Abstract
Aims
The first death in the UK caused by COVID-19 occurred on 5 March 2020. We aim to describe the clinical characteristics and outcomes of major trauma and orthopaedic patients admitted in the early COVID-19 era.
Methods
A prospective trauma registry was reviewed at a Level 1 Major Trauma Centre. We divided patients into Group A, 40 days prior to 5 March 2020, and into Group B, 40 days after.
Results
A total of 657 consecutive trauma and orthopaedic patients were identified with a mean age of 55 years (8 to 98; standard deviation (SD) 22.52) and 393 (59.8%) were males. In all, 344 (approximately 50%) of admissions were major trauma. Group A had 421 patients, decreasing to 236 patients in Group B (36%). Mechanism of injury (MOI) was commonly a fall in 351 (52.4%) patients, but road traffic accidents (RTAs) increased from 56 (13.3%) in group A to 51 (21.6%) in group B (p = 0.030). ICU admissions decreased from 26 (6.2%) in group A to 5 (2.1%) in group B. Overall, 39 patients tested positive for COVID-19 with mean age of 73 years (28 to 98; SD 17.99) and 22 (56.4%) males. Common symptoms were dyspnoea, dry cough, and pyrexia. Of these patients, 27 (69.2%) were nosocomial infections and two (5.1%) of these patients required intensive care unit (ICU) admission with 8/39 mortality (20.5%). Of the patients who died, 50% were older and had underlying comorbidities (hypertension and cardiovascular disease, dementia, arthritis).
Conclusion
Trauma admissions decreased in the lockdown phase with an increased incidence of RTAs. Nosocomial infection was common in 27 (69.2%) of those with COVID-19. Symptoms and comorbidities were consistent with previous reports with noted inclusion of dementia and arthritis. The mortality rate of trauma and COVID-19 was 20.5%, mainly in octogenarians, and COVID-19 surgical mortality was 15.4%.
Cite this article: Bone Joint Open 2020;1-7:330–338.
Introduction
On 30 January 2020, the World Health Organization (WHO) declared the coronavirus disease 2019 (COVID-19) as an outbreak of Public Health Emergency and International Concern.1 On 5 March 2020, over 100 cases were identified and the first official death in the UK occurred.2 On 11 March 2020, the Director General of the WHO characterized COVID-19 as a global pandemic caused by the viral pathogen Severe Acute Respiratory Syndrome Coronavirus 2 (SAR-CoV-2).1
Due to the highly infectious nature of the virus, some hospitals and healthcare systems have been overwhelmed.3 The UK government implemented social lockdown with measures including social distancing and self-isolation. The rapidly changing situation caused hospitals to continually update their healthcare management policies, including surgical management and ward based treatment. Elective surgery was suspended, with emergency and time-critical surgery, including trauma surgery, continuing.4 The British Orthopaedic Association (BOA) published guidelines suggesting changes in approach to standard management practices so as to minimize patient exposure to the virus and reduce overall impact on resources.5
Currently there is a paucity of data on the clinical characteristics and outcomes of the traumatically injured patient with COVID-19 infection. This is of importance in order to understand risk factors, comorbidities, multiplicity of infection (MOI), the anatomy at risk, and the surgical sub-specialties required. Redistribution of resources had an unanticipated impact on the healthcare system and the importance of understanding the clinical characteristics of traumatically injured COVID-19 patients could help to protect patients and staff. The challenge of the pandemic is the need to balance resources while considering the individual, the population, the economy, and the supply of personal protective equipment (PPE) to prevent the possibility of overwhelming healthcare systems.
The aim of this paper is to analyze and present the clinical characteristics of trauma patients presenting to a Level 1 major trauma centre during the early stages of the COVID-19 pandemic in the UK.
Methods
This single-centre, observational study was designed and reported according to STROBE guidance.6 A retrospective analysis of a prospectively collected trauma database was performed at our Level 1 trauma hospital in south west London providing specialist care for 3.5 million people. All clinical notes of major trauma and orthopaedic patients from 26 January 2020 to 14 April 2020 were reviewed. The first confirmed case of COVID-19 in the UK was on 28 February 2020. It is known that many asymptomatic patients had travelled from infected regions into the UK prior to this. We refer to this period as the incubation phase. When SARS-CoV-2 severe pneumonia started to cause mortality on 5 March 2020, the virus was spreading exponentially, requiring governments around the world to begin imposing lockdown strategies. UK official lockdown commenced on 23 March 2020. Even prior to official quarantine, many companies and professional bodies were commencing their own lockdown. This time period is referred to as the lockdown phase. Cases were divided into two groups: Group A (incubation phase), 40 days before the first UK recorded death, and Group B (lockdown phase), 40 days after. No patient was directly involved in this study.
Inclusion criteria for major trauma was activation of the major trauma call or patients with orthopaedic injuries requiring admission. Diagnosis of COVID-19 was by the hospital’s standard swabbing process of the nose and throat and on-site laboratory RNA confirmation. Clinical records were analyzed for diagnostics, pathology results, treatments and outcomes. COVID-19 cases were designated as Group C and non-COVID-19 cases as Group D.
Statistical analysis
Following verification of distributional assumptions of normality, groups were compared in continuous variables using the independent-samples t-test. When equality of variances could not be assumed between groups, Satterwaite’s approximation to the degrees of freedom was made. When distributional assumptions could not be made, groups were compared in continuous variables using the Mann-Whitney U test (test statistic denoted by U). Groups were compared in categorical variables using the chi-squared test, and when invalid by using Fisher’s exact test. Traditional statistical hypothesis testing with a two-sided alternative was employed, with a critical level of significance of p < 0.05. No adjustments were made for multiple hypothesis testing. Analyses were performed using SPSS v. 26 (IBM, Armonk, New York, USA).
Results
Overall, 657 consecutive trauma patients were included in this study. A total of 421 (64.1%) patients were admitted within the first 40 days (Group A: early incubation phase) versus 36 (35.9%) patients in the following 40 days (Group B: early lockdown phase). There was a progressive decline in the number of patients per week in both groups (Figure 1).
