Abstract
According to a report by Millennium Research Group in January 2011, the US orthopaedic extremity device market will generate over $4.6 billion in revenue by 2015.1 With an ageing demographic and increasing demand for better quality of life into old age, there is clearly a commercial drive for the orthopaedic device community to develop new and innovative solutions to bone and joint problems. Devising such solutions is one thing; protecting them, so that research investment can be rewarded, is another. How is such protection achieved? The judicious use of intellectual property rights plays a key role, and this article aims to provide some information about the use of patents to protect innovation.
Principles of patenting
The word 'patent' originates from the Latin patere, which means 'to lay open' (i.e. to make available to the public). It is a shortened version of “Letters Patent” that, in times pre-dating the modern patent system, was a legal instrument in the form of a published written order, granted by royal decree, which bestowed exclusive rights on a person.
In modern terms, a patent is an exclusive right which is granted to an inventor or their assignee for a limited period of time in exchange for the public disclosure of the invention. Typically, this period is 20 years from the filing date of the patent application, subject to the payment of maintenance fees.
A patent does not confer the right to practise or use the invention. Rather, a patent provides the right to exclude others from carrying out certain acts in relation to the invention, thereby providing a market monopoly for the period of time that the patent is in force. In simple terms, these acts (or infringing activities) constitute making, selling, using or importing a patented product, or using or offering for use a patented process.
To obtain a granted patent for an invention, the invention must be new; it must involve an inventive step (in that it is not obvious to a person familiar in the field to which the invention pertains); and it must be capable of industrial applicability.
It is crucial to note that the requirement for the invention to be new means that it must not have been publicly disclosed, even by the inventor to colleagues at a research meeting, or to friends in the pub.
In addition, there are certain categories of excluded subject matter for which a patent shall not be granted. In the UK2 and Europe3 these categories include scientific theories and mathematical discoveries; inventions which are contrary to public policy or morality; and, of most relevance here, inventions which relate to a method for treatment of the human or animal body by surgery or therapy and diagnostic methods practised on the human or animal body.
These latter provisions appear prohibitive with regards to obtaining patent protection for inventions relating to those categories of subject matter. However, it is clearly stated in the relevant legal provisions that the exclusions do not apply to products, including substances and compositions, for use in the excluded methods. Hence, drugs, orthopaedic devices and surgical instruments, for example, are not considered to be matter excluded from patentability per se.
The intention of the medical, surgical and diagnostic methods exclusions is to protect medical and veterinary practitioners, by ensuring that they are not impeded in their practice as a result of patents. The meaning of the terms used in these provisions has been scrutinised, to try to ensure that the exclusions befit this purpose.
For example, in the G1/074 decision the Enlarged Board of Appeal of the European Patent Office (EBA) considered the meaning of "treatment by surgery". In particular, the EBA was asked to consider whether a physical intervention practised on the human body which was carried out as part of an imaging method, causes that method to be excluded from patent protection as encompassing a method of treatment by surgery, even if the physical intervention step was not intended to treat the patient.
The case in question concerned an imaging method that required the injection of a contrasting agent into the heart of a patient.
The EBA considered that such an injection represented a substantial physical intervention on the body which entailed a health risk and required professional medical expertise to be carried out, and, as such, it could be regarded as a method for the treatment of the human or animal body by surgery and hence excluded from patentability. This was the case, irrespective of the fact that the physical intervention on the body was not itself aimed at maintaining life and health.
Inventorship and ownership
Under UK law, an inventor is defined as the actual deviser of the invention.5 An invention can have multiple inventors, as long as the parties concerned are jointly responsible for devising the inventive concept; as Christopher Floyd QC, sitting at the time as a Deputy Judge in the Patents Court stated: “If A discloses a new idea to B, whose only suggestion is to paint it pink, B should not be a joint inventor of a patent for A's product painted pink… the additional feature [of pink paint] does not really create a new inventive concept”.
