Abstract
Aims
To identify the minimum set of outcomes that should be collected in clinical practice and reported in research related to the care of children with idiopathic congenital talipes equinovarus (CTEV).
Methods
A list of outcome measurement tools (OMTs) was obtained from the literature through a systematic review. Further outcomes were collected from patients and families through a questionnaire and interview process. The combined list, as well as the appropriate follow-up timepoint, was rated for importance in a two-round Delphi process that included an international group of orthopaedic surgeons, physiotherapists, nurse practitioners, patients, and families. Outcomes that reached no consensus during the Delphi process were further discussed and scored for inclusion/exclusion in a final consensus meeting involving international stakeholder representatives of practitioners, families, and patient charities.
Results
In total, 39 OMTs were included from the systematic review. Two additional OMTs were identified from the interviews and questionnaires, and four were added after round one Delphi. Overall, 22 OMTs reached ‘consensus in’ during the Delphi and two reached ‘consensus out’; 21 OMTs reached ‘no consensus’ and were included in the final consensus meeting. In all, 21 participants attended the consensus meeting, including a wide diversity of clubfoot practitioners, parent/patient representative, and an independent chair. A total of 21 outcomes were discussed and voted upon; six were voted ‘in’ and 15 were voted ‘out’. The final COS document includes nine OMTs and two existing outcome scores with a total of 31 outcome parameters to be collected after a minimum follow-up of five years. It incorporates static and dynamic clinical findings, patient-reported outcome measures, and a definition of CTEV relapse.
Conclusion
We have defined a minimum set of outcomes to draw comparisons between centres and studies in the treatment of CTEV. With the use of these outcomes, we hope to allow more meaningful research and a better clinical management of CTEV.
Cite this article: Bone Jt Open 2022;3(1):98–106.
Take home message
We have identified a minimum set of outcomes to draw comparisons between centres and studies in the treatment of congenital talipes equinovarus (CTEV). These 31 outcome parameters will be collected after a minimum follow-up of five years.
It incorporates static and dynamic clinical findings, patient-reported outcome measures, and a definition of CTEV relapse.
With the use of these outcomes, we hope to allow more meaningful research and a better clinical management of CTEV.
Introduction
Congenital talipes equinovarus (CTEV), also known as clubfoot, is the most common congenital limb deformity.1,2 The gold standard of primary treatment of CTEV is the Ponseti method, consisting of serial manipulation and casting with percutaneous Achilles tenotomy, followed by long-term use of a foot abduction brace.3-8 The Ponseti method has been implemented worldwide with an excellent primary correction rate reported.7-13
The natural history of the corrected foot throughout childhood includes a significant risk of relapse requiring further input.14-16 This is a dynamic and progressive deformity with regular monitoring required throughout the child’s growth period. The prescribed treatments vary from repeated casting, limited to extensive soft-tissue releases, osteotomies, guided growth, and gradual soft-tissue or callus distraction using circular frame devices. The management of CTEV following the initial Ponseti correction is controversial, and the approach to treatment varies depending on severity, age, geography, and preference.16-18
Outcome reporting in the literature varies both in terms of the outcome tools used and the length of follow-up.11 As relapse of the deformity continues to be identified after the age of four years,11 it is important to define what is the most appropriate age at which outcome reporting is considered meaningful.
A core outcome set (COS) is the minimum set of outcomes to be reported in studies investigating a specific condition. It facilitates comparisons between studies, allows meaningful meta-analyses, and significant decision-making regarding treatment and management.18,19
The use of COSs is well-established in adult orthopaedic research,20-22 and has recently become more common in paediatric orthopaedic surgery.23-25
The aim of this study was to identify key outcomes of CTEV management that could be used routinely in both research and clinical practice using the COMET (Core Outcome Measures in Effectiveness Trials) guidelines.19
Methods
The protocol for development of a COS for idiopathic clubfoot management has been previously published.26 A four-stage process was followed:
-
Identification of key outcomes reported in the literature through a systematic review;
-
Identification of key outcomes relevant to patients and their families through a consultation process involving interviews and a questionnaire;
-
Scoring of the list of outcomes obtained from the previous stages through an international Delphi process; and
-
A final consensus meeting.
A summary of these stages is reported below. The collected outcomes, the stage of collection, and the vote during the Delphi process is presented in Table I.
Table I.
