Abstract
Aims
To analyze outcomes reported in trials of childhood fractures.
Methods
OVID MEDLINE, Embase, and Cochrane CENTRAL databases were searched on the eighth August 2019. A manual search of trial registries, bibliographic review and internet search was used to identify additional studies. 11,476 studies were screened following PRISMA guidelines. 100 trials were included in the analysis. Data extraction was completed by two researchers for each trial. Study quality was not evaluated. Outcomes reported by trials were mapped onto domains in the World Health Organization (WHO) International Classification of Function framework.
Results
In all, 525 outcomes were identified representing 52 WHO domains. Four domains were reported in more than 50% of trials: structure of upper/lower limb, sensation of pain, mobility of joint function, and health services, systems and policies. The Activities Scale for Kids performance (ASK-p) score was the most common outcome score reported in 6/72 upper limb and 4/28 lower limb trials.
Conclusion
There is a diverse range of outcomes reported in trials of childhood fractures covering all areas in the International Classification of Functioning, Disability and Health (ICF) framework. There were three common upper limb and three common lower limb outcomes. In the absence of a core outcome set, we recommend that upper limb trials report pain, range of movement and radiograph appearance of the arm and lower limb trials report pain, radiograph appearance of the leg and healthcare costs to improve consistency of reporting in future trials.
Cite this article: Bone Joint Open 2020;1-5:167–174.
Strengths and limitations
-
Selection of outcomes is vital for the delivery of impactful research studies that are relevant to all stakeholders and can be combined in meaningful meta-analysis.
-
Sensation of pain, mobility of joints and structure of limb (i.e. appearance on rayradiograph) are measured in more than 40% of upper limb trials.
-
Sensation of pain, structure of limb (i.e. appearance on rayradiograph) and impact on health services, systems and policies are measured in more than 40% of lower limb trials.
-
Researchers designing trials should consider measuring the structure of upper limb, mobility of joint functions and sensation of pain in upper limb trials and structure of lower limb, sensation of pain and impact on health services, systems and policies to maximize consistency in reporting and reduce research waste.
Introduction
A third of children will sustain a fracture by their 17th birthday.1,2 Not all children appear to fully recovery after their injury, with up to 9% of children with mild injuries and 15% of children with moderate injuries achieving less than full recovery.3 Measuring recovery is greatly hindered by a lack of understanding as to the expected eventual outcome or agreed outcomes to measure to compare treatments. There is uncertainty surrounding this failure to recover and the required interventional studies are hampered by a lack of agreed outcomes to measure.
Core Outcome Sets (COSs) have been developed for several musculoskeletal conditions,4–6 but not for childhood fractures. A COS provides a minimum requirement for trials relating to childhood fractures to include measurement of certain broad outcome domains (e.g. pain or function). At present, there is not an agreed set of outcomes that should be measured in trials relating to these injuries. Trialists and methodologists therefore must select outcomes to measure based on local feedback and their personal experience. Heterogeneity in outcomes that are measured and reported not only represent research wastage through a loss of opportunity to conduct meta-analysis but also risk inappropriate trial design and the reporting of outcomes with limited value.7
In the absence of a COS, we have applied principles from the COMET initiative to identify the outcomes reported in trials of childhood fractures.4 These outcomes are analyzed by grouping into outcome domains using an internationally recognized framework5 and also through evaluation of outcome tools that have been used to capture patient or surgeon reported outcomes.
Methods
This study followed COMET initiative methodology and PRISMA guidelines to identify, classify, and report outcomes reported in clinical trials.4,6 The systematic review was prospectively registered on the PROSPERO database CRD420181066058 and the study protocol has been published.9 English language randomized and quasi-randomized controlled trials were included with no date restriction. Trial participants were children with fractures of any bone in the appendicular skeleton (i.e. excluding skull, spine, and ribs).
Electronic databases were searched on 8 August 2019. Studies were identified from the OVID MEDLINE, OVID Embase, and the Cochrane Central Register of Controlled Trial (CENTRAL) databases using the search strategy included in supplementary material. The search was devised to identify fractures and included the Cochrane search filter for child health studies10 and randomized trials.11 Additional studies were identified through review of the reference lists of relevant systematic reviews and included trials and through text search of Google Scholar. A manual search of clinicaltrials.gov, the ISRCTN registry, and World Health Organization (WHO) ICTRP registry was completed on the 28 January 2019 in line with Cochrane guidelines to identify relevant unpublished studies and protocols.12
Titles and abstracts were sequentially screened by one researcher to identify potential full text articles. Data extraction and classification into second level WHO International Classification of Functioning, Disability and Health (ICF) domains was performed independently by two researchers and any discrepancies resolved through consensus. Classification of outcomes from patient reported outcomes into second level WHO ICF domains was completed by two researchers through consensus.