UK law then provides that, while the right to the grant of a patent belongs primarily to the inventor, this may be overridden by any rule of law or any legally enforceable agreement existing at the time the invention was made. The most common way in which this proviso would cause the rights to pass from the inventor is when the invention was made in the course of employment.5
In this regard, the provisions of UK law relating to inventions made by employees6 statethat if an employee makes an invention in the course of normal or specifically assigned duties, and the circumstances are such that an invention might reasonably be expected to result from those duties (for example, an employee working in Research and Development; or an employee who has been specifically assigned to improve a product or process), the invention belongs to the employer. In addition, if the invention is made in the course of duties that impose a special obligation to further the interests of the employer (e.g. the inventor is a Director of the employer company), the invention belongs to the employer. In other circumstances the invention typically belongs to the employee.
It is beyond the scope of this article to comment on ownership issues in particular circumstances, such as those surrounding intellectual property arising from employees within the NHS. However, the NHS Institute for Innovation and Improvement, established in July 2005, included the National Innovation Centre (NIC), which aimed to “provide a range of innovation development support to innovators from the NHS, Industry and Academia”. The NIC website7 appeared to encourage the submission of innovative ideas by employees of the NHS, and provided a helpful list of IP related 'frequently asked questions'. The NHS Institute for Innovation and Improvement closed, however, on 31 March 2013, to be replaced immediately by NHS Improving Quality,8 the remit of which, with regard to ideas arising from the workforce of the NHS, appears to be less clear.
Patent application filing and prosecutionIn order to obtain a patent for an invention an application must be filed with the Intellectual Property Office in the country for which patent protection is sought. The application comprises a request for the grant of a patent for the invention and a patent specification, which comprises a detailed description and, where appropriate, drawings of the invention, and a set of claims.
The claims aim to define the invention in a way that encompasses the underlying concept of the invention, rather than a particular embodiment of that concept. For example, say the invention is a new hip implant with a cross-hatched surface on the acetabular shell, which gives rise to improved osteo-integration of the shell with the acetabulum. It would seem reasonable to assume that alternative texturing of the acetabulum shell could give rise to similarly beneficial results. As such, a claim in the patent specification should be directed to a hip implant with the feature of a textured acetabular shell (rather than limited to a hip implant with the specific feature of cross-hatching on the acetabular shell).
Such a claim, which aims to define the crux of the invention in the broadest possible terms, is called an 'independent' claim, in that it stands on its own. The claims will then include multiple 'dependent' claims, which refer to the independent claim, and define the invention more narrowly. In our example, a dependent claim could be directed to an implant wherein the textured surface on the acetabulum shell is provided by cross-hatching.
Following filing of the patent application, the application is searched by a Patent Examiner, with a view to establishing the subject matter that really does constitute a new and innovative development in view of what is already known.
To do this, the Examiner searches databases of published information in the field(s) relating to the invention, including existing patents and applications and journal articles. The Examiner will report any relevant documents that they find to the applicant (or their appointed patent attorney) with an explanation of why this 'prior art' is relevant to the subject matter recited in the claims of the patent application.
The cited documents need to be reviewed, and an opinion formed as to what differences there may be between what is already known, and the invention. A response to the Patent Examiner is formulated which points out these differences. In addition, it may be necessary to amend the claims of the patent application so that they are directed to the feature(s) of the invention which are different and innovative over what is already known; the subject matter of the dependent claims can be useful in this regard.
To use our earlier example, a document may come to light, during a search carried out by the Patent Examiner, which discloses a hip implant with unidirectional lines marked on the surface of the acetabular shell. Our independent claim (directed to a hip implant with a textured acetabular shell) encompasses the known implant, and so the claim would need to be amended to, for example, the more limited embodiment of a hip implant wherein the textured acetabular surface is provided by cross-hatching. A reply to the Patent Examiner could then be formulated pointing out that our implant, with its cross-hatched surface, is different to the known implant (and therefore novel); and, if it can be shown that the cross-hatching imparts superior results and that these superior results wouldn't have been expected, we can also argue that our invention is non-obvious, and therefore inventive, over the known implant.
It is typical for there to be several rounds of correspondence (known as 'patent prosecution') with the Patent Examiner, and the time taken and the number of reports issued by the Examiner differs from country to country and case to case. The patent application is also published around 18 months after the date it was filed (provided it hasn't been withdrawn in advance of that date), thereby disclosing the invention to the public.