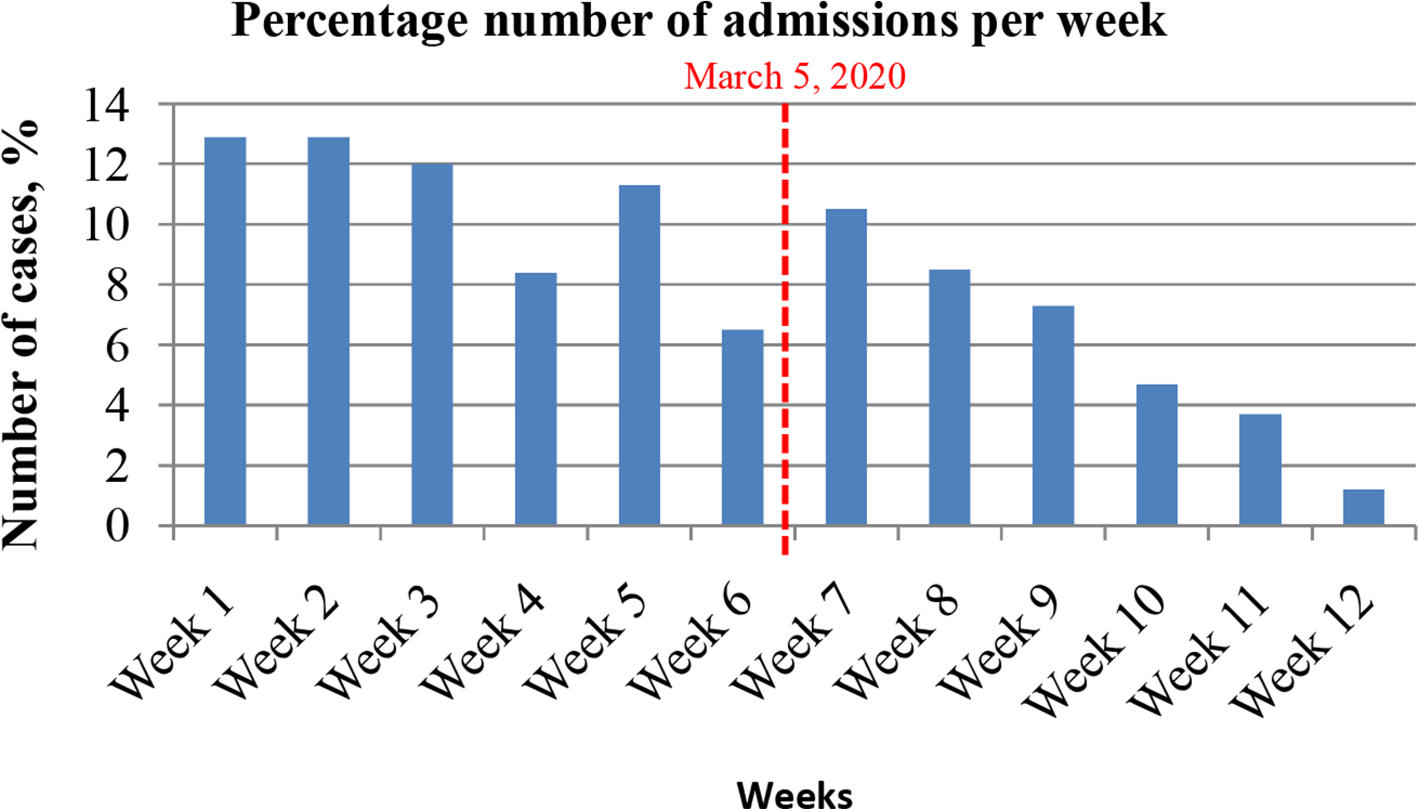
Fig. 1
Number of cases admitted under major trauma and orthopaedics (T&O) during the COVID-19 pandemic. Group A (weeks 1 to 6) 40 days before 5 March 2020 vs Group B (weeks 7 to 12) 40 days after 5 March 2020.
Table I shows the baseline characteristics of patients admitted under major trauma and orthopaedics (T & O) during the onset of the COVID-19 pandemic. Injury and demographics are shown. Both groups had a higher percentage of male patients, with falls and RTA’s being the most common mechanism of injury. Group B showed higher incidence of pelvis, chest and thorax, and spine injuries (p < 0.050). ICU admissions were fewer in the lockdown phase: Group A (26; 6.2%) compared to group B (2.1%); p = 0.020.
Table I.
Baseline characteristics of patients admitted under Orthopaedics during the onset of the COVID-19 pandemic.
| Group A (n = 421) | Group B (n = 236) | Total (n = 657) | p-value | |
|---|---|---|---|---|
| Mean age, yrs (range) | 55.6 (16 to 98) | 54.6 (8 to 98) | 55.3 (8 to 98) | 0.598 |
| Age, n (%) | ||||
| < 60 yrs | 223 (53.0) | 139 (58.9) | 362 (55.1) | 0.165 |
| > 60 yrs | 198 (47.0) | 97 (41.1) | 295 (44.9) | 0.165 |
| Sex, n (%) | ||||
| Female | 169 (40.1) | 95 (40.3) | 264 (40.2) | 0.978 |
| Male | 252 (59.9) | 141 (59.7) | 393 (59.8) | 0.978 |
| Admission type, n (%) | ||||
| Major trauma | 217 (51.5) | 127 (53.8) | 344 (52.4) | 0.576 |
| Orthopaedic | 204 (48.5) | 109 (46.2) | 313 (47.6) | 0.576 |
| MOI | 0.036 | |||
| Fall | 230 (54.6) | 121 (51.3) | 351 (53.4) | |
| RTA | 56 (13.3) | 51 (21.6) | 107 (16.3) | |
| Pain | 38 (9.0) | 16 (6.8) | 54 (8.2) | |
| Assault | 31 (7.4) | 14 (5.9) | 45 (6.8) | |
| Self-harm | 18 (4.3) | 6 (2.5) | 24 (3.7) | |
| Wound infection | 11 (2.6) | 6 (2.5) | 17 (2.6) | |
| Accidental | 5 (1.2) | 9 (3.8) | 14 (2.1) | |
| Sport | 8 (1.9) | 3 (1.3) | 11 (1.7) | |
| Twisting | 3 (0.7) | 3 (1.3) | 6 (0.9) | |
| Malignancy | 1 (0.2) | 2 (0.8) | 3 (0.5) | |
| Unknown | 20 (4.8) | 5 (2.1) | 25 (3.8) | |
| Injury anatomy | ||||
| LL | 120 (28.5) | 53 (22.5) | 173 (26.3) | 0.091 |
| UL | 97 (23.0) | 65 (27.5) | 162 (24.7) | 0.199 |
| Thorax and abdomen | 78 (18.5) | 60 (25.4) | 138 (21.0) | 0.037 |
| Pelvic | 65 (15.4) | 53 (22.5) | 118 (18.0) | 0.025 |
| Head and neck | 63 (15.0) | 49 (20.8) | 112 (17.0) | 0.058 |
| Spinal | 45 (10.7) | 43 (18.2) | 88 (13.4) | 0.007 |
| Intervention | ||||
| Conservative | 250 (59.4) | 144 (61.0) | 394 (60.0) | 0.682 |
| Surgical | 171 (40.6) | 92 (39.0) | 263 (40.0) | 0.682 |
| Surgical speciality | ||||
| Orthopaedic | 158 (37.5) | 75 (31.8) | 233 (35.5) | 0.139 |
| Plastics | 7 (1.7) | 8 (3.4) | 15 (2.3) | 0.155 |
| Neurosurgery | 1 (0.2) | 4 (1.7) | 5 (0.8) | 0.059† |
| General surgery | 3 (0.7) | 2 (0.8) | 5 (0.8) | 1.000† |
| Maxillofacial | 1 (0.2) | 2 (0.8) | 3 (0.5) | 0.294† |
| Cardiothoracic | 1 (0.2) | 0 (0.0) | 1 (0.2) | 1.000† |
| ENT | 0 (0.0) | 1 (0.4) | 1 (0.2) | 0.359† |
| ICU admissions | 26 (6.2) | 5 (2.1) | 31 (4.7) | 0.020 |
| Mortality | 15 (3.6) | 16 (6.8) | 31 (4.7) | 0.083 |
| COVID-19 positive | 10 (2.4) | 29 (12.3) | 39 (5.9) | < 0.001 |
| Community infection | 0 (0.0) | 12 (5.1) | 12 (1.8) | 0.017† |
| Nosocomial infection | 10 (2.4) | 17 (7.2) | 27 (4.1) | 0.017† |
-
*
