Abstract
Aims
To describe the incidence of adverse clinical outcomes related to COVID-19 infection following corticosteroid injections (CSI) during the COVID-19 pandemic. To describe the incidence of positive SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) testing, positive SARS-COV2 IgG antibody testing or positive imaging findings following CSI at our institution during the COVID-19 pandemic.
Methods
A retrospective observational study was undertaken of consecutive patients who had CSI in our local hospitals between 1 February and 30June 2020. Electronic patient medical records (EPR) and radiology information system (RIS) database were reviewed. SARS-CoV-2 RT-PCR testing, SARS-COV2 IgG antibody testing, radiological investigations, patient management, and clinical outcomes were recorded. Lung findings were categorized according to the British Society of Thoracic Imaging (BSTI) guidelines. Reference was made to the incidence of lab-confirmed COVID-19 cases in our region.
Results
Overall, 1,656 lab-confirmed COVID-19 cases were identified in our upper tier local authority (UTLA), a rate of 306.6 per 100,000, as of 30June 2020. A total of 504 CSI injections were performed on 443 patients between 1 February and 30June 2020. A total of 11 RT-PCR tests were performed on nine patients (2% of those who had CSI), all of which were negative for SARS-CoV-2 RNA, and five patients (1.1%) received an SARS-CoV-2 IgG antibody test, of which 2 (0.5%) were positive consistent with prior COVID-19 infection, however both patients were asymptomatic. Seven patients (1.6%) had radiological investigations for respiratory symptoms. One patient with indeterminate ground glass change was identified.
Conclusion
The incidence of positive COVID-19 infection following corticosteroid injections was very low in our cohort and no adverse clinical outcomes related to COVID-19 infection following CSI were identified. Our findings are consistent with CSI likely being low risk during the COVID-19 pandemic. The results of this small observational study are supportive of the current multi-society guidelines regarding the judicious use of CSI.
Cite this article: Bone Joint Open 2020;1-9:605–611.
Take home message
Low incidence of positive COVID-19 testing in our cohort of patients following corticosteroid injections (CSI).
No adverse outcomes secondary to COVID infection were observed in our cohort of patients following CSI.
Introduction
Corticosteroid injections (CSI) are a key treatment for a wide range of musculoskeletal conditions. At the onset of the COVID-19 pandemic, national guidelines were published cautioning against the use of CSI for several reasons.1-4 These were in part due to the well-recognised immunosuppressive effects of CSI5,6 and previous research on the use of systemic corticosteroids during previous epidemics, namely Middle East Respiratory Syndrome (MERS), Severe Acute Respiratory Syndrome (SARS) and influenza.1-4,7 Previous studies have also shown that corticosteroids had the effect of suppressing innate immune responses to rhinovirus and influenza infection, leading to increased virus replication.8-10 However, a protective effect of CSI has been reported in vitro for the seasonal coronavirus 229E11 and SARS-CoV-2.12
An important further consideration at the onset of the pandemic was to minimize throughput of nonurgent patients into acute hospitals. These circumstances resulted a significant reduction in the number of CSI being performed and a large backlog of patients awaiting treatment for their symptoms. This has been further exacerbated by the lack of surgical management of orthopaedic conditions during the pandemic and ongoing limited capacity for elective surgery. While the nationally published guidelines are clear that CSI should be considered for patients with significant disease activity, severe symptoms, or when there are no effective alternatives,13 uncertainty has remained regarding the potential risk of adverse COVID-19 related outcome following CSI during the pandemic.
The present study describes the results of a retrospective observational study into the clinical outcomes of UK patients who received CSI during the height of the pandemic to better understand the risk of CSI in clinical practice.
Methods
A retrospective review was performed of patients in our region who received CSI between 1 February 2020 and 30 June 2020 with patient follow-up extending to 31 July 2020. The project received institutional approval as a service improvement audit and informed consent was waived.
Patient population
The radiology information system (RIS) was used to identify patients who had image guided CSI in radiology during the study period. In parallel, the Medway Electronic Patient Record (EPR) and Electronic Clinic Outcome Forms (eCOF) were used to identify patients who had CSI in clinic during the study period. Patient demographics were recorded alongside clinical indication, site of injection, and dose of steroid.
