Abstract
Aims
In the context of tendon degenerative disorders, the need for innovative conservative treatments that can improve the intrinsic healing potential of tendon tissue is progressively increasing. In this study, the role of pulsed electromagnetic fields (PEMFs) in improving the tendon healing process was evaluated in a rat model of collagenase-induced Achilles tendinopathy.
Methods
A total of 68 Sprague Dawley rats received a single injection of type I collagenase in Achilles tendons to induce the tendinopathy and then were daily exposed to PEMFs (1.5 mT and 75 Hz) for up to 14 days - starting 1, 7, or 15 days after the injection - to identify the best treatment option with respect to the phase of the disease. Then, 7 and 14 days of PEMF exposure were compared to identify the most effective protocol.
Results
The daily exposure to PEMFs generally provided an improvement in the fibre organization, a decrease in cell density, vascularity, and fat deposition, and a restoration of the physiological cell morphology compared to untreated tendons. These improvements were more evident when the tendons were exposed to PEMFs during the mid-acute phase of the pathology (7 days after induction) rather than during the early (1 day after induction) or the late acute phase (15 days after induction). Moreover, the exposure to PEMFs for 14 days during the mid-acute phase was more effective than for 7 days.
Conclusion
PEMFs exerted a positive role in the tendon healing process, thus representing a promising conservative treatment for tendinopathy, although further investigations regarding the clinical evaluation are needed.
Cite this article: Bone Joint Res 2020;9(9):613–622.
Article focus
-
To evaluate the efficacy of pulsed electromagnetic field (PEMF) daily exposure in enhancing the tendon healing process in a rat model of Achilles tendinopathy.
-
To identify the best performing protocol of PEMF daily exposure in terms of treatment starting point and duration.
Key messages
-
The daily exposure to PEMFs during the mid-acute phase of the tendinopathy enhanced the restoration of the physiological structure of the tissue.
-
A more effective outcome was provided by the longer exposure to PEMFs.
Strengths and limitations
-
This study aimed to investigate not only the effects of specific parameters (1.5 mT and 75 Hz) of PEMF exposure in the treatment of tendinopathy, but also to provide significant insights in the identification of the best performing conditions of intervention for potential future clinical applications.
-
It provided preliminary outcomes mainly focused on the histological analysis of the tissue since the number of animals and the amount of evaluations was restricted.
Introduction
Achilles tendinopathy (AT) is a clinical syndrome characterized by the combination of pain, swelling, and impaired performance,1 originally connected with overuse but also characterized by inflammatory events.2,3 The tendon healing process is often slow and incomplete, with an augmented incidence of degenerative events associated with a poor response to treatments.4 Much attention is currently paid to the development of innovative and effective conservative approaches suitable as adjuvants to surgical intervention to promote tissue healing.5
Biophysical stimulations with pulsed electromagnetic fields (PEMFs) represent a noninvasive, cost-effective, and safe conservative treatment already approved by the Food and Drug Administration (FDA) for the treatment of delayed union and nonunion fractures.6
The interest in PEMF therapy is growing rapidly with consistent evidence of its therapeutic efficacy in different musculoskeletal disorders;7-17 a few studies have investigated the role of PEMFs in the healing process of the tendon tissue. In vitro studies on human tendon cells stimulated with PEMFs showed an increased cell proliferation, as well as cytokines (interleukin (IL)-6 and IL-10) and growth factor (TGF-β) release,5,18 together with an up-regulation of specific tenogenic gene transcription (scleraxis and type I collagen).18,19 In vivo, PEMFs positively affected the biomechanical and histological outcomes during the healing of rat rotator cuff tendons,20 the tensile strength at the repair site,21 as well as the rotator cuff tendon-to-bone healing,22 and they may potentially serve as an adjuvant therapy to improve clinical outcomes in rotator cuff tendon repairs.23
In 1984 the first double-blind randomized controlled trial on the beneficial use of PEMF in rotator cuff tendinitis was conducted on patients whose symptoms were refractory to steroid injection and other conventional conservative measures.24 More recently, another clinical randomized study demonstrated that PEMF treatment is an effective adjuvant therapy after rotator cuff arthroscopic repair, reducing inflammation, swelling, and pain at a short-term follow-up25.
Here, we wanted to determine the efficacy of PEMF treatment on tendon healing in a previously established rat model of collagenase-induced Achilles tendinopathy.26 To manage this induced pathological condition, previous in vitro studies allowed for the identification of the parameters of PEMF application, in terms of length of the stimulation ( 8 hours),18 field intensity, and frequency (1.5 mT, 75 Hz).19
Hence, with the aim to identify the most effective PEMF protocol to treat tendinopathy, two main aspects have been investigated in the present research: the effects of PEMF treatment according to the phases of acute tendinopathy process (early, medium, and late phases of the acute process) in which they were applied, and the possible benefits of a short or long duration of the PEMF treatment (7 and 14 days). Given the mechanism of action of PEMF on tendon cells reported by previous studies, our hypothesis is that beginning the PEMF treatment in the very early phase of tendinopathy should be avoided since the high levels of inflammation might impair their mechanisms of action.
Methods
Ethics statement
The study was approved by Mario Negri Institute for Pharmacological Research (IRFMN) Animal Care and Use Committee (IACUC) (Permit N. 363/2015-PR). The IRFMN adheres to the principles set out in the following laws, regulations, and policies governing the care and use of laboratory animals: Italian Governing Law (D.lgs 26/2014; Authorization n.19/2008-A issued 6 March 2008 by Ministry of Health); Mario Negri Institutional Regulations and Policies providing internal authorization for persons conducting animal experiments (Quality Management System Certificate–UNI EN ISO 9001:2008 –Reg. N. 6121); the NIH Guide for the Care and Use of Laboratory Animals (2011 edition) and EU directives and guidelines (EEC Council Directive 2010/63/UE). The experiments were conducted under the Animal Welfare Assurance #A5023-01. Animal housing was provided in a conventional facility with light and dark cycles every 12 hours. The animals were regularly checked by a certified veterinarian responsible for health monitoring, animal welfare supervision, experimental protocols, and procedure revision. All surgeries were performed under general anaesthesia as previously described,26 and all efforts were made to minimize suffering.
Study design
The study was designed to identify the best conditions of PEMF applications in terms of: 1) the effects of daily PEMF treatment according to the phases of acute tendinopathy process, i.e. during the early acute phase (EAP), mid-acute phase (MAP) and late acute phase (LAP) of tendinopathy; and 2) the most effective duration of daily PEMF treatment (7 or 14 days).
In accordance with the Three R's principle (Replacement, Reduction, and Refinement),27 the second part of the study was conducted only on the groups showing the best response to PEMF treatment identified during the first experimental step.
A group of 68 male Sprague Dawley rats (Rattus norvegicus; 12-week-old, mean body weight 250 to 300 g) (Envigo RMS Srl, San Pietro al Natisone, Italy) were used in this study. Specifically, all rats were monolaterally induced for tendinopathy by type I collagenase injection, and then divided into differently treated (+PEMF) and corresponding untreated (-PEMF) groups (Figure 1 and Table I).