An assessment of study quality and risk of bias was not completed as part of this review as it was determined not to be relevant. The objective of this review is to identify all previously reported outcomes, regardless of trial quality.
Heterogeneity was evaluated empirically through injuries included and by definitions of child age that were included in each trial. Due to the purpose and aims of this systematic review a meta-analysis was not appropriate. Instead, reported outcomes were integrated using a narrative synthesis approach and calculation of frequency of outcomes reported. A subgroup analysis for upper and lower limb injuries was completed.
The WHO's ICF framework was selected as a robust framework for analysis to map outcomes reported into descriptive domains. This framework is the international standard for measuring outcomes. The framework includes four constructs: body functions (b); activity and participation (d); body structure (s); and environmental factors (e). Constructs are further subdivided into Chapters second level, third level, and fourth level domains to describe impairment or disability.5
Results
The PRISMA flow diagram is shown in Figure 1. Searches yielded 11,476 candidate articles that were reduced to 9,763 on removal of duplicates. 176 full text articles and 297 trial registrations were reviewed. Outcomes were extracted by two independent researchers from 100 included trials.
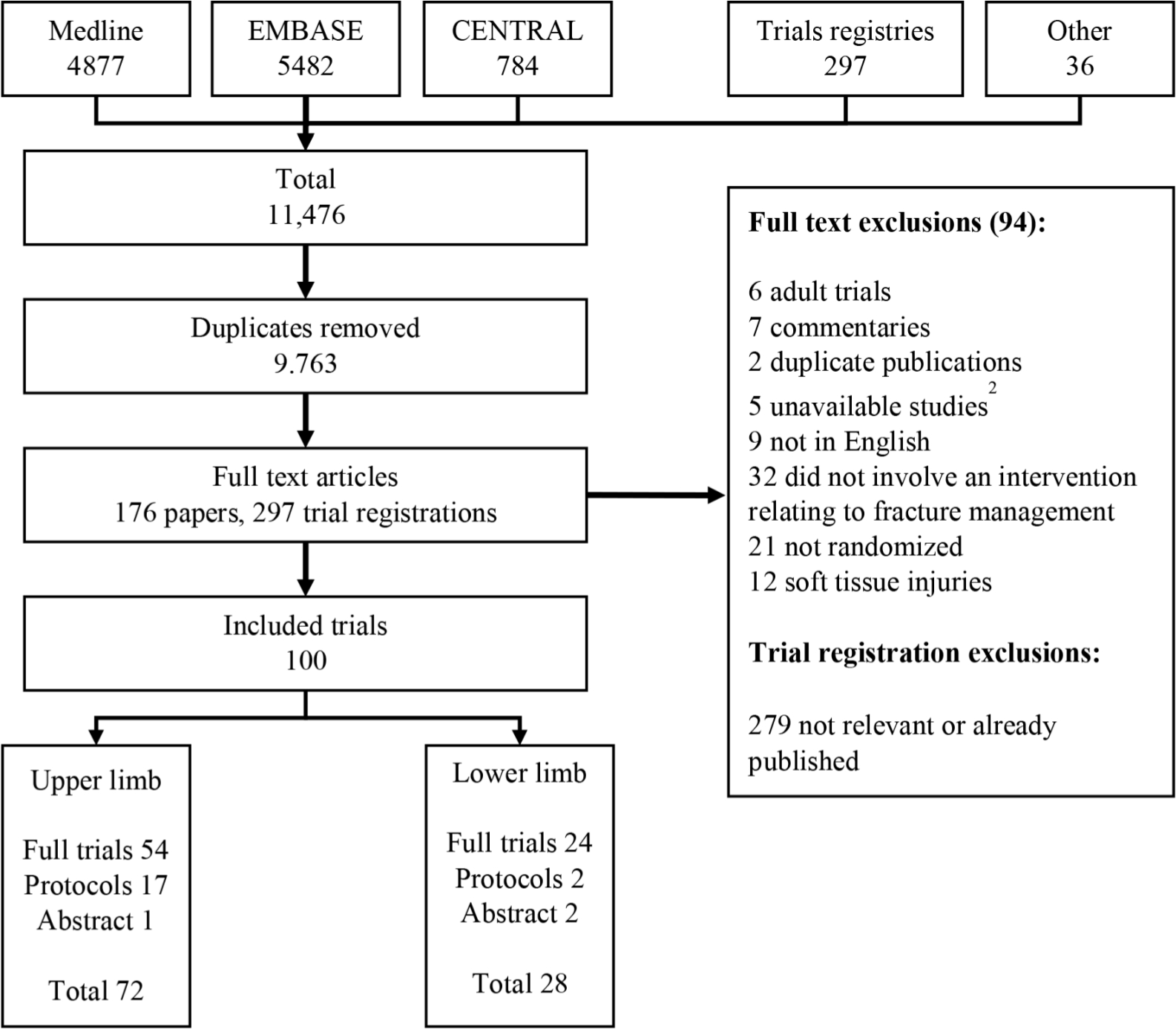
Fig. 1
PRISMA flow diagram. 1: protocols included 1 published protocol and 18 trial registration entries. 2: unavailable studies included 3 unpublished abstracts and 2 trials not available from the British Library or contacting the journal editor.