When all of the Examiner's objections have been addressed, the patent application will proceed to grant. It is only at this point that there is a patent for the invention: at any preceding point, a patent application is merely pending.
Enforcement and revenue generation
The carrying out of any infringing activity without the permission of the patent proprietor, in the territory for which the patent has been granted, constitutes an infringement of the patent.
The territorial aspect of the patent is important: infringement is only possible in a country where a patent is in force. Using our earlier example, if a patent is granted for our hip implant in the UK (only), then our patent can be enforced against anyone making that hip implant in the UK, however, people outside the UK are free to make and sell the hip implant in their countries.
Enforcement of patent rights can be big business: reports of the case between Samsung and Apple made the broadsheets in August 2012, when a court in California ruled that Samsung infringed a number of Apple's patent and designs rights. The patents in question related to user interface elements of Apple's iOS, which Apple alleged were infringed by several of Samsung's smartphones and tablets. Apple was ultimately awarded over $1 billion in damages.9
More recently, Zimmer Holdings Inc. (Zimmer) and Stryker Corp. (Stryker), two orthopaedic device giants, have locked horns over patent rights. A court in Michigan held that Zimmer's Pulsavac wound debridement system, which uses pulsing liquid to loosen debris and remove it by suction during joint replacement surgery, infringed three of Stryker's patents, and ordered Zimmer to pay $70 million to Stryker.10
However, not all patent litigation involves such high costs. The Patents County Court in London, for example, deals with intellectual property disputes, including cases of patent infringement and has a limit on damages of £500 000.
In a competitive field, it's inevitable that a successful product will attract the attention of competitors. But taking action before the courts for infringement of a patent is not the only way to proceed. Revenue from a patent can be generated by assigning (i.e. selling) the rights in the patent, or granting a licence to carry out one or more of the acts that would otherwise constitute an infringement of the patent. A summary flowchart of the process cans be seen in Figure 1.