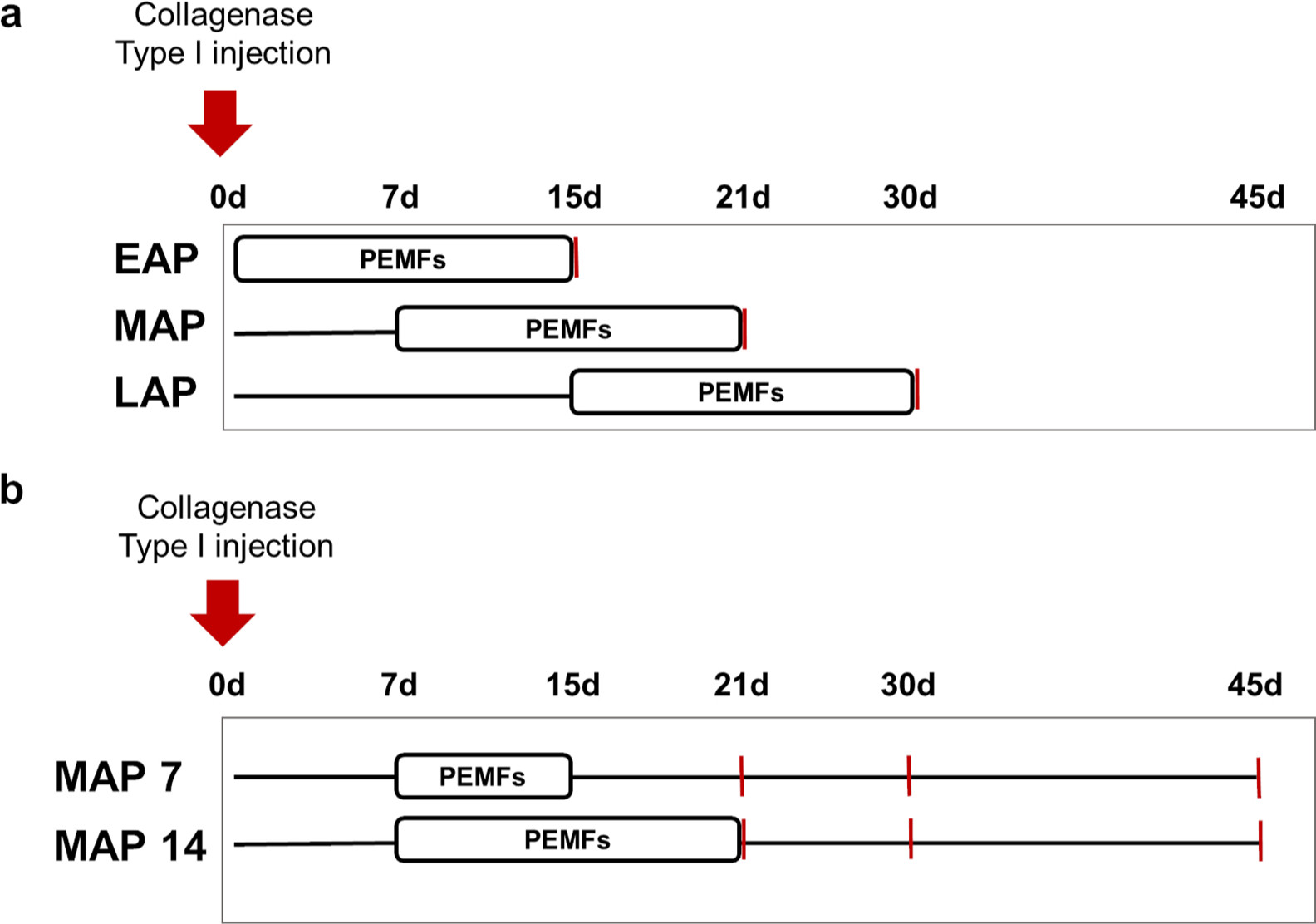
Fig. 1
Study design for a) the evaluation of the most effective start of pulsed electromagnetic field (PEMF) treatment and b) for the most effective duration of PEMF exposure. Red lines correspond to time of sacrifice and tendon explant. EAP, early acute phase; LAP, late acute phase; MAP, mid-acute phase.
Table I.
Animal grouping and respective treatments.
| Group name | Start of PEMF treatment (days after collagenase injection) |
PEMF duration, days | Timepoints (sample size) | |
|---|---|---|---|---|
| +PEMF | EAP | Day 1 | 14 | Day 15 (4) |
| MAP 7 | Day 7 | 7 | Day 21 (6) | |
| Day 30 (6) | ||||
| Day 45 (6) | ||||
| MAP 14 | Day 7 | 14 | Day 21 (6) | |
| Day 30 (6) | ||||
| Day 45 (6) | ||||
| LAP | Day 15 | 14 | Day 30 (6) | |
| -PEMF | N/A | N/A | N/A | Day 15 (4) |
| Day 21 (6) | ||||
| Day 30 (6) | ||||
| Day 45 (6) | ||||
-
EAP, early acute phase; MAP, mid-acute phase; LAP, late acute phase; PEMF, pulsed electromagnetic field; N/A, not applicable.
Achilles tendinopathy was determined under general anaesthesia by injecting 3 mg/ml of type I collagenase (Clostridium histolyticum 185 IU/mg; Worthington, Lakewood, New Jersey, USA) dissolved in sterile saline solution, in tendons exposed through a medial skin incision, as previously described.26 After skin closure and recovery from anaesthesia, rats were free for weight-bearing. No adverse events were observed after surgery or during the follow-up period.
Treatment with PEMFs
To expose animals to PEMFs, a custom-made coil (40 cm × 18 cm) was placed at the bottom of the cages and connected to a PEMF generator system (IGEA SpA, Carpi, Italy).
Rats were exposed to PEMFs (1.5 mT SD 0.2; 75 Hz) for eight hours/day (night-time) for 7 or 14 days, according to the different groups (Figure 2).