The test statistics are for the null hypothesis significance test for the comparison of Group A with Group B. ‡ Fisher’s Exact test conducted due to invalid Chi-Squared test. Group A, incubation phase; Group B, lockdown phase. Statistically significant.
-
MOI, mechanism of injury; RTA, road traffic accident; LL, lower limb; UL, upper limb; ENT, ear, nose and throat; ICU, intensive care unit; NA, not applicable; CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease
A total of 39 patients tested positive for COVID-19 by RNA swab tests with ten patients in Group A and 29 in Group B. It is noted that there was 0% community acquired COVID-19 in Group A and 12 patients (5.1%) in Group B. Overall, there were 2.4% nosocomial infections in Group A and 7.2% in Group B. Within the COVID-19 group, 69.2% (n = 27) were nosocomial infections.
Table II depicts the baseline characteristics of patients with COVID-19 infection (Group C) admitted under T & O in comparison to all non-COVID-19 (Group D) patients. The mean age in Group C was 73.2 years (28 to 98; SD 17.99) versus 54.1 years (8 to 98; SD 22.3) in Group D. Group C shows that 32 (82.1%) patients were > 60 years versus 263 (42.6%) in Group D. There was no difference in ratio of sex between groups. Group C (COVID-19) had significantly higher percentage of comorbidities than Group D (non-COVID-19 patients): hypertension in 18 (46.2%), CVD in 14 (35.9%), arthritis in nine (23.1%), dementia in seven (17.9%), and malignancy in six (15.4%). Diabetes (p = 0.072; Fisher's Exact test) and renal disease (p = 0.001; Fisher's Exact test) were not significantly different. Surgical intervention occurred less in Group C with 33.3% compared to 40.5% in Group D. Surgical mortality was higher in Group C (15.4%) compared to group D (2.8%).
Table II.
Baseline characteristics of patients with COVID-19 infection admitted under Orthopaedics.
| Total (n = 657) | Group C: COVID-19 (n = 39) | Group D: non-COVID-19 (n = 618) | p-value | |
|---|---|---|---|---|
| Age, yrs | ||||
| Mean (SD; range) | 55.3 (22.53; 8 to 98) | 73.2 (17.99; 28 to 98) | 54.1 (22.3) | <0.001$* |
| Median | 57.0 (95% CI 13.0 to 25.2) | 73.2 | 55.0 | < 0.001 |
| < 60 years, n (%) | 362 (55.1) | 7 (17.9) | 355 (57.4) | < 0.001 |
| > 60 years, n (%) | 295 (44.9) | 32 (82.1) | 263 (42.6) | < 0.001 |
| Sex n (%) | ||||
| Female | 264 (40.2) | 17 (43.6) | 247 (40.0) | 0.737 |
| Male | 393 (59.8) | 22 (56.4) | 371 (60.0) | 0.737 |
| Comorbidity, n (%) | ||||
| Hypertension | 153 (23.3) | 18 (46.2) | 135 (21.8) | 0.001 |
| CVD | 90 (13.7) | 14 (35.9) | 76 (12.3) | < 0.001 |
| Arthritis | 70 (10.7) | 9 (23.1) | 61 (9.9) | 0.027‡ |
| Diabetes | 75 (11.4) | 8 (20.5) | 67 (10.8) | 0.072‡ |
| Dementia | 52 (7.9) | 7 (17.9) | 45 (7.3) | 0.027‡ |
| Malignancy | 43 (6.5) | 6 (15.4) | 37 (6.0) | 0.035‡ |
| COPD | 29 (4.4) | 4 (10.3) | 25 (4.0) | 0.086‡ |
| Kidney disease | 121 (18.4) | 4 (10.3) | 117 (18.9) | 0.206 |
| Asthma | 99 (15.1) | 2 (5.1) | 97 (15.7) | 0.102 |
| Intervention, n (%) | ||||
| Surgical | 263 (40.0) | 13 (33.3) | 250 (40.5) | 0.405 |
| Conservative | 394 (60.0) | 26 (66.7) | 368 (59.5) | 0.405 |
| Surgical Mortality | 9 (3.4) | 2 (15.4) | 7 (2.8) | 0.089‡ |
-
*
The test statistics are for the null hypothesis significance test for thecomparison of the COVID patients with non-COVID patients. $Satterwaite’s approximation to the degrees of freedom used; ‡Fisher’s Exact test conducted due to invalid Chi-Squared test; Statisitically significant.