Trials included in the analysis composed of 68 randomized trials, 10 quasi-randomized trials and 22 unpublished trials (trial registrations, protocols or published in abstract form only). There were 72 trials relating to upper limb fractures, 28 trials relating to lower limb fractures. Study characteristics and references are shown in supplementary materials.
525 distinct outcomes were reported. The median number of outcomes reported per trial was four (interquartile range (IQR) 3 to 6). These outcomes mapped onto 52 second level outcome domains across all four components of the ICF framework.5 Overall, 18 body function (b), 26 activity and participation (d), two environmental (e), and six body structural (s) level domains were identified. Complications were reported in 49 upper limb and 20 lower limb trials and were mapped onto the ICF framework.
The distribution of ICF framework outcome domains for upper and lower limb trials is shown in Tables I and II. The most common ICF framework domains reported in upper limb trials of children were structure of upper limb (s730, 74.6%), mobility of joint (b710, 52.1%) and sensation of pain (b280, 46.5%). The most common domains reported in lower limb trials in children were structure of lower limb (s750, 92.9%), sensation of pain (b280, 57.1%), and health services, systems and policies (e580, 57.1%).
Table I.
Distribution of outcomes reported in trials of upper limb childhood fractures mapped onto ICF outcome domains.
| ICF outcome domain | Upper limb trials, n (%) | ICF outcome domain | Upper limb trials, n (%) |
|---|---|---|---|
| Body function | Activities and participation | ||
| b710 Mobility of joint functions | 37 (51.3) | d510 Washing oneself | 18 (25.0) |
| b280 Sensation of pain | 33 (45.8) | d540 Dressing | 14 (19.4) |
| b435 Immunological system functions | 18 (25.0) | d920 Recreation and leisure | 14 (19.4) |
| b180 Experience of self and time functions | 14 (19.4) | d430 Lifting and carrying objects | 13 (18.1) |
| b789 Movement function [immobilization) | 10 (13.9) | d550 Eating | 12 (16.7) |
| b299 Sensory functions and pain, other | 8 (11.1) | d299 General tasks and demands, other | 9 (12.5) |
| b730 Muscle power functions | 6 (8.3) | d170 Writing | 8 (11.1) |
| b152 Emotional functions | 3 (4.2) | d520 Caring for body parts | 8 (11.1) |
| b729 Function of the joints and bones, other (growth/heterotopic ossification) | 3 (4.2) | d820 School education | 8 (11.1) |
| d560 Drinking | 7 (9.7) | ||
| b715 Stability of joint function | 3 (4.2) | d530 Toileting | 7 (9.7) |
| b840 Sensation relating to skin | 3 (4.2) | d445 Hand and arm use | 7 (9.7) |
| b134 Sleep functions | 2 (2.8) | d640 Doing housework | 7 (9.7) |
| b430 Haematological system functions | 1 (1.4) | d850 Remunerative employment | 6 (8.3) |
| b820 Repair functions of the skin | 1 (1.4) | d420 Transferring oneself | 6 (8.3) |
| Body structure | d570 Looking after one's health | 6 (8.3) | |
| s730 Structure of upper limb | 54 (75.0) | d440 Fine hand use | 6 (8.3) |
| s810 Structure of areas of the skin | 21 (29.2) | d230 Carrying out daily routine | 4 (5.6) |
| s199 Structure of the nervous system | 20 (27.8) | d450 Walking | 3 (4.2) |
| s770 Additional musculoskeletal structures | 9 (12.5) | d410 Changing basic body position | 2 (2.8) |
| s410 Structure of cardiovascular system | 6 (8.3) | d455 Moving around | 1 (1.4) |
| Environmental factors | |||
| e580 Health services, systems and policies | 24 (33.3) | ||
| e240 Light (radiation dose) | 2 (2.8) |
Table II.