The collected outcomes, the stage of collection, and the vote during the Delphi process.
| Outcome | Stage of collection* | Vote Delphi stage 1† |
Vote Delphi stage 2† |
|---|---|---|---|
| 1. Dynamic deformity | Systematic review | No consensus | No consensus |
| 2. Fixed deformity | Systematic review | Consensus in | N/A |
| 3. Relapse | Systematic review | Consensus in | N/A |
| 4. Comfortable foot | Systematic review | Consensus in | N/A |
| 5. Pain | Systematic review | Consensus in | N/A |
| 6. Child’s ability to play with their peers | Systematic review | Consensus in | N/A |
| 7. Leisure activities | Systematic review | Consensus in | N/A |
| 8. Overall happiness with the foot/feet | Systematic review | Consensus in | N/A |
| 9. Ability to squat | Systematic review | No consensus | No consensus |
| 10. Ability to hop on the affected leg | Systematic review | No consensus | No consensus |
| 11. Absence of limping | Systematic review | Consensus in | N/A |
| 12. Ability to climb stairs | Systematic review | Consensus in | N/A |
| 13. Ability to fit a good range of footwear | Systematic review | Consensus in | N/A |
| 14. Need for any additional treatment (e.g. casting; minor/major surgery) | Systematic review | Consensus in | N/A |
| 15. Tightness in foot | Systematic review | No consensus | No consensus |
| 16. Tightness in calf | Systematic review | No consensus | No consensus |
| 17. Heel position | Systematic review | Consensus in | N/A |
| 18. Forefoot position | Systematic review | Consensus in | N/A |
| 19. Overall foot position in standing | Systematic review | Consensus in | N/A |
| 20. Plantigrade foot | Systematic review | Consensus in | N/A |
| 21. Overall position in walking | Systematic review | Consensus in | N/A |
| 22. Appropriate ROM at ankle joint | Systematic review | Consensus in | N/A |
| 23. Appropriate ROM at subtalar joint | Systematic review | Consensus in | N/A |
| 24. Strong and balanced muscle activity | Systematic review | Consensus in | N/A |
| 25. Harold and Walker classification | Systematic review | No consensus | Consensus out |
| 26. Catterall Score | Systematic review | No consensus | Consensus out |
| 27. PBS score | Systematic review | No consensus | No consensus |
| 28. Bangla Score | Systematic review | No consensus | No consensus |
| 29. Bhaskar Score | Systematic review | No consensus | No consensus |
| 30. ACT tool | Systematic review | No consensus | No consensus |
| 31. IMAR score | Systematic review | No consensus | No consensus |
| 32. Richard score | Systematic review | No consensus | No consensus |
| 33. Clubfoot Assessment Protocol score | Systematic review | No consensus | No consensus |
| 34. International Clubfoot Study Group score | Systematic review | No consensus | No consensus |
| 35. Roye’s score | Systematic review | No consensus | No consensus |
| 36. Ezra score | Systematic review | No consensus | No consensus |
| 37. Dimeglio score | Systematic review | No consensus | No consensus |
| 38. Laaveg and Ponseti score | Systematic Review | No consensus | No consensus |
| 39. Pirani score | Systematic Review | Consensus in | N/A |
| 40. Pirani sscore for the walking child | Round 1 Delphi | n/a | No consensus |
| 41. The Oxford Ankle Foot Questionnaire | Round 1 Delphi | N/A | No consensus |
| 42. Intoeing | Round 1 Delphi | N/A | No consensus |
| 43. Child’s ability to participate in low-impact sport activities at a level comparable to their peers | Round 1 Delphi | N/A | Consensus in |
| 44. Child’s ability to walk for long distances | Questionnaire/interview | Consensus in | N/A |
| 45. Child’s ability to walk freely | Questionnaire/interview | Consensus in | N/A |
-
*
Stage of collection options: Systematic review, questionnaire, and round one Delphi.
-
†
Voting options: ‘Consensus in’, ‘consensus out’, ‘no consensus’, and not applicable (N/A).
Ethics approval and consent to participate
Consultation with the institutional R&D offices has deemed this project as a “patient service evaluation study” with no requirement for ethical approval. Registration and approval for audit and service evaluation was granted in both hospitals (Great Ormond Street Hospital for Children, UK; and St George's Hospital, UK).
Systematic review
An initial list of outcomes reported in the literature was identified through a systematic review and has been published.27
Questionnaires and qualitative interviews
Qualitative semi-structured interviews were held with parents and children during routine outpatient visits by the treating clinician. These were designed to identify the key outcomes of CTEV among families using study-specific questionnaires designed for both patients and carers. A purposive sampling strategy was used to help identify outcomes normally not considered in clinical and research settings. The questionnaires for the parents were either completed as a self-reported questionnaire or used as a semi-structured interview schedule to be completed with the researcher, who read the questions and took note of the parents’ answers using them as a prompt for further discussion. The questionnaires for the children were grouped according to age and were completed by the patients with the parents’ help when needed. The methods were reported in the study protocol.26 Any novel outcome identified from the questionnaires and interviews was added to the list of outcomes obtained from the systematic review.