-
CVD, cardiovascular disease; COPD, chronic obstructive pulmonary disease
Table III shows the type of orthopaedic procedures and surgical specialties that were involved with patients with COVID-19 compared to non-COVID-19 patients. Two patients (5.1%) required ICU admission and both recovered. In total, 13 patients (33.3%) with COVID-19 infection underwent orthopaedic surgery with five (12.9%) requiring hip surgery. Neurosurgery was required in 12 (30.8%) and cardiothoracic surgery in five (12.8%) patients with COVID-19 infection. Patients with COVID-19 infection had a significantly higher mortality 20.5% (n = 8) versus 3.7% (n = 23) in non-COVID-19 patients (p < 0.010).
Table III.
Types of surgery and specialties involved.
| Surgery | Total (n = 657) | Group C: COVID-19 (n = 39) | Group D: non-COVID-19 (n = 618) | p-value |
|---|---|---|---|---|
| Orthopaedic procedures, n (%) | ||||
| ORIF | 99 (15.1) | 4 (10.3) | 95 (15.4) | 0.493 |
| Hemi | 20 (3.0) | 4 (10.3) | 16 (2.6) | 0.026‡ |
| DHS | 15 (2.3) | 1 (2.6) | 14 (2.3) | 0.605‡ |
| Intramedullary nail | 31 (4.7) | 2 (5.1) | 29 (4.7) | 0.705‡ |
| Exploration | 17 (2.6) | 0 (0.0) | 17 (2.8) | 0.616‡ |
| Debridement & washout | 31 (4.7) | 2 (5.1) | 29 (4.7) | 0.705‡ |
| MUA | 2 (0.3) | 0 (0.0) | 2 (0.3) | 1.000‡ |
| Ex-fix | 5 (0.8) | 0 (0.0) | 5 (0.8) | 1.000‡ |
| Tendon repair | 9 (1.4) | 0 (0.0) | 9 (1.5) | 1.000‡ |
| Arthroscopy | 4 (0.6) | 0 (0.0) | 4 (0.6) | 1.000‡ |
| Total | 233 (35.4) | 13 (33.3) | 220 (35.6) | 1.000‡ |
| Specialities involved, n (%) | ||||
| Plastics | 24 (3.7) | 0 (0.0) | 24 (3.9) | 0.389‡ |
| Cardiothoracic | 55 (8.4) | 5 (12.8) | 50 (8.1) | 0.363‡ |
| Neurosurgery/spine | 129 (19.6) | 12 (30.8) | 117 (18.9) | 0.094‡ |
| General surgery | 41 (6.2) | 2 (5.1) | 39 (6.3) | 1.000‡ |
| Maxillofacial | 19 (2.9) | 2 (5.1) | 17 (2.8) | 0.313‡ |
| ENT | 4 (0.6) | 0 (0.0) | 4 (0.6) | 1.000‡ |
| ICU admissions | 31 (4.7) | 2 (5.1) | 29 (4.7) | 0.693‡ |
| Mortality | 31 (4.7) | 8 (20.5) | 23 (3.7) | < 0.000‡ |
-
*
The test statistics are for the null hypothesis significance test for thecomparison of the COVID patients with non-COVID patients. ‡Fisher’s Exact test conducted due to invalid Chi-Squared test. Statistically significant.
-
ORIF, open reduction internal fixation; DHS, dynamic hip screw; MUA, manipulation under anaesthesia; ENT, ear, nose, throat; ICU, intensive care unit.
Table IV shows signs, symptoms and laboratory findings of COVID–19 patients. The most common symptoms were dyspnoea (56.4%), fever (20.5%) and dry cough (15.4%). Antibiotic treatment, oxygen support and laboratory findings between survivors and non-survivors are shown.
Table IV.
Signs, symptoms and laboratory findings of COVID -19-positive patients.
| Reference range | Total (n = 39) | Survival (n = 31) | Non-survival (8) | p-value | |
|---|---|---|---|---|---|
| Median age, yrs (range) | 80 (28 to 98) | 76 (28 to 90) | 89 (62 to 98) | 0.047 | |
| Age, n (%) | |||||
| < 60 years | NA | 7 (17.9) | 7 (22.6) | 0 (0) | 0.308 |
| > 60 years | NA | 32 (82.1) | 24 (77.4) | 8 (100) | 0.308 |
| Signs and symptoms, n (%) | |||||
| Fever | NA | 8 (20.5%) | 7 (22.6%) | 3 (37.5%) | 0.399‡ |
| Dry cough | NA | 6 (15.4%) | 8 (25.8%) | 3 (57.5%) | 0.663‡ |
| Dyspnoea | NA | 22 (56.4%) | 16 (51.6%) | 7 (87.5%) | 0.109‡ |
| Fatigue | NA | 4 (10.3%) | 11 (35.9%) | 5 (62.5%) | 0.235‡ |
| Onset of infection, n (%) | |||||
| Community | NA | 12 (30.8) | 11 (35.5) | 1 (12.5) | 0.394‡ |
| Nosocomial | NA | 27 (69.2) | 20 (64.5) | 7 (87.5) | 0.394‡ |
| Median laboratory findings (range) | |||||
| White cell count × 10^9/l | 3.6 to 11.0 | 7.5 (5.4 to 9.4) | 7.1 ( 5.4 to 8.4) | 8.6 (5.3 to 14.6) | 0.401 |
| Neutrophil count × 10^9/l | 1.8 to 7.5 | 4.4 (3.6 to 6.5) | 4.4 (3.5 to 6.4) | 7.3 (3.8 to 12.6) | 0.132 |
| Lymphocyte count × 10^9/l | 1.0 to 4.0 | 0.9 (0.7 to 1.0) | 0.9 (0.7 to 1.1) | 0.8 (0.6 to 0.9) | 0.346 |
| Monocyte count x 10^9/l | 0.2 to 0.8 | 0.5 (0.4 to 0.7) | 0.5 (0.4 to 0.7) | 0.6 (0.3 to 1.1) | 0.527 |
| Platelet count × 10^9/l | 140 to 400 | 230 (139 to 313) | 220 (134 to 294) | 314.5 (230.5 to 477.3) | 0.052 |
| Creatine, umol//l | 60 to 110 | 75.0 (51 to 98) | 72 (51 to 96) | 109.5 (46.5 to 327.0) | 0.249 |
| C-reactive protein, mg/l | < 5 | 74.0 (37 to 135) | 56 (32 to 119) | 196 (129.0 to 326.5) | < 0.001 |
| INR | 0.8 to 1.2 | 1.1 (1.0 to 1.3) | 1.1 (1.0 to 1.3) | 1.3 (1.1 to 1.7) | 0.044 |
| Urea, mmol/L | 2.5 to 7.8 | 5.9 (4.5 to 9.0) | 5.8 (4.3 to 7.7) | 14.75 (5.2 to 34.6) | 0.048 |
| Bilirubin, umol/L | < 21 | 8 (6 to 14) | 8 (6 to 11) | 11 (5.8 to 15) | 0.462 |
| Alanine transaminase, U/L | < 36 | 30 (21 to 45) | 27 (19 to 38) | 44 (33.8 to 56) | 0.099 |