Distribution of outcomes reported in trials of lower limb childhood fractures mapped onto ICF outcome domains.
| ICF outcome domain | Lower limb trials, n (%) | ICF outcome domain | Lower limb trials, n (%) |
|---|---|---|---|
| Body function | Activities and participation | ||
| b280 Sensation of pain | 16 (57.1) | d450 Walking | 10 (35.7) |
| b710 Mobility of joint functions | 11 (39.3) | d850 Remunerative employment | 7 (25.0) |
| b435 Immunological system functions | 11 (39.3) | d540 Dressing | 6 (21.4) |
| b789 Movement function (immobilization] | 3 (10.7) | d520 Caring for body parts | 6 (21.4) |
| b730 Muscle power functions | 3 (10.7) | d430 Lifting and carrying objects | 5 (17.9) |
| b152 Emotional functions | 2 (7.1) | d420 Transferring oneself | 5 (17.9) |
| b134 Sleep functions | 2 (7.1) | d470 Moving around using transportation | 5 (17.9) |
| b430 Haematological system functions | 2 (7.1) | d550 Eating | 4 (14.3) |
| b729 Function of the joints and bones, other (heterotopic ossification) | 1 (3.6) | d170 Writing | 4 (14.3) |
| d560 Drinking | 4 (14.3) | ||
| b130 Energy and drive function | 1 (3.6) | d570 Looking after one's health | 4 (14.3) |
| b140 Attention function | 1 (3.6) | d760 Family relationships | 4 (14.3) |
| b164 Higher-level cognitive functions | 1 (3.6) | d299 General tasks and demands, other | 3 (10.7) |
| b770 Gait pattern function | 1 (3.6) | d820 School education | 3 (10.7) |
| Body Structure | d530 Toileting | 2 (7.1) | |
| s750 Structure of lower limb | 26 (92.9) | d445 Hand and arm use | 1 (3.6) |
| s810 Structure of areas of the skin | 3 (10.7) | d640 Doing housework | 1 (3.6) |
| s199 Structure of the nervous system | 1 (3.6) | d230 Carrying out daily routine | 1 (3.6) |
| s770 Additional musculoskeletal structures | 1 (3.6) | d455 Moving around | 1 (3.6) |
| Environmental factors | d360 Conversation | 1 (3.6) | |
| e580 Health services, systems and policies | 16 (57.1) | d460 Moving around in different locations | 1 (3.6) |
| e240 Light (radiation dose) | 2 (7.1) | d750 Informal social relationships | 1 (3.6) |
Patient or parent satisfaction were reported in 19 (26.8%) upper limb trials and six (21.4%) lower limb trials. Satisfaction was most frequently measured using a visual analogue score (VAS) (eight trials). A ten-point VAS was used in four trials and a four-point scale used in two trials. Seven trials reported a binary “yes” or “no” response. Satisfaction could not be mapped onto the current ICF framework, and may represent personal factors which do not currently have an agreed classification within the ICF.13
The distribution of outcomes domains reported as the primary outcome is shown in Table III. In all, 63 (88.7%) upper limb trials identified a primary outcome. The most common primary outcome for upper limb trials was structure of upper limb (angulation) (s730, 25.6%). This was typically measured radiologically. 17 (70.8%) lower limb trials identified a primary outcome. In lower limb trials, the most common primary outcome was structure of upper or lower limb (union) (s750, 29.2%) which was also usually measured radiologically.
Table III.
Distribution of outcome domains reported as primary outcome. ASK-P* includes modifications of the ASK-P score.
| Primary outcome domain or score | Upper limb trials, n (%) |
|---|---|
| s730 Structure of upper limb [angulation] | 18 (25.6) |
| b710 Mobility of joint function | 10 (14.1) |
| b280 sensation of pain | 8 (11.3) |
| Parent or patient satisfaction | 7 (9.9) |
| Functional outcome score: ASK-p* score | 5 (7.0) |
| b435 Immunological system functions | 3 (4.2) |
| e580 Health services, systems and policies | 3 (4.2) |
| s810 Structure of areas of skin [pressure damage] | 2 (2.8) |
| Functional outcome score: Flynn-s | 2 (2.8) |
| b430 Haematological system functions | 1 (1.4) |
| s730 Structure of upper limb [union] | 1 (1.4) |
| Functional outcome score: CHAQ Score | 1 (1.4) |
| Functional outcome score: Mayo score | 1 (1.4) |
| Composite of other domains | 1 (1.4) |
| Primary outcome not stated | 8 (11.3) |
| Primary outcome domain or score | Lower limb trials, n (%) |
| s750 Structure of lower limb (union) | 7 (29.2) |
| e580 Health services, systems and policies | 4 (16.7) |
| Composite of other domains | 3 (12.5) |
| s730 Structure of upper limb (angulation) | 2 (8.3) |
| Functional outcome score: ASK-p* core | 2 (8.3) |
| d450 Walking | 1 (4.2) |
| Functional outcome score: C&C score | 1 (4.2) |
| Functional outcome score: Flynn-f | 1 (4.2) |
| Primary outcome not stated | 7 (29.2) |
Patient or surgeon reported outcome instruments were reported in 28 (39.4%) upper limb trials and 16 (66.7%) lower limb trials. Characteristics of outcome instruments reported are shown in Table IV. The most frequently reported instrument is the Flynn score for supracondylar elbow fractures. This elbow-specific score is calculated by a clinician and includes carrying angle and range of movement of the elbow. The ASK-p14 and ABILHAND-kids15 scores were reported in six (8.5%) and five (7.0%) trials respectively.
