Abstract
Aims
This study sought to estimate the clinical outcomes and describe the nationwide variation in practice, as part of the feasibility workup for a National Institute for Health and Care Excellence (NICE) recommended randomized clinical trial to determine the optimal treatment of torus fractures of the distal radius in children.
Methods
Prospective data collection on torus fractures presenting to our emergency department. Patient consent and study information, including a copy of the Wong-Baker Faces pain score, was issued at the first patient contact. An automated text message service recorded pain scores at days 0, 3, 7, 21, and 42 postinjury. A cross-sectional survey of current accident and emergency practice in the UK was also undertaken to gauge current practice following the publication of NICE guidance.
Results
In all, 30 patients with a mean age of 8.9 years were enrolled over a six-week period. Of the 150 potential data points, data was captured in 146, making the data 97.3% complete. Pain scores were recorded at day 0 (mean 6.5 (95% confidence interval (CI) 5.7 to 7.3)), day 3 (4.4 (95% CI 3.5 to 5.2)), day 7 (3.0 (95% CI 2.3 to 3.6)), day 21 (1.2 (95% CI 0.7 to 1.7)) and day 42 (0.4 (95% CI 0.1 to 0.7)). Of the 100 units who participated in the nationwide survey, 38% were unaware of any local or national protocols regarding torus fractures, 41% treated torus fractures with cast immobilization, and over 60% of patients had follow-up arranged, both contradictory to national guidelines.
Conclusion
We have demonstrated the severity, recovery trajectory, and variation in pain scores among children with torus fractures. We demonstrate excellent follow-up of patient outcomes using text messages. Despite national guidelines, there is significant variation in practice. This data directly informed the development of an ongoing nationwide randomized clinical trial – the FORearm Fracture Recovery in Children Evaluation (FORCE) study.
Article focus
-
To assess the severity, variability, and recovery trajectory of pain symptoms following torus fractures.
-
To ascertain if text messages can be used successfully in patient-reported outcome measure (PROM) collection.
-
To describe the variation in practice in the management of torus fractures of the wrist throughout the UK.
Key messages
-
Pain rapidly improved over the first seven days following injury.
-
By 21 days postinjury, the majority of patients were nearing pain-free status.
-
Using text messages as a data collection yielded a 97.3% completion rate of data points.
Strengths and limitations
-
Small study sample (n = 30).
-
Minimal loss to follow-up.
Introduction
Torus fractures of the forearm account for an estimated 500,000 emergency department attendances a year in the UK.1 They occur when the radius and/or ulna bone buckles, such that there is cortical deformation but no break in the cortex. Torus fractures are very low risk injuries for complications or deformity in the skeletally immature, and these fractures universally heal well.2
There is considerable variation in the management of this injury. Treatment varies from soft bandaging, to the use of a removable splint, and rigid plaster cast immobilization. The variation in practice has arisen from a longstanding doctrine of rigid cast immobilization for fractures,3 tempered with newer evidence to suggest that simpler treatment methods are frequently as effective, or perhaps even more effective.2-7 The proponents of rigid immobilization (i.e. cast/rigid splint) argue that this maximizes pain relief, and minimizes the occurrence of complications, i.e. fracture displacement. However, there is growing evidence to support the absence of complications in these fractures, with widespread acceptance that patients may safely be discharged at diagnosis,2-4 and that rigid immobilization may not improve pain control but will unduly restrict function.3-7
The recent National Institute for Health and Care Excellence (NICE) non-complex fracture guidelines made recommendations on the management of these injuries,1 advocating that they should not be immobilized in a plaster, and that they should be discharged from the emergency department without a subsequent follow-up. The NICE review, which was unable to advise the best form of immobilization owing to the poor quality of the available evidence, and the recommendation that a trial of treatment interventions was necessary. This study informs the feasibility of a randomized controlled trial by assessing the variation in pain scores, the variation and duration of symptoms, and engagement with an innovative text service as a data capture tool.
Methods
Patients with torus fractures of the distal radius were identified on presentation to Alder Hey Children’s Hospital over a six-week period (November to December 2016). Patients were either seen in the accident and emergency department, whereby patients were discharged at initial contact, or at the orthopaedic fracture clinic, where patients were referred from neighbouring minor injury units. All children were immobilized in a rigid wrist splint, with the advice that they should wear the splint for three weeks and then remove it, but to avoid sports for a further three weeks thereafter.