| Alkaline phosphatase, IU/L | 30 to 130 | 100 (76 to 178) | 96 (74 to 154) | 159 (91.3 to 257.3) | 0.550 |
| Albumin, g/L | 35 to 50 | 25 (21.8 to 31.0) | 25.5 (23 to 31.5) | 20 (18.3 to 24) | 0.132 |
| Haemoglobin, g/L | 115 to 180 | 108 (92 to 125) | 108 (92 to 108) | 101.5 (86.5 to 120) | 0.021 |
| Treatment, n (%) | |||||
| Antibiotics | N/A | 13 (33.3) | 9 (29) | 4 (50) | 0.402‡ |
| Antiviral therapy | N/A | 2 (5.1) | 2 (6.5) | 0 | 1.000‡ |
| Steroids | N/A | 2 (5.1) | 2 (6.5) | 0 | 1.000‡ |
| Oxygen support, n (%) | |||||
| Nasal cannula | N/A | 21 (53.8) | 11 (35.5) | 8 (100) | < 0.001‡ |
| Noninvasive ventilator | N/A | 10 (25.6) | 5 (16.1) | 5 (62.5) | < 0.001‡ |
| Ventilator | N/A | 3 (7.7) | 2 (6.4) | 1 (12.5) | < 0.001‡ |
| HLOS | NA | 22 (11 to 28) | 24 (11 to 28) | 17.5 (9.3 to 34.3) | 0.670 |
-
*
For laboratory findings and HLOS, the median plus interquartile range is presented. The test statistics are for the null hypothesis significance test for the comparison of the survival with the non-survival patients. ‡Fisher’s Exact test conducted due to invalid Chi-Squared test. Statistically significant.
-
N/A, not applicable; HLOS, hospital length of stay; INR, international normalized ratio.
Table V depicts clinical characteristics of eight patients with COVID-19 infection who died. 87.5% (7 out of 8) were nosocomial infections. These patients were elderly with a mean age of 84.4 years (p = 0.047) with 25% receiving surgery and no ICU admissions. Discussion
Table V.
Clinical characteristics of eight non-survival patients with COVID-19 infection.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | n (%) | |
|---|---|---|---|---|---|---|---|---|---|
| DOA | 19 March | 27 February | 9 March | 29 February | 10 March | 26 January | 25 March | 7 March | N/A |
| Age, yrs | 62 | 73 | 80 | 89 | 89 | 90 | 94 | 98 | N/A |
| Sex, male/female | Male | Male | Male | Female | Female | Female | Male | Female | 4:4 |
| Comorbidities | |||||||||
| CVD | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 6 |
| HTN | Yes | No | No | No | No | No | No | Yes | 2 |
| Diabetes | Yes | No | No | No | No | No | Yes | No | 2 |
| Kidney disease | Yes | No | Yes | No | No | Yes | No | No | 3 |
| COPD | No | Yes | No | No | No | No | No | No | 1 |
| Dementia | No | No | Yes | Yes | No | Yes | No | No | 3 |
| Admission type | |||||||||
| Trauma | No | No | Yes | Yes | Yes | No | Yes | No | 4 |
| Orthopaedics | Yes | Yes | No | No | No | Yes | No | Yes | 4 |
| MOI | Pain | Pain | Fall | Fall | Fall | Fall | Fall | Fall | N/A |
| Injury | |||||||||
| Head & neck | No | No | Yes | No | Yes | No | No | Yes | 3 |
| Thorax | No | No | No | Yes | Yes | No | No | No | 2 |
| Spinal | No | No | No | Yes | Yes | No | Yes | No | 3 |
| Pelvic | Yes | No | No | No | Yes | No | No | Yes | 3 |
| UL | No | No | No | Yes | No | Yes | No | No | 2 |
| LL | No | Yes | No | No | No | No | No | No | 1 |
| Intervention | |||||||||
| Surgery | No | No | No | No | Yes | No | No | Yes | 2 |
| Conservative | Yes | Yes | Yes | Yes | No | Yes | Yes | No | 6 |
| Surgical Intervention | N/A | N/A | N/A | N/A | Sacral fixation | N/A | N/A | Hemi | 2 |
| Date of surgery | N/A | N/A | N/A | N/A | 11 March | N/A | N/A | 8 March | N/A |
| COVID-19 onset | |||||||||
| Community | No | No | No | No | No | No | Yes | No | 1 |
| Nosocomial | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 |
| First symptom | Fever | Dyspnoea | Dyspnoea | Fever | Dyspnoea | Cough | Cough | Dyspnoea | N/A |
| Date of swab test | 21 March | 27 March | 25 March | 15 March | 25 March | 11 March | 26 March | 15 March | N/A |
| Complication | ARDS | ARDS | ACS | ARDS | Resp failure | Resp failure | ARDS | Resp failure | N/A |
| Date of death | 20 March | 6 April | 6 March | 20 March | 28 March | 21 March | 1 April | 20 March | N/A |
| HLOS | 8 | 39 | 17 | 20 | 18 | 55 | 7 | 13 | N/A |
-
CVD, cardiovascular disease; HTN, hypertension; MOI, mechanism of injury; UL, upper limb; LL, lower limb; HLOS, hospital length of stay; Hemi, hemiarthroplasty; Resp, respiratory; ACS, acute coronary syndrome; ARDS, acute respiratory distress syndrome
This is, to our knowledge, the first study describing the baseline clinical characteristics and outcomes of major trauma and orthopaedic injured patients with COVID-19 infection. We chose the pivotal timepoint of 5 March 2020 as it represented the day of the first UK death from COVID-19 infection. The first known positive test in the UK was 31 January 20207 and Group A represents the early incubation period in this region. After the first death, measures were undertaken, though not stringent, until the official UK lockdown commenced on 23 March 20208. There was a decrease of 56% in trauma admissions between these two times.