Table IV.
Patient and surgeon reported outcome scores reported in trials of childhood fractures. ‡RAND score has five items for children aged 0 to four, and 13 for children aged five to 13. Instruments may be patient- or proxy-reported (PROM) or require input from a clinician/surgeon (SROM) to complete.
| Outcome instrument | Type of tool | Number of trials | Items | Original target condition | Original construct |
|---|---|---|---|---|---|
| Upper limb | |||||
| Flynn (supracondylar fracture)16 | SROM | 8 | 2 | Elbow fractures | Physical function |
| ASK-p17 | PROM | 6 | 30 | Physical disability | Physical function |
| ABILHAND-Kids15 | PROM | 5 | 21 | Cerebral palsy | Physical function |
| EQ-5D-Y18 | PROM | 2 | 6 | All children | Quality of life |
| Price19 | SROM | 2 | 2 | Forearm fractures | Physical function |
| PROMIS Upper Extremity20 | PROM | 1 | 29 | All children | Physical function |
| Mayo21 | SROM | 1 | 4 | Elbow arthroplasty | Physical function |
| Constant22 | SROM | 1 | 5 | Shoulder function | Physical function |
| Upper limb functional index23 | PROM | 1 | 20 | Upper limb | Physical function |
| PODCI24 | PROM | 1 | 83 | Functional health | Quality of life |
| Paediatric disability score25 | SROM | 1 | 5 | Wrist fractures | Physical function |
| CHAQ26 | PROM | 1 | 36 | JRA | Physical function |
| Lower limb | |||||
| Flynn (femur fracture)27 | SROM | 9 | 4 | Femoral fractures | Physical function |
| ASK-p17 | PROM | 4 | 30 | Physical disability | Physical function |
| Care and comfort28 | PROM | 1 | 27 | Cerebral palsy | Physical function |
| Post-Hospitalization Behaviour Questionnaire29 | PROM | 1 | 27 | Child behaviour | Emotional function |
| RAND30 | PROM | 1 | ‡5/13 | Physical health | Physical function |
| AOFAS31 | SROM | 1 | 9 | Ankle surgery | Physical function alignment and pain |
Five outcome instruments were identified that had been reported in lower limb trials. The most common instrument was the Flynn criteria for femoral fractures which is a clinician reported outcome comprising of limb length discrepancy, malalignment, pain and presence of complications.27 The ASK-p14 was reported in four (16.7%) of lower limb trials.
Discussion
This systematic review has demonstrated that a wide range of outcomes covering all components of the WHO ICF have been reported in trials of childhood fractures. There is some limited homogeneity in reporting between trials. In the upper limb, sensation of pain, mobility of joints and structure of upper limb (radiograph appearance) were reported in more than 40% of trials. In the lower limb, the impact on health services, systems and policies (i.e. healthcare cost or length of stay), sensation of pain and structure of lower limb (radiograph appearance) were reported in more than 40% of trials. There was much more heterogeneity in the reporting of activity and participation outcomes, with 26 participation domains reported.
The most common outcome reported in trials was the radiological evaluation of bone structure. This included angulation, loss of reduction and union. These parameters may be measured using radiograph images but the impact of a radiological change on function is unclear. Only three trials have reported follow-up longer than one year which is significant as children have the capacity to remodel some of the residual deformity left following treatment as they continue to grow. Radiological changes are therefore often dynamic and dependent on the follow-up duration.32,33
Outcomes in upper limb trials were recorded using seven patient or proxy reported outcome instruments and five clinician reported outcome instruments. Lower limb trials have reported outcomes using six instruments including four patient- or proxy-reported outcome instruments and two clinician reported outcomes.
Four of these scores (AOFAS, Mayo, Constant, and Upper Limb Functional Index) were designed for adult patients and have been used without modification in the paediatric population.21,23,34 Of the remaining patient- or proxy-reported outcome instruments, none were developed specifically for childhood fractures or injuries and further study is required to identify the measurement properties of these instruments. A systematic review to identify the measurement properties for these PROMs has been registered on the PROSPERO database.35
The paediatric PROMIS upper limb score has been validated in a cohort of 964 children with upper limb fractures to evaluate correlation with PROMIS mobility, pain interference and peer relationship scores, and floor and ceiling effects.36 The PROMIS upper limb score correlates with mobility and pain. However, 8.3% of children reporting a ceiling effect with maximum scores at presentation with their acute fractures.
Construct validity for the Paediatric Outcomes Data Collection Instrument (PODCI) and ASK-p score has been analyzed in a cohort of 166 children including 35 (21%) with fractures.37 Celling effects were found in 10% and 14% children completing PODCI and ASK-p with moderate to good correlation with a seven-point global rating completed by parents.