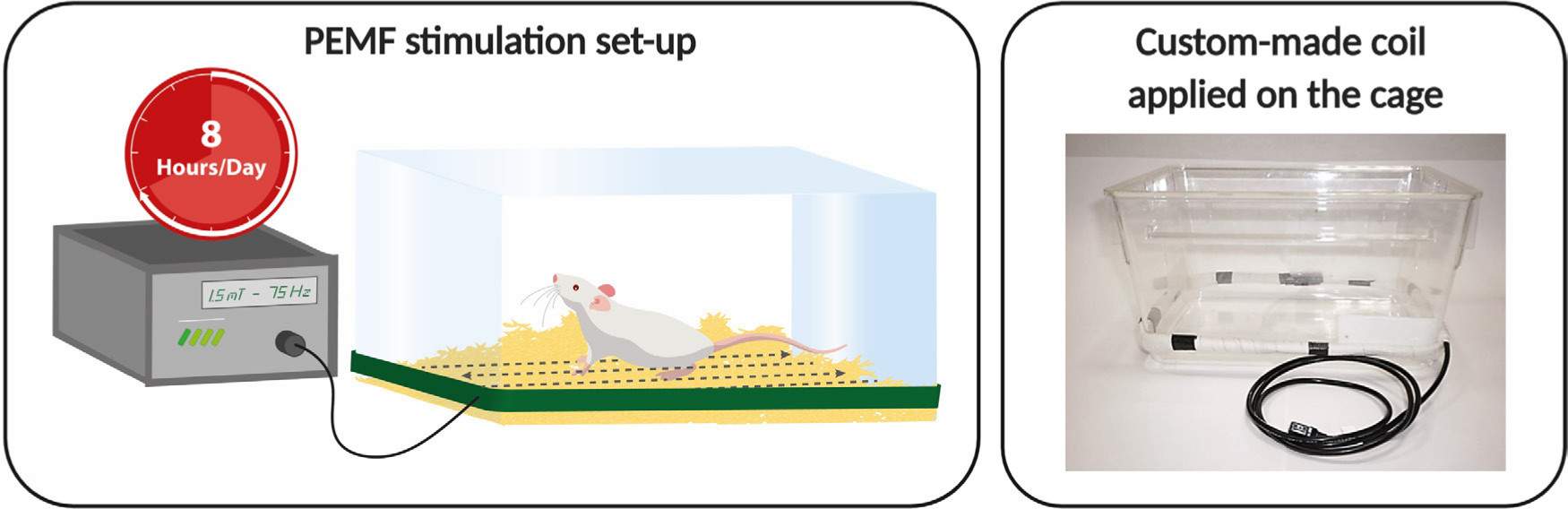
Fig. 2
Pulsed electromagnetic field (PEMF) stimulation set-up: PEMF generator is connected to the solenoid fixed at the bottom of the rat cages. Each PEMF generator is automatically activated eight hours/day by using a timer. The picture is representative of the positioning of the custom-made coil on the cage.
The control groups (-PEMF) were housed without applying the device to the cages, did not receive PEMF treatment, and were killed at the same timepoints.
Histological analysis and CD31 localization
At the selected timepoints, animals were sacrificed by CO2 inhalation and the Achilles tendons were harvested for histological investigations. Tendon specimens were explanted, fixed in 10% formalin, and then processed for dehydration and paraffin embedding. Sample sections were stained with haematoxylin and eosin (H&E) and evaluated by three blinded observers (CPO, ABL, LDG) to assess fibre structure, cell density, cell appearance, presence of inflammatory infiltration, increase of vascularity, and fatty deposits, as previously reported.26 Each parameter was scored with a value comprised between 0 and 3, where 0 identified healthy tissue and 3 damaged tissue. The total score of the tissue - between 0 and 18 - was obtained as the sum of the partial scores.
CD31, known as platelet endothelial cell adhesion molecule 1 (PECAM-1), is a transmembrane glycoprotein expressed on platelets, inflammatory cells, and endothelial cells. It mediates both leucocyte and platelet/endothelial cell adhesion and transendothelial migration during inflammation. To localize CD31, tissue sections were deparaffinized and antigen was retrieved through an overnight incubation at 60°C in Dewax and HIER Buffer H (Thermo Fisher Scientific, Waltham, Massachusetts, USA). The sections were then washed and incubated with anti-CD31 rabbit antibody (Abcam, Cambridge, UK) for one hour at room temperature. The sections were further washed and labelled with anti-rabbit secondary antibody (Vector, Burlingame, California, USA). Diaminobenzidine (DAB; Sigma-Aldrich, St. Louis, Missouri, USA) was used as a chromogenic substrate of the peroxidase reaction.
Statistical analysis
Statistical analyses were performed with GraphPad Prism 5 software (GraphPad Software, San Diego, California, USA). Shapiro-Wilk test was used to assess the distribution of data. Normally distributed data were analyzed with independent-samples t-test or one-way analysis of variance (ANOVA) coupled with Bonferroni’s post hoc test; for nonparametric data, Mann-Whitney test or Kruskal-Wallis test coupled with Dunns’ test were performed. Values of p < 0.05 were considered significant. Data are expressed as means and standard deviations (SD).
The sample size was calculated on the basis of a previous study,26 through a paired t-test with α error = 0.05% and 80% power (G*Power 3.1 software, Düsseldorf, Germany).28 The interobserver reliability of histopathological score was calculated by R software, version 3.5.0 using ICC package (R Foundation for Statistical Computing, Vienna, Austria). The interclass r (ICC) among observers was 0.504 (moderate reliability).29
Results
Exposure to PEMF did not cause any obvious signs of suffering, such as poor water intake and body weight loss, nor changes in behaviour, such as self-injuries and aggression, nor impaired motility. As expected, our overall evaluations indicated that there was no impact on the behaviour and welfare of animals experiencing PEMF exposure over a maximum two-week period.
The outcomes of 14 days of PEMF treatment depend on the time of application
PEMF stimulation during the EAP of tendinopathy did not ameliorate the tissue structure. An overall tissue degeneration was evident and characterized by high cellularity, alteration in cell morphology, and a strong disruption of the fibre structure (Figure 3), without significant differences of the mean total histological score between untreated and treated groups (-PEMF = 12.4 (SD 0.5) vs +PEMF = 12.3 (SD 1.3)).