The primary outcome used was the Wong Baker Faces Pain Scale,8 a validated self-reported tool, and an ordinal assessment of pain outcomes using a series of six facial expressions to illustrate the degree of pain intensity. It has been validated as a self-reporting tool for use among children over three years of age, including in the paediatric emergency department setting, and has been identified to be an excellent measure of pain when estimating the effect of treatment intervention.9,10
Patients were eligible for the study if they met the following inclusion criteria:
Inclusion criteria
-
The patient had sustained a torus/buckle fracture of the distal radius and/or ulna, whereby there was a cortical deformation within the distal third of the radius and/or ulna but no break in the cortex of either bone.
-
The patient was aged between three and 16 years, with three years of age being the lower age limit for which the primary outcome tool may be used.
Exclusion criteria
-
The injury was more than 48 hours old.
-
There was cortical disruption (i.e. greenstick fracture).
-
There was evidence that the patient would be unable to adhere to trial procedures or complete follow-up, such as cognitive impairment or no access by parents to a mobile telephone.
Patient consent and study information, including a copy of the Wong Baker Faces Pain Scale,8 was issued to the patient at first contact. Parents were taught to read the pain scale instructions to their child to enable follow-up at home, inviting the child to select the face that best depicted their level of pain.
If the child had attended the clinic after 24 hours, the child was asked to recall how much pain they had after the injury to produce timepoint ‘0’.
Permission for the evaluation was granted by the R&D department of Alder Hey Children’s Hospital as a service evaluation. Pain scores were collected at the initial visit (timepoint 0), and at four subsequent time points (three, seven, 21, and 42 days postinjury) via an automated online text messaging system.
Text messages, including a hyperlink to the Wong Baker Faces Pain Scale, were sent to the parents of children via a UK-based online text messaging service (Firetext, Cornwall, UK). They were queued to automatically send at 4:00 pm; the time was selected because the National Institute for Health Research (NIHR) young person’s advisory group suggested that this was a convenient time to respond after collection of children from school.
Parents were asked to reply to the text, indicating the response of their child (using the number beneath the relevant face to indicate the child’s pain score). A second text was sent after 24 hours if there was no response to the first. If there was no response after a further 24 hours, a telephone call was made to participants.
A cross-sectional survey of current accident and emergency department practice in the UK was also undertaken to gauge current practice following the recent publication of NICE guidance.1 Clinicians were contacted via telephone and asked three questions:
-
Does you unit have a protocol for managing torus fractures?
-
How do you routinely immobilize torus fractures?
-
What follow-up do you arrange for torus fractures?
The list of accident and emergency units was attained via a freedom of information request.
Results
A total of 30 patients (mean age 8.9, 4 to 15) were enrol over a six-week period. Pain scores were recorded at day 0 (6.5 (95% confidence interval (CI) 5.7 to 7.3)), day 3 (4.4 (95% CI 3.5 to 5.2)), day 7 (3.0 (95% CI 2.3 to 3.6)), day 21 (1.2 (95% CI 0.7 to 1.7)), and day 42 (0.4 (95% CI 0.1 to 0.7)). Responses to ‘first attempt’ text messages allowed data completion of 91.3% (107 out of 120 first attempt text messages sent). In all, 30 data points were at day 0 on enrolment into study. Of the 150 potential data points, data was captured in 146 (97.3% completion).
Figure 1 illustrates the pain scores among the 30 children treated using rigid splint immobilization.