Previous attempts to evaluate the reporting of outcomes in elective and emergency paediatric orthopaedics have focused on patient reported outcomes from studies from six journals. Two studies have been completed, both including studies from the wider orthopaedic literature and not specific to fractures. These studies found that 2.7% to 11.5% of all studies reported a patient reported outcome instrument, with the PODCI and Scoliosis Research Society scores being the most common reported outcome instruments.38,39
When the patient reported outcome instruments were mapped onto the ICF framework, four tools evaluated ten or more second-level domains. The Care and Comfort (C&C) questionnaire and PODCI score cover ten domains across body function and participation. The ASK-p score covers 11 ICF participation domains and is the most frequently reported outcome in trials.
This systematic review has some limitations. The objective was to identify as many reported outcomes from the international literature as possible. Unfortunately, we had to exclude non-English articles due to lack of translating resources. Trials included in this review were identified from 27 different countries from Europe, America, Asia, Africa, and Australasia and from high- and low-income countries. There was an underrepresentation of trials for hand, clavicle, and foot fractures which contribute to 18%, 8% and 8% of childhood fractures respectively. Equally, there was an over-representation of trials for femoral shaft fractures, which contributes to 2% of childhood fractures.1 This may in part be explained as femoral fractures are a top ten research priority due the ongoing uncertainties in interventions.40 Upper limb fractures contribute to approximately 70% of the fracture burden in children which is comparable to the 71% of trials relating to upper limb fractures identified in this review.
An additional limitation of this study is the restriction of this review to randomized controlled trials. This methodology is consistent with reviews of outcomes in other fields,41-43 but does risk omission of important outcomes that have been reported exclusively in case-controlled or cohort studies. The list of ICF outcome domains identified in this study are the outcomes that have been reported by orthopaedic trialists but will require supplementation by further study with other stakeholders including families and clinicians to develop into a comprehensive list of important outcomes following childhood fractures.4
At the design phase, it was decided not to include any evaluation of study quality in the protocol for this review.8 This is because the lack of study quality evaluation does not change the identification and assessment of outcomes reported in previous trials.
While this review has identified four common outcome domains, there is an urgent need to evaluate the other outcomes and understand which are most important for inclusion in future trials. A COS with input from relevant stakeholders (parents, children, clinicians and triallists) would be valuable for the design of future trials and promote more coherent and consistent reporting of outcomes. This would reduce the heterogeneity of outcome reporting and permit more reliable meta-analysis in the future to improve the quality of care for children with these injuries.
In the absence of a core outcome set, the results from this study would suggest that researchers designing future trials should consider measuring the structure of upper limb, mobility of joint functions and sensation of pain in upper limb trials and structure of lower limb, sensation of pain and impact on health services, systems, and policies to maximize consistency in reporting and reduce research waste.
The further identification and evaluation of validated patient reported outcomes for this patient group needs to be performed in a separate review using COnsensus-based Standards for the selection of health Measurement Instruments (COSMIN) guidelines.44 This is urgently required before any recommendations regarding selection of outcome instruments for use in future research can be made.
References
1. Cooper C , Dennison EM , Leufkens HGM , Bishop N , van Staa TP . Epidemiology of childhood fractures in Britain: a study using the general practice research database . J Bone Miner Res . 2004 ; 19 ( 12 ): 1976 – 1981 . Crossref PubMed Google Scholar
2. Lyons RA , Delahunty AM , Kraus D , et al. Children's fractures: a population based study . Inj Prev . 1999 ; 5 ( 2 ): 129 – 132 . Crossref PubMed Google Scholar
3. Kendrick D , Vinogradova Y , Coupland C , et al. Recovery from injury: the UK burden of injury multicentre longitudinal study . Inj Prev . 2007 ; 2013 ( 19 ): 370 – 381 . Crossref PubMed Google Scholar