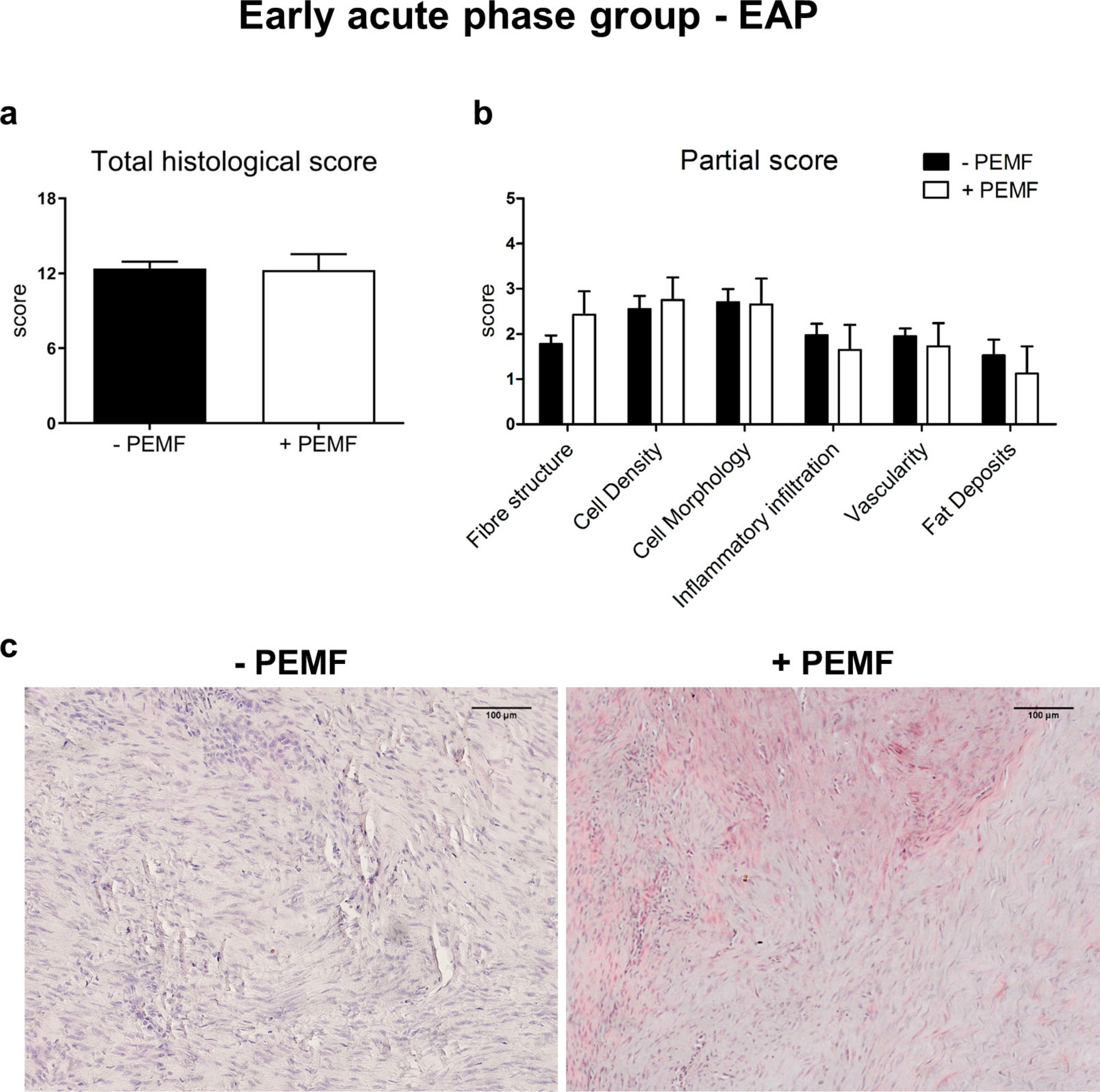
Fig. 3
a) Total and b) partial histological scores (0 = healthy tissue; 18 = completely damaged tissue) and c) representative images for haematoxylin and eosin staining of early acute phase (EAP) group (10×). Data are expressed as mean and SD. PEMF, pulsed electromagnetic field.
When PEMF stimulation was applied during the MAP of tendinopathy, a relevant improvement of the tissue structure was observed (Figure 4). Significant differences (all calculated using independent-samples t-test) were found between untreated and treated groups in the mean histological total score (-PEMF = 12.7 (SD 3.2) vs +PEMF = 6.6 (SD 2.7); p = 0.017) and in almost every category of the mean partial score. In particular, PEMF exposure significantly improved the restoration of physiological fibre structure and cell morphology, and the decrease of cell number and inflammatory infiltrates (fibre structure: -PEMF = 2.1 (SD 0.5) vs +PEMF = 0.9 (SD 0.7), p = 0.022; cell density: -PEMF = 2.5 (SD 0.4); vs +PEMF = 1.5 (SD 0.6), p = 0.021; cell morphology: -PEMF = 2.3 (SD 0.4) vs +PEMF = 1.5 (SD 0.5), p = 0.049; inflammatory infiltration: -PEMF = 1.9 (SD 0.7) vs +PEMF = 0.9 (SD 0.5), p = 0.046). Likewise, a significant decrease of vascularity was observed (-PEMF = 2.2 (SD 0.6) vs +PEMF = 0.7 (SD 0.4), p = 0.004).
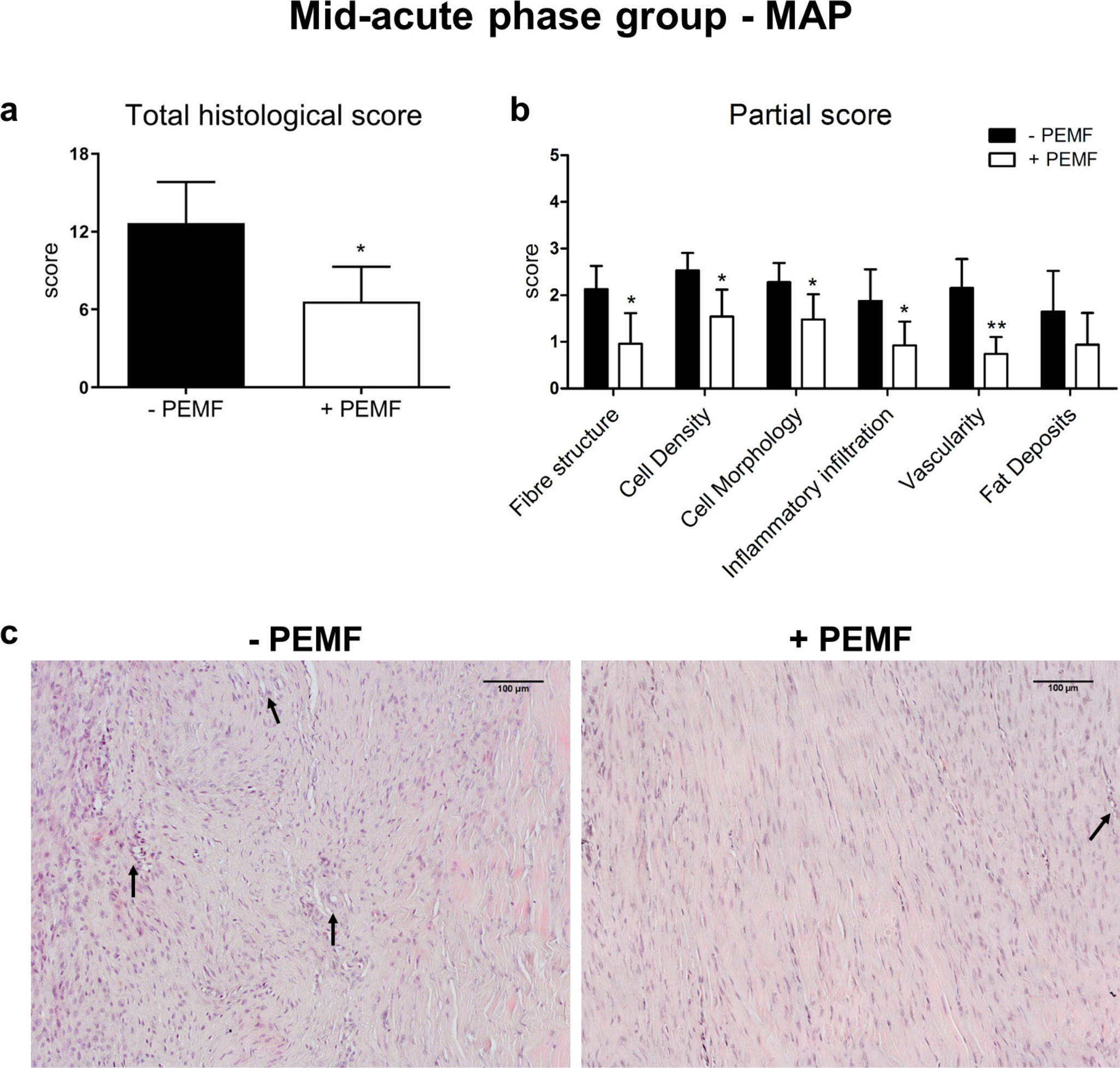
Fig. 4
a) Total and b) partial histological scores, and c) representative images for haematoxylin and eosin staining of mid-acute phase (MAP) group (arrows indicate small vessels) (10×). Data are expressed as mean and SD. PEMF, pulsed electromagnetic field; +PEMF versus -PEMF: *p < 0.05; **p < 0.01 (unpaired t test).