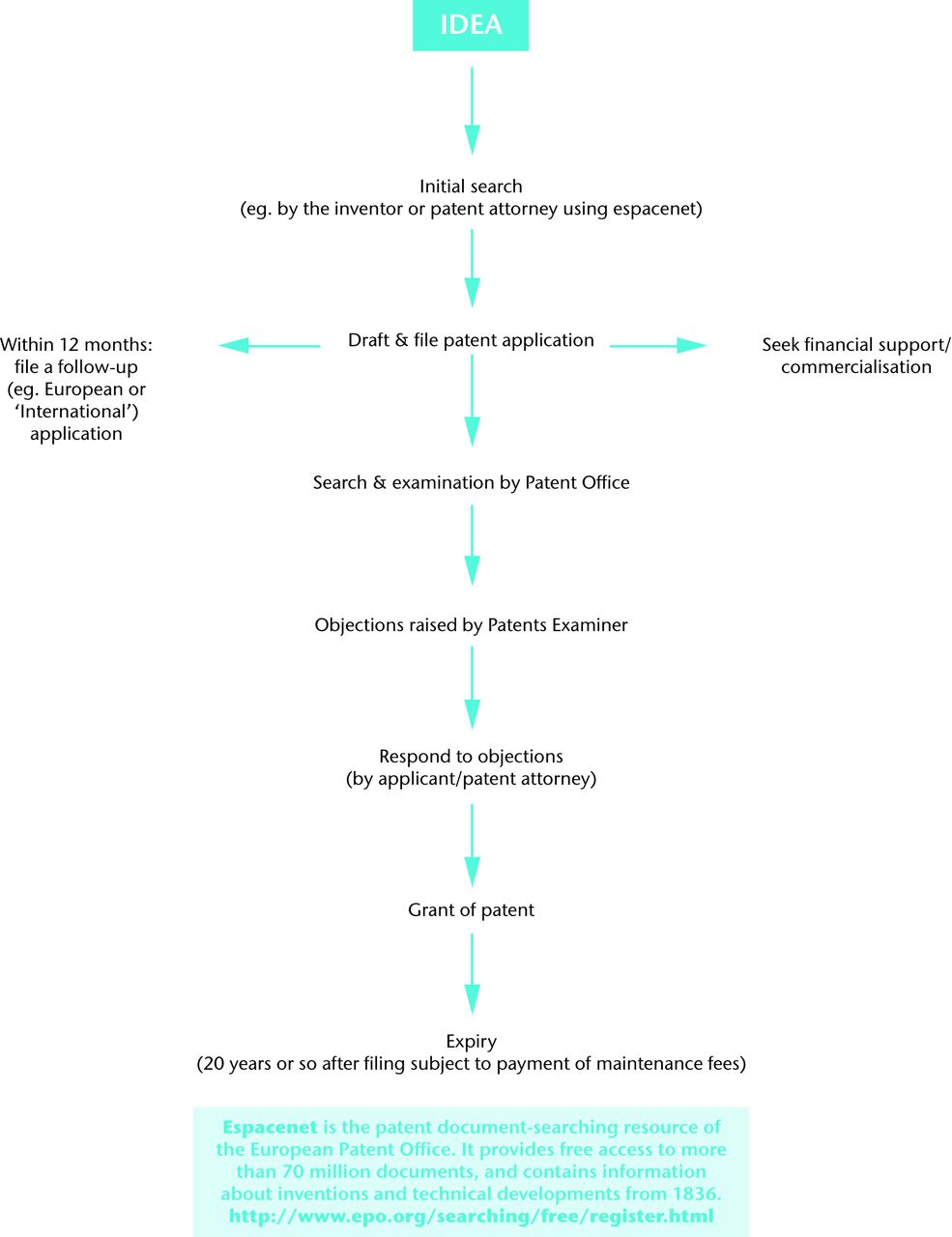
Fig. 1
Flow chart of the patent process.
Conclusion
The patent system, although not without controversy, is believed to provide an incentive to innovate: to design around and improve upon existing technology; and to disseminate the resulting advancement of ideas. It is clearly a system which plays an important role in the development of new orthopaedic devices, providing legal protection for innovative developments in the field, and the means of recouping research investment or attracting the investment needed to take a product to market.
1 Jaganathan A. US Markets for Orthopedic Extremity Devices 2011. http://mrg.net/Products-and-Services/Syndicated-Report.aspx?r=RPUS21OE10 (date last accessed 26 April 2013). Google Scholar
2 No authors listed. The Patents Act 1977 (as amended): Section 4A. http://www.ipo.gov.uk/patentsact1977.pdf (date last accessed 26 April 2013). Google Scholar
3 No authors listed. European Patent Convention: Article 53. http://www.epo.org/law-practice/legal-texts/html/epc/2010/e/ar53.html (date last accessed 26 April 2013). Google Scholar
4 No authors listed. European Patents Office. http://www.epo.org/law-practice/case-law-appeals/eba/number.html (date last accessed 26 April 2013). Google Scholar
5 No authors listed. The Patents Act 1977 (as amended): Section 7. http://www.ipo.gov.uk/patentsact1977.pdf (date last accessed 26 April 2013). Google Scholar
6 No authors listed. The Patents Act 1977 (as amended): Section 39. http://www.ipo.gov.uk/patentsact1977.pdf (date last accessed 26 April 2013). Google Scholar
7 The NHS National Innovation Centre. http://www.nic.nhs.uk/ (date last accessed 26 April 2013). Google Scholar
8 NHS Improving Quality. http://www.england.nhs.uk/ourwork/qual-clin-lead/nhsiq/ (date last accessed 26 April 2013). Google Scholar
9 Blackden R. Apple wins $1.05bn from arch rival Samsung in patent case. The Telegraph, 25 August 2012. http://www.telegraph.co.uk/technology/apple/9498541/Apple-wins-1.05bn-from-arch-rival-Samsung-in-patent-case.html (date last accessed 16 April 2013). Google Scholar
10 Decker S. Zimmer Told to Pay Stryker $70 Million in Patent Case. Bloomberg, 6 February 2013. http://www.bloomberg.com/news/2013-02-05/zimmer-told-to-pay-stryker-70-million-for-surgical-tool-patents.html (date last accessed 26 April 2013). Google Scholar