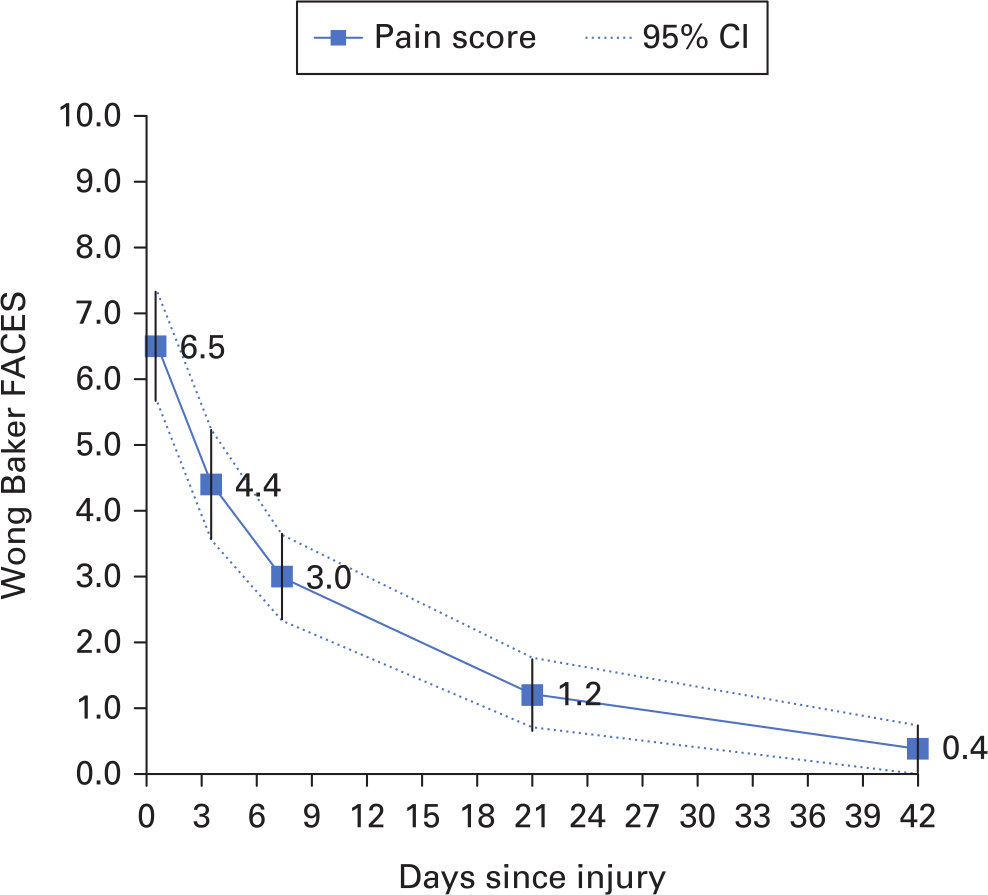
Fig. 1
Wong Baker Faces pain scores of 30 children with a torus fracture of the distal radius. The scale has six ordinal facial expressions; 0, 2, 4, 6, 8, and 10.
Contact telephone numbers were obtained for 175 accident and emergency units across the UK. Consultants, senior doctors, or senior nurses at 100 units agreed to answer our short survey. Of the respondents, 38% of the units did not have a protocol for patients diagnosed with torus fractures, 41% of units immobilized such injuries in a plaster cast, 54% of the units used a rigid splint, and 5% used soft cast. With regards to follow-up, one unit had local follow-up in the emergency department, the majority (60%) organized follow-up with their local orthopaedic clinic, and 39% had no follow-up (Figure 2). In total, 36% of units treated torus fractures in accordance with the recent guidance, without both cast immobilization or follow-up.