When animals were exposed to PEMFs during the LAP of tendinopathy, a general improvement of the tissue structure was observed; even though it was less evident with respect to the one observed when the treatment was applied during the MAP (Figure 5). Fibres were more organized, and cell density and appearance returned to a more physiological status when compared to untreated tendons. The mean total histological score revealed a significant difference between untreated and treated groups (-PEMF = 10.2 (SD 3.2) vs + PEMF = 6.4 (SD 1.7); p = 0.026 (unpaired t test)), whereas the mean partial histological score revealed a significant difference only in term of cell density and cell morphology (cell density: -PEMF = 2.4 (SD 0.5) vs +PEMF = 1.7 (SD 0.3), p = 0.014 (unpaired t test); cell morphology: -PEMF = 2.1 (SD 0.6) vs +PEMF = 1.6 (SD 0.5), p = 0.048 (unpaired t test)).
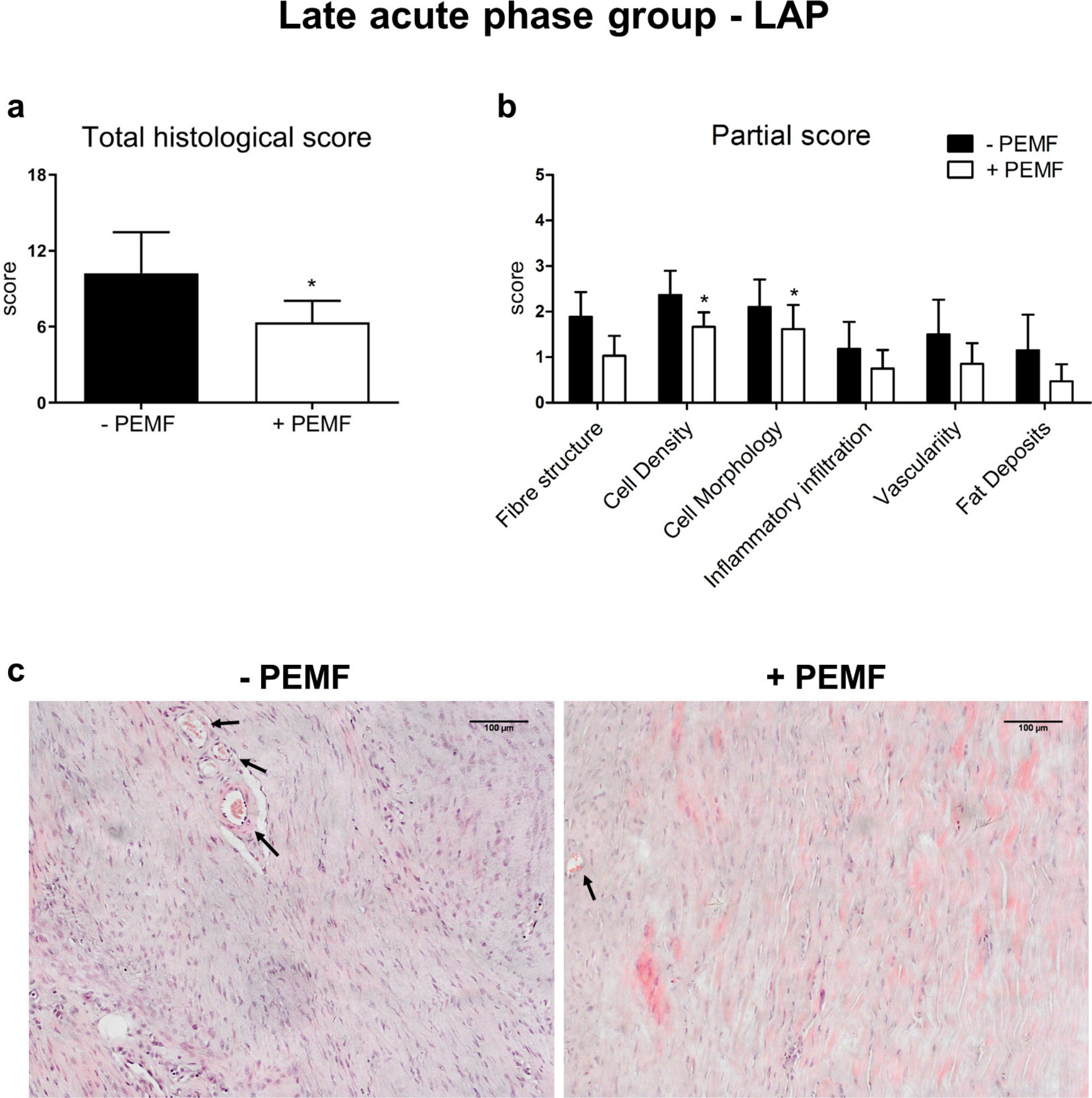
Fig. 5
a) Total and b) partial histological scores, and c) representative images for haematoxylin and eosin staining of late acute phase (LAP) group (arrows indicate vessels) (10×). Data are expressed as mean and SD. PEMF, pulsed electromagnetic field; +PEMF versus -PEMF: *p < 0.05 (unpaired t test).
Duration of PEMF treatment influences the outcomes
Application of PEMF stimulation for 7 or 14 days during MAP (7 days after collagenase injection) did not reveal significant differences at days 30 and 45, with total score values comparable to the untreated group. At day 21, significant differences were observed in the 14-day MAP group (mean +PEMF = 6.6 (SD 2.7); p = 0.017 (one-way ANOVA with Bonferroni's post hoc test)), and only a slight improvement in the 7-day MAP group (mean +PEMF = 8.4 (SD 3.4); p = 0.080 (one-way ANOVA with Bonferroni's post hoc test)) compared to the untreated group (mean -PEMF = 12.7 (SD 3.1)) (Figure 6a).
