when comparing long-term outcomes. However, sampled study population samples were drawn from more economically developed countries.31-36 While limiting the applicability of study findings to patients in less economically developed countries, this contributes to homogeneity when comparing studies in this synthesis. Furthermore, all but one study was actively recruiting patients between 1999 and 2009, this temporal distribution again supporting internal validity.31-36
Although sampling databases from inception, and thus including Bucholz et al,31 may have introduced some historical practice variation, this study explored hydroxyapatite as a synthetic bone graft, which is still used contemporaneously.,64,65 Finally, a wide variety of graft options have been included in this review, possibly introducing heterogeneity to the synthetic treatment group. These factors, in combination with our small number of assimilated studies, may limit the power of our analysis and increase exposure to publication bias. However, pragmatically, this synthesis still presents the highest quality (OCEBM, Level 1) evidence in this setting.50,63
In conclusion, this meta-analysis challenges the long-held paradigm that the gold standard for void management in tibial plateau fractures is ABG.14-16 Our findings indicate that if a surgeon selects a synthetic bone graft to supplement fixation of a tibial plateau fracture, they can expect an equivalent accuracy of initial reduction and maintenance of long-term reduction, alongside minor reductions in operating time and blood loss. Subsequently, in this setting, surgeons should select void fillers while considering patient morbidity and operating time in surgical care provision. However, future research is needed to elucidate the optimal method in the surgical management of bone voids in tibial plateau fractures.
References
1. Elsoe R , Larsen P , Nielsen NPH , Swenne J , Rasmussen S , Ostgaard SE . Population-based epidemiology of tibial plateau fractures . Orthopedics . 2015 ; 38 ( 9 ): e780 - 6 . Crossref PubMed Google Scholar
2. Moore TM , Patzakis MJ , Harvey JP . Tibial plateau fractures: definition, demographics, treatment rationale, and long-term results of closed traction management or operative reduction . J Orthop Trauma . 1987 ; 1 ( 2 ): 97 – 119 . Google Scholar
3. Lafferty P , Cole P . Skeletal Trauma: Basic Science, Management, and Reconstruction . In : Tibial Plateau Fractures . Vol . 2020 . 6th ed . Elsevier , : 2181 – 2276 . Google Scholar
4. Papagelopoulos PJ , Partsinevelos AA , Themistocleous GS , Mavrogenis AF , Korres DS , Soucacos PN . Complications after tibia plateau fracture surgery . Injury . 2006 ; 37 ( 6 ): 475 – 484 . Crossref PubMed Google Scholar
5. Young MJ , Barrack RL . Complications of internal fixation of tibial plateau fractures . Orthop Rev . 1994 ; 23 ( 2 ): 149 – 154 . PubMed Google Scholar
6. Schatzker J , McBroom R , Bruce D . The tibial plateau fracture. The Toronto experience 1968--1975 . Clin Orthop Relat Res . 1979 ; 138 : 94 – 104 . PubMed Google Scholar
7. Dirschl DR , Dawson PA . Injury severity assessment in tibial plateau fractures . Clin Orthop Relat Res . 2004 ; 423 : 85 – 92 . Crossref PubMed Google Scholar
8. Zeltser DW , Leopold SS . Classifications in brief: Schatzker classification of tibial plateau fractures . Clin Orthop Relat Res . 2013 ; 471 ( 2 ): 371 – 374 . Crossref PubMed Google Scholar
9. Orthopaedic Trauma Association . Fracture and dislocation compendium. orthopaedic trauma association committee for coding and classification . J Orthop Trauma . 1996 ; 10 ( Suppl 1 v-ix ): 1 – 154 . PubMed Google Scholar
10. Yao X , Zhou K , Lv B , et al. 3D mapping and classification of tibial plateau fractures . Bone Joint Res . 2020 ; 9 ( 6 ): 258 – 267 . Crossref PubMed Google Scholar
11. Gardner MJ , Yacoubian S , Geller D , et al. Prediction of soft-tissue injuries in Schatzker II tibial plateau fractures based on measurements of plain radiographs . J Trauma . 2006 ; 60 ( 2 ): 319 – 323 ; . Crossref PubMed Google Scholar
12. Bennett WF , Browner B . Tibial plateau fractures: a study of associated soft tissue injuries . J Orthop Trauma . 1994 ; 8 ( 3 ): 183 – 188 . PubMed Google Scholar