Our local guidelines for steroid injections evolved over the course of the pandemic. From 10 June 2020, local guidelines were produced which specified that CSI would only be considered in patients with high levels of pain and disability who had failed first-line measures and when the referring clinicians judged that continuation of those symptoms would have a significant negative effect on patients health and wellbeing. No patients with symptoms of infection – such a fever or a persistent cough – were considered for injection. Furthermore, high-risk patients were not considered for injection. High risk conditions included: chronic respiratory diseases, such as asthma, chronic obstructive pulmonary disease (COPD), emphysema, cystic fibrosis, or bronchitis; chronic heart disease; chronic kidney disease; chronic liver disease; chronic neurological conditions, such as Parkinson’s disease, motor neurone disease, multiple sclerosis (MS), a learning disability or cerebral palsy; diabetes; immunosuppression as the result of conditions such as HIV and AIDS, or medical treatments such as chemotherapy; a body mass index (BMI) of 40 or above; patients who were pregnant; organ transplant patients; patients undergoing active chemotherapy or radiotherapy; or patients with haematological malignancy.
The Electronic Patient Record (EPR) for each patient was reviewed for SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) and IgG antibody test results. The radiology information system (RIS) records were reviewed for each patient to identify radiological investigations for suspected COVID-19 infection, or incidental findings consistent with COVID-19 infection following the CSI. Radiology reports were reviewed by authors (DM, JP) who were blinded to any RT-PCR or IgG antibody result. Reports were categorized as: normal or classic/probable COVID-19, indeterminate for COVID-19, or an alternative diagnosis in accordance with the British Society of Thoracic Imaging (BSTI) guidelines.14 Based on BSTI guidelines, classic/probable COVID-19 features included lower lobe predominant, peripheral, multiple, bilateral foci of ground-glass opacification or bronchocentric, and peripheral consolidation. Indeterminate for COVID-19 was used for cases that had some of the above patterns but which did not entirely fit into the classic/probable category or when an alternative diagnosis, such as interstitial lung disease, were contributory.14
During the initial period of our study, SARS-CoV-2 RT-PCR testing was not routinely used at our institution and not all patients presenting to hospital were tested. When a RT-PCR test was obtained, it was via a combined nose and throat swab, taken using a flocked swab and transported to the laboratory in viral transport medium. RNA extraction was carried out on the Thermofisher KingFisher platform (Waltham, Massachusetts, USA) and amplification of SARS-CoV-2 RNA was carried out on the Thermofisher Quantstudio 5 using either the Bosphore Novel Coronavirus Detection Kit or Thermofisher TaqPath COVID-19 CE-IVD RT-PCR kit. Antibody screening tests were available to local NHS staff from June 2020 using Ab- SARS-CoV-2 IgG on Abbot Architect (Thermofisher).
In patients with either post-CSI radiological investigations, RT-PCR tests, or IgG antibody tests, further data were extracted on presenting symptoms, management, and patient outcomes.
Results
Between 1 February and 30 June 2020, 504 steroid injections were performed in our region on a total of 443 patients. Of these, 281 (63%) were female and the median age was 57 (interquartile range 47 to 69). Overall, 27 patients (6.1%) were from a Black, Asian, and minority ethnic or minority ethnic (BAME) background. Injection types are listed in Table I, with the timeline of injections described in Figure 1 relative to the lab-confirmed COVID-19 cases in our upper tier local authority (UTLA) described in Figures 2 and 3.
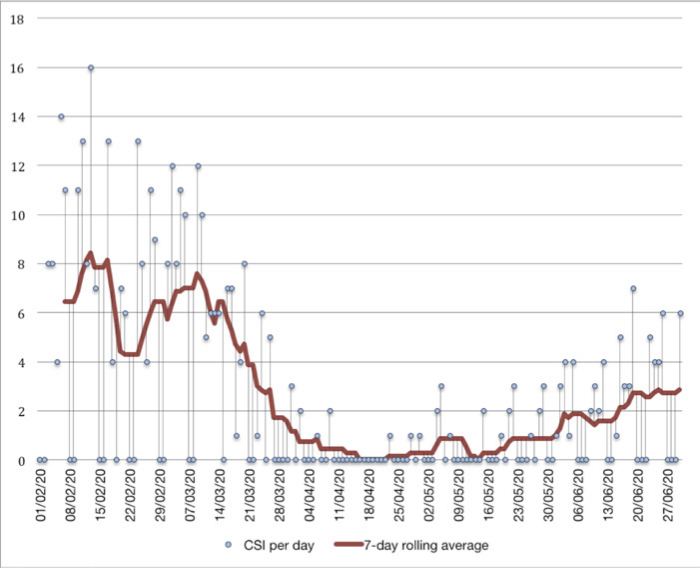
Fig. 1
Date of corticosteroid injections performed from 1 February to 30 June 2020. CSI, corticosteroid injections.