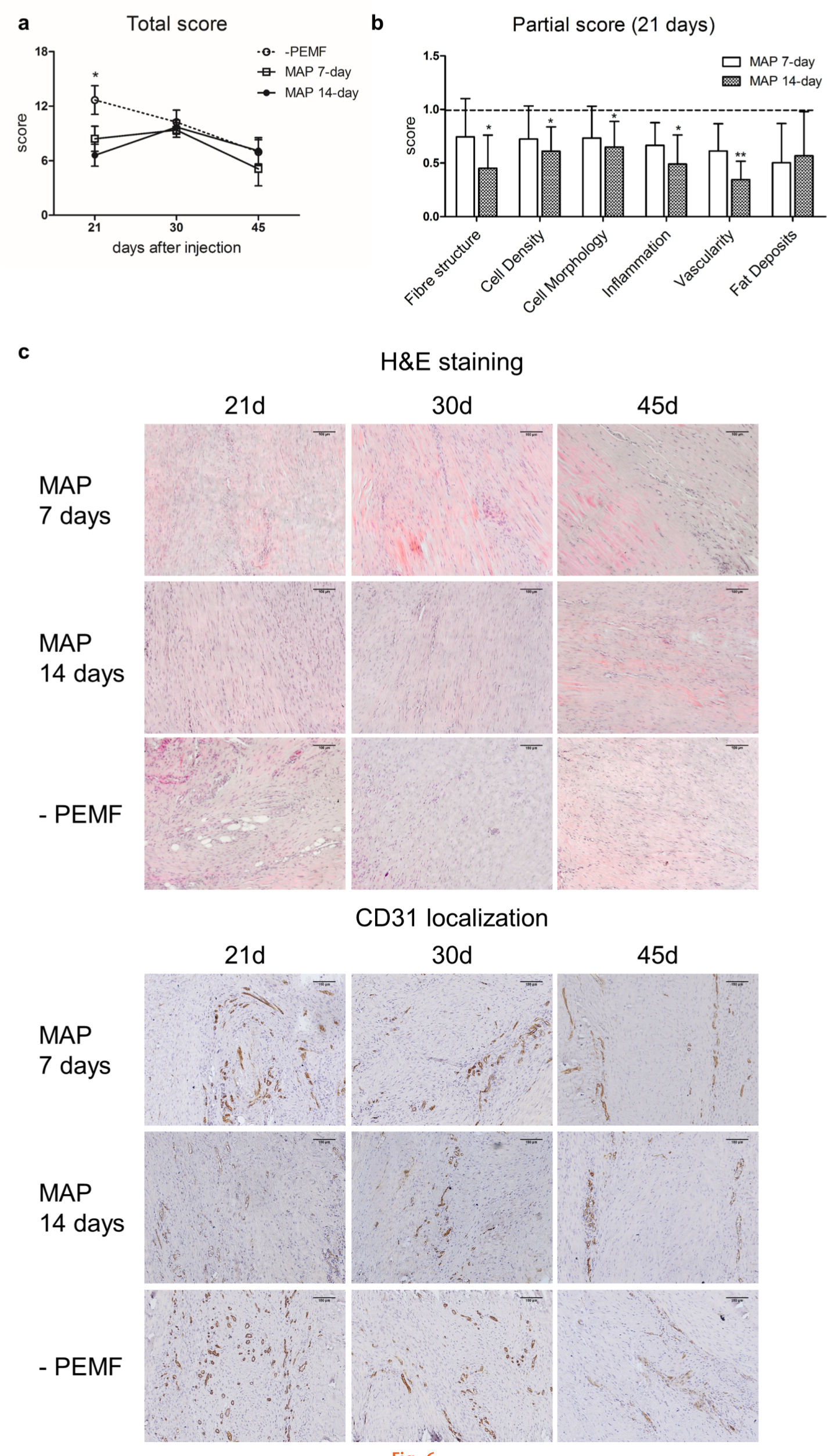
Fig. 6
a) Total score of haematoxylin and eosin (H&E)-stained mid-acute phase (MAP) group, stimulated with pulsed electromagnetic fields (PEMFs) (7 and 14 days) and untreated at 21, 30, and 45 days. Data are expressed as mean and SD; *p < 0.05 (one-way ANOVA with Bonferroni's post hoc test). b) Partial score expressed as mean fold change values with SD of MAP after 7 and 14 days of stimulation calculated on the untreated group (-PEMF) at 21 days. *p < 0.05; **p < 0.01 (one-way ANOVA with Bonferroni's post hoc test). c) Representative images of H&E- and d) cluster of differentiation 31 (CD31)-stained samples of MAP after 7 and 14 days of stimulation and untreated group at 21, 30, and 45 days (both 10× magnification).
At day 21, the partial score analysis of the 7 day MAP group revealed a decreasing trend of each parameter with respect to the untreated group (Figure 6b) while in the 14 day MAP group significant differences in the partial score were observed in almost each category.
Comparing 7 and 14 day MAP groups, no significant differences were observed in term of total and partial scores, suggesting that even a short PEMF stimulation is able to stimulate a positive tissue response, further increasing over time. However, the CD31 localization revealed the permanence of vascularity in the 7 day MAP group compared to the 14 day MAP group, even though lower than the untreated group (Figure 6c).
Discussion
This in vivo study investigated for the first time the effects of PEMF exposure (1.5 mT and 75 Hz) to support Achilles tendon healing, and also identified the best performing conditions in terms of time of intervention and duration of the treatment. PEMFs have already been shown to be a valuable option in clinical practice as a conservative treatment for bone and cartilage tissues.14,30 More recently, PEMFs have also been indicated as a promising treatment for tendons.6,18,19,22 Indeed, the potential of PEMF stimulation on tendon cells has already been evaluated and reported in the literature.18,19 However, few in vivo studies have been performed so far to confirm its effectiveness and to identify a condition of the treatment that can be translated to the clinical environment.20,22
In this study, the induction of the Achilles tendinopathy by a single injection of 3 mg/ml of type I collagenase was performed as a reliable model that allows observation of the progression of different pathological phases, as previously demonstrated.26
Overall, the results of this study revealed that the exposure to PEMFs exerted a positive effect on damaged tendons, enhancing the healing of the tissue, as confirmed by restored tendon structure and physiological cell morphology, accompanied by a decrease of the hypercellularity and vascularity.
An initial hypothesis suggests that the PEMF stimulation provokes a cellular and molecular response intervening in the resolution of the inflammatory response.6 Previous in vitro studies demonstrated that the daily exposure of tendon cells to PEMFs increases the release of anti-inflammatory cytokines.18 However, a wide investigation of the transcriptome responses of IL-1β-primed rat Achilles tendon cells to PEMF treatment revealed that PEMFs do not exert a significant impact on metalloprotease expression,31 and thus on the degradation of collagen in the very early inflammatory phase. These findings are in accordance with what we observed in this study concerning PEMF treatment during EAP. The absence of significant improvements of tissue appearance, such as structure and cell morphology, and the persistence of hypercellularity, suggest that the treatment was not sufficiently effective to counteract the tissue response in the very first phases of an acute injury.