13. McNamara IR , Smith TO , Shepherd KL , et al. Surgical fixation methods for tibial plateau fractures . Cochrane Database Syst Rev . 2015 ; 9 : CD009679 . Crossref PubMed Google Scholar
14. Koval KJ , Helfet DL . Tibial plateau fractures: evaluation and treatment . J Am Acad Orthop Surg . 1995 ; 3 ( 2 ): 86 – 94 . Crossref PubMed Google Scholar
15. Azi ML , Aprato A , Santi I , Kfuri M , Masse A , Joeris A . Autologous bone graft in the treatment of post-traumatic bone defects: a systematic review and meta-analysis . BMC Musculoskelet Disord . 2016 ; 17 ( 1 ): 465 . Crossref PubMed Google Scholar
16. Tscherne H , Lobenhoffer P . Tibial plateau fractures: management and expected results . Clin Orthop Relat Res . 1993 ; 292 : 87 – 100 . Google Scholar
17. Shaw KA , Griffith MS , Shaw VM , Devine JG , Gloystein DM . Harvesting autogenous cancellous bone graft from the anterior iliac crest . JBJS Essent Surg Tech . 2018 ; 8 ( 3 ): e20 . Crossref PubMed Google Scholar
18. Younger EM , Chapman MW . Morbidity at bone graft donor sites . J Orthop Trauma . 1989 ; 3 ( 3 ): 192 – 195 . Crossref PubMed Google Scholar
19. Goulet JA , Senunas LE , DeSilva GL , Greenfield ML . Autogenous iliac crest bone graft. complications and functional assessment . Clin Orthop Relat Res . 1997 ; 339 : 76 – 81 . Crossref PubMed Google Scholar
20. Koons GL , Diba M , Mikos AG . Materials design for bone-tissue engineering . Nat Rev Mater . 2020 ; 5 ( 8 ): 584 – 603 . Crossref Google Scholar
21. Welch RD , Zhang H , Bronson DG . Experimental tibial plateau fractures augmented with calcium phosphate cement or autologous bone graft . J Bone Joint Surg Am . 2003 ; 85-A ( 2 ): 222 – 231 . Crossref Google Scholar
22. Trenholm A , Landry S , McLaughlin K , et al. Comparative fixation of tibial plateau fractures using alpha-BSM, a calcium phosphate cement, versus cancellous bone graft . J Orthop Trauma . 2005 ; 19 ( 10 ): 698 – 702 . Crossref PubMed Google Scholar
23. Page MJ , McKenzie JE , Bossuyt PM , et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews . BMJ . 2021 ; 372 : 71 . Crossref PubMed Google Scholar
24. Higgins JPT , Thomas J , Chandler J , Cumpston M , Li T , Page MJ . Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 . https://training.cochrane.org/handbook ( date last accessed 14 February 2022 ). Google Scholar
25. Cohen J . A coefficient of agreement for nominal scales . Educ Psychol Meas . 2016 ; 20 ( 1 ): 37 – 46 . Crossref Google Scholar
26. Sterne JAC , Savović J , Page MJ , et al. RoB 2: a revised tool for assessing risk of bias in randomised trials . BMJ . 2019 ; 366 : l4898 . Crossref PubMed Google Scholar
27. Guyatt GH , Oxman AD , Vist GE , et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations . BMJ . 2008 ; 336 ( 7650 ): 924 – 926 . Crossref PubMed Google Scholar
28. Chinn S . A simple method for converting an odds ratio to effect size for use in meta-analysis . Stat Med . 2000 ; 19 ( 22 ): 3127 – 3131 . Crossref PubMed Google Scholar
29. Wan X , Wang W , Liu J , Tong T . Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range . BMC Med Res Methodol . 2014 ; 14 ( 1 ): 135 . Crossref PubMed Google Scholar
30. The Cochrane Collaboration . Review Manager (RevMan) (5.4.1) . https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman Google Scholar
31. Bucholz RW , Carlton A , Holmes R . Interporous hydroxyapatite as a bone graft substitute in tibial plateau fractures . Clin Orthop Relat Res . 1989 ; 240 : 53 – 62 . PubMed Google Scholar
32. Russell TA , Leighton RK , Alpha-BSM Tibial Plateau Fracture Study Group . Comparison of autogenous bone graft and endothermic calcium phosphate cement for defect augmentation in tibial plateau fractures. A multicenter, prospective, randomized study . J Bone Joint Surg Am . 2008 ; 90-A ( 10 ): 2057 – 2061 . Crossref PubMed Google Scholar
33. Heikkilä JT , Kukkonen J , Aho AJ , Moisander S , Kyyrönen T , Mattila K . Bioactive glass granules: a suitable bone substitute material in the operative treatment of depressed lateral tibial plateau fractures: a prospective, randomized 1 year follow-up study . J Mater Sci Mater Med . 2011 ; 22 ( 4 ): 1073 – 1080 . Crossref PubMed Google Scholar