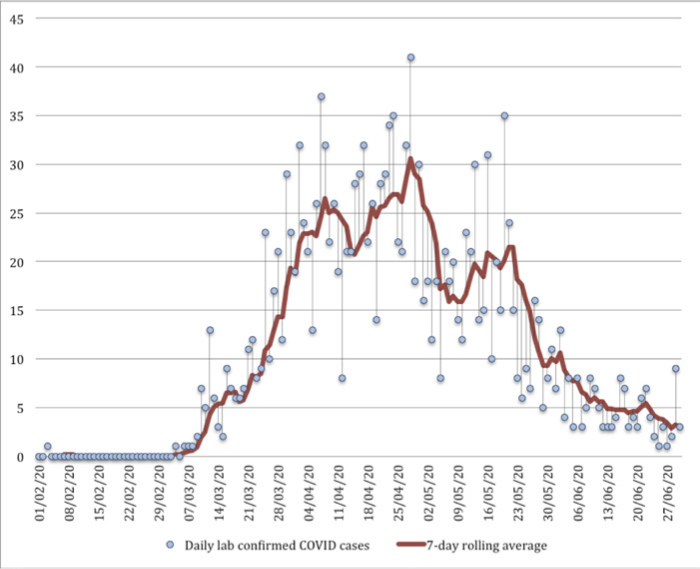
Fig. 2
Date of lab confirmed COVID-19 cases in Buckinghamshire upper tier local authority. Lab-confirmed positive cases are attributed to the day the first specimen was taken from the person being tested (the specimen date).
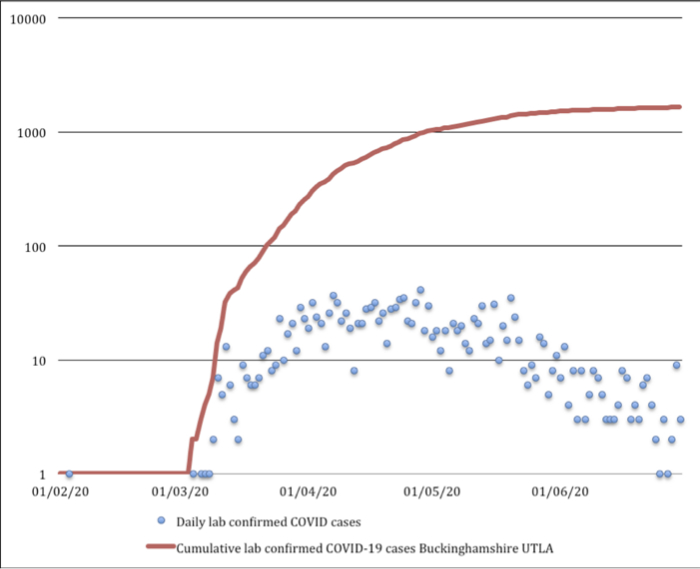
Fig. 3
Cumulative lab-confirmed COVID-19 cases in Buckinghamshire upper tier local authority (UTLA) 1 February to 30 June 2020. Lab-confirmed positive cases are attributed to the day the first specimen was taken from the person being tested (the specimen date).
Table I.
Site of corticosteroid injections performed from February 2020 to.June 2020.
| Type of infection | n |
|---|---|
| CT-guided | |
| Cervical nerve root | 12 |
| Lumbar nerve root | 31 |
| Ultrasound-guided | |
| Ankle | 11 |
| Elbow | 9 |
| Foot | 32 |
| Hand | 26 |
| Hip | 93 |
| Knee | 42 |
| Shoulder | 130 |
| Wrist | 21 |
| Rheumatology clinic | |
| Ankle | 5 |
| Elbow | 3 |
| Foot | 9 |
| Hand | 13 |
| Hip | 1 |
| Knee | 32 |
| Shoulder | 20 |
| Wrist | 13 |
| Paraspinal soft tissue | 1 |
Of these 504 injections, 407 were performed in radiology under imaging guidance. For these image guided injections standardized injectate solutions were administered. Nerve root injections received 6.6 mg dexamethasone; facet joint injections received 40 mg triamcinolone acetate; small joints (joints of the wrists, hands, ankles and feet) received 40 mg depomedrone; and large joints (elbow, shoulder, hip and knee) received 40 mg triamcinolone. The injected solutions were diluted with a variable volume of 0.25% bupivacaine depending on the joint targeted.
A total of 97 injections were performed in rheumatology outpatient clinics on 69 patients. Steroid used included triamcinolone acetate and depomedrone. Doses administered varied dependent on the joint targeted with an equivalent mean dose of 37 mg depomedrone administered per injection (range 10mg to 80mg depomedrone). Electronic patient medical records were analysed and all investigations for SARS-CoV-2 were identified (Figure 4).