The Delphi survey
The list of outcomes obtained from stages one and two formed the basis for an international Delphi process consisting of two rounds of voting, each lasting three weeks. During round one, participants’ demographic data (contact email, stakeholder group, country, and country’s income group) were collected. The participants were then asked to score the list of suggested outcomes from 1 to 9 (with 1 to 3 being not relevant, 4 to 6 being important but not critical, and 7 to 9 being extremely relevant). Participants were also given the opportunity to suggest additional outcomes of relevance that were not already listed and were asked what is the minimum follow-up time required for COS collection to be meaningful. The data obtained from round one were then analyzed using bar charts stratified by stakeholder group. The data were then summarized as ‘consensus in’, ‘consensus out’, or ‘no consensus’ based on the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) guidelines,28 where:
-
Consensus in was defined as the agreement of the vast majority (> 70% of the group) on considering the outcome extremely relevant (7 to 9 points), with only a minority (< 15% of the group) considering the outcome not relevant (1 to 3 points).
-
Consensus out was defined as the agreement of the vast majority (> 70% of the group) on considering the outcome not relevant (1 to 3 points), with only a minority (< 15% of the group) considering the outcome extremely relevant (7 to 9 points).
Outcomes that reached ‘consensus in’ were added to the final core outcome list. Outcomes that reached ‘consensus out’ were removed from the list and outcomes that reached ‘no consensus’ were moved forward to round two of the survey, along with the additional outcomes suggested during round one.
During round two, participants were invited to again score the outcomes that did not reach ‘consensus in’ during round one using the same descriptors and with the option of changing their scores if they choose to. Data obtained from round two were then summarized using the GRADE guidelines in the same format as in round one.
Following round two, all outcomes that reached ‘consensus out’ were removed, outcomes that reached ‘consensus in’ were added to the final core outcome list, and outcomes that reached ‘no consensus’ were moved forward to the final consensus meeting.
To maximize the engagement of lay people (i.e. children, parents, and families), the initial section did not include medical terminology or specific technical details. All participants were encouraged to participate in this section, whereas only healthcare professionals were asked to complete the subsequent section relating to technical outcomes of success.
Final consensus meeting
The list of outcomes obtained from the Delphi survey was presented in a final consensus meeting which was held in June 2021. The meeting was attended by 21 international stakeholder representatives, including orthopaedic surgeons, physiotherapists, nurse practitioners, and parents/patients’ representatives, along with an independent chair, who did not participate in the voting procedure. First, the full list of outcomes included in the Delphi survey were presented, with outcomes divided according to whether they were ‘consensus in’, ‘consensus out’, or ‘no consensus’. There was an opportunity for an open discussion related to all outcomes. Outcomes that did not reach consensus were discussed and voted upon with an “inclusion yes/inclusion no” procedure, where the score of the majority (51% of the voting participants) decided the outcomes inclusion or exclusion from the final set. Participants scored each outcome anonymously, using the VoxVote online platform (VoXVote, the Netherlands),29 to determine the outcomes to be included in the final COS.
Results
The list of collected outcomes, the stage of collection, and the decision following the Delphi process is presented in Table I. A flowchart of the number of outcomes collected and voted upon at every stage is presented in Figure 1.
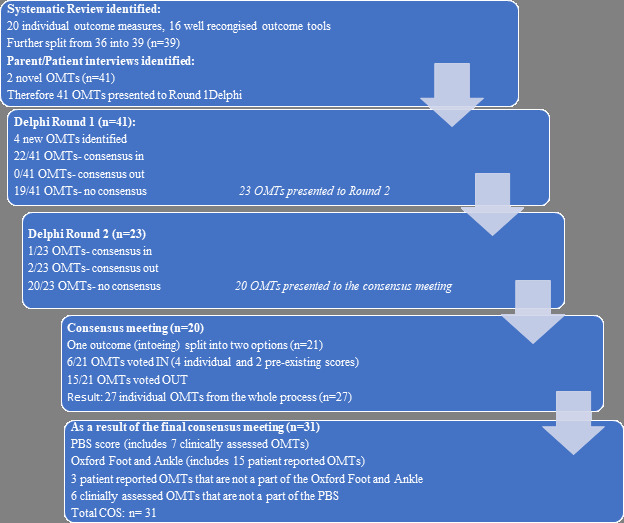
Fig. 1
Flowchart of the core outcome set process, and the outcomes identified.
Systematic review
The results of the systematic review were published in 2020.27 In summary, after data extraction 20 individual OMTs and 16 pre-determined outcome scores were identified and categorized in domains according to the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) modified filter.18 For ease of scoring, these outcomes were split further before round one Delphi (n = 39).
Questionnaires and qualitative interviews
In all, 14 parents and children participated in the interviews and questionnaires process as a part of their appointment at the Great Ormond Street Hospital for Children, UK; and St George's Hospital, UK, clubfoot clinics between the January 2021 and April 2021. Two novel OMTs were identified at this stage and added to the outcomes list (Table I).