34. Pernaa K , Koski I , Mattila K , et al. Bioactive glass S53P4 and autograft bone in treatment of depressed tibial plateau fractures - a prospective randomized 11-year follow-up . J Long Term Eff Med Implants . 2011 ; 21 ( 2 ): 139 – 148 . Crossref PubMed Google Scholar
35. Jónsson BY , Mjöberg B . Porous titanium granules are better than autograft bone as a bone void filler in lateral tibial plateau fractures: A randomised trial . Bone Joint J . 2015 ; 97-B ( 6 ): 836 – 841 . Crossref PubMed Google Scholar
36. Hofmann A , Gorbulev S , Guehring T , et al. Autologous iliac bone graft compared with biphasic hydroxyapatite and calcium sulfate cement for the treatment of bone defects in tibial plateau fractures: a prospective, randomized, open-label, multicenter study . J Bone Joint Surg Am . 2020 ; 102-A ( 3 ): 179 – 193 . Crossref PubMed Google Scholar
37. Blokker CP , Rorabeck CH , Bourne RB . Tibial plateau fractures. An analysis of the results of treatment in 60 patients . Clin Orthop Relat Res . 1984 ; 182 : 193 – 199 . PubMed Google Scholar
38. Waddell JP , Johnston DW , Neidre A . Fractures of the tibial plateau: a review of ninety-five patients and comparison of treatment methods . J Trauma . 1981 ; 21 ( 5 ): 376 – 381 . Crossref PubMed Google Scholar
39. Li Y , Chen S-K , Li L , Qin L , Wang X-L , Lai Y-X . Bone defect animal models for testing efficacy of bone substitute biomaterials . J Orthop Translat . 2015 ; 3 ( 3 ): 95 – 104 . Crossref PubMed Google Scholar
40. Goff T , Kanakaris NK , Giannoudis PV . Use of bone graft substitutes in the management of tibial plateau fractures . Injury . 2013 ; 44 Suppl 1 : S86 - 94 . Crossref PubMed Google Scholar
41. Schmidt AH . Autologous bone graft: is it still the gold standard? Injury . 2021 ; 52 Suppl 2 : S18 – S22 . Crossref PubMed Google Scholar
42. Greenwald AS , Boden SD , Goldberg VM , et al. Bone-graft substitutes: facts, fictions, and applications . J Bone Joint Surg Am . 2001 ; 83-A Suppl 2 Pt 2 : 98 – 103 . Crossref PubMed Google Scholar
43. Giannoudis PV , Einhorn TA , Marsh D . Fracture healing: the diamond concept . Injury . 2007 ; 38 Suppl 4 : S3 - 6 . Crossref PubMed Google Scholar
44. Almaiman M , Al-Bargi HH , Manson P . Complication of anterior iliac bone graft harvesting in 372 adult patients from may 2006 to may 2011 and a literature review . Craniomaxillofac Trauma Reconstr . 2013 ; 6 ( 4 ): 257 – 266 . Crossref PubMed Google Scholar
45. Kamal M , Gremse F , Rosenhain S , et al. Comparison of bone grafts from various donor sites in human bone specimens . J Craniofac Surg . 2018 ; 29 ( 6 ): 1661 – 1665 . Crossref PubMed Google Scholar
46. Mott A , Mitchell A , McDaid C , et al. Systematic review assessing the evidence for the use of stem cells in fracture healing . Bone Jt Open . 2020 ; 1 ( 10 ): 628 – 638 . Crossref PubMed Google Scholar
47. Simpson D , Keating JF . Outcome of tibial plateau fractures managed with calcium phosphate cement . Injury . 2004 ; 35 ( 9 ): 913 – 918 . Crossref PubMed Google Scholar
48. Drosos GI , Ververidis A , Babourda EC , Kakagia D , Verettas D-A . Calcium sulfate cement in contained traumatic metaphyseal bone defects . Surg Technol Int . 2012 ; 22 : 313 – 319 . PubMed Google Scholar
49. Iundusi R , Gasbarra E , D’Arienzo M , Piccioli A , Tarantino U . Three year follow-up from a prospective study . BMC Musculoskelet Disord . 2015 ; 16 : 115 . Crossref Google Scholar
50. OCEBM Levels of Evidence Working Group . The Oxford 2011 Levels of Evidence . http://www.cebm.net/index.aspx?o=5653 ( date last accessed 14 February 2022 ). Google Scholar
51. Betz RR . Limitations of autograft and allograft: new synthetic solutions . Orthopedics . 2002 ; 25 ( 5 Suppl ): s561 - 70 . Crossref PubMed Google Scholar
52. Brodt MD , Swan CC , Brown TD . Mechanical behavior of human morselized cancellous bone in triaxial compression testing . J Orthop Res . 1998 ; 16 ( 1 ): 43 – 49 . Crossref PubMed Google Scholar
53. Bajammal SS , Zlowodzki M , Lelwica A , et al. The use of calcium phosphate bone cement in fracture treatment. A meta-analysis of randomized trials . J Bone Joint Surg Am . 2008 ; 90-A ( 6 ): 1186 – 1196 . Crossref PubMed Google Scholar