Falls remained the most common MOI, however the percentage of RTAs was significantly higher (p = 0.036). Anecdotal evidence suggested that empty roads were associated with higher speeding and there has been a call to lower speed limits to 20mph in urban areas.9
A contributing factor to decreased tertiary admissions lay in the recommendations from national guidelines to limit referrals to major trauma centres. Novel telemedicine or video conferencing allowed expert specialist opinions to deliver care locally and prevent transfer where appropriate.10
Surgery itself did not seem to be a risk factor for SARS-CoV-2 pneumonia. The incidence of surgery was 40% (n = 263) across 657 patients, with one-third of COVID-19 patients and one-quarter of COVID-19 deaths undergoing surgery. In contrast to this, a study of 34 operative patients in Wuhan during the incubation period of COVID-19 suggests that surgery may have accelerated and exacerbated the disease progression due to patients developing symptoms shortly after surgery.11
There was a significantly higher ICU admission rate in Group A (6.2%) versus Group B (2.1%). These figures may reflect bed pressures. We had our peak of inpatient admissions (310 COVID-19 inpatients) on 2 April and our hospital peaked with 83 ICU patients on 19 April (Figure 2).
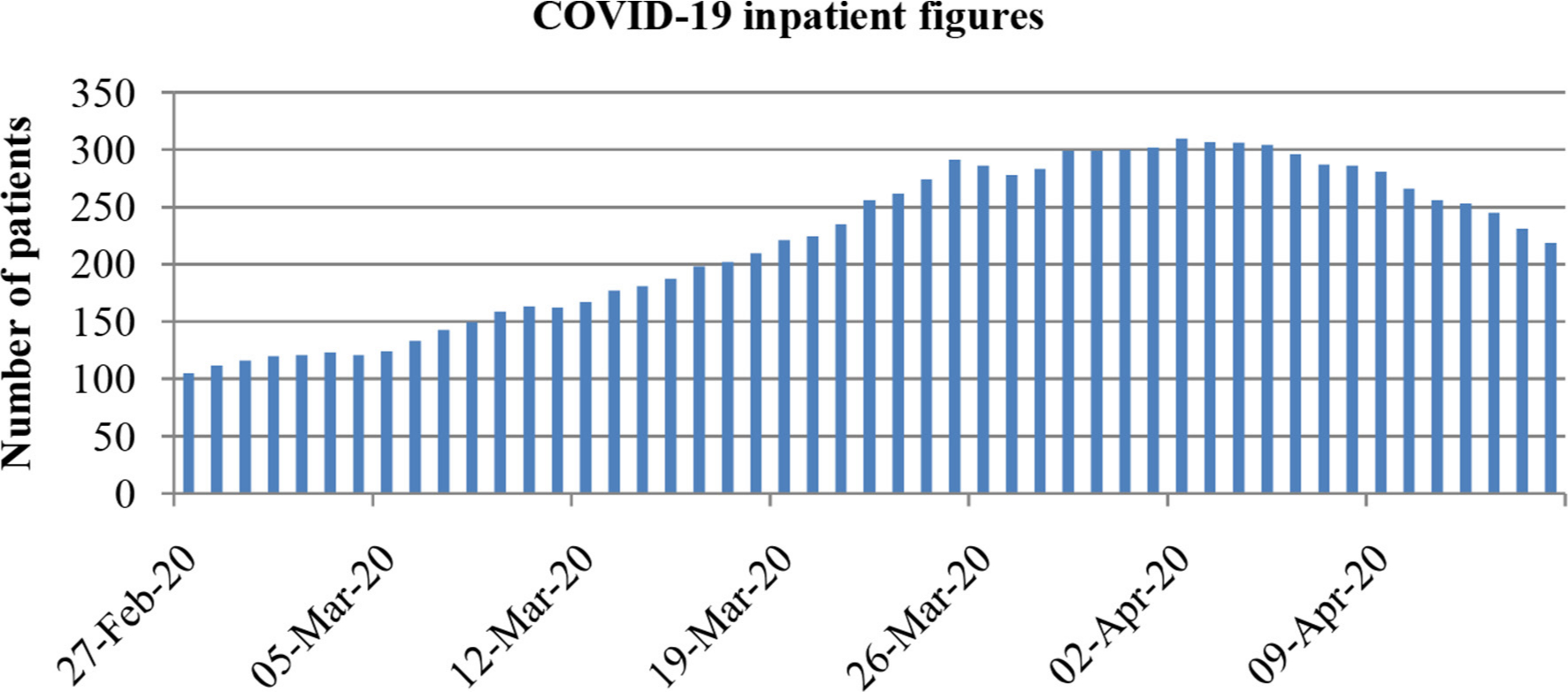
Fig. 2
Total number of COVID-19 positive inpatient figures at our hospital during the period of 26 February to 14 April 2020.
Stringent treatment escalation plans may also have persuaded families and patients to avoid ICU admission which caused nursing staff to issue warnings against blanket DNACPR notices.12
COVID-19 in our cohort had a similar preponderance for sex which correlates to a study in China on 135 patients,13 whereas other reports have quoted males at higher risk.14 The comorbidities in COVID-19 patients compared to noninfected patients were hypertension, cardiovascular disease, dementia, cancer, and arthritis. Arthritis has not previously been reported. Unlike other reports diabetes was not prevalent. While body mass index and ethnicity are noted to be factors influencing outcome in COVID-19, we were not able to include these due to incomplete data.15
Trauma patients with COVID-19 infection were significantly (p < 0.001) older (mean 73.2 years; 28 to 98) and had one or more underlying comorbidities. Jordan et al16 reported that 25% of people are labelled high risk in the UK, including adults aged over 70 years in addition to people with underlying health conditions such as cardiovascular, respiratory disease, and cancer. Based on data in our study, no children were discovered with coronavirus and we show that during this time major trauma was uncommon in children. In support of these findings, reports from the Chinese Centre for Disease Control and Prevention showed that among 44,672 confirmed cases, most patients were 30 to 79 years, 1% were aged nine years or younger, 1% were aged ten to 19 years, and 3% were aged 80 years or older.17
The 39 patients in our study who developed COVID-19 had similar symptoms and presentation to those reported in other studies.11,18
Patients received antibiotics if indicative with the SAR-CoV-2 severe pneumonia. Supportive therapy was the main course of action including non-invasive ventilation (NIV) (25.6%; n = 10) and three patients ventilated (7.7%). There is currently no medication for the treatment of COVID-19 and the therapeutic strategies are only supportive.