Delphi survey
Round one
Round one of the Delphi survey consisted of 68 participants, including ten patients/parents (15%), and 58 healthcare professionals (85%) from 12 countries. The participants were from the UK, Israel, USA, Canada, New Zealand, Brazil, Romania, Turkey, Austria, Mercy Ships UK (a charity), India, and Italy (Figure 2). Overall, 59% of the participants were from high-income countries, 33% from middle-income countries, and 8% from low-income countries. The second round of the Delphi had a dropout rate of 9%. The final number of participants responding to both rounds of the survey was 62, which was made up of 84% healthcare professionals and 16% patients/parents. The participant distribution is presented in Table II. The list of outcomes presented in round one of the Delphi was composed of the outcomes collected from the systematic review (n = 36), some of which were split further to make them easier to score during round one (n = 39 outcomes), together with the two outcomes identified during the patients/parents’ questionnaire/interview, for a total of 41 outcomes included in Table I.
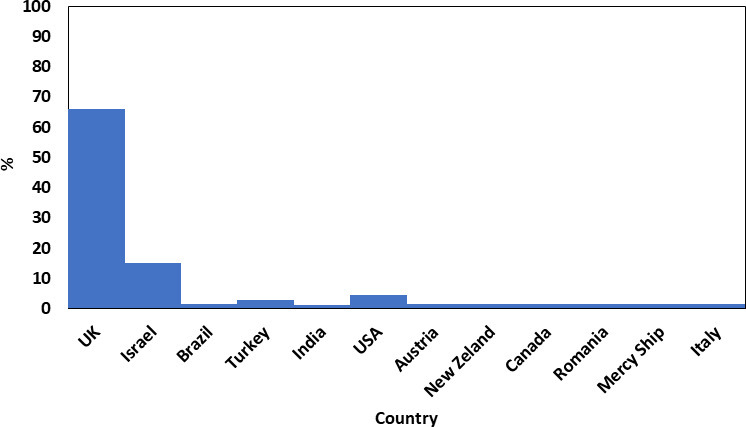
Fig. 2
Countries that participated in the Delphi process (n = 68).
Table II.
Delphi survey participants’ groups.
| Role | Total, n (%) |
|---|---|
| Paediatric orthopaedic surgeon | 42 (63.2) |
| Physiotherapist | 13 (19.1) |
| Allied health professional | 1 (1.5) |
| Advanced nurse practitioner | 1 (1.5) |
| Plaster technician | 1 (1.5) |
| Patients/parents | 10 (14.7) |
Round two
Overall, 62 participants (91% of round one) participated in round two. Three additional OMTs identified from round one were included in round two. In round two, we also sought to identify the ideal time for collection of meaningful definitive outcomes as a few of the outcome tools were appropriate for the infant stage only. Of the total 45 items for scoring, 22 obtained ‘consensus in’, two obtained ‘consensus out’, and 20 obtained ‘no consensus’. The list of 22 outcomes that reached ‘consensus in’, after the two round Delphi process, is included in Table I. The Pirani score was scored twice; in round two, looking specifically at its use after walking age. The minimum time for follow-up was determined as five years.
Final consensus meeting
The meeting was a hybrid meeting including 21 participants with five attending face to face and 16 attending virtually. The meeting participants were from the UK, Israel, India, Romania, Austria, Italy, and MercyShips UK, and included 15 CTEV practitioners, one parent/ patient representative, and four patient charity representatives. The meeting was facilitated by an independent chair, who did not participate in the voting procedure.
The parent representative participated only in the voting procedure related to non-technical outcomes, which included areas of adverse events, life impact, and pathophysiological manifestations. One outcome (intoe-ing) was subdivided so that 21 outcomes were discussed in the final consensus meeting, 15 were voted as ‘consensus out’, and six were voted as ‘consensus in’. The outcomes that were voted upon and their results are presented in Table III.
Table III.
Final consensus meeting, with outcome measurement tool (OMT) and scores list.
| OMTs and scores (n = 21) | Consensus vote (in/out) |
|---|---|
| 1. Dynamic deformity | In |
| 2. Squatting | In |
| 3. Hopping | In |
| 4. Tightness in foot | Out |
| 5. Tightness in calf | Out |
| 6. Intoeing-general | Out |
| 7. Intoeing originating from the foot | In |
| 8. Pirani score | Out |
| 9. Dimeglio score | Out |
| 10. Richard score | Out |
| 11. IMAR | Out |
| 12. Clubfoot Assessment Protocol | Out |
| 13. International Clubfoot Study Group | Out |
| 14. Laaveg and Ponseti | Out |
| 15. ACT | Out |
| 16. Ezra | Out |
| 17. Roye’s | Out |
| 18. OxAFQ-C | In |
| 19. Bangla score | Out |
| 20. Bhaskar score | Out |
| 21. PBS | In |
-
ACT, assessing clubfoot treatment; IMAR, Institution of Motion Analysis and Research; OxAFQ-C, Oxford Ankle Foot Questionnaire for Children; PBS, Pirani/Bohm/Sinclair.
None of the outcome scores collected from the systematic review reached ‘consensus in’ during the Delphi process. The popular Pirani score30 reached ‘no consensus’ when the minimum follow-up time was specified as five years, and voted out in the consensus meeting as it was developed for the infant foot and felt not to be appropriate for the walking child and for definitive evidence of treatment success or failure.