When tendons were exposed to PEMFs at later phases of tendinopathy, significant differences between the treated and untreated groups were observed. The most evident histological improvements were observed when the treatment was started during MAP. In this experimental setting, daily PEMF exposure significantly enhanced the recovery of a more organized fibre structure, as well as decrease of cellularity and vascularity. As previously observed in a rat model of Achilles tendinopathy, seven days after injury, a few occasional inflammatory cells were found in the tissue matrix; whereas fibroblasts with rounded nuclei were still present.32 These observations further confirm the end of the hyper-acute inflammatory response of the tissue in favour of a milder inflammatory environment and the beginning of the reparation phase, as observed in the present study.
The tissue was still macroscopically abnormal 15 days after the collagenase injection, with marked alterations of the fibre structure32 and increased cell density, probably caused by the activation of resident cells. These observations suggest that the tissue is entering the proliferative/remodelling phase of the healing process.33 While PEMFs applied 15 days after the induction of tendinopathy (LAP group) provided satisfactory outcomes comparable to those obtained from the MAP group, the anticipation of the treatment before the remodelling phase may prevent the development of fibrosis typical of tissues undergoing a reparative process. Nevertheless, it should be considered that, in this model, a spontaneous healing was observed starting around day 30 after collagenase injection,26 thus possibly masking the effect of PEMF application in LAP, as the evaluations were carried out in this timeframe.
For all these reasons, the investigation regarding the effects of PEMF duration was conducted during MAP. The short PEMF exposure (7 days) demonstrated similar effects at the tissue level with respect to the 14-day-long treatment, suggesting that a satisfactory outcome can be obtained even with a shorter exposure time. However, the improvements obtained in the 14 days MAP group were more marked, thus, in the view of future clinical applications, this outcome should be taken into account to carefully balance efficacy and feasibility.
Limitations of this study include the limited number of animals for each experimental group, as well as the lack of biomechanical analysis. However, in keeping with the Three R's principle, we opted to focus on the evaluation of PEMF effectiveness in different conditions which represent the novelty and therefore one of the main strengths of the study. Together with the previous findings on the mechanism of action of PEMFs on tendon cells,18,19 the results of the present study are crucial to designing future clinical trials.
In conclusion, our findings support the hypothesis that PEMFs enhance tendon tissue healing. In particular, PEMFs exerted their best effectiveness when applied during the mid-acute phase of tendinopathy for 14 days. Considering the compelling need for new conservative approaches to treat tendon disorders that provide high safety profile and evidences of effectiveness, the present study supports further investigations for the implementation of PEMF-based treatments for tendinopathy.
References
1. Li HY , Hua YH . Achilles tendinopathy: current concepts about the basic science and clinical treatments . Biomed Res Int . 2016 ; 2016 : 6492597 . Crossref PubMed Google Scholar
2. Millar NL , Hueber AJ , Reilly JH , et al. Inflammation is present in early human tendinopathy . Am J Sports Med . 2010 ; 38 ( 10 ): 2085 – 2091 . Crossref PubMed Google Scholar
3. Ueda Y , Inui A , Mifune Y , et al. The effects of high glucose condition on rat tenocytes in vitro and rat Achilles tendon in vivo . Bone Joint Res . 2018 ; 7 ( 5 ): 362 – 372 . Crossref PubMed Google Scholar
4. Benca E , Willegger M , Wenzel F , et al. Biomechanical evaluation of two methods of fixation of a flexor hallucis longus tendon graft . Bone Joint J . 2018 ; 100-B ( 9 ): 1175 – 1181 . Crossref PubMed Google Scholar
5. Rosso F , Bonasia DE , Marmotti A , Cottino U , Rossi R . Mechanical Stimulation (Pulsed Electromagnetic Fields "PEMF" and Extracorporeal Shock Wave Therapy "ESWT") and Tendon Regeneration: A Possible Alternative . Front Aging Neurosci . 2015 ; 7 : 211 . Crossref PubMed Google Scholar
6. Viganò M , Sansone V , d'Agostino MC , Romeo P , Perucca Orfei C , de Girolamo L . Mesenchymal stem cells as therapeutic target of biophysical stimulation for the treatment of musculoskeletal disorders . J Orthop Surg Res . 2016 ; 11 ( 1 ): 163 . Crossref PubMed Google Scholar
7. Galli C , Pedrazzi G , Guizzardi S . The cellular effects of pulsed electromagnetic fields on osteoblasts: a review . Bioelectromagnetics . 2019 ; 40 ( 4 ): 211 – 233 . Crossref PubMed Google Scholar
8. Wang P , Tang C , Wu J , et al. Pulsed electromagnetic fields regulate osteocyte apoptosis, RANKL/OPG expression, and its control of osteoclastogenesis depending on the presence of primary cilia . J Cell Physiol . 2018 ; 234 ( 7 ): 10588 – 10601 . Crossref PubMed Google Scholar
9. Iwasa K , Reddi AH . Pulsed electromagnetic fields and tissue engineering of the joints . Tissue Eng Part B Rev . 2018 ; 24 ( 2 ): 144 – 154 . Crossref PubMed Google Scholar
10. Ongaro A , Varani K , Masieri FF , et al. Electromagnetic Fields (EMFs) and Adenosine Receptors Modulate Prostaglandin E(2) and Cytokine Release in Human Osteoarthritic Synovial Fibroblasts . J Cell Physiol . 2012 ; 227 ( 6 ): 2461 – 2469 . Google Scholar
11. Veronesi F , Cadossi M , Giavaresi G , et al. Pulsed electromagnetic fields combined with a collagenous scaffold and bone marrow concentrate enhance osteochondral regeneration: an in vivo study . BMC Musculoskelet Disord . 2015 ; 16 ( 1 ): 233 . Crossref PubMed Google Scholar
12. Miller SL , Coughlin DG , Waldorff EI , Ryaby JT , Lotz JC . Pulsed electromagnetic field (PEMF) treatment reduces expression of genes associated with disc degeneration in human intervertebral disc cells . Spine J . 2016 ; 16 ( 6 ): 770 – 776 . Crossref PubMed Google Scholar
13. Wang C , Liu Y , Wang Y , et al. Low-frequency pulsed electromagnetic field promotes functional recovery, reduces inflammation and oxidative stress, and enhances HSP70 expression following spinal cord injury . Mol Med Rep . 2019 ; 19 ( 3 ): 1687 – 1693 . Google Scholar
14. Massari L , Benazzo F , Falez F , et al. Biophysical stimulation of bone and cartilage: state of the art and future perspectives . Int Orthop . 2019 ; 43 ( 3 ): 539 – 551 . Crossref PubMed Google Scholar