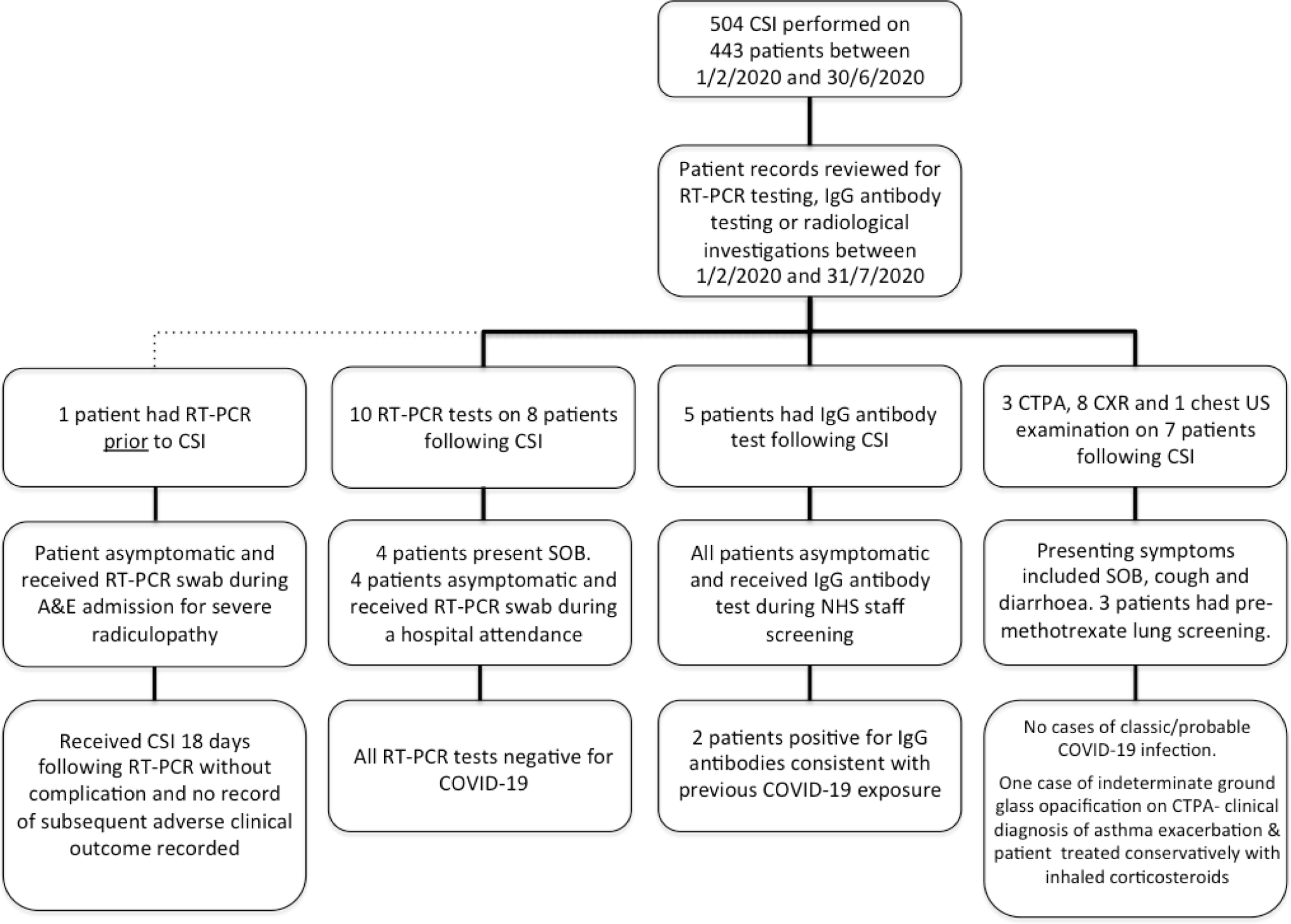
Fig. 4
Investigations for COVID-19 in patients who received corticosteroid injections between February and June 2020. CTPA, CT pulmonary angiogram; CSI, corticosteroid injection;CXR, chest x-ray; US, ultrasound; RT-PCR, reverse transcriptase polymerasechain reaction; SOB, short of breath.
A total of 11 RT-PCR tests were performed on nine patients (2%) following their CSI, all of which were negative. Four of these patients presented with symptoms of minor shortness of breath but had negative RT-PCR tests and normal chest radiographs. One patient had a RT-PCR swab in A&E after presenting with endophthalmitis for which they were treated with topical and oral steroids; two patients were swabbed during their preoperative assessment for coronary stent insertion and hernia repair; one patient had a precautionary swab as they were the parent of a paediatric inpatient. One patient received a RT-PCR test prior to their CSI having presented to A&E with severe radicular pain and received at RT-PCR swab which was negative for SARS-CoV-2 RNA; 18 days later they had a CT guided L4 nerve root injection without complication with no further record of COVID-19 related investigations or adverse clinical outcome. Five patients (1.1%) were NHS staff and received a screening IgG antibody test, of which two (0.5%) were positive for prior COVID-19 exposure. Both of these patients were asymptomatic.