Grouping the OMTs into two established scores
The consensus group agreed that several of the individual OMTs that reached ‘consensus in’ could be grouped together and would then represent part of two established outcome scores. The PBS score,31 which includes OMTs such as muscle strength, ankle, and subtalar range of motion, foot position and gait, represents the musculoskeletal domain under the core area of pathophysiological manifestations. Five additional clinical OMTs that are not a part of the PBS score have reached ‘consensus in’, of which one is static and four are dynamic. The outcome ‘presenting with a fixed deformity‘ falls under the domain of adverse events. The ability to run, walk, and hop on examination represented the musculoskeletal domain under the core area of pathophysiological manifestations. The outcome of in-toeing was added to the list from the interviews and questionnaires. It reached ‘no consensus’ following the two-round Delphi survey, and during the consensus meeting was discussed and added following a small change in phrasing assuring the in-toeing was originating from the foot.
The Oxford Foot and Ankle Questionnaire (OXAFQ)32 incorporates several patient-reported outcome measures (PROMs), such as the ability to stand, walk, run, participate in various activities, pain, footwear, and foot appearance. These outcomes represent the quality-of-life domain and fall under the core area of life impact.
Three additional individual PROMs that are not a part of the OXAFQ reached ‘consensus in’ during the Delphi survey. The ability to take part in a long walk was added to the Delphi survey following the interviews and questionnaires, and reached ‘consensus in’ during the Delphi survey. Stair-climbing in a reciprocal fashion reached ‘consensus in’ during the survey. The ability to squat reached ‘no consensus’ during the Delphi process but was voted in during the final meeting due to a better appreciation of its importance in some cultures/countries.
Recurrence as an outcome
The outcome ‘recurrence that requires further intervention’ reached ‘consensus in’ during the first round of the Delphi survey. The definition was refined during the consensus meeting to be ‘any change in clinical presentation of the fully corrected foot (following initial management) that requires further treatment’ in order to distinguish it from a dynamic supination that presents with muscle imbalance from infancy. This outcome falls both under the domain of adverse events, as well as under the domain resources use, when further intervention is required.
In total, 21 OMTs were presented to the consensus group and 27 were included in the final COS. These items, grouped by their domains, are presented in Table IV. As it was decided in the consensus meeting that the complete OXAFQ is appropriate to be used to represent the quality-of-life domain it was included in its entirety and therefore contributed four additional PROMs (26, 27, 29, and 30 in Table V). The complete COS assessment form (n = 31 items) is presented in Table V.
Table IV.
The final core outcomes set.
| Core area | Core domain | OMTs |
|---|---|---|
| Adverse events | Adverse events | Deformity (fixed and dynamic); relapse. |
| Life impact | Physical/social/emotional/ cognitive/health-related quality of life | Pain; child’s ability to play with their peers; leisure activities participations; sport participations; overall happiness with their foot/feet; ability to walk for long distances; ability to walk freely; child’s ability to squat; child’s ability to hop on the affected leg; absence of limping; ability to climb stairs; ability to fit a good range of footwear; comfortable foot. |
| Resource use | Economical/hospital/need for intervention/social burden | Need for any additional treatment (e.g. casting, minor surgery) |
| Pathophysiological manifestations | Musculoskeletal | Heel position; foot in-toeing; forefoot position; overall foot position in standing; overall position in walking; plantigrade foot; appropriate ROM at ankle joint; appropriate ROM at subtalar joint; strong and balanced muscle activity. |
| Death | N/A | N/A |
| Technical considerations | Technical/surgical considerations | Appropriate scale/questionnaire for assessment (e.g. PBS; Oxford Ankle Foot Questionnaire). |
-
N/A, not applicable; OMTs, cutcome measurement tools; PBS, Pirani/Bohm/Sinclair.
Table V.
The core outcome set (COS) assessment form.