54. Phan K , Kim JS , Kim JH , et al. Anesthesia duration as an independent risk factor for early postoperative complications in adults undergoing elective ACDF . Global Spine J . 2017 ; 7 ( 8 ): 727 – 734 . Crossref PubMed Google Scholar
55. Wilson JM , Schwartz AM , Farley KX , Roberson JR , Bradbury TL , Guild GN . Quantifying the backlog of total hip and knee arthroplasty cases: predicting the impact of COVID-19 . HSS J . 2020 ; 16 ( Suppl 1 ): 1 – 7 . Crossref PubMed Google Scholar
56. McGain F , Muret J , Lawson C , Sherman JD . Environmental sustainability in anaesthesia and critical care . Br J Anaesth . 2020 ; 125 ( 5 ): 680 – 692 . Crossref PubMed Google Scholar
57. Hampton M , Riley E , Garneti N , Anderson A , Wembridge K . The orthopaedic waiting list crisis . Bone Jt Open . 2021 ; 2 ( 7 ): 530 – 534 . Crossref PubMed Google Scholar
58. Driban JB , Stout AC , Duryea J , et al. Coronal tibial slope is associated with accelerated knee osteoarthritis: data from the Osteoarthritis Initiative . BMC Musculoskelet Disord . 2016 ; 17 ( 1 ): 299 . Crossref PubMed Google Scholar
59. Wang W , Yeung KWK . Bone grafts and biomaterials substitutes for bone defect repair: a review . Bioact Mater . 2017 ; 2 ( 4 ): 224 – 247 . Crossref Google Scholar
60. Gagnier JJ . Patient reported outcomes in orthopaedics . J Orthop Res . 2017 ; 35 ( 10 ): 2098 – 2108 . Crossref PubMed Google Scholar
61. Brown TD , Anderson DD , Nepola JV , Singerman RJ , Pedersen DR , Brand RA . Contact stress aberrations following imprecise reduction of simple tibial plateau fractures . J Orthop Res . 1988 ; 6 ( 6 ): 851 – 862 . Crossref PubMed Google Scholar
62. Russell N , Tamblyn P , Jaarsma R . Tibial plateau fractures treated with plate fixation: to lock or not to lock . Eur J Orthop Surg Traumatol . 2008 ; 19 ( 2 ): 75 – 82 . Crossref Google Scholar
63. Delgado-Rodríguez M , Llorca J . Bias . J Epidemiol Community Health . 2004 ; 58 ( 8 ): 635 – 641 . Crossref PubMed Google Scholar
64. Lobenhoffer P , Gerich T , Witte F , Tscherne H . Use of an injectable calcium phosphate bone cement in the treatment of tibial plateau fractures: a prospective study of twenty-six cases with twenty-month mean follow-up . J Orthop Trauma . 2002 ; 16 ( 3 ): 143 – 149 . Crossref PubMed Google Scholar
65. Ong JCY , Kennedy MT , Mitra A , Harty JA . Fixation of tibial plateau fractures with synthetic bone graft versus natural bone graft: a comparison study . Ir J Med Sci . 2012 ; 181 ( 2 ): 247 – 252 . Crossref PubMed Google Scholar
Author contributions
G. M. Cooper: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing.
M. J. Kennedy: Investigation, Methodology, Writing – original draft, Writing – review & editing.
B. Jamal: Conceptualization, Writing – review & editing.
D. W. Shields: Conceptualization, Data curation, Project administration, Supervision, Investigation, Methodology, Writing – review & editing.
Funding statement
The authors received no financial or material support for the research, authorship, and/or publication of this article.
ICMJE COI statement
D. W. Shields and B. Jamal report a research grant from Biocomposites, unrelated to this study. B. Jamal also reports a speaker payment from Biocomposites, unrelated to this study. B. Jamal is also a council member of the Royal College of Physicians and Surgeons of Glasgow Executive committee, the British Limb Reconstruction Society Trauma Committee, and the British Orthopaedic Association.
Open access funding
The authors confirm that the open access funding for this study was self-funded.
Follow G. M. Cooper @GCooper_2000
Follow M. J. Kennedy @mjkennedy_1
Follow D. W. Shields @dwshields
Supplementary material
The supplementary material contains additional information on our statistical methodology and outlines the literature search undertaken. It also summarises the excluded studies, the risk of bias evaluations (ROB2) of included studies and the certainty of evidence (GRADE) evaluation for synthesised outcomes. Finally, the complete list of adverse events from our literature sample is presented.
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.