Seven patients had chest imaging following their CSI with eight chest plain film examinations, three CT pulmonary angiogram (CTPA), and one pleural ultrasound examination being performed. No classic/probable cases of COVID-19 were identified. One patient had indeterminate chest imaging findings. They were a known asthmatic presenting with shortness of breath; however, a CT pulmonary angiogram (CTPA) was requested by the clinical team to exclude a pulmonary embolism (PE). This scan was negative for PE but did demonstrate a small focus of peripheral non-specific ground glass opacification in the right lower lobe of indeterminate significance. This patient was treated conservatively for a clinical diagnosis of asthma exacerbation with a course of inhaled steroids and discharged home where they made a full recovery. Other incidental findings included one case of basal atelectasis and one patient with basal consolidation and associated pleural effusion who was treated successfully with oral antibiotics.
There were a total number of 1,656 lab-confirmed COVID-19 cases by 30 June 2020 in our UTLA, a rate of 306.6 per 100,00015 (Figures 2 and 3). The first lab-confirmed case in our region was on 3 February 2020; however, the second lab-confirmed case did not occur until 4 March 2020 and the third on 6 March 2020. Subsequently, cases were confirmed every day until 30 June 2020, with a daily peak of 41 cases diagnosed on 28 April 2020. The Coronavirus (COVID-19) Infection Survey from IQVIA, Oxford University, and UK Biocentre modelled that the incidence rate of the community testing positive for COVID-19 in our region fell during the latter part of our study period.16 The percentage of the population testing positive for COVID-19 in our region was modelled as 0.49% (95% credible interval 0.15 to 1.13) on 26 April 2020, when the Coronavirus (COVID-19) Infection Survey began, and 0.03% on 27 June 2020 (95% credible interval 0.00 to 0.09). In this survey, as of 29 June 2020, 6.3% (95% confidence interval (CI) 4.7% to 8.1%) of individuals from whom blood samples were taken tested positive for COVID-19 antibodies.16
Discussion
This study describes the very low incidence of symptomatic COVID-19 in our UK patient cohort following CSI. No adverse outcomes related to SARS-CoV-2 infection were identified following 504 CSI in 443 patients. These finding suggest CSI is likely a low risk treatment option for the majority of low risk patients during the COVID-19 crisis.
The disruption from the pandemic to normal musculoskeletal services has been huge for several reasons, one of which has been the marked reduction in CSI injections performed during recent months. CSI can be an important treatment for those suffering severe painful and debilitating musculoskeletal conditions.17 Indeed it is widely considered good practice to perform a diagnostic/therapeutic CSI before committing to orthopaedic surgery.
Guidance at the onset of the pandemic1-4 appropriately raised questions regarding the potential risk of immunosuppression secondary to CSI based on the available research. Friedly et al18 reported 32 patients (20.3% of the cohort treated with corticosteroid) experienced cortisol reduction at three weeks of > 50%, although reported that only one of 149 patients suffered an adverse clinical event - pneumonia - that may have been related to immunosuppression. Youssef et al5 reviewed the infection risk of corticosteroid usage in rheumatology patients and reported that evidence from randomized controlled trials showed ‘no significant increased risk of infection was noted in the corticosteroid arms in most of the trials’. Systma et al19 reported that CSI can increase the risk of influenza infection with an influenza infection rate of 1.08% in 43,236 vaccinated control patients, 1.64% in 15,018 vaccinated patients who also had at least one CSI, and 1.70% in 4,804 unvaccinated patients who received at least one CSI. Of note, is that the mean dose-equivalent of methylprednisolone administered with each CSI in this study was 65.9 mg, higher than the dose typically used for a single intra-articular joint injection in the UK.
Since the onset of the pandemic, new research has emerged regarding the role glucocorticoid steroids may play in modulating the immunoresponse during COVID-19 infection. Rheumatology patients may be considered at particular risk for serious infections due to their immunocompromised state resulting from their underlying immune conditions and use of targeted immune-modulating therapies such as biologics. Gianfrancesco et al20 performed multivariate logistic regression on 600 patients from the COVID-19 Global Rheumatology Alliance registry. Their multivariable-adjusted models demonstrated that a prednisone dose of ≥ 10 mg/day was associated with higher odds of hospitalisation (OR 2.05 (95% CI 1.06 to 3.96)). However, this is a far higher cumulative glucocorticoid steroid dose than that routinely used for single intra-articular joint injection. An active surveillance project with USA/Canada Infectious Disease specialists via the Emerging Infections Network (EIN) screened 2500 cases of PCR-confirmed COVID-19 infection, from which 77 (3%) were identified to be using immunomodulatory drugs.21 Only nine patients were identified using corticosteroids alone with a median daily dose 15 mg prednisolone (2–60 mg). Of these, 7 patients were admitted to Intensive Care Unit, 6 patients required ventilator support and one patient died. The small numbers of patients identified in this publication highlighting the challenges for future population-based studies which will be necessary to understand the risk of disease-modifying anti-rheumatic drugs (DMARDs) with COVID-19.