| Definition |
|---|
|
The corrected foot :
is one in which the talar head is covered, the heel is in neutral or valgus, the anterior process of the os calcis has rotated out from under the talus and where the ankle has 15° of dorsiflexion. is one that fits comfortably into the boots and bar. |
| Timing |
| The minimum follow-up for meaningful COS reporting is five years. |
| Outcome measures to be assessed, yes/no |
| General |
| 1. Recurrence: any change in clinical presentation of the fully corrected foot (following initial management) that requires further treatment. |
| Clinical outcome |
|
Static: 2. Is there any fixed deformity? Dynamic: Can the child: 3. Walk without a limp 4. Run 5. Hop 6. Is there any in-toeing originating from the foot? |
|
PBS31
7. Hindfoot varus in standing 8. Supination in standing 9. Swing phase supination in walking 10. Early heel rise in stance when tibia perpendicular in walking 11. Passive ankle dorsiflexion in sitting 12. Active ankle dorsiflexion in sitting 13. Subtalar abduction in sitting |
| PROMs |
| Is the child able to: 14. Stair-climb in a reciprocal fashion (one foot after another) 15. Take part in a long walk (for example more than one to two miles and/or more than an hour) 16. Squat |
|
Oxford Foot and Ankle Questionnaire32
17. Does the child have difficulty in walking because of the foot or ankle? 18. Does the child have difficulty in running because of the foot or ankle? 19. Does the child have difficulty in standing up for a long period because of the foot or ankle? 20. Does the child have pain in the foot or ankle? 21. Has the child’s legs been sore or ached after walking or running? 22. Has the child been tired because of the foot or ankle? 23. Has the foot or ankle stopped them joining in with others in the playground? 24. Has the foot or ankle stopped them playing outside or in the park? 25. Has the foot or ankle stopped them taking part in PE? 26. Has the foot or ankle stopped them from taking part in any other lesson at school? 27. Has the foot or ankle appearance bothered them? 28. Has their walk bothered them? 29. Have they been embarrassed because of the foot or ankle? 30. Has anyone been unkind because of the foot and ankle? 31. Has it stopped them wearing any shoes they wanted to wear? |
-
PE, physical education; PROMs, patient-reported outcome measures.
Discussion
We have derived a COS and defined the minimum duration of follow-up to record meaningful definitive outcomes. The use of this COS as a minimum set of outcomes to be reported among all children with CTEV will allow meaningful comparisons to be made between patients. This will enable centre outcomes to be compared and will facilitate evidence synthesis in research.
The wide variety of outcomes reported in CTEV is made clear when so many OMTs and scores have been reported in the literature over the years, with each study using a different outcome measure. There is no consistency in the choice of outcomes, the definition of deformity recurrence, and the minimum follow-up time used so that no meaningful, transferrable outcome reporting actually occurs.11 The lack of an acceptable outcome measure(s) has probably contributed to the wide range of treatment options selected for children with CTEV.16,17
We have developed a COS for CTEV according to the COMET initiative guidelines,18,19 to be measured and reported in research and treatment of patients with CTEV. This is the minimal essential recommended list of OMTs but it can be complemented by any additional outcome measure that offers more information and is appropriate for an individual unit in terms of availability of time, resources, and interest.
This document begins with the building-block to the COS assessment form, which is a definition of the corrected foot. The Delphi process defined a minimum follow-up period of five years from treatment onset, after which outcome reporting was felt to be meaningful. The assessment form describes 31 OMTs, which are all to be assessed on a simple ‘yes’ or ‘no’ basis. The list includes 13 clinician-reported OMTs and 18 patient-reported OMTs . The clinician-reported OMTs include a detailed description and illustrations attached to the COS assessment form to promote standardization.
The strengths of this study are the robust methodology of COS development according to the COMET initiative,19 and OMERACT guidelines,22 the involvement of a diverse international group of stakeholders from both low-middle income countries and high income countries, and a low dropout rate during the Delphi process. The study was successful in implementing all stages of the protocol.26 Another significant strength is the detail in which the OMTs are defined in order to provide simplicity and reproducibility in every setting.
We acknowledge that the representation of parents in the final consensus meeting was low, as there was only a single representative. The patients and parents were heavily involved in the previous stages, and only a few outcomes relevant to patients were discussed in the consensus meeting. Many of the outcomes discussed in the final meeting were clinically based, and thus a stronger presence of practitioners was appropriate. We also had international parents charity representatives with a strong insight into patients and parents priorities and concerns particularly in low income countries.
The aim of this study was to establish a list of OMTs that reached consensus among stakeholders, as well as to create a multifaceted tool that includes both static and dynamic clinical examination and PROM, was easy to use and adaptable to different cultural settings.27 The final COS fulfils these requirements, allowing it to serve as a building block for outcome reporting in research.
In conclusion, in this study we have developed a COS for idiopathic CTEV for use by all professionals who manage this condition. This COS include 31 OMTs based on a four-stage process, including input from the literature, from parents and patients, and from treating clinicians. We hope that use of this COS will facilitate better research and improved decision-making when treating children with CTEV.