4. Williamson PR , Altman DG , Bagley H , et al. The comet Handbook: version 1.0 . Trials . 2017 ; 18 ( Suppl 3 ): 280 . Crossref PubMed Google Scholar
5. WHO . International Classification of Functioning, Disability and Health (ICF). WHO . http://www.who.int/classifications/icf/en/ (date last accessed 5 September 2018 ). Google Scholar
6. Moher D , Liberati A , Tetzlaff J , Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement . PLoS Med . 2009 ; 6 ( 7 ): e1000097 . Crossref PubMed Google Scholar
7. Gargon E , Gorst SL , Harman NL , et al. Choosing important health outcomes for comparative effectiveness research: 4th annual update to a systematic review of core outcome sets for research . PLoS One . 2018 ; 13 ( 12 ): e0209869 . Crossref PubMed Google Scholar
8. Marson BA , Deshmukh SR , Grindlay D , Ollivere B . A systematic review of outcomes measured in trials of treatments for fractures in children. PROSPERO 2018: CRD42018106605. PROSPERO . http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018106605 (date last accessed 3 October 2018 ). Google Scholar
9. Marson BA , Manning JC , James M , et al. CORE-Kids: a protocol for the development of a core outcome set for childhood fractures . BMJ Open . In Press . 2020 ; 10 ( 2 ): e036224 . Crossref PubMed Google Scholar
10. Leclercq E , Leeflang MMG , van Dalen EC , Kremer LCM . Validation of search filters for identifying pediatric studies in PubMed . J Pediatr . 2013 ; 162 ( 3 ): 629 – 634 . Crossref PubMed Google Scholar
11. Cochrane collaboration . RCT filters for different databases . https://work.cochrane.org/rct-filters-different-databases Google Scholar
12. Lefebvre C , Glanville J , Briscoe S , Higgins J , et al. Chapter 4: Searching for and selecting studies | Cochrane Training . In : Higgins J , Thomas J , Chandler J , Page M . Cochrane Handb. Syst. rev. Interv. 6.0 . Oxford : Cochrane , 2019 . Google Scholar
13. Üstün TB . The ICF: an overview introducing the ICF . Geneva , 2010 . Google Scholar
14. Young NL , Williams JI , Yoshida KK , Wright JG . Measurement properties of the activities scale for kids . J Clin Epidemiol . 2000 ; 53 ( 2 ): 125 – 137 . Crossref PubMed Google Scholar
15. Arnould C , Penta M , Renders A , Thonnard J-L . ABILHAND-Kids: a measure of manual ability in children with cerebral palsy . Neurology . 2004 ; 63 ( 6 ): 1045 – 1052 . Crossref PubMed Google Scholar
16. Flynn JC , Matthews JG , Benoit RL . Blind pinning of displaced supracondylar fractures of the humerus in children. Sixteen years’ experience with long-term follow-up . J Bone Jt Surg - Ser A . 1974 ; 56 ( 2 ): 263 – 272 . Google Scholar
17. Young NL , Yoshida KK , Williams JI , Bombardier C , Wright JG . The Role of Children in Reporting Their Physical Disability , 1995 . Google Scholar
18. Wille N , Badia X , Bonsel G , et al. Development of the EQ-5D-Y: a child-friendly version of the EQ-5D . Qual Life Res . 2010 ; 19 ( 6 ): 875 – 886 . Crossref PubMed Google Scholar
19. Price CT , Scott DS , Kurzner ME , Flynn JC . Malunited forearm fractures in children . J Pediatr Orthop . 1990 ; 10 ( 6 ): 705 – 712 . Crossref PubMed Google Scholar
20. Irwin DE , Gross HE , Stucky BD , et al. Development of six PROMIS pediatrics proxy-report item banks . Health Qual Life Outcomes . 2012 ; 10 ( 1 ): 22 . Crossref PubMed Google Scholar
21. Morrey BF , Adams RA . Semiconstrained arthroplasty for the treatment of rheumatoid arthritis of the elbow . J Bone Joint Surg Am . 1992 ; 74 ( 4 ): 479 – 490 . PubMed Google Scholar
22. Constant CR , Murley AH . A clinical method of functional assessment of the shoulder . Clin Orthop Relat Res . 1987 ; 214 ( 214 ): 160???164 – 164 . PubMed Google Scholar
23. Stratford PW , Binkley JM , Stratford DM . Development and initial validation of the upper extremity functional index . Physiother Canada . 2001 ; 53 : 259 – 267 . Google Scholar
24. Daltroy LH , Liang MH , Fossel AH , Goldberg MJ . The POSNA pediatric musculoskeletal functional health questionnaire: report on reliability, validity, and sensitivity to change. pediatric outcomes instrument development group. pediatric orthopaedic Society of North America . J Pediatr Orthop . 1998 ; 18 ( 5 ): 561 – 571 . Crossref PubMed Google Scholar
25. Pountos I , Clegg J , Siddiqui A . Diagnosis and treatment of greenstick and Torus fractures of the distal radius in children: a prospective randomised single blind study . J Child Orthop . 2010 ; 4 ( 4 ): 321 – 326 . Crossref PubMed Google Scholar
26. Singh G , Athreya BH , Fries JF , Goldsmith DP . Measurement of health status in children with juvenile rheumatoid arthritis . Arthritis Rheum . 1994 ; 37 ( 12 ): 1761 – 1769 . Crossref PubMed Google Scholar