Researchers have reported that the prevalence of asthma and COPD among hospitalised COVID-19 patients may be lower than in the general population22 and recent research by Finney et al23 has demonstrated using human and animal in vitro and in vivo disease models that inhaled corticosteroid (ICS) administration attenuates pulmonary expression of the SARS-CoV2 viral entry receptor angiotensin-converting enzyme (ACE)-2 which it is postulated may result in reduced susceptibility to COVID-19 infection. Systemic steroids may also mediate suppression of IFN and attenuate ACE2 expression. However, it is important to note that the systemic effect of peripheral CSI upon endogenous cortisol levels is relatively short-lived – Habib24 reported that the systemic effect of CSI on endogenous cortisol levels was maximal after 48 hours with recovery to baseline levels within one to four weeks.
Research from the RECOVERY collaborative group has reported that in patients hospitalized with COVID-19, the use of dexamethasone resulted in lower 28-day mortality among those who were receiving either invasive mechanical ventilation or oxygen alone. These findings have transformed the treatment of COVID-19 patients internationally and were in contrast to the earlier paper by Huang et al25 and the Lancet commentary by Russell et al7 which had advised against the use of corticosteroids in the treatment of COVID-19 infection. This highlights how quickly guidance may change as our understanding progresses. The RECOVERY collaborative group data did not show a clear effect of dexamethasone among patients who were not receiving any respiratory support at randomization.26 However this cohort of hospitalized patients represents a very different patient group to the asymptomatic community based patients who are typical the recipients of low dose intra-articular CSI.
A recent editorial from Little et al17 emphasized the importance of CSI in the management of musculoskeletal conditions and stressed the need for a balanced assessment of the available evidence and careful consideration of the potential risk and benefits of CSI for individual patients.
Subsequently, the most recent revised multisociety UK guidance has highlighted the important role CSI can play in treating musculoskeletal conditions albeit with some caution. This guidance advocates using intra-articular injections for inflammatory joints only where there is active synovitis± effusion, and to consider using lowest clinically effective doses.13 Periarticular CSI for musculoskeletal pain, such as for carpal tunnel syndrome or de Quervain’s tenosynovitis, may be considered if a patient has high levels of pain and disability and has failed first-line measures such as simple analgesia, activity modification, or splinting.13 CSI for spinal radiculopathy can be offered for severe symptoms and as an alternative to surgery in patients who have failed to respond to all available non-invasive treatments.13
There are several limitations to this study that should be discussed. Firstly, the authors recognize that this small observational study is not sufficiently powered to definitively determine the absolute risk of CSI. A comparison with a cohort who did not receive CSI has not been analyzed and we are unable to calculate of the relative risk of CSI over the general population. It is clear that future large population-based studies and meta-analyses will be necessary to fully understand the risk of corticosteroid injections with COVID-19.
Our data collection was limited to our local patients, which may introduce biases based on the demographics of the local population including race and socioeconomic status. It is possible that our enhanced written consent process for CSI during this period, which included lengthy discussion of the potential risk of corticosteroid induced immunosuppression during the on-going crisis, resulted in patients taking more effective precautionary physical distancing measures which reduced their risk of COVID-19 infection.
Our data collection does not include private sector testing and it may be that some patient results have not been included. It is possible that patients presented to hospitals outside our region; however, given the national lockdown restrictions in place during the period of this study, the likelihood of patients moving outside the region is small. Our Radiology Information System (RIS) is down datastream from our patient administration system (Medway) which in turn is down data stream from the national NHS data Spine. This includes information relevant to patient admission, discharge and radiology investigations allowing for robust data capture.
As not all patients were tested, we are unable to comment on the overall incidence of COVID-19 in our patient cohort and it is important to note that a large number of COVID-19 infections are asymptomatic. Lavezzo et al27 reported that 42.5% of confirmed COVID-19 infections were asymptomatic across the two nasopharyngeal swabs surveys for 85.9% and 71.5% of the population of the Italian municipality of Vo’.27
Given these significant limitations, the the authors would caution against over-interpretation of the findings. However, the results from this small retrospective study appear consistent with CSI likely being low risk for the majority of patients in the setting of COVID-19.17 The authors believe this study provides helpful information and have found discussion of our findings extremely useful when discussing the potential risk of CSI when obtaining informed patient consent, particularly when contextualised with the current incidence of COVID-19 cases regionally in comparison with the incidence at the peak of the pandemic.