There was increased C reactive protein in COVID-19 patients and in addition, the non-survival group had more deranged urea and Hb. White cell, neutrophil, and lymphocyte counts were normal. Biomarkers have been explored in the early stage of COVID-19 to determine the severity. Results showed CRP levels were significantly compared to the severity of illness and greater size of lung lesions. C reactive protein reflects disease severity in the early stages.19 Our data shows a marked increase in later stages associated with severe SAR-CoV-2 pneumonia deaths.
The mortality rate increased between groups in the incubation phase and lockdown phase (3.6% (n = 15) and 6.8% (n = 16)), but this did not reveal statistical significance. However, the mortality of patients infected by COVID-19 was 20.5% compared to non-infected patients of 3.7% which was significantly higher. COVID-19 surgical mortality is as high as 27.3% with thoracic surgery.20 Our surgical mortality in patients with COVID-19 was 15.4% (n = 2) in contrast to Bhangu et al21 who reported on 1,128 patients in 24 countries with a mortality rate of 23.8%, Of note, their mortality rate was composed of 75% emergency and 25% elective surgeries, whereas our data only represents emergencies. In contrast to our COVID-19 surgical mortality, the non-COVID-19 surgical mortality was only 2.8% (n = 7).
ICU admission rate during the lockdown phase was 2.1% (n = 5), which was significantly (p = 0.020) lower than patients during incubation phase (6.2%). Only 5.1% (n = 2) of COVID-19 patients were admitted to ICU. Critical care triage to allow the rationing of scarce ICU resources have been required in some countries.22–24 We know from our hospital data that we were never overwhelmed by the pandemic. Ward inpatients peaked at over 300 and almost 90 ICU admissions, yet our capacity was 420 ventilated beds with a further 100 possible. Therefore, a rise in mortality but decrease in ICU admissions for our cohort of traumatically injured was not an ICU bed capacity/resource issue.
Overall, 39 (5.9%) of patients in our study tested positive for COVID-19 infection. Of these 39 patients, 27 (69.2%) patients acquired their infection during their admission in hospital. Nosocomial infection was to be expected given the contagious infectivity.25 Our figure is higher than the reported literature with a meta-analysis showing a COVID-19 nosocomial infection rate of 44%.26 It is interesting to see a 70% nosocomial infection within the coronavirus cohort, but perhaps not surprising given their risk factors of age, comorbidities, as well as the immunosuppression associated with major trauma.
COVID-19 tests were not initially performed on asymptomatic admissions in our institution nor were healthcare providers routinely screened. Only five (12.8%) had definite positive swabs on admission and positive swabs occurred in an average of 12.3 days (mean 12.3; 0 to 45). In contrast, Wong et al27 showed that with vigilant contact tracing nosocomial transmission may be controlled and was unlikely an airborne disease. COVID-19 virus is primarily transmitted between people through respiratory droplets (> 5 um) and contact routes.28 Airborne transmission may occur if aerosol generating medical procedures (AGMP) transmit particles which may remain airborne longer. Rivett et al29 showed only 3% of asymptomatic healthcare workers tested positive, suggesting our assumed asymptomatic patients on admission were true nosocomial infections. With multiple transmission routes, there are multiple factors to contribute to our high nosocomial infection rate. Recommendations of adequate indoor ventilation to remove virus laden respiratory droplets have been made.30
Our study reveals that eight trauma patients died with COVID-19 compared to 31 patients who survived. We show that patients who died were older (mean 84.4 years) and had multiple comorbidities (cardiovascular disease, hypertension, dementia, diabetes). In one meta-analysis of seven studies it was reported that hypertension, respiratory system disease and cardiovascular disease were risk factors.31 In another study, Lei et al11 showed that hypertension and cardiovascular disease were the most common comorbidities of seven patients that died of COVID-19, in keeping with our study.
The limitations of this study include the small sample size of positive COVID-19 patients. However, this figure is in keeping with international figures, if not higher than from some of the highest incidence centres in the literature.11 Numbers may have decreased at our centre secondary to national guidelines to reduce referrals to major trauma centres; however, suitable cases were always transferred.10 National and local guidelines dictated that only symptomatic patients were tested on admission.32 Lack of PPE in this country did not seem to be a major issue at our hospital although the frequently changing protocols may have contributed. Aerosol and contact transmission may play a role in the hospital environment but are difficult to measure. Statistical relevance must be carefully considered due to the small sample numbers but benefit from the large comparison cohort.
Major trauma will inevitably still occur in a pandemic where lockdown and social distancing have been imposed. While the numbers may decline, major trauma and orthopaedic injuries will account for regular admissions to hospital. This has implications for the spread of COVID-19. Our data shows that the total number of trauma patients with COVID-19 (5.9%; n = 39) was small, but rose from 2.4% (n = 10) in the incubation phase to 12.3% (n = 29) during the lockdown phase. It affects equal preponderance of males to females and the older age group (over 70 years). Their most common injuries were pelvic, chest and spine caused mainly by falls but also from an increased incidence of RTA. One-third were treated surgically. Our study confirms that symptoms mainly included dyspnoea, dry cough, and pyrexia. Common comorbidities included hypertension, CVD, dementia, arthritis, and malignancy, but not diabetes. Trauma patients with COVID-19 have a high incidence of nosocomial infection (69.2%; n = 27) but also a high mortality rate (20.5%) in octogenarians. Our surgical COVID-19 mortality was 15.3%, moderately lower in comparison to other reports, but five-times greater than our non-COVID-19 surgical mortality, underlining the risk of COVID-19 infection with surgery.