References
1. Dobbs MB , Gurnett CA . Update on clubfoot: etiology and treatment . Clin Orthop Relat Res . 2009 ; 467 ( 5 ): 1146 – 1153 . Crossref PubMed Google Scholar
2. Smythe T , Kuper H , Macleod D , Foster A , Lavy C . Birth prevalence of congenital talipes equinovarus in low- and middle-income countries: a systematic review and meta-analysis . Trop Med Int Health . 2017 ; 22 ( 3 ): 269 – 285 . Crossref PubMed Google Scholar
3. Švehlík M , Floh U , Steinwender G , Sperl M , Novak M , Kraus T , et al. Ponseti method is superior to surgical treatment in clubfoot - Long-term, randomized, prospective trial . Gait Posture . 2017 ; 58 : 346 – 351 . Crossref PubMed Google Scholar
4. Laaveg SJ , Ponseti IV . Long-term results of treatment of congenital club foot . J Bone Joint Surg Am . 1980 ; 62-A ( 1 ): 23 – 31 . PubMed Google Scholar
5. Morcuende JA , Dolan LA , Dietz FR , Ponseti IV . Radical reduction in the rate of extensive corrective surgery for clubfoot using the Ponseti method . Pediatrics . 2004 ; 113 ( 2 ): 376 – 380 . Crossref PubMed Google Scholar
6. Sætersdal C , Fevang JM , Bjørlykke JA , Engesæter LB . Ponseti method compared to previous treatment of clubfoot in Norway. A multicenter study of 205 children followed for 8-11 years . J Child Orthop . 2016 ; 10 ( 5 ): 445 – 452 . Google Scholar
7. Jowett CR , Morcuende JA , Ramachandran M . Management of congenital talipes equinovarus using the Ponseti method: a systematic review . J Bone Joint Surg Br . 2011 ; 93-B ( 9 ): 1160 – 1164 . Crossref PubMed Google Scholar
8. Shack N , Eastwood DM . Early results of a physiotherapist-delivered Ponseti service for the management of idiopathic congenital talipes equinovarus foot deformity . J Bone Joint Surg Br . 2006 ; 88-B ( 8 ): 1085 – 1089 . Crossref PubMed Google Scholar
9. Herzenberg JE , Radler C , Bor N . Ponseti versus traditional methods of casting for idiopathic clubfoot . J Pediatr Orthop . 2002 ; 22 ( 4 ): 517 – 521 . PubMed Google Scholar
10. Ganesan B , Luximon A , Al-Jumaily A , Balasankar SK , Naik GR . Ponseti method in the management of clubfoot under 2 years of age: A systematic review . PLoS One . 2017 ; 12 ( 6 ): e0178299 . Crossref PubMed Google Scholar
11. Gelfer Y , Wientroub S , Hughes K , Fontalis A , Eastwood DM . Congenital talipes equinovarus: a systematic review of relapse as a primary outcome of the Ponseti method . Bone Joint J . 2019 ; 101-B ( 6 ): 639 – 645 . Crossref PubMed Google Scholar
12. Radler C . The Ponseti method for the treatment of congenital club foot: review of the current literature and treatment recommendations . Int Orthop . 2013 ; 37 ( 9 ): 1747 – 1753 . Crossref PubMed Google Scholar
13. Dunkley M , Gelfer Y , Jackson D , et al. Mid-term results of a physiotherapist-led Ponseti service for the management of non-idiopathic and idiopathic clubfoot . J Child Orthop . 2015 ; 9 ( 3 ): 183 – 189 . Crossref PubMed Google Scholar
14. Gelfer Y , Dunkley M , Jackson D , et al. Evertor muscle activity as a predictor of the mid-term outcome following treatment of the idiopathic and non-idiopathic clubfoot . Bone Joint J . 2014 ; 96-B ( 9 ): 1264 – 1268 . Crossref PubMed Google Scholar
15. Bhaskar A , Patni P . Classification of relapse pattern in clubfoot treated with Ponseti technique . Indian J Orthop . 2013 ; 47 ( 4 ): 370 – 376 . Crossref PubMed Google Scholar
16. Eidelman M , Kotlarsky P , Herzenberg JE . Treatment of relapsed, residual and neglected clubfoot: adjunctive surgery . J Child Orthop . 2019 ; 13 ( 3 ): 293 – 303 . Crossref PubMed Google Scholar
17. Murphy D , Raza M , Khan H , Eastwood DM , Gelfer Y . What is the optimal treatment for equinus deformity in walking-age children with clubfoot? A systematic review . EFORT Open Rev . 2021 ; 6 ( 5 ): 354 – 363 . Crossref PubMed Google Scholar
18. Boers M , Kirwan JR , Wells G , et al. Developing core outcome measurement sets for clinical trials: OMERACT filter 2.0 . J Clin Epidemiol . 2014 ; 67 ( 7 ): 745 – 753 . Crossref PubMed Google Scholar
19. Williamson PR , Altman DG , Bagley H , et al. The COMET Handbook: version 1.0 . Trials . 2017 ; 18 ( Suppl 3 ): 280 . Crossref PubMed Google Scholar
20. Haywood KL , Griffin XL , Achten J , Costa ML . Developing a core outcome set for hip fracture trials . Bone Joint J . 2014 ; 96-B ( 8 ): 1016 – 1023 . Crossref PubMed Google Scholar
21. Chiarotto A , Deyo RA , Terwee CB , et al. Core outcome domains for clinical trials in non-specific low back pain . Eur Spine J . 2015 ; 24 ( 6 ): 1127 – 1142 . Crossref PubMed Google Scholar