Our findings are supportive of the previous1-4 and current13 national multisociety guidance which advocate the use of CSI where appropriate. Musculoskeletal complaints represent a substantial health burden, with pain the reason for presentation in approximately one in four cases in primary care.28,29 CSI are likely to be a particularly important therapeutic option during the current period given the long waiting lists for elective orthopaedic surgery17 and judicious use of these minimally invasive treatments may help vulnerable patients during the current crisis, alleviating patient pain and improving quality of life.
Future large population studies and meta-analyses will be required to better understand the complex interaction between the host immune response, CSI and risk from COVID-19 infection. These future studies may address more nuanced questions, for example to discover whether risks relating to the specific timing of CSI in relation to COVID-19 exposure exist and how the complex interplay of host immune response affects the risk of contracting SARS-CoV-2 infection, or the risk of severe COVID-19 disease sequelae such as cytokine storm. Despite these acknowledged limitations, this study provides the first description of patient outcomes related to COVID-19 following CSI during the pandemic in the UK.
Our retrospective observational study adds to the available literature by providing the first description of clinical outcomes related to COVID-19 in UK patients who received CSI during the pandemic. No adverse clinical outcomes related to COVID-19 infection following CSI administered during months of February to June 2020 were identified. Our findings support the careful use of CSI during the COVID-19 pandemic, consistent with the latest multi-society guidance. This important therapeutic option may be of even greater relevance given the restricted access to elective surgical options during the current crisis.
Acknowledgements
This paper and the research behind it would not have been possible without the exceptional support of the Buckinghamshire Healthcare NHS Trust Orthopaedic Department. We would especially like to thank Mr Bernard McElroy for his invaluable guidance and insight during the preparation of this manuscript.
Author contributions
D. McKean: Inception, Carried out background research, Analyzed the data, Wrote the manuscript.
S. L. Chung: Collected the data, Wrote and reviewed the manuscript.
R. Fairhead: Collected the data, Wrote and reviewed the manuscript.
O. Bannister: Collected the data, Reviewed the manuscript.
M. Magliano: Collected the data, Reviewed the manuscript.
J. Papanikitas: Analyzed the data, Wrote and reviewed the manuscript.
T. H. N. Wong: Collected the data, Wrote and reviewed the manuscript.
R. Hughes: Inception, Carried out background research, Wrote and reviewed the manuscript.
Funding statement
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Ethical review statement
The project received institutional approval as a service improvement audit and informed consent was waived.
Follow Buckinghamshire Healthcare Radiology @BucksRadiology
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attributions licence (CC-BY-NC-ND), which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.
References
1. Faculty of Pain Medicine of the Royal College of anaesthetists . FPM response to concern related to the safety of steroids injected as part of pain procedures during the current COVID-19 virus pandemic . 2020 . https://fpm.ac.uk/sites/fpm/files/documents/2020-03/FPM-COVID-19-Steroid-Statement-2020.pdf Google Scholar
2. British Society of Skeletal Radiology . The safety of corticosteroid injections during the COVID-19 global pandemic . 2020 . https://www.bssr.org.uk/static/uploads/forum/Musculoskeletal_Radiology_during_the_COVID-19_Global_Pandemic.pdf Google Scholar
3. NHSE & British Society of Rheumatology . Clinical guide for the management of patients with musculoskeletal and rheumatic conditions on corticosteroids during the coronavirus pandemic . https://www.rheumatology.org.uk/news-policy/details/Covid19-Coronavirus-update-members Google Scholar
4. The British Pain Society . Pain management during COVID-19 viral infection . 2020 . https://www.britishpainsociety.org/static/uploads/resources/files/Pain_Management_during_COVID-19_viral_infection.pdf Google Scholar
5. Youssef J , Novosad SA , Winthrop KL . Infection risk and safety of corticosteroid use . Rheum Dis Clin North Am . 2016 ; 42 ( 1 ): 157 – 176 . Crossref PubMed Google Scholar