15. Fini M , Giavaresi G , Torricelli P , et al. Pulsed electromagnetic fields reduce knee osteoarthritic lesion progression in the aged Dunkin Hartley guinea pig . J Orthop Res . 2005 ; 23 ( 4 ): 899 – 908 . Crossref PubMed Google Scholar
16. Fini M , Cadossi R , Canè V , et al. The effect of pulsed electromagnetic fields on the osteointegration of hydroxyapatite implants in cancellous bone: a morphologic and microstructural in vivo study . J Orthop Res . 2002 ; 20 ( 4 ): 756 – 763 . Crossref PubMed Google Scholar
17. Coric D , Bullard DE , Patel VV , et al. Pulsed electromagnetic field stimulation may improve fusion rates in cervical arthrodesis in high-risk populations . Bone Joint Res . 2018 ; 7 ( 2 ): 124 – 130 . Crossref PubMed Google Scholar
18. de Girolamo L , Stanco D , Galliera E , et al. Low frequency pulsed electromagnetic field affects proliferation, tissue-specific gene expression, and cytokines release of human tendon cells . Cell Biochem Biophys . 2013 ; 66 ( 3 ): 697 – 708 . Crossref PubMed Google Scholar
19. de Girolamo L , Viganò M , Galliera E , et al. In vitro functional response of human tendon cells to different dosages of low-frequency pulsed electromagnetic field . Knee Surg Sports Traumatol Arthrosc . 2015 ; 23 ( 11 ): 3443 – 3453 . Crossref PubMed Google Scholar
20. Tucker JJ , Cirone JM , Morris TR , et al. Pulsed electromagnetic field therapy improves tendon-to-bone healing in a rat rotator cuff repair model . J Orthop Res . 2017 ; 35 ( 4 ): 902 – 909 . Crossref PubMed Google Scholar
21. Strauch B , Patel MK , Rosen DJ , Mahadevia S , Brindzei N , Pilla AA . Pulsed magnetic field therapy increases tensile strength in a rat Achilles' tendon repair model . J Hand Surg Am . 2006 ; 31 ( 7 ): 1131 – 1135 . Crossref PubMed Google Scholar
22. Huegel J , Choi DS , Nuss CA , et al. Effects of pulsed electromagnetic field therapy at different frequencies and durations on rotator cuff tendon-to-bone healing in a rat model . J Shoulder Elbow Surg . 2018 ; 27 ( 3 ): 553 – 560 . Crossref PubMed Google Scholar
23. Liu M , Lee C , Laron D , et al. Role of pulsed electromagnetic fields (PEMF) on tenocytes and myoblasts-potential application for treating rotator cuff tears . J Orthop Res . 2017 ; 35 ( 5 ): 956 – 964 . Crossref PubMed Google Scholar
24. Binder A , Parr G , Hazleman B , Fitton-Jackson S . Pulsed electromagnetic field therapy of persistent rotator cuff tendinitis. A double-blind controlled assessment . Lancet . 1984 ; 1 ( 8379 ): 695 – 698 . Crossref PubMed Google Scholar
25. Osti L , Buono AD , Maffulli N . Pulsed electromagnetic fields after rotator cuff repair: a randomized, controlled study . Orthopedics . 2015 ; 38 ( 3 ): e223 – e228 . Crossref PubMed Google Scholar
26. Perucca Orfei C , Lovati AB , Viganò M , et al. Dose-Related and time-dependent development of collagenase-induced tendinopathy in rats . PLoS One . 2016 ; 11 ( 8 ): e0161590 . Crossref PubMed Google Scholar
27. Russell WMS , Burch RL . The Principles of Humane Experimental Technique . 1st ed . London, UK : Methuen , 1959 . Google Scholar
28. Faul F , Erdfelder E , Lang AG , Buchner A . G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences . Behav Res Methods . 2007 ; 39 ( 2 ): 175 – 191 . Crossref PubMed Google Scholar
29. Fleiss JL , Levin B , Paik MC . Statistical Methods for Rates and Proportions . 3rd ed . New York, NY : John Wiley , 1981 . Google Scholar
30. Cadossi M , Frizziero A , Vulpiani MC , et al. Clinical Application of Biophysical Stimulation on Bone in Europe . In : Rosch PJ , Paul J . Bioelectromagnetic and Subtle Energy Medicine . 24 . Second Edition : CRC Press , 2014 : 267 – 278 . Google Scholar
31. Gehwolf R , Schwemberger B , Jessen M , et al. Global responses of Il-1β-Primed 3D tendon constructs to treatment with pulsed electromagnetic fields . Cells . 2019 ; 8 ( 5 ): 399 . Crossref PubMed Google Scholar
32. Lee EW , Maffulli N , Li CK , Chan KM . Pulsed magnetic and electromagnetic fields in experimental Achilles tendonitis in the rat: a prospective randomized study . Arch Phys Med Rehabil . 1997 ; 78 ( 4 ): 399 – 404 . Crossref PubMed Google Scholar
33. Schneider M , Angele P , Järvinen TAH , Docheva D . Rescue plan for Achilles: therapeutics steering the fate and functions of stem cells in tendon wound healing . Adv Drug Deliv Rev . 2018 ; 129 : 352 – 375 . Crossref PubMed Google Scholar
Author contributions
C. Perucca Orfei: Performed the in vivo study, Collected and analyzed the data, Wrote and reviewed the manuscript.
A. B. Lovati: Designed and performed the in vivo study, Collected the tissue samples, Drafted and reviewed the manuscript.
G. Lugano: Performed the in vivo study, Collected and analyzed the data, Reviewed the manuscript.
M. Viganò: Collected and analyzed the data, Reviewed the manuscript.
M. Bottagisio: Performed the in vivo study, Reviewed the manuscript.
D. D’Arrigo: Performed the in vivo study, Reviewed the manuscript.
V. Sansone: Analyzed the data, Reviewed the manuscript.
S. Setti: Performed the in vivo study, Reviewed the manuscript.
L. de Girolamo: Designed the study, Collected the tissue samples, Drafted and reviewed the manuscript.
Funding statement
This work was supported by IGEA SpA and the Italian Ministry of Health, "Ricerca Corrente L1018”.
ICMJE COI statement
IGEA SpA provided a research grant and free equipment to the IRCCS Istituto Ortopedico Galeazzi to conduct the study.
S. Setti is an employee of IGEA SpA Clinical Biophysics.
A. B. Lovati reports an ERA-NET-ENM3 grant from the European Union's Horizon 2020 research and innovation programme, and a patent (102020000004081), both unrelated to this study.
L. de Girolamo reports consultancy payments from Lipogems SpA, unrelated to this study.
Acknowledgements
The authors thank Alessia Di Giancamillo for her initial contribution in setting up the histological sample preparation.
Ethical review statement
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research. The study was approved by Mario Negri Institute for Pharmacological Research (IRFMN) Animal Care and Use Committee (IACUC) and by the Italian Ministry of Health (Permit N. 363/2015-PR). The in vivo analyses of this work were performed at the animal facility of the IRCCS Istituto di Ricerche Farmacologiche Mario Negri, Milan, Italy, and the ex vivo analyses at the IRCCS Istituto Ortopedico Galeazzi, Milan, Italy.
© 2020 Author(s) et al. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (CC BY-NC-ND 4.0) licence, which permits the copying and redistribution of the work only, and provided the original author and source are credited. See https://creativecommons.org/licenses/by-nc-nd/4.0/.