27. Flynn JM , Hresko T , Reynolds RA , et al. Titanium elastic nails for pediatric femur fractures: a multicenter study of early results with analysis of complications . J Pediatr Orthop . 2001 ; 21 ( 1 ): 4 – 8 . Crossref PubMed Google Scholar
28. Nemer McCoy R , Blasco PA , Russman BS , O'Malley JP . Validation of a care and comfort hypertonicity questionnaire . Dev Med Child Neurol . 2006 ; 48 ( 3 ): 181 . Crossref PubMed Google Scholar
29. Vernon DT , Schulman JL , Foley JM . Changes in children's behavior after hospitalization. Some dimensions of response and their correlates . Am J Dis Child . 1966 ; 111 ( 6 ): 581 – 593 . Crossref PubMed Google Scholar
30. Marvin E , Donald CA , Ware JE , Brook RH . Conceptualization and measurement of health for children in the health insurance study. . Santa Monica . 1980 . Google Scholar
31. Kitaoka HB , Alexander IJ , Adelaar RS , et al. Clinical rating systems for the Ankle-Hindfoot, Midfoot, hallux, and lesser toes . Foot Ankle Int. . 1994 ; 15 ( 7 ): 349 – 353 . Crossref PubMed Google Scholar
32. Morrissy RT , Weinstein SL . Lovell and Winter’s Pediatric Orthopaedics . 6th ed. . Philadelphia, PA : Lippincott Williams & Wilkins, , 2006 . Google Scholar
33. Wilkins KE . Principles of fracture remodeling in children . Injury . 2005 ; 36 ( 1 ): S3 – S11 . Crossref PubMed Google Scholar
34. Constant CR , Murley AH . A clinical method of functional assessment of the shoulder . Clin Orthop Relat Res . 1987 ( 214 ): 160???164. . PubMed Google Scholar
35. Marson BA , Craxford S , Deshmukh SR , Grindlay DJC , Ollivere BJ . Patient-reported outcomes for childhood fractures. PROSPERO . https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=159911 (date last accessed 27 November 2019 ). Google Scholar
36. Gerull WD , Okoroafor UC , Guattery J , et al. Performance of pediatric PROMIS cats in children with upper extremity fractures . Hand . 2020 ; 15 ( 2 ): 194-200 . Crossref PubMed Google Scholar
37. Pencharz J , Young NL , Owen JL , Wright JG . Comparison of three outcomes instruments in children . J Pediatr Orthop . 2001 ; 21 ( 4 ): 425 – 432 . PubMed Google Scholar
38. Truong WH , Price MJ , Agarwal KN , et al. Utilization of a Wide Array of Nonvalidated Outcome Scales in Pediatric Orthopaedic Publications: Can't We All Measure the Same Thing? J Pediatr Orthop . 2019 ; 39 ( 2 ): e153 – e158 . Crossref PubMed Google Scholar
39. Phillips L , Carsen S , Vasireddi A , Mulpuri K . Use of patient-reported outcome measures in pediatric orthopaedic literature . J Pediatr Orthop . 2018 ; 38 ( 8 ): 393 – 397 . Crossref PubMed Google Scholar
40. Perry DC , Wright JG , Cooke S , et al. A consensus exercise identifying priorities for research into clinical effectiveness among children’s orthopaedic surgeons in the United Kingdom . Bone Joint J . 2018 ; 100-B ( 5 ): 680 – 684 . Google Scholar
41. Duncan PW , Jorgensen HS , Wade DT . Outcome measures in acute stroke trials . Stroke . 2000 ; 31 ( 6 ): 1429 – 1438 . Crossref PubMed Google Scholar
42. Evans JR , de Silva SR , Ziaei M , Kirthi V , Leyland MD . Outcomes in randomised controlled trials of multifocal lenses in cataract surgery: the case for development of a core outcome set . Br J Ophthalmol . 2020 : bjophthalmol-2019-315410 . bjophthalmol-2019-315410 . Crossref PubMed Google Scholar
43. Sinha IP , Williamson PR , Smyth RL . Outcomes in clinical trials of inhaled corticosteroids for children with asthma are narrowly focussed on short term disease activity . PLoS One . 2009 ; 4 ( 7 ): e6276 . Crossref PubMed Google Scholar
44. Prinsen CAC , Mokkink LB , Bouter LM , et al. COSMIN guideline for systematic reviews of patient-reported outcome measures . Qual Life Res . 2018 ; 27 ( 5 ): 1147 – 1157 . Crossref PubMed Google Scholar
Author contributions
B. A. Marson: Designed study, Conducted search, Screened results, Conducted analysis, Wrote first draft, Edited the manuscript.
S. Craxford: Screened results, Conducted analysis, Edited the manuscript.
S. R. Deshmukh: Screened results, Conducted analysis, Edited the manuscript.
D. Grindlay: Designed and optimized search stratergy, Conducted search, Edited the manuscript
J. Manning: Designed study, Supervised analysis, Edited the manuscript.
B. J. Ollivere: Designed study, Supervised analysis, Edited the manuscript.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose.
Potential Conflicts of Interest
The authors have no conflicts of interest relevant to this article to disclose.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attributions licence (CC-BY-NC-ND), which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