6. Smitten AL , Choi HK , Hochberg MC , et al. . The risk of hospitalized infection in patients with rheumatoid arthritis . J Rheumatol . 2008 ; 35 ( 3 ): 387 – 393 . PubMed Google Scholar
7. Russell CD , Millar JE , Baillie JK . Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury . The Lancet . 2020 ; 395 ( 10223 ): 473 – 475 . Google Scholar
8. Singanayagam A , Glanville N , Girkin JL , et al. . Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations . Nat Commun . 2018 ; 9 ( 1 ): 2229 . Crossref PubMed Google Scholar
9. Thomas BJ , Porritt RA , Hertzog PJ , Bardin PG , Tate MD . Glucocorticosteroids enhance replication of respiratory viruses: effect of adjuvant interferon . Sci Rep . 2015 ; 4 ( 1 ): 7176 . Crossref PubMed Google Scholar
10. Kan-O K , Ramirez R , MacDonald MI , et al. . Human metapneumovirus infection in chronic obstructive pulmonary disease: impact of glucocorticosteroids and interferon . J Infect Dis . 2017 ; 215 ( 10 ): 1536 – 1545 . Crossref PubMed Google Scholar
11. Yamaya M , Nishimura H , Deng X , et al. . Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells . Respir Investig . 2020 ; 58 ( 3 ): 155 – 168 . Crossref PubMed Google Scholar
12. Matsuyama S , Kawase M , Nao N . The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15 [Internet] . Microbiology . 2020 . Google Scholar
13. BSR BOA BASS RCGP BSIR FPM BPS CSP . Management of patients with musculoskeletal and rheumatic conditions who: are on corticosteroids; require initiation of oral/IV corticosteroids; require a corticosteroid injection . 2020 . https://www.boa.ac.uk/uploads/assets/3767f092-abfb-40c8-bab2c711a81306d5/MSKcorticosteroidguidance.pdf Google Scholar
14. BSTI . The British Society of Thoracic Imaging COVID-19 BSTI Reporting templates. [Internet] . 2020 . https://www.bsti.org.uk/covid-19-resources/covid-19-bsti-reporting-templates/ Google Scholar
15. GOV.UK . Coronavirus (COVID-19) in the UK [Internet] . 2020 . https://coronavirus.data.gov.uk/ Google Scholar
16. ONS . Coronavirus (COVID-19) Infection Survey pilot [Internet] . 2020 . https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/31july2020 Google Scholar
17. Little CP , Birks ME , Horwitz MD , Ng CY , Warwick D . COVID-19: a rethink of corticosteroid injection? Bone & Joint Open . 2020 ; 1 ( 6 ): 253 – 256 . Crossref PubMed Google Scholar
18. Friedly JL , Comstock BA , Heagerty PJ , et al. . Systemic effects of epidural steroid injections for spinal stenosis . Pain . 2018 ; 159 ( 5 ): 876 – 883 . Crossref PubMed Google Scholar
19. Sytsma TT , Greenlund LK , Greenlund LS . Joint corticosteroid injection associated with increased influenza risk . Mayo Clin Proc Innov Qual Outcomes . 2018 ; 2 : 194 – 198 . Crossref PubMed Google Scholar
20. Gianfrancesco M , Hyrich KL , Al-Adely S , et al. . Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry . Ann Rheum Dis . 2020 ; 79 ( 7 ): 859 – 866 . Crossref PubMed Google Scholar
21. Winthrop KL , Brunton AE , Beekmann S , et al. . Sars CoV-2 infection among patients using immunomodulatory therapies . Ann Rheum Dis . 2020 : annrheumdis-2020-218580 . Crossref PubMed Google Scholar
22. Halpin DMG , Faner R , Sibila O , Badia JR , Agusti A . Do chronic respiratory diseases or their treatment affect the risk of SARS-CoV-2 infection? Lancet Respir Med . 2020 ; 8 ( 5 ): 436 – 438 . Google Scholar
23. Finney LJ , Glanville N , Farne H . Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon [Internet] . Immunology . 2020 . Google Scholar
24. Habib GS . Systemic effects of intra-articular corticosteroids . Clin Rheumatol . 2009 ; 28 ( 7 ): 749 – 756 . Crossref PubMed Google Scholar
25. Huang C , Wang Y , Li X , et al. . Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China . The Lancet . 2020 ; 395 ( 10223 ): 497 – 506 . Crossref PubMed Google Scholar
26. RECOVERY Collaborative Group , Horby P , Lim WS , et al. . Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report . N Engl J Med . 2020 . Google Scholar
27. Lavezzo E , Franchin E , Ciavarella C , et al. . Suppression of a SARS-CoV-2 outbreak in the Italian municipality of VO' . Nature . 2020 ; 584 ( 7821 ): 425 – 429 . Crossref PubMed Google Scholar
28. Rekola KE , Keinänen-Kiukaanniemi S , Takala J . Use of primary health services in sparsely populated country districts by patients with musculoskeletal symptoms: consultations with a physician . Journal of Epidemiology & Community Health . 1993 ; 47 ( 2 ): 153 – 157 . Crossref PubMed Google Scholar
29. Mäntyselkä P , Kumpusalo E , Ahonen R , et al. . Pain as a reason to visit the doctor: a study in Finnish . primary health care: Pain . 2001 ; 89 ( 2 ): 175 – 180 . Crossref PubMed Google Scholar