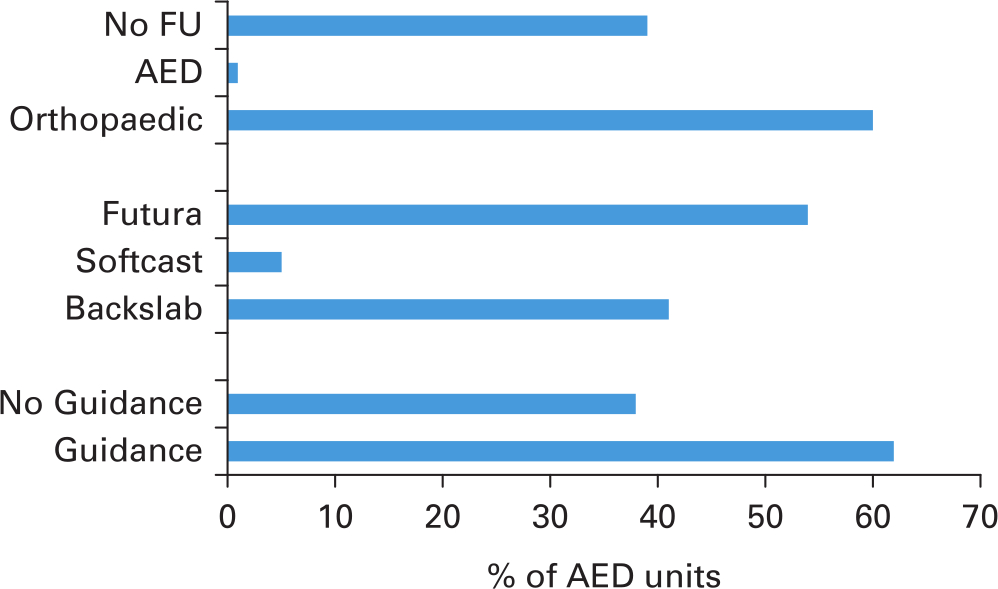
Fig. 2
Results of 100 accident and emergency units surveyed in this study assessing gauging current practice in treatment of torus fractures.
Discussion
Our study has demonstrated the severity, recovery trajectory, and variation in pain scores among children with torus fractures of the distal radius. The majority of pain suffered is within the first seven days, with symptoms nearing resolution by three weeks. The study also demonstrated that the use of text messages was a very efficient means of follow-up.
Following the recommendation from NICE for randomized clinical trials to ascertain the optimal treatments for torus fracture of the distal radius, this feasibility study provides robust information to inform the design of a definitive trial. Key components required prior to a randomized clinical trial are knowledge of the effect size, population variance, pattern of recovery, and optimal data collection tool.11-13 Furthermore, this study also offers evidence of uncertainty within the clinical community with marked variation in the treatment of this simple fracture. However, none of the units chose to treat these injuries in a simple bandage as recommended by NICE.
The use of the automated text message follow-up service undoubtedly contributed to the high levels of data capture. The time of 4.00 pm was decided upon by a patient advisory group advocating that this would coincide with pick up from school and hence be a timely reminder. It would be interesting to see if the data capture rate had varied if texts were sent at different times of day. It has been cited previously that text messaging services have been used, to good effect, to improve both adherence to medical treatment and clinic appointments.14-16 There is little in the literature to showcase the use of text messages as a fruitful data collection tool for PROMs. We believe our study highlights the potential of texts being used in this manner, and indeed plan to utilize a similar data collection method in the randomized clinical trial planned in light of the findings from this feasibility study.
We also highlight the level of variation in the UK regarding torus fracture management. Given the frequency of the injury, even minor modification to the care pathway of such a common fracture could have large financial implications across the UK NHS.
Our study includes a relatively small sample size of participants, collects a single outcome measure, and does not make comparisons between different methods of treatment. The results in isolation therefore only give a flavour of fracture severity and recovery, though provide important data for forthcoming work. Furthermore, the novelty of the follow-up approach is of importance to the design of future randomized clinical trials in trauma surgery.
Torus fractures are a common injury. We now know the magnitude and chronology of pain associated with these injuries when being treated with a rigid splint. We also know that, despite national guidelines, significant variation in practice for the treatment of these injuries remains. The use of simpler treatments (i.e. bandage), recommended by NICE, is not currently employed in the UK. Consequently, the UK is now engaged in a nationwide randomized controlled trial that used the information from this feasibility work as the cornerstone of the work; the Forearm Fracture Recovery in Children Evaluation (FORCE) study, a multicentre prospective randomized equivalence trial of an optional soft bandage and immediate discharge versus current treatment with rigid immobilization for torus fractures of the distal radius in children and is due to report in 2021.