22. Singh JA , Dohm M , Choong PF . Consensus on draft OMERACT core domains for clinical trials of Total Joint Replacement outcome by orthopaedic surgeons: a report from the International consensus on outcome measures in TJR trials (I-COMiTT) group . BMC Musculoskelet Disord . 2017 ; 18 ( 1 ): 1 . Crossref PubMed Google Scholar
23. Crosby BT , Behbahani A , Olujohungbe O , Cottam B , Perry D . Developing a core outcome set for paediatric wrist fractures: a systematic review of prior outcomes . Bone Jt Open . 2020 ; 1 ( 5 ): 121 – 130 . Crossref PubMed Google Scholar
24. Dorman SL , Shelton JA , Stevenson RA , Linkman K , Kirkham J , Perry DC , et al. Management of medial humeral epicondyle fractures in children: a structured review protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey . Trials . 2018 ; 19 ( 1 ): 119 . Crossref PubMed Google Scholar
25. Leo DG , Jones H , Murphy R , et al. The outcomes of Perthes’ disease . Bone Joint J . 2020 ; 102-B ( 5 ): 611 – 617 . Google Scholar
26. Leo DG , Russell A , Bridgens A , Perry DC , Eastwood DM , Gelfer Y . Development of a core outcome set for idiopathic clubfoot management . Bone Jt Open . 2021 ; 2 ( 4 ): 255 – 260 . Crossref PubMed Google Scholar
27. Gelfer Y , Hughes KP , Fontalis A , Wientroub S , Eastwood DM . A systematic review of reported outcomes following Ponseti correction of idiopathic club foot . Bone Jt Open . 2020 ; 1 ( 8 ): 457 – 464 . Crossref PubMed Google Scholar
28. Balshem H , Helfand M , Schünemann HJ , et al. GRADE guidelines: 3. Rating the quality of evidence . J Clin Epidemiol . 2011 ; 64 ( 4 ): 401 – 406 . Crossref PubMed Google Scholar
29. VoxVote . Free and easy mobile voting tool for any speaker or teacher . 2014 . https://www.voxvote.com/ ( date last accessed 25 January 2022 ). Google Scholar
30. Pirani S , Hodges D . A reliable & valid method of assessing the amount of deformity in the congenital clubfoot deformity . Orthop Proc . 2008 ; 90-B ( Supp1 ): 53 . Google Scholar
31. Böhm S , Sinclair MF . The PBS Score - a clinical assessment tool for the ambulatory and recurrent clubfoot . J Child Orthop . 2019 ; 13 ( 3 ): 282 – 292 . Crossref PubMed Google Scholar
32. Morris C , Doll HA , Wainwright A , Theologis T , Fitzpatrick R . The Oxford Ankle Foot Questionnaire for Children: scaling, reliability and validity . J Bone Joint Surg Br . 2008 ; 90-B ( 11 ): 1451 – 1456 . Crossref PubMed Google Scholar
Author contributions
Y. Gelfer: Conceptualization, Visualization, Formal analysis, Project administration, Writing – original draft, Writing – review & editing.
D. G. Leo: Methodology, Investigation, Formal analysis, Writing – review & editing.
A. Russell: Resources, Methodology, Writing – review & editing.
A. Bridgens: Resources, Methodology, Writing – review & editing.
D. C. Perry: Conceptualization, Writing – review & editing.
D. M. Eastwood: Conceptualization, Visualization, Formal analysis, Project administration, Writing – original draft, Writing – review & editing.
Funding statement
The author(s) received no financial or material support for the research, authorship, and/or publication of this artic
ICMJE COI statement
D. M. Eastwood reports royalties from Oxford University Press, lecture fees and fees for a skeletal dysplasia advisory board workshop from Biomarin, and being on the council of management for the Editorial Society Bone and Joint Surgery, all of which are unrelated to this article.
Acknowledgements
We would like to thank and acknowledge Professor Duncan Tennent for chairing the final consensus meeting. We would also like to thank and acknowledge the following practitioners who participated in the final consensus meeting (in alphabetic order): Alaric Aroojis, Jose Blanco, Dan Cosma, Nicola Cox, Naomi Davis, Christine Douglas, Mia Dunkley, Vicky Easton, Ehud Lebel, Kerry McGarrity, Anna Peek, Sally Tennant, Chris Radler, Denise Watson, Shlomo Wientroub, and Elizabeth Wright. Finally, we would like to acknowledge all the trainees, practitioners, parents, and patients who participated in all the stages of the study.
Ethical review statement
Consultation with the institutional R&D offices has deemed this project as a “patient service evaluation study” with no requirement for ethical approval. Registration and approval for audit and service evaluation was granted in both hospitals (St George’s University Hospitals NHS Foundation Trust and Great Ormond Street Hospital for Children NHS Foundation Trust, UK).
Follow Y. Gelfer @yaelgelfer
Follow D. C Perry @MrDanPerry
Follow D. M Eastwood @deboraheastwood
© 2022 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/