References
1. Sohrabi C , Alsafi Z , O'Neill N , et al. World Health organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) . Int J Surg . 2020 ; 76 : 71 – 76 . Crossref PubMed Google Scholar
2. Mahase E . Covid-19: UK records first death, as world’s cases exceed 100 000 . BMJ . 2020 : m943 . Google Scholar
3. Strengthening the health system response to COVID-19 . World Health Organization . 2020 . https://www.euro.who.int/__data/assets/pdf_file/0007/436354/strengthening-health-systems-response-COVID-19-technical-guidance-1.pdf (date last accessed 1 July 2020 ). Google Scholar
4. Ashford RU , Nichols JS , Mangwani J . Annotation: The COVID-19 pandemic and clinical orthopaedic and trauma surgery. . J Clin Orthop Trauma. 2 . 020 . Crossref PubMed Google Scholar
5. No authors listed . Management of patients with urgent orthopaedic conditions and trauma during the coronavirus pandemic [Internet] . 2020 . https://www.england.nhs.uk/coronavirus/publication/specialty-guides/ (date last accessed 12 May 2020 ). Google Scholar
6. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies . EQUATOR network . https://www.equator-network.org/reporting-guidelines/strobe/ (date last accessed 1 July 2020 ). Crossref PubMed Google Scholar
7. Lillie PJ , Samson A , Li A , et al. Novel coronavirus disease (Covid-19): the first two patients in the UK with person to person transmission . J Infect . 2020 ; 80 ( 5 ): 578 – 606 . Crossref PubMed Google Scholar
8. No authors listed . UK lockdown is “crucial” to saving lives, say doctors and scientists [Internet]. [cited 2020 May 9]. . Bmj . https://blogs.bmj.com/bmj/2020/03/24/can-we-improve-the-nhss-ability-to-tackle-covid-19-through-emergency-public-health-interventions/ (date last accessed 18 June 2020 ). Google Scholar
9. No authors listed . Can we improve the NHS’s ability to tackle covid-19 through emergency public health interventions? . BMJ . 2020 . https://blogs.bmj.com/bmj/2020/03/24/can-we-improve-the-nhss-ability-to-tackle-covid-19-through-emergency-public-health-interventions/ (date last accessed 18 June 2020 ). Google Scholar
10. Mauffrey C , Trompeter A . Lead the way or leave the way: leading a department of Orthopedics through the COVID-19 pandemic . Eur J Orthop Surg Traumatol . 2020 ; 30 ( 4 ): 555 – 557 . Google Scholar
11. Lei S , Jiang F , Su W , et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection . EClinicalMedicine . 2020 . Crossref PubMed Google Scholar
12. No authors listed . Warning against “blanket” DNACPR notices . RCNi . 2002 . https://rcni.com/nursing-standard/newsroom/news/covid-19-warning-against-blanket-dnacpr-notices-160096 (date last accessed 9 May 2020 ). Google Scholar
13. Wan S , Xiang Y , Fang W , et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing . J Med Virol . 2020 ; 92 ( 7 ): 797 . Crossref PubMed Google Scholar
14. Jin J-M , Bai P , He W , et al. Gender differences in patients with COVID-19: Focus on severity and mortality . Front Public Health . 2020 ; 8 : 152 . Crossref PubMed Google Scholar
15. Chakravorty I , Daga S , Dave S , et al. An online survey of healthcare professionals in the COVID-19 . Pandemic in the UK : Sushruta J Heal Policy Opin , 2020 . Google Scholar
16. Jordan RE , Adab P , Cheng KK . Covid-19: risk factors for severe disease and death . BMJ . 2020 ; 368 : m1198 . Crossref PubMed Google Scholar
17. Wu Z , McGoogan JM . Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China . JAMA . 2020 ; 323 ( 13 ): 1239 . Crossref PubMed Google Scholar
18. Huang C , Wang Y , Li X , et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China . Lancet . 2020 . Crossref PubMed Google Scholar
19. Wang L . C-Reactive protein levels in the early stage of COVID-19 . Med Mal Infect . 2020 ; 50 ( 4 ): 332 – 334 . Crossref PubMed Google Scholar
20. Zhang Y , Chen H . Commentary: challenges to thoracic surgeons in the global coronavirus pandemic . J Thorac Cardiovasc Surg . 2020 . Crossref PubMed Google Scholar
21. Bhangu A , COVIDSurg Collaborative . Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study . Lancet . 2020 . Crossref PubMed Google Scholar
22. Phua J , Weng L , Ling L , et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations . Lancet Respir Med . 2020 ; 8 ( 5 ): 506 – 517 . Crossref PubMed Google Scholar
23. Li R , Rivers C , Tan Q , et al. The demand for inpatient and ICU beds for COVID-19 in the US: lessons from Chinese cities . medRxiv . 2020 : 2020.03.09.20033241 . Crossref PubMed Google Scholar
24. Branas CC , Rundle A , Pei S , et al. Flattening the curve before it flattens us: Hospital critical care capacity limits and mortality from novel coronavirus (SARS-CoV2) cases in US counties . medRxiv . 2020 . Google Scholar
25. Liu D . Reflection on the work of nosocomial infection management during the coronavirus disease (COVID-19) outbreak . Chongqing Med . 2020 . Google Scholar
26. Zhou Q , Gao Y , Wang X , et al. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis . medRxiv . 2020 . Crossref PubMed Google Scholar
27. Wong SCY , Kwong RTS , TC W , et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong . J Hosp Infect . 2020 . Crossref PubMed Google Scholar
28. No authors listed . World Health Organization W. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations . World Health organization . 2020 . https://apps.who.int/iris/bitstream/handle/10665/331601/WHO-2019-nCoV-Sci_Brief-Transmission_modes-2020.1-eng.pdf (date last accessed 18 June 2020 ). Google Scholar
29. Rivett L , Sridhar S , Sparkes D , et al. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission . Elife . 2020 ; 9 . Crossref PubMed Google Scholar
30. Morawska L , Cao J . Airborne transmission of SARS-CoV-2: the world should face the reality . Environ Int . 2020 ; 139 : 105730 . Crossref PubMed Google Scholar
31. Yang J , Zheng Y , Gou X , et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: A systematic review and meta-analysis . Int J Infect Dis . 2020 . Google Scholar
32. No authors listed . Interim Guidance: Healthcare Professionals 2019-nCoV . Cdc . 2020 . https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html (date last accessed 9 May 2020 ). Google Scholar
Author contributions
B. Ajayi: Collected the data, Searched the literature, Analyzed the data, Created the figures and tables, Wrote the manuscript.
Designed the study, Analysed the data, Edited the manuscript.
Designed the study, Edited the manuscript.
Performed the statistical analysis.
Designed the study, Analyzed and interpreted the data, Edited the manuscript.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
A. Trompeter reports consultancy from Stryker, expert testimony for medicolegal work, payment for lectures (including service on speakers bureaus) and development of educational presentations from Stryker, Smith & Nephew, and Orthofix, royalties from Oxford University Press and JP Medical, all of which are unrelated to this article. D. F. Lui reports consultancy from Stryker and Zimmer Biomet, which are unrelated to this article.
Follow B. Ajayi @Bee_Aj1
Follow D. F. Lui @Darren_Lui
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attributions licence (CC-BY-NC-ND), which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.