References
1. No authors listed . Fractures (non-complex): assessment and management. National Institute for Health and Care Excellence (NICE) (UK). NICE guideline [NG38], February 2016 . https://www.nice.org.uk/guidance/ng38 (date last accessed 18 December 2019 ). Google Scholar
2. Solan MC , Rees R , Daly K . Current management of Torus fractures of the distal radius . Injury . 2002 ; 33 ( 6 ): 503 – 505 . Crossref PubMed Google Scholar
3. Davidson JS , Brown DJ , Barnes SN , Bruce CE . Simple treatment for torus fractures of the distal radius . J Bone Joint Surg Br . 2001 ; 83-B ( 8 ): 1173 – 1175 . PubMed Google Scholar
4. Symons S , Rowsell M , Bhowal B , Dias JJ . Hospital versus home management of children with buckle fractures of the distal radius. A prospective, randomized trial . J Bone Joint Surg Br . 2001 ; 83-B ( 4 ): 556 – 560 . Google Scholar
5. Williams KG , Smith G , Luhmann SJ , et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference . Pediatr Emerg Care . 2013 ; 29 ( 5 ): 555 – 559 . Crossref PubMed Google Scholar
6. Plint AC , Perry JJ , Correll R , Gaboury I , Lawton L . A randomized, controlled trial of removable splinting versus casting for wrist buckle fractures in children . Pediatrics . 2006 ; 117 ( 3 ): 691 – 697 . Crossref PubMed Google Scholar
7. Oakley EA , Ooi KS , Barnett PLJ . A randomized controlled trial of 2 methods of immobilizing Torus fractures of the distal forearm . Pediatr Emerg Care . 2008 ; 24 ( 2 ): 65 – 70 . Crossref PubMed Google Scholar
8. Wong DL , Baker CM . Pain in children: comparison of assessment scales . Pediatr Nurs . 1988 ; 14 ( 1 ): 9 – 17 . PubMed Google Scholar
9. Garra G , Singer AJ , Taira BR , et al. Validation of the Wong-Baker FACES pain rating scale in pediatric emergency department patients . Acad Emerg Med . 2010 ; 17 ( 1 ): 50 – 54 . Crossref PubMed Google Scholar
10. Tomlinson D , von Baeyer CL , Stinson JN , Sung L . A systematic review of faces scales for the self-report of pain intensity in children . Pediatrics . 2010 ; 126 ( 5 ): e1168 – e1198 . Crossref PubMed Google Scholar
11. Perry DC , Griffin XL , Parsons N , Costa ML . Designing clinical trials in trauma surgery: overcoming research barriers . Bone Joint Res . 2014 ; 3 ( 4 ): 123 – 129 . Crossref PubMed Google Scholar
12. Haddad FS . PROMS are great value, but sometimes we need more information . Bone Joint J . 2018 ; 100-B ( 5 ): 557 – 558 . Crossref PubMed Google Scholar
13. Simpson AHRW , Frost H , Norrie J . Pragmatic surgical studies: are they the new gold standard . Bone Joint J . 2018 ; 100-B ( 11 ): 1407 – 1408 . Crossref PubMed Google Scholar
14. Agboola S , Jethwani K , Lopez L , et al. Text to move: a randomized controlled trial of a Text-Messaging program to improve physical activity behaviors in patients with type 2 diabetes mellitus . J Med Internet Res . 2016 ; 18 ( 11 ): e307 . Crossref PubMed Google Scholar
15. Buis L , Hirzel L , Dawood RM , et al. Text messaging to improve hypertension medication adherence in African Americans from primary care and emergency department settings: results from two randomized feasibility studies . JMIR Mhealth Uhealth . 2017 ; 5 ( 2 ): e9 . Crossref PubMed Google Scholar
16. Lin C-L , Mistry N , Boneh J , Li H , Lazebnik R . Text message reminders increase appointment adherence in a pediatric clinic: a randomized controlled trial . Int J Pediatr . 2016 ; 2016 : 8487378. Crossref PubMed Google Scholar
Author contributions
J. Widnall: Data collection, Data analysis, Wrote manuscript.
T. Capstick: Data collection.
M. Wijesekera: Data collection.
S. Messahel: Data collection, Data analysis.
D. C. Perry: Project lead.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
ICMJE COI statement
The authors have no conflict of interest.
Ethical review statement
This study did not require ethical approval.
©2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attributions licence (CC-BY-NC-ND), which permits unrestricted use, distribution, and reproduction in any medium, but not for commercial gain, provided the original author and source are credited.










